Abstract
Background
Nasal masks and nasal prongs are used as interfaces for providing continuous positive airway pressure (CPAP) for preterm infants with or at risk of respiratory distress, either as primary support after birth or as ongoing support after endotracheal extubation from mechanical ventilation. It is unclear which type of interface is associated with lower rates of CPAP treatment failure, nasal trauma, or mortality and other morbidity.
Objectives
To assess the benefits and harms of nasal masks versus nasal prongs for reducing CPAP treatment failure, nasal trauma, or mortality and other morbidity in newborn preterm infants with or at risk of respiratory distress.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was October 2021.
Selection criteria
We included randomised controlled trials comparing masks versus prongs as interfaces for delivery of nasal CPAP in newborn preterm infants (less than 37 weeks' gestation) with or at risk of respiratory distress.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. treatment failure, 2. all‐cause mortality, and 3. neurodevelopmental impairment. Our secondary outcomes were 4. pneumothorax, 5. moderate–severe nasal trauma, 6. bronchopulmonary dysplasia, 7. duration of CPAP use, 8. duration of oxygen supplementation, 9. duration of hospitalisation, 10. patent ductus arteriosus receiving medical or surgical treatment, 11. necrotising enterocolitis, 12. severe intraventricular haemorrhage, and 13. severe retinopathy of prematurity. We used the GRADE approach to assess the certainty of the evidence.
Main results
We included 12 trials with 1604 infants. All trials were small (median number of participants 118). The trials occurred after 2001 in care facilities internationally, predominantly in India (eight trials). Most participants were preterm infants of 26 to 34 weeks' gestation who received nasal CPAP as the primary form of respiratory support after birth. The studied interfaces included commonly used commercially available masks and prongs. Lack of measures to blind caregivers or investigators was a potential source of performance and detection bias in all the trials.
Meta‐analyses suggested that use of masks compared with prongs may reduce CPAP treatment failure (risk ratio (RR) 0.72, 95% confidence interval (CI) 0.58 to 0.90; 8 trials, 919 infants; low certainty). The type of interface may not affect mortality prior to hospital discharge (RR 0.83, 95% CI 0.56 to 1.22; 7 trials, 814 infants; low certainty). There are no data on neurodevelopmental impairment. Meta‐analyses suggest that the choice of interface may result in little or no difference in the risk of pneumothorax (RR 0.93, 95% CI 0.45 to 1.93; 5 trials, 625 infants; low certainty). Use of masks rather than prongs may reduce the risk of moderate–severe nasal injury (RR 0.55, 95% CI 0.44 to 0.71; 10 trials, 1058 infants; low certainty). The evidence is very uncertain about the effect on bronchopulmonary dysplasia (RR 0.69, 95% CI 0.46 to 1.03; 7 trials, 843 infants; very low certainty).
Authors' conclusions
The available trial data provide low‐certainty evidence that use of masks compared with prongs as the nasal CPAP interface may reduce treatment failure and nasal injury, and may have little or no effect on mortality or the risk of pneumothorax in newborn preterm infants with or at risk of respiratory distress. The effect on bronchopulmonary dysplasia is very uncertain. Large, high‐quality trials would be needed to provide evidence of sufficient validity and applicability to inform policy and practice.
Keywords: Humans; Infant, Newborn; Bronchopulmonary Dysplasia; Bronchopulmonary Dysplasia/etiology; Bronchopulmonary Dysplasia/prevention & control; Continuous Positive Airway Pressure; Continuous Positive Airway Pressure/adverse effects; Continuous Positive Airway Pressure/methods; Infant, Premature; Masks; Masks/adverse effects; Pneumothorax; Pneumothorax/etiology; Respiratory Distress Syndrome
Plain language summary
Nasal masks versus nasal prongs for continuous positive airway pressure in preterm infants
Key messages
Masks rather than nasal prongs may reduce the risk of continuous positive airway pressure (CPAP) treatment failure and nasal injury but may have little or no impact on the risk of death or other complications associated with premature birth.
What is continuous positive airway pressure treatment?
Nasal CPAP is a form of breathing support that is less invasive than mechanical ventilation (where a breathing tube is placed into a baby's windpipe). Nasal CPAP delivers oxygen to a baby through prongs into the nose or a soft face mask that covers the nose. It can be used after weaning a baby from ventilation (extubation), or to help babies who need help for lung problems, but do not need ventilation.
What did we want to find out?
We assessed whether there was evidence to favour masks versus prongs for reducing the rates of CPAP treatment failure (that is, the baby's condition worsening or the baby needing mechanical ventilation), and reducing complications and harms.
What did we do?
We searched medical databases for clinical trials up to October 2021.
What did we find?
We included 12 trials that compared use of masks versus prongs for CPAP in 1604 babies born more than three weeks before their estimated due date. The trials were mostly small, and had design flaws that might bias their findings.
Key results
Analyses showed that using masks rather than prongs may reduce the risk of CPAP treatment failure and nasal injury but may have little or no impact on the risk of death or other complications associated with premature birth. None of the studies assessed the effect on disability or developmental outcomes.
What are the limitations of the evidence?
The quality of the evidence for the effects of masks versus prongs for CPAP in preterm babies is low or very low because of concerns that the methods used in the included trials may have introduced biases and there were limited amounts of data from the trials. Consequently, our confidence in the results is limited, and the true effects may be substantially different from what we found.
Summary of findings
Summary of findings 1. Mask versus prongs for nasal continuous positive airway pressure for preterm infants.
| Masks compared to prongs for nasal continuous positive airway pressure (CPAP) in preterm infants | ||||||
| Patient or population: preterm infants receiving nasal CPAP Setting: neonatal care facilities internationally (India, Malaysia, Turkey, Ireland, USA) Intervention: nasal mask CPAP Comparison: nasal prongs CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Assessment of heterogeneity | |
| Risk with prongs | Risk with mask | |||||
| Treatment failure | Study population | RR 0.72 (0.58 to 0.90) | 919 (8 studies) | ⊕⊕⊝⊝ Lowa,b | Heterogeneity: I² = 25% | |
| 295 per 1000 | 212 per 1000 (171 to 266) | |||||
| All‐cause mortality | Study population | RR 0.83 (0.56 to 1.22) | 814 (7 studies) | ⊕⊕⊝⊝ Lowa,b | Heterogeneity: I² = 0% | |
| 120 per 1000 | 100 per 1000 (67 to 147) | |||||
| Neurodevelopmental impairment | Not assessed in any included trials | |||||
| Pneumothorax | Study population | RR 0.93 (0.45 to 1.93) | 625 (5 studies) | ⊕⊕⊝⊝ Lowa,b | Heterogeneity: I² = 0% | |
| 45 per 1000 | 42 per 1000 (20 to 87) | |||||
| Moderate–severe nasal injury | Study population | RR 0.55 (0.44 to 0.71) | 1058 (10 studies) | ⊕⊕⊝⊝ Lowa,c | Heterogeneity: I² = 73% Subgroup difference by:
|
|
| 248 per 1000 | 136 per 1000 (109 to 176) | |||||
| Bronchopulmonary dysplasia | Study population | RR 0.69 (0.46 to 1.03) | 843 (7 studies) | ⊕⊝⊝⊝ Very lowa,b,c | Heterogeneity: I² = 51% Subgroup difference by:
|
|
| 120 per 1000 | 83 per 1000 (55 to 124) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPAP: continuous positive airway pressure; df: degrees of freedom; HIC: high‐income country; LMIC: low‐ or middle‐income country; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded one level for serious study design limitations (high risk of bias due to lack of blinding of clinicians and outcome assessment) in all trials. b Downgraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial risk of harm or of benefit). c Downgraded one level for serious inconsistency (moderate or high heterogeneity).
Background
Nasal continuous positive airway pressure (CPAP) is a recommended and widely used type of non‐invasive respiratory support for spontaneously breathing newborn preterm infants with or at risk of respiratory distress (Beltempo 2018; Lissauer 2017; Sweet 2019). The most common interfaces for CPAP are nasal masks and short binasal prongs. These interfaces may differ in how well infants tolerate them, how efficient the nasal seal is, the degree of resistance to air flow, and consequently the effectiveness of CPAP delivery (Green 2019).
This review focused on examining whether using masks versus prongs affects the risk of CPAP treatment failure and associated mortality and morbidity in preterm infants. Other Cochrane Reviews assessed the effects of different CPAP devices and levels in preterm infants (Bamat 2021; De Paoli 2008), and the effects of newer forms of non‐invasive ventilation adapted from CPAP including bilevel positive airway pressure and non‐invasive positive pressure ventilation (Lemyre 2016; Lemyre 2017). The use of short, thin nasal cannulae as the interface for delivering heated and humidified air or supplemental oxygen at high flow rates to generate a distending pressure is also the subject of a separate Cochrane Review (Wilkinson 2016).
Description of the condition
Respiratory distress syndrome (RDS) is a major cause of morbidity and mortality in preterm infants (Fraser 2004). Primarily, RDS is caused by deficiency of surfactant, a complex mixture of phospholipids and proteins that reduces alveolar surface tension and maintains alveolar stability. As most surfactant is produced after about 32 weeks' gestation, very preterm infants born before then are at high risk of developing RDS. The incidence and severity of RDS increases with decreasing gestational age at birth, occurring in more than 80% of extremely preterm infants born before 28 weeks' gestation (Stoll 2015). If untreated, the structurally immature and surfactant‐deficient lungs have a tendency to segmental collapse and atelectasis, ventilation–perfusion mismatch, and pulmonary hypertension that worsens hypoxia and hypercarbia. Consequently, infants with severe RDS can become fatigued and apnoeic and require supplemental oxygen and assisted ventilation (Sweet 2019). Mechanical ventilation via an endotracheal tube, especially if associated with high airway pressures and high concentrations of oxygen, may cause iatrogenic injuries that contribute to the pathogenesis of bronchopulmonary dysplasia (Laughon 2011). Preterm infants who experience severe RDS are at high risk of other neonatal morbidities including pneumothorax, persistent patent ductus arteriosus, severe intraventricular haemorrhage, retinopathy of prematurity, and necrotising enterocolitis, that are associated with a prolonged need for respiratory support and hospitalisation, and with mortality and neurodevelopmental impairment (Horbar 2012).
Two major advances in perinatal care – antenatal corticosteroids to promote endogenous surfactant production and exogenous surfactant replacement – have improved outcomes for preterm infants, particularly very preterm infants (Curstedt 2015; McGoldrick 2020). Following the widespread adoption of these interventions in well‐resourced settings over the past several decades, the principal form of respiratory support for preterm infants with or at risk of RDS has moved from mechanical ventilation via an endotracheal tube to non‐invasive ventilation, most commonly via nasal CPAP devices (Soll 2019; Stoll 2015). Nasal CPAP maintains low pressure distension of the lungs when infants are breathing spontaneously and thereby increases functional residual capacity and improves oxygenation (Wright 2016). Other effects include conserving surfactant and reducing alveolar fluid, dilating the larynx to reduce supraglottic airway resistance, synchronising respiratory thoraco‐abdominal movements, and enhancing the Hering‐Breuer inflation reflex following airway occlusion (Gaon 1999; Krouskop 1975; Locke 1991; Martin 1977; Miller 1985; Richardson 1978; Yu 1977). Evidence exists that use of nasal CPAP (compared to spontaneous breathing) reduces the risk of respiratory failure, receipt of mechanical ventilation, and mortality in preterm infants with respiratory distress (Ho 2020).
Treatment failure
Nasal CPAP and other modalities of non‐invasive respiratory support aim to prevent the iatrogenic problems associated with mechanical ventilation via an endotracheal tube and minimise ventilator‐induced lung injury and other complications (Glaser 2021). Evidence from randomised controlled trials suggests that using nasal CPAP (compared to mechanical ventilation) for primary respiratory support reduces the risk of bronchopulmonary dysplasia in preterm infants, and reduces the need for endotracheal re‐intubation in preterm infants following a period of mechanical ventilation (Davis 2003; Subramaniam 2016). However, the effect size of these benefits is limited due to the high rate of CPAP 'treatment failure' (features such as increasing work of breathing or oxygen requirement, or frequent apnoeic pauses that meet criteria for endotracheal intubation and mechanical ventilation). Almost half of all very preterm infants treated with nasal CPAP require endotracheal intubation and mechanical ventilation during in the first week after birth (Dargaville 2016). Treatment failure occurs more commonly in extremely preterm infants, and prolongs the need for respiratory support and supplemental oxygen, and is associated with an increased risk of death or bronchopulmonary dysplasia (Dargaville 2013).
Several factors are considered to affect the risk of treatment failure and associated complications in preterm infants including the CPAP pressure source (bubble CPAP versus ventilator or Infant Flow Driver) and pressure levels ('low' (5 cmH2O or less) versus 'moderate–high' (greater than 5 cmH2O)). These are considered in separate Cochrane Reviews (Bamat 2021; De Paoli 2021). This review focused on assessing the trial evidence for the effect of different nasal interfaces on treatment failure, nasal trauma, or mortality and other morbidity in newborn preterm infants with or at risk of respiratory distress.
Description of the intervention
Nasal masks and nasal prongs are the recommended and most commonly used interfaces for providing CPAP for preterm infants with or at risk of RDS (Sweet 2019).
Nasal prongs
Short binasal prongs designed to fit into the infant's nostrils with minimal leakage have lower resistance than nasopharyngeal prongs and are more effective than single nasal or nasopharyngeal prongs in reducing treatment failure and the need for re‐intubation after a period of mechanical ventilation (De Paoli 2002; De Paoli 2008). Several types of binasal prong devices are available commercially including Argyle prongs, Hudson prongs, and INCA prongs (Gupta 2016). Other short binasal prong systems, such as those for Infant Flow Driver devices, have been engineered to allow sufficient flow to the infant on inspiration while minimising expiratory resistance and may reduce work of breathing slightly compared with conventional devices (Pandit 1999). Modified standard oxygen cannulae (usually as part of a bubble CPAP system) are an alternative nasal interface used in limited‐resource settings in some low‐ or middle‐income countries (Lissauer 2017).
Concern exists that pressure generation can be variable and ventilation suboptimal if the seal is ineffective or the prongs are poorly tolerated by infants (Morley 2004). Furthermore, binasal prongs have been associated with nasal trauma including bleeding, ulceration or erosion, excoriation or necrosis, septal injury, and distortion of the nares (Robertson 1996; Sreenan 2001). Moderate or severe nasal trauma has been reported as occurring in more than one‐third of very preterm infants receiving CPAP with nasal prongs (Imbulana 2018).
Nasal masks
Nasal masks were commonly used interfaces for CPAP in preterm infants during the 1970s, but these lost popularity (and were superseded by nasal prongs in most settings) because of the difficulty in maintaining an adequate seal and a tendency to cause nasal airway obstruction (Chernick 1973; Cox 1974; Kattwinkel 1973). However, concern about the risk of nasal trauma associated with nasal prongs has led to the development of 'new‐generation', more anatomically appropriate, soft silicone‐ or gel‐based nasal masks. These masks are available with several CPAP systems (including Fisher‐Paykel, Drager BabyFlow, and Infant Flow Driver) and are promoted as being able to provide a comfortable and stable nasal seal, and a less traumatic fit than previously available masks, so improving CPAP delivery while reducing the risk of nasal injury (Green 2019).
How the intervention might work
Interfaces may differ in how well infants tolerate them, how efficient the nasal seal is, the degree of resistance to air flow, and consequently the effectiveness of CPAP delivery. There is considerable variation in the measured resistance of available CPAP interfaces at gas flows commonly applied in neonatal care. The degree of leak around the nasal interface and the resistance of the interface may contribute substantially to pressure loss (De Paoli 2005). This varies between interfaces with masks having lower intrinsic resistance than short binasal prongs (Green 2019). Interfaces with high resistance may lower the delivered airway pressure (compared to the set circuit pressure) so reducing CPAP effectiveness, and increasing the risk of treatment failure and associated complications. A related concern is interface comfort, fit, and the risk of nasal trauma. If nasal masks are more comfortable and less likely to cause injury than nasal prongs, then potentially this may increase tolerance and adherence, improve CPAP delivery, and reduce treatment failure (Imbulana 2018).
Why it is important to do this review
International policy statements that exist to guide practice do not make unconditional recommendations about which nasal interface to use in providing CPAP for preterm infants (Committee on Fetus and Newborn 2014). Given the possibility and plausibility that the choice of nasal interface for delivering CPAP may affect the risk of treatment failure, nasal trauma, or mortality and other morbidity in newborn preterm infants with or at risk of respiratory distress, appraising and synthesising the trial evidence could inform practice, policy, and research.
Objectives
To assess the benefits and harms of nasal masks versus nasal prongs for reducing CPAP treatment failure, nasal trauma, or mortality and other morbidity in newborn preterm infants with or at risk of respiratory distress.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (including cluster‐randomised controlled trials).
Cross‐over studies were not eligible for inclusion.
Types of participants
Preterm infants (less than 37 weeks' gestation) supported with nasal CPAP, either as primary treatment for respiratory distress after birth, or following a period of mechanical ventilation (postextubation).
Types of interventions
A previous version of this review concluded that short binasal prongs are more effective than nasopharyngeal prong(s) in reducing the rate of treatment failure (De Paoli 2008). Consequently, this updated review focused on the comparison of nasal masks (e.g. Fisher‐Paykel, Infant Flow Driver devices) versus nasal prongs (e.g. Hudson prongs, Argyle prongs, Infant Flow Driver devices, INCA prongs, or other prong interfaces such as modified nasal cannulae or RAM cannulae).
High‐flow nasal cannulae is not a CPAP system that has an intrinsic pressure monitoring or pressure relief/blow‐off system and is considered in another Cochrane Review (Wilkinson 2016).
Types of outcome measures
We focused on assessing effects on infant‐ and family‐important outcomes, principally CPAP treatment failure and neonatal morbidities that plausibly affect rates of mortality or neurodevelopmental impairment. We did not include surrogate outcomes such as physiological measures of respiratory function.
Primary outcomes
Treatment failure indicated by recurrent apnoea, hypoxia, hypercarbia, increasing oxygen requirement, or the receipt of mechanical ventilation within 72 hours after initiation of nasal CPAP
All‐cause mortality prior to hospital discharge
Neurodevelopmental impairment assessed by a validated test after 12 months' post‐term: neurological evaluations, developmental scores, and classifications of disability, including cerebral palsy and auditory and visual impairment
Secondary outcomes
Pneumothorax (including pneumomediastinum, pneumopericardium) before hospital discharge
Moderate–severe nasal injury defined by trial investigators including septal injury, septal necrosis, and scarring before hospital discharge
Bronchopulmonary dysplasia: oxygen or respiratory support requirement at 36 weeks' postmenstrual age (Ehrenkranz 2005; Jobe 2001)
Duration of CPAP use (days)
Duration of oxygen supplementation (days)
Duration of hospitalisation (days)
Patent ductus arteriosus receiving medical or surgical treatment
Necrotising enterocolitis (Bell stage 2 or greater) (Bell 1978; Walsh 2004)
Severe intraventricular haemorrhage (Papile 1978)
Severe retinopathy of prematurity (ICROP 2005)
Search methods for identification of studies
An Information Specialist developed search strategies in consultation with the authors.
Electronic searches
We searched the following databases in October 2021 with language or date restrictions:
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 10) in the Cochrane Library (Wiley);
MEDLINE Ovid (1946 to 25 October 2021);
Embase Ovid (1974 to 25 October 2021);
Maternity & Infant Care Database Ovid (1971 to 19 October 2021);
Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 26 October 2021).
Search strategies combined controlled vocabulary and text words; complete strategies are available in Appendix 1; Appendix 2; Appendix 3; Appendix 4; and Appendix 5. We used clinical trial filters as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review.
We searched the following clinical trials registries for ongoing or recently completed trials; strategies available in Appendix 6.
US National Library of Medicine registry (clinicaltrials.gov).
World Health Organization's International Trial Registry and Platform (www.who.int/clinical-trials-registry-platform).
The ISRCTN Registry (www.isrctn.com/).
Data collection and analysis
We used the standard methods of Cochrane Neonatal (neonatal.cochrane.org).
Selection of studies
Two review authors (WM and RP or WM and SO) independently screened title/abstracts and assessed full‐texts. We resolved disagreements regarding inclusion/exclusion by discussion or by involving a third review author (ADP).
Data extraction and management
Two review authors (SO and WM) independently extracted data using a data collection form on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We discussed disagreements until we reached consensus. If data from the trial reports were insufficient, we contacted trialists for further information.
Assessment of risk of bias in included studies
Two review authors (SO and WM or SO and RP) independently assessed risk of bias in included trials using the Cochrane RoB 1 tool (Higgins 2011) for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with the third review author.
See Appendix 7 for a description of risk of bias for each domain.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). When we deemed it appropriate to combine two or more study arms, we obtained treatment effects from combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We determined the number needed to treat for an additional beneficial outcome (NNTB) for outcomes with a detected RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. For cluster‐randomised controlled trials (had we identified any for inclusion), we planned to undertake analyses at the level of the individual while accounting for clustering in the data using methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
Dealing with missing data
We requested additional data from trial investigators when data on important outcomes were missing or were reported unclearly. If more than 20% of outcome data remained missing, we planned to examine the impact on effect size estimates by performing sensitivity analyses.
Assessment of heterogeneity
We examined treatment effects in individual trials and heterogeneity between trial results by inspecting forest plots if there was more than one trial included in a meta‐analysis. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may have been due to heterogeneity rather than to sampling error. If we detected moderate or high levels of heterogeneity (I² > 50%), we explored possible causes by performing prespecified subgroup analyses (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes and reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. We documented studies using the interventions in a potentially eligible infant population but not reporting on any of the primary and secondary outcomes in the Characteristics of included studies table. We planned to use funnel plots to screen for publication bias where there were a sufficient number of trials (at least 10) reporting the outcome. If publication bias was suggested by asymmetry of the funnel plot on visual assessment, we planned to assess this statistically use Harbord's modification of Egger's test (Harbord 2006).
Data synthesis
We used a fixed‐effect model inverse variance meta‐analysis for combining data where trials examined the same intervention and the populations and methods of the trials were judged to be similar.
Subgroup analysis and investigation of heterogeneity
We planned to explore moderate or high levels of heterogeneity (I² > 50%) in subgroup analyses stratified by:
timing of nasal CPAP: primary support after birth versus after postextubation;
gestation or birth weight: preterm or low birth weight versus very preterm (less than 32 weeks' gestation at birth) or very low birth weight (less than 1500 g);
pressure source for CPAP: bubble versus ventilator or Infant Flow Driver;
setting: low‐ and middle‐income versus high‐income countries (World Bank 2021).
Sensitivity analysis
We planned to perform sensitivity analyses if:
there was unexplained high heterogeneity (I² > 75%) by removing the outlying trial or trials;
a trial with high risk of bias (including high level of missing outcome data) was included in the meta‐analysis of an outcome where the other studies had low risk of bias (removed the study with high risk of bias).
Summary of findings and assessment of the certainty of the evidence
Two review authors (SO and WM or SO and RP) used the GRADE approach as outlined in the GRADE Handbook to assess the certainty of the evidence for the following outcomes.
Treatment failure
All‐cause mortality
Neurodevelopmental impairment
Pneumothorax
Moderate‐severe nasal injury
Bronchopulmonary dysplasia
We considered evidence from randomised controlled trials as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon: design (risk of bias); consistency across trials; directness of the evidence; precision of estimates; and presence of publication bias (Schünemann 2013; Walsh 2021). We used GRADEpro GDT to create a summary of findings table to report the certainty of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
Database searches identified 9226 references and trial registry searches identified 770 records. After removing 3698 duplicates, 6298 were available for screening. We excluded 6269 based on title/abstract review, assessed 28 full‐texts, and excluded 16 full texts. We included 12 RCTs in the qualitative synthesis. The search identified one ongoing study. Details are provided in Figure 1.
1.
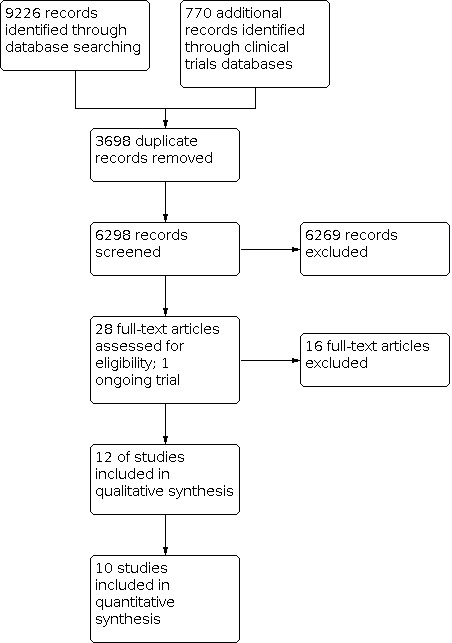
Study flow diagram: review update.
Included studies
We included 12 trials (see Characteristics of included studies table). These were conducted from 2001 onwards in neonatal centres in India (Bashir 2019; Chandrasekaran 2017; Goel 2015; Kumar 2017; Prakash 2019; Sharma 2021; Singh 2017; Solanki 2019), Malaysia (Yong 2005), Turkey (Say 2016), Ireland (Kieran 2012), and the USA (Newnam 2015). Individual infants were allocated randomly to intervention or control groups in all the trials. None used a cluster‐randomised design.
Population
In total, 1604 infants participated in the trials. The median number of participants in trials was 118 (range 56 to 457).
Gestational age at birth or birth weight was a primary inclusion criterion for 11 trials:
less than 31 weeks' (Bashir 2019; Kieran 2012);
26 to 32 weeks' (Chandrasekaran 2017; Say 2016; Sharma 2021);
27 to 34 weeks' (Goel 2015);
1000 g to 2500 g (Kumar 2017);
less than 1500 g (Newnam 2015; Yong 2005);
28 to 34 weeks' (Prakash 2019);
28 to 36 weeks' (Solanki 2019).
One trial recruited preterm and term infants (Singh 2017). Subgroup data by gestation were not available. Because the mean gestation at birth was about 33 weeks', and the mean birth weight about 1800 g, we included this trial.
In all trials, participants were infants with respiratory distress for whom non‐invasive respiratory support was offered, typically within six hours after birth. In five trials, participants included infants offered nasal CPAP as continuing respiratory support immediately following endotracheal extubation at the end of a period of mechanical ventilation (Kieran 2012; Kumar 2017; Newnam 2015; Singh 2017; Yong 2005). Subgroup data by indication (primary versus postextubation) were available only for Kieran 2012.
All trials excluded infants with severe congenital anomalies from participating.
Interventions
Ten trials were two‐arm, parallel group designs comparing nasal masks versus short binasal prongs. Two trials allocated infants to an additional option of rotation between mask and prongs groups (Bashir 2019; Newnam 2015). We excluded the rotation arms from the analyses.
The masks used in the trials were:
Drager BabyFlow (Bashir 2019);
Fisher‐Paykel (Chandrasekaran 2017; Goel 2015; Sharma 2021);
Infant Flow Driver (Kieran 2012; Yong 2005);
Cardinal AirLife (Newnam 2015);
SLE EasyFlow (Say 2016).
The prongs used were:
Hudson (Bashir 2019; Goel 2015; Sharma 2021);
Argyle (Chandrasekaran 2017);
Infant Flow Driver (Kieran 2012; Yong 2005);
Cardinal AirLife (Newnam 2015);
INCA cannula (Say 2016).
Four trials did not state which mask or prongs were used (Kumar 2017; Prakash 2019; Singh 2017; Solanki 2019).
The pressure generation devices used were:
Bubble CPAP (Bashir 2019; Chandrasekaran 2017; Goel 2015; Prakash 2019; Sharma 2021; Solanki 2019);
Infant Flow Drivers (Kieran 2012; Newnam 2015; Yong 2005);
Ventilator CPAP (Say 2016; Singh 2017).
One trial report did not state the pressure generation device used (Kumar 2017).
Most trials used initial nasal CPAP pressures of about 5 cmH2O with the option of increasing the pressure to 7 cmH2O to 9 cmH2O based on clinical assessment and level of oxygen needed to avoid hypoxia.
Outcomes
Eight trials reported 'treatment failure', defined as receipt of mechanical ventilation within 72 hours of nasal CPAP (Bashir 2019; Chandrasekaran 2017; Goel 2015; Kieran 2012; Kumar 2017; Prakash 2019; Say 2016; Sharma 2021).
Criteria for endotracheal intubation for surfactant administration and mechanical ventilation varied between trials. In addition to clinical features of treatment failure (worsening respiratory distress, prolonged or frequent apnoea, severe acidaemia, and shock), most specified a fraction of inspired oxygen (FiO2) needed to avoid hypoxia (typically, transcutaneous oxygen saturation (SpO2) less than 90% when CPAP pressures were 7 cmH2O) to 9 cmH2O):
FiO2 greater than 0.4 (Kieran 2012);
FiO2 greater than 0.5 (Kumar 2017);
FiO2 greater than 0.6 (Bashir 2019; Goel 2015);
FiO2 greater than 0.7 (Chandrasekaran 2017; Sharma 2021);
FiO2 greater than 0.8 (Singh 2017).
Five trial reports did not state the threshold FiO2 for mechanical ventilation (Newnam 2015; Prakash 2019; Say 2016; Solanki 2019; Yong 2005).
Seven trials reported mortality prior to hospital discharge (Bashir 2019; Chandrasekaran 2017; Goel 2015; Kieran 2012; Kumar 2017; Say 2016; Sharma 2021).
No trials reported neurodevelopmental impairment.
Of the secondary outcomes, those most commonly reported were moderate–severe nasal injury (10 trials) and duration of CPAP use (11 trials).
Excluded studies
We excluded 16 reports (see Characteristics of excluded studies table). The most common reasons were wrong study design (non‐randomised, or cross‐over) or wrong intervention (did not include CPAP via mask as a comparison group).
Ongoing studies
We identified one ongoing study (see Characteristics of ongoing studies table).
Risk of bias in included studies
Methodological quality varied between the trials (Figure 2). All had unclear or high risk of bias in at least one domain.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Trials were mostly at low risk of selection bias. Random sequence was generated through computer or web‐based programmes and allocation concealed through sealed, opaque envelopes. In five trials, the methods used to generate the random sequence or conceal allocation (or both) were not described (unclear risk; random sequence: Newnam 2015; Say 2016; Singh 2017; allocation concealment: Kumar 2017; Prakash 2019; Singh 2017).
Blinding
All trials were "open label" and parents, clinicians, or investigators were not blinded (high risk).
Incomplete outcome data
Most trials reported complete or near‐complete assessments of primary outcomes. The risk of attrition bias was high for two trials with high (greater than 20%) levels of exclusion postrandomisation (Prakash 2019; Solanki 2019). We were unable to assess attrition for one trial (unclear risk; Singh 2017).
Selective reporting
Most trials reported a comprehensive group of infant‐important outcomes (low risk). Three trials were at high risk of reporting bias. One trial did not report any of the prespecified outcomes for this review (Solanki 2019). One did not report any data for any outcomes apart from nasal trauma (Singh 2017).
Other potential sources of bias
We did not find evidence of between‐group baseline differences in participant characteristics or demographics in most of the trials (low risk). In one trial, the mean birth weight and mean gestational age were substantially lower in the mask group than the prongs group (high risk; Singh 2017). In another trial, there is concern about postrandomisation reallocation (high risk; Newnam 2015).
Effects of interventions
See: Table 1
Primary outcomes
Treatment failure
Meta‐analysis of data from eight trials (919 infants) suggested that masks may reduce the risk of treatment failure (RR 0.72, 95% CI 0.58 to 0.90; I² = 25%; RD −0.08, 95% CI −0.14 to −0.03; NNTB 12, 95% CI 7 to 33; Analysis 1.1; Figure 3). We assessed the certainty of evidence as low, downgraded one level for serious study design limitations (lack of blinding) and one level for imprecision (Table 1).
1.1. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 1: Treatment failure
3.

Forest plot of comparison: 1 Mask versus prongs nasal CPAP, outcome: 1.1 Nasal CPAP (treatment) failure.
All‐cause mortality prior to hospital discharge
Meta‐analysis of data from seven trials (814 infants) suggested that masks may not affect the risk of mortality prior to hospital discharge (RR 0.83, 95% CI 0.56 to 1.22; I² = 0%; RD −0.02, 95% CI −0.06 to 0.02; Analysis 1.2; Figure 4). We assessed the certainty of evidence as low, downgraded one level for serious study design limitations (lack of blinding) and one level for imprecision (Table 1).
1.2. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 2: All‐cause mortality
4.

Forest plot of comparison: 1 Mask versus prongs nasal CPAP, outcome: 1.2 All‐cause mortality.
Neurodevelopmental impairment
None of the trials assessed neurodevelopmental outcomes.
Secondary outcomes
Pneumothorax
Meta‐analysis of data from five trials (625 infants) suggested that masks may not affect the risk of pneumothorax (RR 0.93, 95% CI 0.45 to 1.93; I² = 0%; RD −0.00, 95% CI −0.04 to 0.03; Analysis 1.3; Figure 5). We assessed the certainty of evidence as low, downgraded one level for serious study design limitations (lack of blinding) and one level for imprecision (Table 1).
1.3. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 3: Pneumothorax
5.

Forest plot of comparison: 1 Mask versus prongs nasal CPAP, outcome: 1.3 Pneumothorax.
Moderate–severe nasal injury
Meta‐analysis of data from 10 trials (1058 infants) suggested that masks may reduce the risk of moderate–severe nasal injury (RR 0.55, 95% CI 0.44 to 0.71; I² = 73%; RD −0.12, 95% CI −0.16 to −0.07; NNTB 8, 95% CI 6 to 14; Analysis 1.4). We assessed the certainty of evidence as low, downgraded one level for serious study design limitations (lack of blinding) and one level for inconsistency (Table 1).
1.4. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 4: Moderate–severe nasal injury
Subgroup analysis for heterogeneity
Subgroup analyses were not possible for timing of CPAP or for gestational age or birth weight categories due to lack of data.
There was evidence of a subgroup difference by pressure source for CPAP (Chi² = 24.68, degrees of freedom (df) = 1 (P < 0.001); I² = 95.9%; Analysis 1.5):
1.5. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 5: Nasal injury – pressure source
bubble: RR 0.11, 95% CI 0.05 to 0.25 (I² = 41%);
ventilator or Infant Flow Driver: RR 0.95, 95% CI 0.69 to 1.31 (I² = 0%).
There was no evidence of a subgroup difference by country income level (Chi² = 0.46, df = 1 (P = 0.50), I² = 0%; Analysis 1.6):
1.6. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 6: Nasal injury – country income level
low‐ and middle‐income countries: RR 0.55, 95% CI 0.43 to 0.70 (I² = 76%; 9 trials);
high‐income countries: RR 1.07, 95% CI 0.16 to 7.34 (1 trial).
Bronchopulmonary dysplasia
Meta‐analysis of data from seven trials (843 infants) provided very uncertain evidence about the effect of masks on the risk of bronchopulmonary dysplasia (RR 0.69, 95% CI 0.46 to 1.03; I² = 51%; RD −0.04, 95% CI −0.08 to 0.00; Analysis 1.7). We assessed the certainty of evidence as very low, downgraded one level for serious study design limitations (lack of blinding), one level for imprecision, and one level for inconsistency (Table 1).
1.7. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 7: Bronchopulmonary dysplasia (BPD)
Subgroup analysis for heterogeneity
Subgroup analyses were not possible for gestational age or birth weight categories due to lack of data.
There was evidence of a subgroup difference by timing of nasal CPAP (Chi² = 6.99, df = 1 (P = 0.008), I² = 85.7%; Analysis 1.8):
1.8. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 8: BPD – timing of CPAP
primary treatment: RR 0.49, 95% CI 0.28 to 0.83 (I² = 21%; 6 trials);
postextubation: RR 1.77, 95% CI 0.80 to 3.90 (1 trial).
There was no evidence of a subgroup difference by pressure source for CPAP (Chi² = 1.41, df = 1 (P = 0.23), I² = 29.3%; Analysis 1.9):
1.9. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 9: BPD – pressure source
bubble: RR 0.50, 95% CI 0.26 to 0.99 (I² = 0%; 4 trials);
ventilator or Infant Flow Driver: RR 0.85, 95% CI 0.51 to 1.42 (I² = 75%; 3 trials).
There was evidence of a subgroup difference by setting (Chi² = 8.92, df = 1 (P = 0.003), I² = 88.8%; Analysis 1.10):
1.10. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 10: BPD – country income level
low‐ and middle‐income countries: RR 0.44, 95% CI 0.26 to 0.76 (I² = 0%; 6 trials);
high‐income countries: RR 1.71, 95% CI 0.85 to 3.46 (1 trial).
Duration of continuous positive airway pressure use
Nine trials found no difference in median duration of CPAP with masks versus prongs (but did not provide 95% CI for inclusion in meta‐analysis) (Bashir 2019: 0.8 days versus 0.8 days; Chandrasekaran 2017: 1.8 days versus 1.3 days; Goel 2015: 6.1 days versus 5.3 days; Kieran 2012: 12.8 days versus 11.7 days; Kumar 2017: 2.0 days versus 1.8 days; Newnam 2015: 4.8 days versus 3.5 days; Prakash 2019: 5.2 days versus 4.5 days; Sharma 2021: 7.2 days versus 6.4 days; Yong 2005: 22.3 days versus 27.7 days).
One trial showed a lower median duration of CPAP use with masks versus prongs (Say 2016: 2 days versus 4 days).
One trial showed a higher median duration of CPAP use with mask versus prongs (Singh 2017: 7.2 days versus 3.6 days).
One trial did not report duration of CPAP use (Solanki 2019).
Duration of oxygen supplementation
Five trials found no difference in median duration of oxygen supplementation with masks versus prongs (but did not provide 95% CI for inclusion in meta‐analysis) (Bashir 2019: 6 days versus 5 days; Chandrasekaran 2017: 3.4 days versus 4.6 days; Goel 2015: 5 days versus 4 days; Say 2016: 4 days versus 7 days; Sharma 2021: 6 days versus 5 days).
Seven trials did not report duration of CPAP use (Kieran 2012; Kumar 2017; Newnam 2015; Prakash 2019; Singh 2017; Solanki 2019; Yong 2005).
Duration of hospitalisation
Seven trials found no difference in median duration of hospitalisation with masks versus prongs (but did not provide 95% CI for inclusion in meta‐analysis) (Bashir 2019: 28 days versus 31 days; Goel 2015: 22 days versus 19 days; Kumar 2017: 11 days versus 9 days; Prakash 2019: 24.6 days versus 21.4 days; Say 2016; 25 days versus 18 days; Sharma 2021; 18.5 days versus 19.2 days; Yong 2005: 60 days versus 56 days).
Five trials did not report duration of hospitalisation (Chandrasekaran 2017; Kieran 2012; Newnam 2015; Singh 2017; Solanki 2019).
Patent ductus arteriosus
Meta‐analysis of data from four trials (467 infants) suggested little or no difference between groups (RR 0.96, 95% CI 0.69 to 1.33; I² = 0%; RD −0.01, 95% CI −0.08 to 0.07; Analysis 1.11).
1.11. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 11: Patent ductus arteriosus
Necrotising enterocolitis
Meta‐analysis of data from six trials (762 infants) suggested little or no difference between groups (RR 1.06, 95% CI 0.63 to 1.80; I² = 0%; RD 0.00, 95% CI −0.03 to 0.04; Analysis 1.12).
1.12. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 12: Necrotising enterocolitis
Severe intraventricular haemorrhage
Meta‐analysis of data from six trials (754 infants) suggested little or no difference between groups (RR 0.66, 95% CI 0.34 to 1.27; I² = 0%; RD −0.02, 95% CI −0.05 to 0.01; Analysis 1.13).
1.13. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 13: Severe intraventricular haemorrhage
Severe retinopathy of prematurity
Meta‐analysis of data from seven trials (827 infants) suggests little or no difference between groups (RR 0.85, 95% CI 0.54 to 1.34; I² = 0%; RD −0.01, 95% CI −0.05 to 0.02; Analysis 1.14).
1.14. Analysis.

Comparison 1: Mask versus prongs nasal continuous positive airway pressure, Outcome 14: Severe retinopathy of prematurity
Sensitivity analyses by risk of bias
We planned sensitivity analyses for:
high heterogeneity (I² > 75%): none of the prespecified meta‐analyses contained high levels of heterogeneity;
risk of bias: none of the prespecified meta‐analyses contained a data from a trial with high risk of bias where the other studies had low risk of bias.
Discussion
Summary of main results
This systematic review of 12 trials, with 1604 participants, suggests that use of masks compared with prongs as the interface for nasal CPAP in preterm infants may reduce the rate of treatment failure by about 25%. The available data suggest that the choice of interface may not affect mortality prior to hospital discharge. There are no data on the effect on neurodevelopmental impairment. Meta‐analyses suggest that the choice of interface may not affect the risk of pneumothorax, but masks may reduce the risk of moderate–severe nasal injury. The evidence about the effect on bronchopulmonary dysplasia is very uncertain. Other outcomes such as major morbidities and duration of CPAP use and hospitalisation appear not to be influenced by interface type. However, the number of participants and trials in meta‐analyses was low and the estimates of effect were imprecise.
Overall completeness and applicability of evidence
The trials were undertaken from 2001 onwards in healthcare facilities internationally, predominantly in India (8 of 12 trials). None of the trials was conducted in sub‐Saharan Africa or in South America (limiting applicability to resource‐limited settings where mechanical ventilation may not be an option in the event of treatment failure). The trials used various pressure sources for CPAP, and the findings appeared broadly applicable to current care practices for very preterm infants receiving bubble or ventilator/Infant Flow Driver CPAP. Most participants were very preterm or very low birth weight, but a few were extremely preterm or extremely low birth weight (limiting applicability for those infants with the highest risk of CPAP treatment failure). Subgroup analyses for heterogeneity were not possible for gestational age or birth weight categories due to paucity of data.
All trial reports described criteria for treatment failure and indications for endotracheal intubation. These were broadly similar, typically specifying receipt of mechanical ventilation via an endotracheal tube within 72 hours of initiation of CPAP as the primary criterion. The indication for intubation and mechanical ventilation, however, did vary between trials, with, for example, the specified threshold level of oxygen supplementation (FiO2 requirement) ranging from 40% to 80%.
The mechanism whereby use of masks may reduce the rate of treatment failure is unclear. Masks may be more comfortable and, therefore, better tolerated than prongs, and may be more effective at transmitting the prescribed pressure to the airway. While the infant's own nasal airway has resistance to air flow, the passage of prongs through the nasal passage reduces the diameter of the airway and increases this resistance. However, recent data from one cross‐over study (preterm infants alternated four‐hourly between mask and prongs CPAP) found no difference in pressure stability of provision of positive airway pressure or in the occurrence of intermittent hypoxia (Poets 2021). Although this practice of rotating masks and prongs every few hours is increasingly used, primarily as a mechanism to reduce the risk of nasal injury, we have not assessed the effect of this strategy here (Bashir 2019; Newnam 2015).
Quality of the evidence
We used GRADE methods to assess the certainty of the evidence for effects on treatment failure, all‐cause mortality, neurodevelopmental impairment (no data), pneumothorax, moderate–severe nasal injury, and bronchopulmonary dysplasia (Table 1). Using this framework, the certainty of evidence was downgraded because of methodological weaknesses (risk of bias) in all trials, principally lack of blinding measures for parents, caregivers, and clinical assessors that may have introduced performance and detection biases. As it is impractical to blind caregivers to the CPAP interface it is possible that bias in the use of co‐interventions may have occurred, for example the use of methylxanthines, or that detection bias was introduced, for example checking for nasal injury more often in infants allocated to prongs versus masks.
The other major reason for downgrading the certainty of evidence across all outcomes was the existence of substantial imprecision in the estimate of effect, with each meta‐analysis generating 95% CIs that included large benefit as well as small or no benefit or harm. Although the total number of participants in the 12 included trials was more than 1600, not all trials contributed data to all outcome estimates (fewer than 10 trials and fewer than 1000 participants contributed to most analyses), and estimates of effect were consequently imprecise, especially for less common outcomes including mortality. For example, although the point estimate for the NNTB for treatment failure was 12 infants, the upper bound of the 95% CI was consistent with an NNTB of 33 infants (Analysis 1.1).
Moderate or high heterogeneity was a further limitation in two analyses. Although our findings suggest that masks versus prongs may reduce moderate–severe nasal injury and bronchopulmonary dysplasia, both meta‐analyses contained moderate or high heterogeneity and the certainty of the evidence was low or very low by GRADE criteria (Table 1). We identified potential sources of heterogeneity in prespecified subgroup analyses. For nasal injury, heterogeneity may have been due in part to differences in the pressure source for CPAP (bubble versus ventilator or Infant Flow Driver), with the larger effect existing for bubble CPAP (Analysis 1.5). It is uncertain whether this finding is robust as the analysis contained residual heterogeneity that may have been due to between‐trial differences in other factors such as setting, indication, or definition of moderate–severe nasal injury (and how subjectively this was assessed). For bronchopulmonary dysplasia, we found subgroup differences for timing of nasal CPAP (larger effect size for primary versus postextubation treatment), and setting (larger effect size in trials conducted in low‐ and middle‐income countries versus high‐income countries). However, these findings should be treated cautiously due to residual confounding and imprecision as only one trial contributed data to the postextubation treatment and high‐income countries subgroups (Kieran 2012).
Potential biases in the review process
An important concern with the review process is the possibility that the findings are subject to publication and other reporting biases (Hopewell 2009). Data from trials that show significant or potentially important effects tend to be more readily available for inclusion in meta‐analyses (Gale 2020). Publication bias, as well as other sources of small‐study bias, is an important contributor to inflation of effect size estimates in meta‐analyses of interventions to improve outcomes in preterm infants (Walsh 2021). We could not assess whether publication bias (or other types of small‐study biases) exaggerated the effect size since the meta‐analyses contained insufficient data points (fewer than 10) to make funnel plot inspection and regression analysis valid and reliable, that is, able to distinguish real asymmetry from chance asymmetry (Higgins 2020). Although we attempted to minimise the threat of publication bias by screening the reference lists of included trials and related reviews and searching the proceedings of the major international perinatal conferences to identify trial reports that are not published in full form in academic journals, we cannot be sure that other trials have been undertaken but not reported.
Agreements and disagreements with other studies or reviews
This review is in broad agreement with three systematic reviews of randomised controlled trials that have assessed the effects of masks versus nasal prongs as CPAP interfaces for preterm infants (Jasani 2018; King 2019; Razak 2020). These reviews included most of the trials identified in this review, and, consistent with our findings, concluded that "compared to binasal prongs, nasal masks may provide a safe and effective alternative by minimising the risk of CPAP failure in preterm infants" (Jasani 2018), with low‐ to moderate‐certainty evidence suggesting that nasal masks are "more effective in preventing intubation and mechanical ventilation" than binasal prongs (King 2019; Razak 2020).
Authors' conclusions
Implications for practice.
Given the low certainty of the evidence generated by these analyses, the implications for practice remain uncertain. Although this review does suggest that use of a nasal mask as the CPAP interface may reduce the risk of treatment failure compared with binasal prongs, because of design limitations and paucity of data (imprecision) and heterogeneity, it remains unclear how or if this translates to effects on other important outcomes including mortality, neurodevelopmental impairment, and other major morbidities including bronchopulmonary dysplasia.
In settings with few and scarce healthcare resources, the infant population most likely to be affected are more mature preterm infants in whom CPAP may be life‐saving in the absence of intensive care and additional therapies including surfactant and mechanical ventilation. In high‐income countries with well‐resourced healthcare facilities, evaluating the comparative effects of different interfaces for CPAP may be particularly relevant to extremely preterm or extremely low birth weight infants at high risk of treatment failure and associated complications including bronchopulmonary dysplasia. However, in these settings research priorities may already have shifted towards comparative studies with the newer forms of non‐invasive ventilation (including nasal intermittent positive pressure ventilation and humidified high flow nasal cannulae) and that are increasingly being adopted in practice. Furthermore, the clinical and research context for non‐invasive ventilation, particularly in well‐resourced facilities, has been affected by other innovations including the early use of "less‐invasive surfactant therapy", which is associated with reduced risk of death or bronchopulmonary dysplasia compared with surfactant therapy via an endotracheal tube and continued mechanical ventilation (Abdel‐Latif 2021).
Implications for research.
Well‐designed trials evaluating this important aspect of a recommended and commonly used neonatal therapy are needed. Trials reporting infant‐important endpoints such as the primary outcomes of this review are of particular need, while including the review outcomes will facilitate future evidence synthesis. Although blinding of clinical investigators to treatment allocation is likely to be unfeasible, trials should aim to minimise performance or detection bias, for example strict and consistent application of protocols for management and criteria for subjective diagnoses.
Acknowledgements
The Methods of this review are based on a standard template used by Cochrane Neonatal.
We thank Cochrane Neonatal: Jane Cracknell and Michelle Fiander, Managing Editors, and Roger Soll, Co‐ordinating editor, who provided editorial and administrative support.
We thank Melissa Harden, Information Specialist, who designed and ran the literature searches.
We thank Colleen Ovelman, previously Managing Editor Cochrane Neonatal, for logistical support and for screening the electronic search records for previous versions of this review
We thank peer reviewers Nicolas Bamat, Children's Hospital of Philadelphia, USA and Souvik Mitra, Dalhousie University and IWK Health Centre, Canada for constructive comments and suggestions.
We thank Anne Lawson, Central Production Service, Cochrane, for copy editing.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL)
via Wiley onlinelibrary.wiley.com/
Date range: Issue 10, October 2021
Date searched: 26 October 2021
Records retrieved: 2522
#1 [mh "Infant, Newborn"] 16781
#2 [mh ^"Premature Birth"] 1617
#3 (neonat* or neo NEXT nat*):ti,ab,kw 23965
#4 (newborn* or new NEXT born* or newly NEXT born*):ti,ab,kw 29310
#5 (preterm or preterms or pre NEXT term*1):ti,ab,kw 14624
#6 (preemie* or premie or premies):ti,ab,kw 53
#7 (prematur* NEAR/3 (birth* or born or deliver*)):ti,ab,kw 3122
#8 (low NEAR/3 (birthweight* or birth NEXT weight*)):ti,ab,kw 5718
#9 low NEXT birthweight*:ti,ab,kw 936
#10 (LBW or VLBW or ELBW):ti,ab,kw 1756
#11 infan*:ti,ab,kw 66527
#12 (baby or babies):ti,ab,kw 9291
#13 {OR #1‐#12} 84598
#14 [mh "Positive‐Pressure Respiration"] 2889
#15 ((((continuous* or positive) NEAR/3 pressure*) or (positive NEXT pressure* or PAP)) and (airway* or air NEXT way* or breath*1 or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)):ti,ab,kw 10166
#16 (((airway* or air NEXT way*) NEAR/3 pressure*) and (breath*1 or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)):ti,ab,kw 5700
#17 ((PPV or CPAP or C NEXT PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (airway* or air NEXT way* or breath*1 or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)):ti,ab,kw 5264
#18 ((((continuous* or positive or airway* or air NEXT way*) NEAR/3 pressure*) or positive NEXT pressure*) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face NEXT mask* or headgear* or head NEXT gear or headbox or head NEXT box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth NEXT piece* or nebuli?er* or prong*1)):ti,ab,kw 3776
#19 ((PAP or PPV or CPAP or C NEXT PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face NEXT mask* or headgear* or head NEXT gear or headbox or head NEXT box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth NEXT piece* or nebuli?er* or prong*1)):ti,ab,kw 2279
#20 {OR #14‐#19} 12820
#21 #13 AND #20 in Trials 2522
Key:
mh = exploded indexing term (MeSH)
mh ^ = indexing term (MeSH)
* = truncation
? = one additional letter
ti,ab,kw = terms in either title or abstract or keyword fields
near/3 = terms within three words of each other (any order)
next = terms are next to each other.
Appendix 2. MEDLINE search strategy
Ovid MEDLINE(R) ALL
via Ovid ovidsp.ovid.com/
Date range searched: 1946 to 25 October 2021
Date searched: 26 October 2021
Records retrieved: 2222
1 exp Infant, Newborn/ (637431)
2 Premature Birth/ (16568)
3 (neonat* or neo nat* or neo‐nat*).ti,ab,kw,kf. (288619)
4 (newborn* or new born* or new‐born* or newly born* or newly‐born*).ti,ab,kw,kf. (190355)
5 (preterm or preterms or pre term or pre terms or pre‐term or pre‐terms).ti,ab,kw,kf. (84264)
6 (preemie* or premie or premies).ti,ab,kw,kf. (196)
7 (prematur* adj3 (birth* or born or deliver*)).ti,ab,kw,kf. (17449)
8 (low adj3 (birthweight* or birth weight* or birth‐weight*)).ti,ab,kw,kf. (37689)
9 low‐birthweight*.ti,ab,kw,kf. (7988)
10 (LBW or VLBW or ELBW).ti,ab,kw,kf. (9342)
11 infan*.ti,ab,kw,kf. (516690)
12 (baby or babies).ti,ab,kw,kf. (75209)
13 or/1‐12 (1154537)
14 exp Positive‐Pressure Respiration/ (27450)
15 ((((continuous* or positive) adj3 pressure*) or (positive‐pressure* or PAP)) and (airway* or air‐way* or breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,kw,kf. (29166)
16 (((airway* or air‐way*) adj3 pressure*) and (breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,kw,kf. (13768)
17 ((PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (airway* or air‐way* or breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,kw,kf. (11708)
18 ((((continuous* or positive or airway* or air‐way*) adj3 pressure*) or positive‐pressure*) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong?)).ti,ab,kw,kf. (7690)
19 ((PAP or PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong?)).ti,ab,kw,kf. (6308)
20 or/14‐19 (50904)
21 13 and 20 (7745)
22 randomized controlled trial.pt. (546951)
23 controlled clinical trial.pt. (94473)
24 randomized.ab. (537599)
25 placebo.ab. (222375)
26 drug therapy.fs. (2388879)
27 randomly.ab. (368111)
28 trial.ab. (572427)
29 groups.ab. (2260913)
30 or/22‐29 (5149590)
31 21 and 30 (2456)
32 exp animals/ not humans.sh. (4900887)
33 31 not 32 (2263)
34 remove duplicates from 33 (2222)
Key:
/ or.sh. = indexing term (Medical Subject Heading: MeSH)
exp = exploded indexing term (MeSH)
$ or * = truncation
? = one additional letter
ti,ab,kw,kf = terms in either title, abstract, keyword heading or keyword heading word fields
fs = floating subheading
adj3 = terms within three words of each other (any order).
pt = publication type
Appendix 3. Embase search strategy
Embase
via Ovid ovidsp.ovid.com/
Date range searched: <1974 to 2021 October 25>
Date searched: 26th October 2021
Records retrieved: 3037
1 exp infant/ (1043193)
2 prematurity/ (111462)
3 (neonat* or neo nat* or neo‐nat*).ti,ab,kw,kf. (375756)
4 (newborn* or new born* or new‐born* or newly born* or newly‐born*).ti,ab,kw,kf. (212794)
5 (preterm or preterms or pre term or pre terms or pre‐term or pre‐terms).ti,ab,kw,kf. (118888)
6 (preemie* or premie or premies).ti,ab,kw,kf. (307)
7 (prematur* adj3 (birth* or born or deliver*)).ti,ab,kw,kf. (24468)
8 (low adj3 (birthweight* or birth weight* or birth‐weight*)).ti,ab,kw,kf. (48417)
9 low‐birthweight*.ti,ab,kw,kf. (9591)
10 (LBW or VLBW or ELBW).ti,ab,kw,kf. (12848)
11 infan*.ti,ab,kw,kf. (542350)
12 (baby or babies).ti,ab,kw,kf. (105078)
13 or/1‐12 (1481919)
14 exp positive pressure ventilation/ (10821)
15 ((((continuous* or positive) adj3 pressure*) or (positive‐pressure* or PAP)) and (airway* or air‐way* or breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,kw,kf. (42487)
16 (((airway* or air‐way*) adj3 pressure*) and (breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,kw,kf. (20406)
17 ((PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (airway* or air‐way* or breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,kw,kf. (21906)
18 ((((continuous* or positive or airway* or air‐way*) adj3 pressure*) or positive‐pressure*) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong?)).ti,ab,kw,kf. (12067)
19 ((PAP or PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong?)).ti,ab,kw,kf. (12054)
20 or/14‐19 (68350)
21 13 and 20 (10471)
22 randomized controlled trial/ (681013)
23 controlled clinical trial/ (464247)
24 Random$.ti,ab,ot. (1718052)
25 randomization/ (92074)
26 intermethod comparison/ (276291)
27 placebo.ti,ab,ot. (331268)
28 (compare or compared or comparison).ti,ot. (549169)
29 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2386565)
30 (open adj label).ti,ab,ot. (91833)
31 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,ot. (249613)
32 double blind procedure/ (188957)
33 parallel group$1.ti,ab,ot. (28272)
34 (crossover or cross over).ti,ab,ot. (113184)
35 ((assign$ or match or matched or allocation) adj5 (alternate or group or groups or intervention or interventions or patient or patients or subject or subjects or participant or participants)).ti,ab,ot. (365313)
36 (assigned or allocated).ti,ab,ot. (430514)
37 (controlled adj7 (study or design or trial)).ti,ab,ot. (390851)
38 (volunteer or volunteers).ti,ab,ot. (261612)
39 human experiment/ (557463)
40 trial.ti,ot. (341840)
41 or/22‐40 (5553114)
42 21 and 41 (3384)
43 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$).ti,ot. and animal experiment/ (1125936)
44 Animal experiment/ not (human experiment/ or human/) (2362745)
45 43 or 44 (2418981)
46 42 not 45 (3130)
47 remove duplicates from 46 (3037)
Key:
/ or.sh. = indexing term (Emtree Subject Heading)
exp = exploded indexing term (Emtree)
$ or * = truncation
? = one additional letter
ti,ab,kw,kf = terms in either title, abstract, keyword heading or keyword heading word fields
adj3 = terms within three words of each other (any order).
pt = publication type
ot = original title
Appendix 4. Maternity & Infant Care Database (MIDIRS) search strategy
Maternity & Infant Care Database (MIDIRS)
via Ovid ovidsp.ovid.com/
Date range searched: 1971 to 19 October 2021
Date searched: 26 October 2021
Records retrieved: 146
1 (neonat* or neo nat* or neo‐nat*).ti,ab,hw,de. (54111)
2 (newborn* or new born* or new‐born* or newly born* or newly‐born*).ti,ab,hw,de. (43599)
3 (preterm or preterms or pre term or pre terms or pre‐term or pre‐terms).ti,ab,hw,de. (30681)
4 (preemie* or premie or premies).ti,ab,hw,de. (61)
5 (prematur* adj3 (birth* or born or deliver*)).ti,ab,hw,de. (7452)
6 (low adj3 (birthweight* or birth weight* or birth‐weight*)).ti,ab,hw,de. (12960)
7 low‐birthweight*.ti,ab,hw,de. (3351)
8 (LBW or VLBW or ELBW).ti,ab,hw,de. (3469)
9 infan*.ti,ab,hw,de. (98052)
10 (baby or babies).ti,ab,hw,de. (31975)
11 or/1‐10 (144990)
12 ((((continuous* or positive) adj3 pressure*) or (positive‐pressure* or PAP)) and (airway* or air‐way* or breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,hw,de. (1375)
13 (((airway* or air‐way*) adj3 pressure*) and (breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,hw,de. (1031)
14 ((PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (airway* or air‐way* or breath? or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)).ti,ab,hw,de. (740)
15 ((((continuous* or positive or airway* or air‐way*) adj3 pressure*) or positive‐pressure*) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong?)).ti,ab,hw,de. (379)
16 ((PAP or PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong?)).ti,ab,hw,de. (241)
17 or/12‐16 (1660)
18 11 and 17 (1601)
19 limit 18 to randomised controlled trial (146)
Key:
/ or.sh. = indexing term (Emtree Subject Heading)
exp = exploded indexing term (Emtree)
$ or * = truncation
? = one additional letter
ti,ab,hw,de = terms in either title, abstract, heading word, or descriptor fields
adj3 = terms within three words of each other (any order).
Appendix 5. CINAHL search strategy
Cumulative Index to Nursing and Allied Health Literature (CINAHL Complete)
via EBSCOHost web.b.ebscohost.com/
Date range: inception–26 October 2021
Date searched: 26 October 2021
Records retrieved: 1299
S47 S21 AND S46 1,299
S46 S37 OR S45 1,490,245
S45 S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 1,170,012
S44 TI before N3 after OR AB before N3 after 87,774
S43 (MH "Controlled Before‐After Studies") 210
S42 (multicentre* or multi‐centre* or multicenter* or multi‐center*) OR AB (multicentre* or multi‐centre* or multicenter* or multi‐center*) 348,828
S41 (MH "Multicenter Studies") 313,630
S40 TI assign* OR AB assign* 86,110
S39 TI (group or groups) OR AB (group or groups) 849,450
S38 (MH "Control Group") 12,667
S37 S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 907,641
S36 AB (cluster W3 RCT) 422
S35 (crossover design) OR MH (comparative studies) 422,225
S34 AB (control W5 group) 127,088
S33 PT (randomized controlled trial) 135,692
S32 MH (placebos) 13,399
S31 MH (sample size) AND AB (assigned OR allocated OR control) 4,253
S30 TI trial 156,367
S29 AB random* 355,992
S28 TI randomised OR randomized 286,827
S27 MH "Cluster Sample" 4,835
S26 MH "Pretest‐Posttest Design" 47,503
S25 MH "Random Assignment" 70,782
S24 MH "Single‐Blind Studies" 15,177
S23 MH "Double‐Blind Studies" 51,726
S22 MH "Randomized Controlled Trials" 122,091
S21 S13 AND S20 2,919
S20 S14 OR S15 OR S16 OR S17 OR S18 OR S19 18,348
S19 TI ((PAP or PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong#)) OR AB ((PAP or PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong#)) 1,919
S18 TI ((((continuous* or positive or airway* or air‐way*) N3 pressure*) or positive‐pressure*) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong#)) OR AB ((((continuous* or positive or airway* or air‐way*) N3 pressure*) or positive‐pressure*) and (source* or device* or interface* or driver* or operator* or generator* or machine* or mask* or face‐mask* or headgear* or head gear or head‐gear or headbox or head box or head‐box or helmet* or bag* or BVM or AMBU or "Infant Flow" or mouthpiece* or mouth piece* or mouth‐piece* or nebuli?er* or prong#)) 2,557
S17 TI ((PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (airway* or air‐way* or breath# or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)) OR AB ((PPV or CPAP or C‐PAP or NCPAP or BiPAP or APRV or IPPB or IPPV) and (airway* or air‐way* or breath# or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)) 3,539
S16 TI (((airway* or air‐way*) N3 pressure*) and (breath# or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)) OR AB (((airway* or air‐way*) N3 pressure*) and (breath# or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)) 4,319
S15 TI ((((continuous* or positive) N3 pressure*) or (positive‐pressure* or PAP)) and (airway* or air‐way* or breath# OR or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)) OR AB ((((continuous* or positive) N3 pressure*) or (positive‐pressure* or PAP)) and (airway* or air‐way* or breath# OR or breathing or ventilat* or respir* or inspir* or inhal* or expir* or exhal*)) 9,329
S14 MH "Positive Pressure Ventilation+" 12,102
S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 283,632
S12 TI (baby or babies) OR AB (baby or babies) 36,343
S11 TI infan* OR AB infan* 123,227
S10 TI (LBW or VLBW or ELBW) OR AB (LBW or VLBW or ELBW) 3,653
S9 TI low‐birthweight* OR AB low‐birthweight* 2,988
S8 TI (low N3 (birthweight* or birth weight* or birth‐weight*)) OR AB (low N3 (birthweight* or birth weight* or birth‐weight*)) 13,346
S7 TI (prematur* N3 (birth* or born or deliver*)) OR AB (prematur* N3 (birth* or born or deliver*)) 5,101
S6 TI (preemie* or premie or premies) OR AB (preemie* or premie or premies) 337
S5 TI (preterm or preterms or pre term or pre terms or pre‐term or pre‐terms) OR AB (preterm or preterms or pre term or pre terms or pre‐term or pre‐terms) 37,264
S4 TI (newborn* or new born* or new‐born* or newly born* or newly‐born*) OR AB (newborn* or new born* or new‐born* or newly born* or newly‐born*) 34,940
S3 TI (neonat* or neo nat* or neo‐nat*) OR AB (neonat* or neo nat* or neo‐nat*) 73,766
S2 MH "Childbirth, Premature" 11,706
S1 MH "Infant, Newborn+" 149,439
Key:
MH + = exploded indexing term (MeSH)
MH = indexing term (MeSH)
* = truncation
# = up to one additional letter
? = one replacement letter
TI = terms in the title
AB = terms in the abstract
N3 = terms near three words of each other (any order).
W5 = terms within three words of each other (specified order).
Appendix 6. Trial registry search strategies
ClinicalTrials.gov
via clinicaltrials.gov/
Date searched: 26 October 2021
Records retrieved: 555
Other Terms: (infan* OR baby OR neonat* OR prematur* OR newborn* OR LBW OR VLBW OR ELBW) AND ((PAP OR PPV OR (positive AND pressure)) AND (airway* OR breathing OR ventilat* OR respir*))
International Clinical Trials Registry Platform (ICTRP)
via trialsearch.who.int/
Date searched: 26 October 2021
Records retrieved: 215 records for 214 trials
Advanced search:
Intervention: ((PAP OR PPV OR (positive AND pressure)) AND (airway OR breathing OR ventilation OR respiration))
Recruitment status: All
Search for clinical trials in children
Appendix 7. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the Characteristics of included studies table. We evaluated the following issues and entered the findings into the risk of bias table.
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants;
low risk, high risk, or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors;
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (less than 20% missing data);
high risk (20% or greater of missing data);
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Mask versus prongs nasal continuous positive airway pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment failure | 8 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.58, 0.90] |
| 1.2 All‐cause mortality | 7 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.56, 1.22] |
| 1.3 Pneumothorax | 5 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.93] |
| 1.4 Moderate–severe nasal injury | 10 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.44, 0.71] |
| 1.5 Nasal injury – pressure source | 8 | 918 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.65] |
| 1.5.1 Bubble | 4 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.25] |
| 1.5.2 Ventilator or Infant Flow Driver | 4 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.31] |
| 1.6 Nasal injury – country income level | 10 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.44, 0.71] |
| 1.6.1 Low‐ and middle‐income countries | 9 | 938 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.43, 0.70] |
| 1.6.2 High‐income countries | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.16, 7.34] |
| 1.7 Bronchopulmonary dysplasia (BPD) | 7 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.03] |
| 1.8 BPD – timing of CPAP | 6 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.06] |
| 1.8.1 Primary | 6 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.28, 0.83] |
| 1.8.2 Postextubation | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.80, 3.90] |
| 1.9 BPD – pressure source | 7 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.03] |
| 1.9.1 Bubble | 4 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.26, 0.99] |
| 1.9.2 Ventilator or Infant Flow Driver | 3 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.42] |
| 1.10 BPD – country income level | 7 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.03] |
| 1.10.1 Low‐ and middle‐income countries | 6 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.26, 0.76] |
| 1.10.2 High‐income countries | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.85, 3.46] |
| 1.11 Patent ductus arteriosus | 4 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.33] |
| 1.12 Necrotising enterocolitis | 6 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.63, 1.80] |
| 1.13 Severe intraventricular haemorrhage | 6 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.34, 1.27] |
| 1.14 Severe retinopathy of prematurity | 7 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.54, 1.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bashir 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 117 newborn infants (< 31 weeks' gestation) with respiratory distress treated with bubble nasal CPAP (Fisher‐Paykel) within 6 hours of birth | |
| Interventions | Mask (Drager BabyFlow): n = 57 Prongs (Hudson): n = 60 Mask/prongs in rotation: n = 58 (not used in this review)a |
|
| Outcomes | Nasal injury Treatment failure (mechanical ventilation within 72 hours of CPAP) Duration of CPAP use Death before discharge Necrotising enterocolitis Intraventricular haemorrhage Chronic lung disease Retinopathy of prematurity Duration of hospitalisation |
|
| Notes | Setting: Hyderabad, India (2016–2018) Funding: no specific funding a2‐stage randomisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label (nasal injury assessed by clinician blinded to group allocation). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reporting complete. |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely (comprehensive). |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Chandrasekaran 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 72 newborn infants (26–32 weeks' gestation) with respiratory distress treated with bubble CPAP (Fisher‐Paykel) within 6 hours of birth | |
| Interventions | Mask (Fisher‐Paykel): n = 37 Prongs (Argyle): n = 35 |
|
| Outcomes | Level of oxygen supplementation until 24 hours of CPAP Treatment failure until 72 hours of CPAP (persistent hypoxia, respiratory distress, prolonged apnoea, shock) Nasal trauma Duration of CPAP use Duration of supplemental oxygen Death before discharge Sepsis/pneumonia Intraventricular haemorrhage Chronic lung disease Retinopathy of prematurity |
|
| Notes | Setting: New Delhi, India (2012–2013) Funding: no specific funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were analysed and reported. |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely (comprehensive). |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Goel 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 118 newborn infants (27–34 weeks' gestation) with respiratory distress treated with bubble CPAP (Fisher‐Paykel) after birth room stabilisation | |
| Interventions | Mask (Fisher‐Paykel): n = 61 Prongs (Hudson RCI): n = 57 |
|
| Outcomes | Treatment failure (mechanical ventilation within 72 hours of CPAP) Duration of CPAP use Duration of supplemental oxygen Pulmonary interstitial emphysema Pneumothorax Patent ductus arteriosus Death before discharge Duration of hospitalisation Intraventricular haemorrhage Chronic lung disease Retinopathy of prematurity Feed intolerance Necrotising enterocolitis aNasal injury |
|
| Notes | Setting: Mumbai, India (2014–2015) Funding: no specific funding aData from authors (August 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were analysed and reported. |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely (comprehensive). |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Kieran 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 120 newborn infants (< 31 weeks' gestation) treated with nasal CPAP via Viasys Infant Flow Driver (as primary support or postextubation) | |
| Interventions | Mask (Viasys): n = 58 Prongs (Viasys): n = 62 |
|
| Outcomes | Treatment failure (mechanical ventilation within 72 hours of CPAP) Death before discharge Nasal trauma sufficiently severe "to prompt clinicians or nursing staff to change the interface" Pneumothorax Necrotising enterocolitis Intraventricular haemorrhage Chronic lung disease Retinopathy of prematurity |
|
| Notes | Setting: Dublin, Ireland (2009–2010) Funding: National Children's Research Centre |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reporting complete. |
| Selective reporting (reporting bias) | Low risk | Unlikely (comprehensive). |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Kumar 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 60 preterm infants (birth weight 1000–2500 g) with respiratory distress treated with CPAP (pressure source not stated) as primary support or postextubation | |
| Interventions | Mask (manufacturer not stated): n = 30 Prongs (manufacturer not stated): n = 30 |
|
| Outcomes | Treatment failure (persistent hypoxia, worsening respiratory distress, frequent apnoea, acidaemia within 72 hours of CPAP) Death before discharge Duration of CPAP use Duration of hospitalisation Nasal trauma Pneumothorax |
|
| Notes | Setting: New Delhi, India (dates not stated, likely early 2010s) Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were analysed and reported. |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely (comprehensive). |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Newnam 2015.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 56 newborn infants (birth weight 500–1500 g) treated with nasal CPAP via Cardinal variable flow driver (as primary support or postextubation) | |
| Interventions | Mask (Cardinal AirLife): n = 35 Prongs (Cardinal AirLife): n = 21 Mask/prongs in rotation: n = 22 (not used in this review) |
|
| Outcomes | "Neonatal Skin Condition Scale" (ano data for moderate–severe nasal trauma) Duration of CPAP use |
|
| Notes | Setting: Virginia, USA (2012–2013) Funding: no specific funding adata not reported or available from authors (contacted August 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reporting complete. |
| Selective reporting (reporting bias) | High risk | No outcomes apart from "Neonatal Skin Condition Scale" reported. |
| Other bias | High risk | Mean gestation at birth lower in mask (26 weeks') vs prongs (27 weeks') group. Mean birth weight lower in mask (826 g) vs prongs (941 g) group. Quote: "[Seven] infants whose size prevented correct fit with nasal prongs according to manufacture guidelines were defaulted to the mask group, regardless of group assignment". |
Prakash 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 113 preterm infants (28–34 weeks' gestation) with respiratory distress treated with bubble CPAP (pressure source not stated) within 6 hours after birth | |
| Interventions | Mask (manufacturer not stated): n = 56 Prongs (manufacturer not stated): n = 57 |
|
| Outcomes | Treatment failure (mechanical ventilation) Duration of CPAP use Duration of hospitalisation Nasal trauma Patent ductus arteriosus Retinopathy of prematurity Necrotising enterocolitis Death before discharge and chronic lung disease not reported |
|
| Notes | Setting: Uttar Pradesh, India (2016–2018) Funding: no specific funding Author contacted 28 August 2021; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33/113 randomised infants excluded because of nasal CPAP treatment failure. |
| Selective reporting (reporting bias) | Unclear risk | Mortality not reported. |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Say 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 149 newborn infants (26–32 weeks' gestation) with respiratory distress treated with nasal CPAP (via SLE 2000 mechanical ventilator) after birth (infants received less invasive surfactant administration if FiO2 > 0.3 to maintain SpO2 > 89%) | |
| Interventions | Mask (SLE EasyFlow): n = 74 Prongs (INCA nasal cannulae): n = 75 |
|
| Outcomes | Treatment failure (mechanical ventilation up to 72 hours of CPAP) Duration of CPAP use Duration of supplemental oxygen Pneumothorax Patent ductus arteriosus Death before discharge Duration of hospitalisation Intraventricular haemorrhage (grade 2–4) Chronic lung disease Retinopathy of prematurity Necrotising enterocolitis Nasal trauma (skin breakdown) |
|
| Notes | Setting: Ankara, Turkey (2014) Funding: not stated ClinicalTrials.gov Identifier: NCT02287116 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were analysed and reported. |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely (comprehensive). |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Sharma 2021.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 178 newborn infants (26–32 weeks' gestation) with respiratory distress treated with bubble CPAP (Fisher‐Paykel) within 6 hours of birth | |
| Interventions | Mask (Fisher‐Paykel): n = 90 Prongs (Hudson): n = 88 |
|
| Outcomes | Treatment failure (mechanical ventilation within 72 h of CPAP) Nasal trauma Death before discharge Duration of CPAP use Duration of supplemental oxygen Pneumothorax Necrotising enterocolitis Chronic lung disease aSevere intraventricular haemorrhage Retinopathy of prematurity Duration of hospitalisation |
|
| Notes | Setting: Jaipur, India (2017–2018) Funding: not stated aUnpublished data courtesy of Dr Sharma (September 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were analysed and reported. |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely (comprehensive). |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Singh 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 75 newborn infants (mean gestation 33 weeks') with respiratory distress treated with ventilator nasal CPAP for > 24 hours (as primary support or postextubation) | |
| Interventions | Mask (manufacturer not stated): n = 38 Prongs (manufacturer not stated): n = 37 |
|
| Outcomes | Nasal trauma aTreatment failure aNecrotising enterocolitis aChronic lung disease aIntraventricular haemorrhage aRetinopathy of prematurity |
|
| Notes | Setting: Hyderabad, India (2011) Funding: not stated aData not reported or available from authors (contacted August 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | aNot stated. |
| Allocation concealment (selection bias) | Unclear risk | aNot stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | aUnable to assess attrition. |
| Selective reporting (reporting bias) | High risk | aData not provided for any outcomes other than nasal trauma. |
| Other bias | High risk | Mean gestation at birth higher in mask (32 weeks') vs prongs (34 weeks') group. Mean birth weight higher in mask (1647 g) vs prongs (1939 g) group. |
Solanki 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 538 newborn infants (28–36 weeks' gestation) with respiratory distress treated with bubble nasal CPAP for > 72 hours (as primary support or postextubation) | |
| Interventions | Mask (manufacturer not stated): n = 276 Prongs (manufacturer not stated): n = 282 |
|
| Outcomes | Septal necrosis (ano data for other nasal trauma) | |
| Notes | Setting: Vadodara, Gujarat, India (2017) Funding: not stated aData not reported or available from authors (contacted August 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 338/538 infants excluded postrandomisation (due to lack of consent or death or receiving CPAP > 72 hours). |
| Selective reporting (reporting bias) | High risk | Data not provided for any outcomes other than nasal trauma. |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
Yong 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 89 newborn very low birth weight infants with respiratory distress treated with nasal CPAP via Infant Flow Driver (as primary support or postextubation) | |
| Interventions | Mask (Infant Flow Driver): n = 41 Prongs (Infant Flow Driver): n = 48 |
|
| Outcomes | Nasal trauma (crusting and excoriation, bleeding, narrowing of the nasal passage) Duration of CPAP use Duration of supplemental oxygen Duration of hospitalisation Bronchopulmonary dysplasia Death before discharge Treatment failure not reported – author contacted September 2021 |
|
| Notes | Setting: Kuala Lumpur, Malaysia (2001–2003) Funding: research grant (FF/28/2001) from the Faculty of Medicine, Universiti Kebangsaan, Malaysia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed opaque envelopes "shuffled randomly". |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were analysed and reported. |
| Selective reporting (reporting bias) | Low risk | No access to protocol but unlikely. |
| Other bias | Low risk | No evidence imbalance in baseline demographics. |
CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; n: number of participants; RCT: randomised controlled trial; SpO2: oxygen saturation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahluwalia 1998 | Randomised study compared binasal vs single prong nasal CPAP. |
| Bhandari 1996 | Compared nasal vs naso‐pharyngeal CPAP and was non‐randomised. |
| Buettiker 2004 | 3‐armed RCT comparing single prong vs binasal (Hudson) prongs vs CPAP delivered via Infant Flow Driver. |
| Campbell 2004 | Compared Infant Flow CPAP with high‐flow nasal cannulae. |
| Davis 2001 | Compared single prong vs binasal (Hudson) prongs. |
| Kaufman 2013 | Observational study of facemask CPAP for stabilisation after preterm birth |
| Kavvadia 2000 | Non‐randomised comparison of single prong and Infant Flow CPAP |
| Mazzella 2001 | Trial comparing bubble CPAP (via nasopharyngeal tube) vs CPAP delivered via Infant Flow Driver |
| Nair 2005 | Trial of high‐flow nasal cannula system vs bubble CPAP (prong type not specified) in preterm infants. |
| Rego 2002 | Trial comparing CPAP delivered via Hudson nasal prongs vs Argyle nasal prongs |
| Roukema 1999a | Compared CPAP delivered via nasopharyngeal tube vs CPAP delivered via Infant Flow Driver |
| Roukema 1999b | Cross‐over study of Infant Flow CPAP vs nasopharyngeal CPAP |
| Sreenan 2001 | Compared high‐flow nasal cannulae vs nasal CPAP |
| Stefanescu 2003 | Compared binasal prongs (INCA system) with nasal CPAP delivered via Infant Flow Driver |
| Sun 1999 | Compared binasal prongs (Medicorp system) with nasal CPAP delivered via Infant Flow Driver |
| Trevisanuto 2005 | Compared Infant Flow nasal CPAP vs CPAP delivered via a polycarbonate helmet |
CPAP: continuous positive airway pressure; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT01989442.
| Study name | Nasal mask and prong use in non‐invasive ventilation for newborns (NIV) |
| Methods | Randomised controlled trial |
| Participants | All newborns who require non‐invasive ventilation as first‐line treatment as respiratory support in delivery room or neonatal intensive care unit |
| Interventions | Nasal masks vs binasal prongs |
| Outcomes | Failure of non‐invasive ventilation (endotracheal intubation) |
| Starting date | 2013 |
| Contact information | Ufuk Cakir, Ankara University, Turkey |
| Notes | Recruitment status: unknown |
Differences between protocol and review
At the request of the World Health Organization (WHO), we created this review on a subtopic of De Paoli 2008 (Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates).
This current review is based on the protocol for De Paoli 2008, but with the following changes.
Updated the background section.
Modified both primary and secondary outcome measures in consultation with authorship team and WHO.
Modified selected subgroup analyses in consultation with authorship team and WHO.
Added risk of bias assessment.
Added GRADE assessment and summary of findings table.
We updated the search strategies for all databases to improve sensitivity and to use most current neonatal population terms and methodological filters.
Contributions of authors
Conceptualised the review: RP, AD, SO, PD, WM.
Screened search results, assessed study eligibility, extracted and synthesised data, assessed risk of bias, and undertook GRADE assessment: RP, SO, WM.
Wrote the review: RP, AD, SO, PD, WM.
Sources of support
Internal sources
-
University of York, UK
Host institution (WM)
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
World Health Organization, Switzerland
Editorial and administrative support for this review has been provided by a grant from World Health Organization to Cochrane Neonatal.
-
National Institute for Health Research, UK
National Institute of Health Research (NIHR) Evidence‐synthesis Programme Grant. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
-
National Health and Medical Research Council, Australia
Peter Davis receives salary support from a National Health and Medical Research Council (NHMRC) grant provided by the Australian Government.
Declarations of interest
RP has no interests to declare.
ADP works as a consultant neonatologist at Royal Hobart Hospital, Tasmania, Australia.
PD: none.
SO works as a health professional at Bradford Teaching Hospitals, UK.
WM is a Co‐ordinating Editor of Cochrane Neonatal. He was not involved in the editorial process for this review.
New
References
References to studies included in this review
Bashir 2019 {published data only}
- Bashir T, Murki S, Kiran S, Reddy VK, Oleti TP. 'Nasal mask' in comparison with 'nasal prongs' or 'rotation of nasal mask with nasal prongs' reduce the incidence of nasal injury in preterm neonates supported on nasal continuous positive airway pressure (nCPAP): a randomized controlled trial. PLOS One 2019;14(1):e0211476. [DOI: 10.1371/journal.pone.0211476] [DOI] [Google Scholar]
Chandrasekaran 2017 {published data only}
- Chandrasekaran A, Thukral A, Jeeva Sankar M, Agarwal R, Paul VK, Deorari AK. Nasal masks or binasal prongs for delivering continuous positive airway pressure in preterm neonates-a randomised trial. European Journal of Pediatrics 2017;176(3):379-86. [DOI: 10.1007/s00431-017-2851-x] [DOI] [Google Scholar]
Goel 2015 {published data only}
- Goel S, Mondkar J, Panchal H, Hegde D, Utture A, Manerkar S. Nasal mask versus nasal prongs for delivering nasal continuous positive airway pressure in preterm infants with respiratory distress: a randomized controlled trial. Indian Pediatrics 2015;52(12):1035-40. [DOI: 10.1007/s13312-015-0769-9] [DOI] [Google Scholar]
Kieran 2012 {published data only}
- Kieran EA, Twomey AR, Molloy EJ, Murphy JF, O'Donnell CP. Randomized trial of prongs or mask for nasal continuous positive airway pressure in preterm infants. Pediatrics 2012;130(5):e1170-6. [DOI: 10.1542/peds.2011-3548] [DOI] [Google Scholar]
Kumar 2017 {published data only}
- Kumar G, Copra M, Copra M. To study effectiveness of nasal prong and nasal mask in nasal continuous positive airway pressure in preterm neonates with respiratory distress. Journal of Medical Science and Clinical Research 2017;5(2):21409-15. [Google Scholar]
Newnam 2015 {published data only}
- Newnam KM, McGrath JM, Salyer J, Estes T, Jallo N, Bass WT. A comparative effectiveness study of continuous positive airway pressure-related skin breakdown when using different nasal interfaces in the extremely low birth weight neonate. Applied Nursing Research 2015;28(1):36-41. [DOI: 10.1016/j.apnr.2014.05.005] [DOI] [Google Scholar]
Prakash 2019 {published data only}
- Dubey A, Malik S, Prakash S. A comparative study of incidence and severity of nasal complications while using nasal prongs and nasal mask as CPAP interface in preterm neonates: a randomized control trial. International Journal of Pediatric Research 2019;6:177-82. [DOI: 10.17511/ijpr.2019.i04.05] [DOI] [Google Scholar]
- Prakash S, Dubey A, Malik S. A comparative study of outcomes of nasal prongs and nasal mask as CPAP interface in preterm neonates: a randomized control trial. Journal of Clinical Neonatology 2019;8(3):147-50. [Google Scholar]
Say 2016 {published data only}
- Say B, Kanmaz Kutman HG, Oguz SS, Oncel MY, Arayici S, Canpolat FE, et al. Binasal prong versus nasal mask for applying CPAP to preterm infants: a randomized controlled trial. Neonatology 2016;109(4):258-64. [DOI: 10.1159/000443263] [DOI] [Google Scholar]
Sharma 2021 {published and unpublished data}
- Sharma D, Kaur A, Farahbakhsh N, Agarwal S. To compare nasal mask with binasal prongs in delivering continuous positive airway pressure for reducing need of invasive ventilation: randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2021;34(12):1890-6. [Google Scholar]
Singh 2017 {published data only}
- Singh J, Bhardwar V, Chirla D. To compare the efficacy and complication of nasal prongs vs nasal mask CPAP in neonates. International Journal of Medical and Dental Sciences 2017;6(1):1392-7. [Google Scholar]
Solanki 2019 {published data only}
- Solanki JR, Bhil DL. Comparative study of nasal mask versus nasal prong in terms of nasal septal necrosis for delivering nasal continuous positive airway pressure in newborns with respiratory distress. Indian Journal of Child Health 2019;26(6):601-4. [Google Scholar]
Yong 2005 {published data only}
- Yong SC, Chen SJ, Boo NY. Incidence of nasal trauma associated with nasal prong versus nasal mask during continuous positive airway pressure treatment in very low birth weight infants: a randomised control study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90:F480-3. [DOI: 10.1136/adc.2004.069351] [DOI] [Google Scholar]
References to studies excluded from this review
Ahluwalia 1998 {published data only}
- Ahluwalia JS, White DK, Morley CJ. Infant Flow Driver or single prong nasal continuous positive airway pressure: short-term physiological effects. Acta Paediatrica 1998;87:325-7. [Google Scholar]
Bhandari 1996 {published data only}
- Bhandari V, Rogerson S, Barfield C, Yu V, Rowe JC. Nasal versus naso-pharyngeal continuous positive airway pressure (CPAP) use in preterm neonates (abstract). Pediatric Research 1996;39(Suppl):196A. [Google Scholar]
Buettiker 2004 {published data only}
- Buettiker V, Hug MI, Baenziger O, Meyer C, Frey B. Advantages and disadvantages of different nasal CPAP systems in newborns. Intensive Care Medicine 2004;30:926-30. [Google Scholar]
Campbell 2004 {published data only}
- Campbell DM, Shah P, Shah V, Kelly E. High flow nasal cannula CPAP versus infant flow nasal CPAP in newly-extubated neonates < 1250 g (abstract). Pediatric Research 2004;56:472A. [Google Scholar]
Davis 2001 {published and unpublished data}
- Davis P, Davies M, Faber B. A randomised controlled trial of two methods of delivering nasal continuous positive airway pressure after extubation to infants weighing less than 1000g: binasal (Hudson) versus single nasal prongs. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85:F82-5. [Google Scholar]
Kaufman 2013 {published data only}
- Kaufman J, Schmölzer GM, Kamlin CO, Davis PG. Mask ventilation of preterm infants in the delivery room. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(5):F405-10. [DOI: 10.1136/archdischild-2012-303313] [DOI] [Google Scholar]
Kavvadia 2000 {published data only}
- Kavvadia V, Greenough A, Dimitriou G. Effect on lung function of continuous positive airway pressure administered either by Infant Flow Driver or a single nasal prong. European Journal of Pediatrics 2000;159:289-92. [Google Scholar]
Mazzella 2001 {published data only}
- Mazzella M, Bellini C, Calevo MG, Campone F, Massocco D, Mezzano P, et al. A randomised control study comparing the Infant Flow Driver with nasal continuous positive airway pressure in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85:F86-90. [Google Scholar]
Nair 2005 {published data only}
- Nair G, Karna P. Comparison of the effects of Vapotherm and nasal CPAP in respiratory distress in preterm infants (abstract). E-PAS 2005;57:2054. [Google Scholar]
Rego 2002 {published data only}
- Rego MA, Martinez FE. Comparison of two nasal prongs for application of continuous positive airway pressure in neonates. Pediatric Critical Care Medicine 2002;3:239-43. [Google Scholar]
Roukema 1999a {published data only}
- Roukema H, O'Brien K, Nesbitt K, Zaw W. A randomized controlled trial of Infant Flow continuous positive airway pressure (CPAP) versus nasopharyngeal CPAP in the extubation of babies ≤ 1250g. Pediatric Research 1999;45:318A. [Google Scholar]
Roukema 1999b {published data only}
- Roukema H, O'Brien K, Nesbitt K, Zaw W. A crossover trial of Infant Flow continuous positive airway pressure versus nasopharyngeal CPAP in the extubation of babies ≤ 1250 grams birthweight (abstract). Pediatric Research 1999;45:317A. [Google Scholar]
Sreenan 2001 {published data only}
- Sreenan C, Lemke RP, Hudson-Mason A, Osiovich H. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001;107:1081-3. [Google Scholar]
Stefanescu 2003 {published data only}
- Stefanescu BM, Murphy WP, Hansell BJ, Fuloria M, Morgan TM, Aschner JL. A randomized, controlled trial comparing two different continuous positive airway pressure systems for the successful extubation of extremely low birth weight infants. Pediatrics 2003;112:1031-8. [Google Scholar]
Sun 1999 {published and unpublished data}
- Sun SC, Tien HC. Randomized controlled trial of two methods of nasal CPAP (NCPAP): Flow Driver vs conventional NCPAP. Pediatric Research 1999;45:322A. [Google Scholar]
Trevisanuto 2005 {published data only}
- Trevisanuto D, Grazzina N, Doglioni N, Ferrarese P, Marzari F, Zanardo V. A new device for administration of continuous positive airway pressure in preterm infants: comparison with a standard nasal CPAP continuous positive airway pressure system. Intensive Care Medicine 2005;31:859-64. [Google Scholar]
References to ongoing studies
NCT01989442 {published data only}
- NCT01989442. Nasal mask and prong use in non-invasive ventilation for newborns (NIV) [Efficacy and safety of nasal mask and prong use in non-invasive ventilation for newborns]. clinicaltrials.gov/ct2/show/NCT01989442 (first received 21 November 2013).
Additional references
Abdel‐Latif 2021
- Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD011672. [DOI: 10.1002/14651858.CD011672.pub2] [DOI] [Google Scholar]
Bamat 2021
- Bamat N, Fierro J, Mukerji A, Wright CJ, Millar D, Kirpalani H. Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD012778. [CENTRAL: CD012778] [DOI: 10.1002/14651858.CD012778.pub2] [DOI] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [MEDLINE: ] [DOI] [Google Scholar]
Beltempo 2018
- Beltempo M, Isayama T, Vento M, Lui K, Kusuda S, Lehtonen L, et al, on behalf of the International Network for Evaluating Outcomes of Neonates. Respiratory management of extremely preterm infants: an international survey. Neonatology 2018;14(1):28-36. [DOI: 10.1159/000487987] [DOI] [Google Scholar]
Chernick 1973
- Chernick V. Continuous distending pressure in hyaline membrane disease: of devices, disadvantages, and a daring study. Pediatrics 1973;52:114-5. [Google Scholar]
Committee on Fetus and Newborn 2014
- Committee on Fetus and Newborn. Respiratory support in preterm infants at birth. Pediatrics 2014;133(1):171-4. [Google Scholar]
Cox 1974
- Cox JM, Boehm JJ, Millare EA. Individual nasal masks and intranasal tubes: a non-invasive neonatal technique for the delivery of continuous positive airway pressure (CPAP). Anaesthesia 1974;29:597-600. [Google Scholar]
Curstedt 2015
- Curstedt T, Halliday HL, Hallman M, Saugstad OD, Speer CP. 30 years of surfactant research – from basic science to new clinical treatments for the preterm infant. Neonatology 2015;107(4):314-6. [DOI: 10.1159/000381160] [DOI] [Google Scholar]
Dargaville 2013
- Dargaville PA, Aiyappan A, De Paoli AG, Dalton RG, Kuschel CA, Kamlin CO, et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology 2013;104(1):8-14. [Google Scholar]
Dargaville 2016
- Dargaville PA, Gerber A, Johansson S, De Paoli AG, Kamlin CO, Orsini F, et al, Australian and New Zealand Neonatal Network. Incidence and outcome of CPAP failure in preterm infants. Pediatrics 2016;138(1):e20153985. [DOI: 10.1542/peds.2015-3985] [DOI] [Google Scholar]
Davis 2003
- Davis P, Henderson-Smart DJ. Nasal continuous positive airway pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD000143. [DOI: 10.1002/14651858.CD000143] [DOI] [Google Scholar]
De Paoli 2002
- De Paoli AG, Morley CJ, Davis PG, Lau R, Hingeley E. In vitro comparison of nasal continuous positive airway pressure devices for neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86:F42-5. [DOI: 10.1136/fn.87.1.f42] [DOI] [Google Scholar]
De Paoli 2005
- De Paoli AG, Lau R, Davis PG, Morley CJ. Pharyngeal pressure in preterm infants receiving nasal continuous positive airway pressure. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(1):F79-81. [DOI: 10.1136/adc.2004.052274] [DOI] [Google Scholar]
De Paoli 2021
- Prakash R, De Paoli AG, Davis PG, Oddie SJ, McGuire W. Pressure sources for nasal continuous positive airway pressure in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD002977. [DOI: 10.1002/14651858.CD002977.pub2] [DOI] [Google Scholar]
Ehrenkranz 2005
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al, National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353-60. [DOI: 10.1542/peds.2005-0249] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fraser 2004
- Fraser J, Walls M, McGuire W. Respiratory complications of preterm birth. BMJ 2004;329(7472):962-5. [DOI: ] [Google Scholar]
Gale 2020
- Gale C, McGuire W, Juszczak E. Randomised controlled trials for informing perinatal care. Neonatology 2020;117(1):8-14. [DOI: 10.1159/000499881] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gaon 1999
- Gaon P, Lee S, Hannan S, Ingram D, Milner AD. Assessment of effect of nasal continuous positive pressure on laryngeal opening using fibre optic laryngoscopy. Archives of Disease in Childhood. Fetal and Neonatal Edition 1999;80:F230-2. [Google Scholar]
Glaser 2021
- Glaser K, Wright CJ. Indications for and risks of noninvasive respiratory support. Neonatology 2021;118(2):235-43. [DOI: 10.1159/000515818] [DOI] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 11 July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Green 2019
- Green EA, Dawson JA, Davis PG, De Paoli AG, Roberts CT. Assessment of resistance of nasal continuous positive airway pressure interfaces. Archives of Disease in Childhood. Fetal and Neonatal Edition 2019;104(5):F535-9. [DOI: 10.1136/archdischild-2018-315838] [DOI] [Google Scholar]
Gupta 2016
- Gupta S, Donn SM. Continuous positive airway pressure: physiology and comparison of devices. Seminars in Fetal and Neonatal Medicine 2016;21(3):204-11. [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI: 10.1002/sim.2380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Ho 2020
- Ho JJ, Subramaniam P, Davis PG. Continuous positive airway pressure (CPAP) for respiratory distress in preterm infants. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD002271. [DOI: 10.1002/14651858.CD002271.pub3] [DOI] [Google Scholar]
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horbar 2012
- Horbar JH, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129(6):1019-26. [DOI: 10.1542/peds.2011-3028] [PMID: ] [DOI] [PubMed] [Google Scholar]
ICROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991-9. [DOI: 10.1001/archopht.123.7.991] [PMID: ] [DOI] [PubMed] [Google Scholar]
Imbulana 2018
- Imbulana DI, Manley BJ, Dawson JA, Davis PG, Owen LS. Nasal injury in preterm infants receiving non-invasive respiratory support: a systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(1):F29-35. [DOI: 10.1136/archdischild-2017-313418] [DOI] [Google Scholar]
Jasani 2018
- Jasani B, Ismail A, Rao S, Patole S. Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants – a systematic review and meta-analysis. Pediatric Pulmonology 2018;53(7):987-92. [DOI: 10.1002/ppul.24014] [DOI] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [DOI: 10.1164/ajrccm.163.7.2011060] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kattwinkel 1973
- Kattwinkel J, Fleming D, Cha CC, Fanaroff AA, Klaus MH. A device for administration of continuous positive airway pressure by the nasal route. Pediatrics 1973;52:131-4. [Google Scholar]
King 2019
- King BC, Gandhi BB, Jackson A, Katakam L, Pammi M, Suresh G. Mask versus prongs for nasal continuous positive airway pressure in preterm infants: a systematic review and meta-analysis. Neonatology 2019;116(2):100-14. [DOI: 10.1159/000496462] [DOI] [Google Scholar]
Krouskop 1975
- Krouskop RW, Brown EG, Sweet AY. The early use of continuous positive airway pressure in the treatment of idiopathic respiratory distress syndrome. Journal of Pediatrics 1975;87:263-7. [Google Scholar]
Laughon 2011
- Laughon M, Bose C, Allred EN, O'Shea TM, Ehrenkranz RA, Marter LJ, et al. Antecedents of chronic lung disease following three patterns of early respiratory disease in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(2):F114-20. [Google Scholar]
Lemyre 2016
- Lemyre B, Laughon M, Bose C, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005384. [DOI: 10.1002/14651858.CD005384.pub2] [DOI] [Google Scholar]
Lemyre 2017
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub3] [DOI] [Google Scholar]
Lissauer 2017
- Lissauer T, Duke T, Mellor K, Molyneux L. Nasal CPAP for neonatal respiratory support in low and middle-income countries. Archives of Disease in Childhood. Fetal and Neonatal Edition 2017;102(3):F194-6. [DOI: 10.1136/archdischild-2016-311653] [DOI] [Google Scholar]
Locke 1991
- Locke R, Greenspan JS, Shaffer TH, Rubenstein SD, Wolfson MR. Effect of nasal CPAP on thoracoabdominal motion in neonates with respiratory insufficiency. Pediatric Pulmonology 1991;11:259-64. [Google Scholar]
Martin 1977
- Martin RJ, Nearman HS, Katona PG, Klaus MH. The effect of a low continuous positive airway pressure on the reflex control of respiration in the preterm infant. Journal of Pediatrics 1977;90:976-81. [Google Scholar]
McGoldrick 2020
- McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD004454. [DOI: 10.1002/14651858.CD004454.pub4] [DOI] [Google Scholar]
Miller 1985
- Miller MJ, Carlo WA, Martin RJ. Continuous positive airway pressure selectively reduces obstructive apnea in preterm infants. Journal of Pediatrics 1985;106:91-4. [Google Scholar]
Morley 2004
- Morley C, Davis P. Continuous positive airway pressure: current controversies. Current Opinion in Pediatrics 2004;16(2):141-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pandit 1999
- Pandit PB, Pyon KH, Courtney SE, Habib RH. Inspiratory work of breathing with a demand flow vs constant flow nasal continuous positive airway pressure device in preterm neonates. Pediatric Research 1999;45:314A. [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [DOI] [Google Scholar]
Poets 2021
- Poets CF, Lim K, Marshall A, Jackson H, Gale TJ, Dargaville PA. Mask versus nasal prong leak and intermittent hypoxia during continuous positive airway pressure in very preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2021;106(1):81-3. [DOI: 10.1136/archdischild-2020-319092] [DOI] [Google Scholar]
Razak 2020
- Razak A, Patel W. Nasal mask vs binasal prongs for nasal continuous positive airway pressure in preterm infants: a systematic review and meta-analysis. Pediatric Pulmonology 2020;55(9):2261-71. [DOI: 10.1002/ppul.24878] [DOI] [Google Scholar]
Richardson 1978
- Richardson CP, Jung AL. Effects of continuous positive airway pressure on pulmonary function and blood gases of infants with respiratory distress syndrome. Pediatric Research 1978;12:771-4. [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Soll 2019
- Soll RF, Barkhuff W. Noninvasive ventilation in the age of surfactant administration. Clinics in Perinatology 2019;46(3):493-516. [DOI: 10.1016/j.clp.2019.05.002] [DOI] [Google Scholar]
Stoll 2015
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;34(10):1039-51. [DOI: 10.1001/jama.2015.10244] [DOI] [Google Scholar]
Subramaniam 2016
- Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD001243. [DOI: 10.1002/14651858.CD001243.pub3] [DOI] [Google Scholar]
Sweet 2019
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, te Pas A, et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology 2019;115:432-50. [DOI: 10.1159/000499361] [DOI] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114:1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2021
- Walsh V, McGuire W, Halliday HL. Evaluation of the quality of perinatal trials: making the GRADE. Neonatology 2021;118(3):378-83. [DOI: 10.1159/000516239] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilkinson 2016
- Wilkinson D, Andersen C, O'Donnell CP, De Paoli AG, Manley BJ. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD006405. [DOI: 10.1002/14651858.CD006405.pub3] [DOI] [Google Scholar]
World Bank 2021
- World Bank. World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 4 May 2021).
Wright 2016
- Wright CJ, Polin RA, Kirpalani H. Continuous positive airway pressure to prevent neonatal lung injury: how did we get here, and how do we improve? Journal of Pediatrics 2016;173:17–e2. [Google Scholar]
Yu 1977
- Yu VY, Rolfe P. Effect of continuous positive airway pressure breathing on cardiorespiratory function in infants with respiratory distress syndrome. Acta Paediatrica Scandinavica 1977;66:59-64. [Google Scholar]
References to other published versions of this review
De Paoli 2008
- De Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD002977. [DOI: 10.1002/14651858.CD002977.pub2] [DOI] [Google Scholar]


