Abstract
The D7 proteins are highly expressed in the saliva of hematophagous Nematocera and bind biogenic amines and eicosanoid compounds produced by the host during blood feeding. These proteins are encoded by gene clusters expressing forms having one or two odorant-binding protein-like domains. Here we examine functional diversity within the D7 group in the genus Anopheles and make structural comparisons with D7 proteins from culicine mosquitoes in order to understand aspects of D7 functional evolution. Two domain long form (D7L) and one domain short form (D7S) proteins from anopheline and culicine mosquitoes were characterized to determine their ligand selectivity and binding pocket structures. We previously showed that a D7L protein from Anopheles stephensi, of the subgenus Cellia, could bind eicosanoids at a site in its N-terminal domain but could not bind biogenic amines in its C-terminal domain as does a D7L1 ortholog from the culicine species Aedes aegypti, raising the question of whether anopheline D7L proteins had lost their ability to bind biogenic amines. Here we find that D7L from anopheline species belonging to two other subgenera, Nyssorhynchus and Anopheles, can bind biogenic amines and have a structure much like the Ae. aegypti ortholog. The unusual D7L, D7L3, can also bind serotonin in the Cellia species An. gambiae. We also show through structural comparisons with culicine forms that the biogenic amine binding function of single domain D7S proteins in the genus Anopheles may have evolved through gene conversion of structurally similar proteins, which did not have biogenic amine binding capability. Collectively, the data indicate that D7L proteins had a biogenic amine and eicosanoid binding function in the common ancestor of anopheline and culicine mosquitoes, and that the D7S proteins may have acquired a biogenic amine binding function in anophelines through a gene conversion process.
Keywords: hematophagy, mosquito, saliva, D7 proteins, odorant-binding protein, biogenic amine, eicosanoids, gene duplication, evolution, protein diversity
Graphical Abstract

1. Introduction
The saliva of blood feeding arthropods contains a cocktail of biomolecules that counteract host hemostatic, immune and inflammatory defenses (reviewed by (Andersen and Ribeiro, 2017; Arca and Ribeiro, 2018; Champagne, 2005; Francischetti, 2010; Francischetti et al., 2009; Ribeiro, 1995; Ribeiro and Arcà, 2009) ) thereby playing a pivotal role in feeding, parasite transmission and vectorial capacity (Abdeladhim et al., 2014; Agarwal et al., 2016; Gillespie et al., 2000; Kamhawi, 2000; Ribeiro, 1987; Rodriguez and Hernandez-Hernandez Fde, 2004; Schneider and Higgs, 2008; Titus and Ribeiro, 1988; Valenzuela, 2004; Volfova et al., 2008). Hematophagy has arisen independently at least 20 times during arthropod evolution, resulting in a huge diversity of mechanisms and molecules involved in the physiological adaptations to blood feeding (Arca and Ribeiro, 2018; Graca-Souza et al., 2006; Mans, 2011; Ribeiro, 1995; Ribeiro et al., 2010; Ribeiro and Arcà, 2009). As a result of this independent evolution, different blood feeding lineages contain proteins representing different families that perform virtually identical functions. For example, representatives of the odorant binding protein (Alvarenga et al., 2010; Andersen, 2010; Calvo et al., 2006; Calvo et al., 2009; Jablonka et al., 2019), lipocalin (Andersen et al., 2003; Andersen et al., 2005; Assumpcao et al., 2010; Jablonka et al., 2016; Ma et al., 2012; Mans and Ribeiro, 2008a, b; Mans et al., 2008; Neelakanta et al., 2018; Ribeiro and Walker, 1994; Xu et al., 2013), CAP (cysteine-rich, antigen V and pathogenesis-related 1 proteins) (Xu et al., 2012) and yellow/major-royal jelly protein (MRJP) families (Xu et al., 2011) have all been found to bind biogenic amines or eicosanoid compounds in different arthropod species.
Salivary protein genes are commonly distributed in gene clusters derived through repeated tandem duplication. Gene dosage effects are probably important in maintaining these duplicated clusters in the genome but individual genes within a cluster also show substantial sequence divergence, suggesting that individual cluster members have evolved modified or novel functions (Arca et al., 2002; Mans, 2011; Mans et al., 2017). Indeed, interspecific comparisons suggest that salivary protein genes evolve at a much faster pace than housekeeping genes (Arca et al., 2017; Arca and Ribeiro, 2018; Arca et al., 2014; Neafsey et al., 2015) with gene duplication followed by rapid divergence allowing the gain and loss of function within members of the same structural protein family.
The D7 salivary protein family belongs to the insect odorant-binding protein (OBP)/Pheromone Binding Protein (PBP) superfamily and its members are divided in short (D7S) or long (D7L) forms, containing one or two OBP-like domains, respectively. Despite relatively low sequence identity to classical OBPs, functional analogy with these proteins led to the suggestion that, like OBPs, D7 proteins would sequester small hydrophobic compounds (Arca et al., 2002). Later, D7 proteins were characterized and shown to bind biogenic amines (Calvo et al., 2006; Calvo et al., 2009; Martin-Martin et al., 2021; Martin-Martin et al., 2020b), cysteinyl leukotrienes (Cys-LTs) (Alvarenga et al., 2010; Calvo et al., 2009; Jablonka et al., 2019; Martin-Martin et al., 2021; Martin-Martin et al., 2020b), thromboxane A2 (TXA2) (Alvarenga et al., 2010; Jablonka et al., 2019; Martin-Martin et al., 2021) and ADP (Martin-Martin et al., 2020a), thereby acting as anti-inflammatory and/or anti-hemostatic proteins facilitating blood feeding. Protein structures determined by X-ray crystallography were crucial to elucidating D7 binding mechanisms and provided insights into alterations of key residues that led to the loss and/or gain of new functions (Alvarenga et al., 2010; Calvo et al., 2009; Jablonka et al., 2019; Mans et al., 2007). For example, D7S (given the names D7r1-r4) from An. gambiae and D7L from Ae. aegypti (AeD7L1)1 were shown to bind biogenic amines (Calvo et al., 2006; Calvo et al., 2009). The only characterized anopheline D7L, AnSte-D7L1 from An. stephensi, as well as D7L from sand flies were unable to bind biogenic amines, but bound cysteinyl leukotrienes and thromboxane A2 (Alvarenga et al., 2010; Jablonka et al., 2019). This raised the question, of whether anopheline D7L proteins had lost their ability to bind biogenic amines, and that this function has passed to the D7S which have become similar to the biogenic amine binding domain of the D7L proteins in Ae. aegypti.
Importantly, all anopheline D7s studied so far belong to subgenus Cellia, while none belonging subgenus Anopheles, and Nyssorhynchus have been characterized. Given the fast pace of salivary protein evolution and divergence, and the fact that Nyssorhynchus, for example, diverged from Cellia more than 100 million years ago (MYA) our knowledge regarding this protein family is limited. In this study we have examined the functional evolution of the D7 salivary protein family in mosquitoes, and addressed how these proteins, encoded by duplicated gene clusters, have evolved from a structural and functional standpoint, with a focus on anopheline mosquitoes. To accomplish this, we have compared the structures of a taxonomically diverse set of D7 proteins determined by X-ray diffraction and molecular modelling using AlphaFold2 (Jumper et al., 2021). We have coupled this with calorimetric binding assays of recombinant protein to evaluate how the numerous observed structural differences in these proteins affect ligand binding function. The results reveal gains and losses of ligand binding function over evolutionary time as well as showing a variety of binding pocket changes that result in differences in ligand selectivity.
2. Materials and Methods
2.1. Reagents
L-arginine, serotonin hydrochloride, tryptamine hydrochloride, L(+)-norepinephrine (+) bitartrate salt monohydrate, epinephrine, dopamine hydrochloride, histamine dihydrochloride, (±)-octopamine hydrochloride and ADP sodium salt were obtained from Sigma-Aldrich. IPTG, reduced (GSH) and oxidized (GSSG) glutathione were purchased from Gold Biotechnology. Ultrapure guanidinium hydrochloride was obtained from Invitrogen. All leukotrienes and prostaglandins as well as U46619 and arachidonic acid were obtained from Cayman Chemical. Crystallization reagents and screening kits were manufactured by Hampton Research and Nextal.
2.2. Sequences, alignment, and analysis
Anopheles gambiae D7 protein sequences (accession numbers listed on Table S1) were used as input to search (BLAST) for orthologues in different Anopheles at VectorBase website. In some cases, like Anopheles darlingi D7L2 (ADAC010080), when sequence on VectorBase was clearly incomplete, the partial sequence available was used to search NCBI database and retrieve the complete sequence. All sequences obtained were checked for the presence of initial methionine and stop codons. Signal peptides were assigned using the SignalP-5.0 server (Almagro Armenteros et al., 2019) and excluded from sequences to provide the mature protein primary structure. Alignments were performed using MegAlign Pro (available at DNAStar core suite, Lasergene).
2.3. Protein expression, refolding and purification
Anopheles darlingi D7L2 (EU934268.1), Anopheles atroparvus D7L1 (AATE004070), Anopheles gambiae D7L3 (AGAP028120), Culex quinquefasciatus D7S (GenBank AAR18437.1) and Aedes aegypti D7S1 (AAEL006406) were cloned into the expression vector pET17b, used to transform BL21(DE3)pLysS E. coli cells, and transformed colonies were selected on LB agar plates containing chloramphenicol and ampicillin (35 and 100 μg/mL, respectively). For expression, cultures were grown at 37°C until they reach OD600nm around 0.6–0.8, then induced with 1 mM IPTG for 3 hr. After expression, inclusion bodies were washed in Triton X-100 as previously described (Andersen et al., 2003) and extracted proteins denatured in 6M guanidinium HCl in 20 mM Tris-HCl pH 8.0 containing 1 mM EDTA and 10 mM DTT.
Refolding of each specific protein was performed by dropwise dilution. AnDar-D7L2 and D7CQS1 were refolded in 0.3 M L-arginine monohydrochloride in 50 mM CAPS buffer pH10.0; AnAtr-D7L1 and Anopheles gambiae D7L3 were refolded in 0.5 M L-arginine, 5 mM GSH and 0.5 mM GSSG in 50 mM CAPS buffer pH 10.0 or 50 mM Tris pH 9.0 with 1 mM EDTA, respectively. AeD7S1 was refolded in 40 mM Tris HCl, pH 8.0 containing 300 mM arginine. After dropwise addition to the refolding buffer, solutions were stirred for 1 hour at room temperature, and subsequently transferred to 4°C overnight. Finally, refolded recombinant proteins were concentrated by tangential flow ultrafiltration followed by diafiltration and purified by Fast Protein Liquid Chromatography (FPLC) using and AKTA purifier 10 system.
After purification, protein concentration was determined by measuring the absorbance of a diluted aliquot of each pure stock at 280 nm, and concentration calculated using their respective molecular extinction coefficient at 280 nm (ε280nm) obtained based on their amino acid sequence.
2.4. Ligand-protein screening and characterization by isothermal titration calorimetry (ITC)
Aliquots of protein stocks were diluted to the desired concentration in 20 mM Tris-HCl 0.15 M NaCl pH 7.4. All compounds used in ITC assays were prepared in this same buffer. Nevertheless, lipidic reagents such as eicosanoids, needed a more elaborate preparation due to their nature and to the fact that their stocks come in organic solvents, and any trace of these could lead to artefacts on calorimetry assays. Aliquots of lipid stock solutions were dried under a stream of nitrogen or helium in a glass vial, then dissolved in 20 mM Tris-HCl 0.15 M NaCl pH 7.4, vortexed and sonicated for 10 minutes. All solutions were degassed prior to use.
Binding experiments were performed on a MicroCal VP-ITC instrument at 30°C. The cell was filled with a solution of the protein and the syringe filled with ligand in which was delivered to the cell in 10 µL injections (20 seconds duration), with spacing interval of 300 seconds and syringe stirring speed of 286 rpm. Subtraction of heats of dilution as well as data analysis were performed using the Microcal Origin software. Measured heats were converted to enthalpies, which were then plotted against molar ratio, and when binding was observed thermodynamic parameters such as N (molar ratio of binding), K (equilibrium association constant), enthalpy change (∆H) and entropy change (∆S) were calculated based on the fitting of the curve using a single-site binding model. The equilibrium dissociation constant (KD), which is the reciprocal of K was calculated based on the experimentally obtained K. The respective protein and ligand concentrations used are described in the figure captions.
2.5. Ligand competition assays using isothermal titration calorimetry (ITC)
To establish whether cysteinyl leukotrienes (LTC4) and serotonin occupy the same or separate binding sites in AnDar-D7L2, competition assays were performed as follows. Aliquots of protein stocks and ligands were prepared as described above, concentrations used were chosen based on ligand screening assays. As a general rule the chosen concentration of protein was mixed with a 2 times molar excess one of the ligands and placed on the ITC cell. A solution of the other ligand 10 times more concentrated than the protein was used to fill the syringe and titrated (10 µL injections) using same ITC experiment parameters described above.
2.6. Platelet aggregation assays
Blood was obtained from medication-free platelet donors using citrate as anticoagulant. Platelet-rich plasma (PRP) was prepared as described in (Assumpcao et al., 2010) and concentration adjusted to 200,000 platelets/μL. Platelets were placed in a cuvette and stirred at 1,200 rpm at 37°C for 1 minute, followed by addition of reagents, indicated by the figure legends. Aggregation response was monitored by turbidimetry using a Lumi-Aggregometer (Chrono-Log Corp).
2.7. Crystallization, data collection, structure solution and refinement.
Protein crystals were obtained using the hanging drop vapor diffusion method. AnDar-D7L2 (protein stock in 10 mM Tris pH 8.0) without ligands crystallized from 30% PEG 5000 MME, 0.2 M ammonium sulfate, 0.1M HEPES pH 7.5. Crystals were flash cooled for data collection in the crystallization buffer described above, containing 25 % glycerol. To obtain AnDar-D7L2 crystals containing bound ligands, U46619 and serotonin bound were prepared in 10 mM Tris-HCl pH 8.0 buffer then pre incubated with the protein for 10 minutes. Importantly, ligands were used in a 1.5 molar excess over protein. The mixture was crystallized by the hanging drop vapor diffusion method in precipitant buffer containing 2 M Ammonium Sulfate, 2% (v/v) PEG 400, 0.1M HEPES Sodium pH 7.5. Crystals were flash cooled for data collection in the crystallization buffer described above, containing 15 % glycerol. D7CQS1 (protein stock in 10 mM Tris pH 8.0) crystallized from 25% (w/v) PEG 5000 MME, 0.2 M lithium sulfate, 0.1M Tris pH 8.5. Crystals were flash cooled for data collection in the crystallization buffer described above, containing 10 % glycerol. AeD7S1 crystallized from 20 % PEG 6000, 0.1 M MES monohydrate pH 6.0. Crystals were flash cooled for data collection in the crystallization buffer containing 10% glycerol.
For the structure of AnDar-D7L2 without ligands, data were collected on a Rigaku MicroMax-007 HF high-flux microfocus rotating anode X-ray generator equipped with a Rigaku SATURN A200 CCD detector using Cu radiation. The data were integrated, reduced, and scaled with XDS (Kabsch, 2010). The structure was determined by molecular replacement using Phaser (McCoy et al., 2007) as implemented in PHENIX (Adams et al., 2010) by employing separate, manually constructed, search models for the N-terminal and C-terminal domains based on the sequence alignment of AnDar-D7L2 to the AnSte-D7L1 sequence and known crystal structure (PDB ID 3NGV). The final model of unligated AnDar-D7L2 was constructed by iterative manual tracing of the chain using the program Coot (Emsley and Cowtan, 2004) after each cycle of refinement with a stepwise increase in the resolution using PHENIX (Adams et al., 2010). The final refinement statistics are presented in Table S2.
For the structure of AnDar-D7L2 complexed with its ligands, D7CQS1 and AeD7S1 diffraction data were collected at beamlines 22-ID and 22-BM at SER-CAT, the Southeast Regional Collaborative Access Team at the Advanced Photon Source, Argonne National Laboratory (Table S2). The data were processed using HKL2000 (Otwinowski and Minor, 1997) or XDS (Kabsch, 2010). The structures of AnDar-D7L2 were solved by molecular replacement using Phaser (McCoy et al., 2007) with AnStD7L1 as a search model for AnDar-D7L2 and a model constructed using AlphaFold2 (Jumper et al., 2021) for D7CQ1. AeD7S1 was solved by single anomalous diffraction methods using a selenomethionine derivative of the protein with SHELX C/D/E (Schneider and Sheldrick, 2002; Sheldrick, 2002) followed by autobuilding of the model using Buccaneer (Cowtan, 2006). Protein models for all of the proteins were rebuilt using Coot (Emsley and Cowtan, 2004) and refined using Phenix.refine (Adams et al., 2010).
Molecular modeling with AlphaFold 2.0 (Jumper et al., 2021) and 2.1 was performed on the Biowulf computing cluster at NIH. All models presented correspond to the “ranked_0” PDB format output.
2.8. Structural data deposition and Protein Data Bank (PDB) accession codes
Coordinates and experimental structure of proteins solved during this study have been deposited in the Protein Data Bank (https://www.rcsb.org/) with accession codes as follows: ligand-free AnDarl-D7L2 (7U1N), AnDar-D7L2 co-crystalized with U46619 and serotonin (7TX8), AeD7S1 (7TVC) and D7CQ1 (7TVY).
3. Results and Discussion
3.1. D7 gene clusters in anopheline and culicine mosquitoes.
The D7 gene cluster is made up of duplicated groups of D7L and D7S genes and is present in anopheline and culicine mosquitoes as well as other groups of blood feeding Nematocera. In anophelines, the D7 cluster consists of either one or two genes, depending on the subgenus (as further discussed in the next session), given the names of D7L1 and D7L2, followed by a D7L3 gene spaced ~7.5–7.9 kb (in most species analyzed) downstream (Fig. 1). D7L3 is generally directly followed by a subcluster of two to five D7S genes (named D7r1-r5) that are transcribed in the opposite direction to the D7L genes and span about 6 kb of chromosomal sequence. In the culicine species Ae. aegypti, there is no D7L3 gene, but the D7 cluster is arranged similarly and extends over larger segment of the chromosome. The two Ae. aegypti D7L genes are separated from each other by ~22 kb and from a subcluster of D7S genes by about 31 kb (Fig. 1). It appears generally true that in An. gambiae and other Anopheles species, D7S are among the most abundant proteins in the saliva while D7L, including D7L3 proteins, are expressed at lower levels (Arca et al., 2017; Arca et al., 2005; Calvo et al., 2006; Francischetti et al., 2002). Conversely, the D7L1/D7L2 proteins of Ae. aegypti are among the most abundant in the saliva while the D7S genes are expressed at somewhat lower levels (Ribeiro et al., 2007; Valenzuela et al., 2002). These differences in expression may reflect functional differences among D7 proteins in the two groups of mosquitoes.
Figure 1. D7 genes are organized in clusters in anopheline and culicine mosquitoes.

Schematic of the representation the pattern of D7 genes distribution and organization in the chromosome maps of six different anopheline species representing various sub genus/series (indicated in parentheses) and the culicine Aedes aegypti. D7L genes are marked with the letter L (1–3) in boxes with purple outline, D7S are represented by the letter “r”(1–5) in boxes with magenta outline and shortened D7S found in Nyssorhynchus species are represented in yellow. In most Anopheles subgenera the D7 cluster contains one or two D7L genes separated by ~7.5 to 7.9 kb from D7L3, which is adjacent to the D7S genes (r1 to r5) that are generally transcribed in the direction (indicated with arrows) opposite to D7L genes. Analysis of available anopheline genomes suggests that all species, regardless of their subgenus, retained D7L2 and D7L3 genes. On the other hand D7L1 genes, believed to arise from D7L2 gene duplication, are just present in series Myzomyia and Pyretophorous from subgenus Cellia and in subgenus Anopheles. The number of D7S genes also varies among the subgenera. All Cellia species retained at least five genes encoding D7S (represented by the letter “r”), and some species (An. stephensi) have a sixth gene, species from the subgenera Anopheles and Nyssorhynchus, consistently show the loss of two and three D7S genes respectively. Details regarding the forms present in each species as well as annotation numbers can be found at Table S1. 3
In Ae. aegypti, representing culicine species, the D7 cluster is arranged similarly but occupies a larger segment of the chromosome. The D7L3 gene present in all Anopheles species is not present in culicines. Culicine D7S were not named like those of Anopheles ones, since the two kinds of D7S are not apparent orthologs.
D7 genes whose products have been already characterized in the literature have their respective boxes filled in green (binding) and/or red (no binding) to represent their capacity to bind eicosanoids (in the N-terminal of D7L) and/or serotonin (C-terminal of D7L and D7S). Boxes with no filling color represent genes producing yet uncharacterized proteins, while those filled with purple represent the ones whose proteins structural and functional characterization are being described in the present work, in addition to the Culex quinquefasciatus D7S (D7CQS1) not represented in this scheme. Figure created with BioRender.com.
3.2. D7 distribution and binding selectivity in Anopheles species
We have shown previously that AnSt-D7L12, the apparent An. stephensi ortholog of AeD7L1 from Ae. aegypti is incapable of binding biogenic amines (Alvarenga et al., 2010). On the other hand, An. gambiae D7S proteins (D7r1-D7r4) were shown to be similar in structure to the C-terminal domain of Ae. aegypti AeD7L1 and to bind biogenic amines in an essentially identical manner (Calvo et al., 2006; Calvo et al., 2009; Mans et al., 2007). These observations suggested that in anopheline species, the biogenic amine binding function was assumed by highly expressed D7S (D7r1-r4) proteins, resulting in loss of this function in D7L proteins. However, these studies were performed only with D7 proteins from species contained in a single subgenus, Cellia. Here, we present a more extensive sequence analysis of Anopheles D7L proteins from the subgenera Cellia, Anopheles and Nyssorhynchus and use our knowledge of the structures of D7L Ae. aegypti (AeD7L1) (Calvo et al., 2009) and An. stephensi (AnSt-D7L1) (Alvarenga et al., 2010), as well as the D7S protein D7r4 from An. gambiae (Mans et al., 2007) to identify sequence determinants of biogenic amine binding that could be used to predict and understand the binding activities of the diverse group of uncharacterized D7s from the three Anopheles subgenera with relevance to medical entomology.
Currently the VectorBase database contains data from eighteen Anopheles species (reference strains) genomes (Neafsey et al., 2015). An. gambiae has eight distinct D7 protein-coding genes previously identified in salivary gland transcriptomes and in the genome. These include three D7L forms (D7L1–3) and five D7S (D7r1–5). The amino acid sequence of each An. gambiae D7 was used to search for orthologs using BLAST. Table S1 summarizes retrieved orthologs and their accession numbers from D7s of each Anopheles species having a representative genome. These are sorted according to their subgenus (and series when applicable), as well as by geographic region. Species belonging to the subgenus Cellia, series Pyretophorous and to the subgenus Anopheles have maintained the three D7L genes, while all the other groups analyzed have retained only D7L2 and D7L3. It is important to note that the only exception to this would be An. stephensi D7L1, whose original annotation was L1, but is more similar and groups with other D7L2 in phylogenetic analysis (Arca et al., 2017).
The lack of biogenic amine binding by AnSt-D7L1 is due to a rearrangement of the C-terminal domain associated with mutation of two cysteine residues resulting in the loss of one of the three disulfide bonds (DS4) in the C-terminal domain (Fig. 2A). DS4 tethers the C-terminus of the protein to second α-helix (B2) of the domain (Fig. 2). When the disulfide bond is present, helix H2 is positioned to form an open binding pocket that accommodates the ligand (Fig. 2B). In the absence of the disulfide, H2 is shifted in its position and helix B2 is unwound causing a collapse of the binding pocket structure (Fig. 2A), with amino acid side chains filling the putative ligand binding site (Fig. 2C). A number of residues that make up the biogenic amine binding pocket in D7r4 (Fig. 2D) are conserved in AnSt-D7L1 (Fig. S1B) but occupy different positions in the structure (Fig. 2C).
Figure 2. The loss of DS4 leads to the lack of ability of subgenus Cellia D7L1 and L2s to bind biogenic amines -.

Comparison of AnSt-D7L1 C-terminal domain and Anopheles gambiae D7r4 published structures (Alvarenga et al., 2010; Mans et al., 2007). A ribbon diagram showing the position of the eight helices (A2-H2) at the C-terminal domain of (A) AnSt-D7L1 and (B) An. gambiae D7r4 containing serotonin. Disulfide bonds are shown as yellow sticks and disulfide bound 4 is labeled as DS4. Detailed view of the amino acids lining (C) AnSt-D7L1 C-terminal domain region and the (D) D7r4 serotonin binding pocket, showing that in AnSt-D7L1 (C) helix H2 is changed in its position and B2 is unwound with R177 partially occupying of the position of serotonin in D7r4 (D). Serotonin is colored magenta, oxygen atoms are shown in red and nitrogen in blue. Hydrogen bonds are represented as dashed red lines.
Alignment of D7L sequences from twelve anopheline species (Fig. S1) shows that only the D7L1 and D7L2 belonging to subgenus Cellia contain the C-terminal domain rearrangement that includes the loss of DS4 as described above (Fig 2A and C), while their orthologs in the subgenera Anopheles and Nyssorhynchus contain DS4, as do anopheline biogenic amine-binding D7S proteins such as An. gambiae D7r4 (Fig. 2B and D) and AeD7L1 from Ae. aegypti. This suggests that D7L from the subgenera Anopheles and Nyssorhynchus contain a functional binding pocket and would be able to bind biogenic amines.
3.3. AnDar-D7L2 binds serotonin with high affinity.
One such D7L molecule predicted to bind biogenic amines is AnDar-D7L2 from An. darlingi (Nyssorhynchus), one of the main malaria vectors in the Americas. We produced recombinant AnDar-D7L2 and assayed its ability to bind serotonin and other biogenic amines. Results of isothermal titration calorimetry (ITC) experiments showed high affinity binding (KD = 17 nM) and relatively high selectivity for serotonin (Fig. 3A). Tryptamine, an indoleamine differing from serotonin only in the absence of a hydroxyl group at position 5 of the indole component, bound 30-fold less tightly (KD = 518 nM) (Fig. 3B) suggesting that protein interactions with the 5-hydroxyl are important. Binding of the catecholamines octopamine, dopamine and norepinephrine (Fig. S2 A–C) was also measurable, but the affinities were considerably lower than for serotonin, suggesting that these are not preferred ligands for the protein. No detectable binding was observed for either epinephrine or histamine (Fig. S2 D–E). These results show that anopheline D7L proteins outside of the subgenus Cellia can bind biogenic amines in the manner of D7S proteins, while biogenic amine binding capability has been lost in the D7L1 and D7L2 proteins of subgenus Cellia. The functional similarity of the AnDar-D7L2 to Ae. aegypti (Calvo et al., 2006; Calvo et al., 2009) and Ae. albopictus (Martin-Martin et al., 2020b) D7L proteins suggests that biogenic amine binding by the C-terminal domain is the ancestral condition existing before the split of culicines and anophelines about 150 MYA, but was lost in the Cellia subgenus of Anopheles.
Figure 3. AnDar-D7L2 binds serotonin, cysteinyl leukotrienes and the TXA2 analog U46619 with high affinity and inhibits TXA2-mediated platelet aggregation.
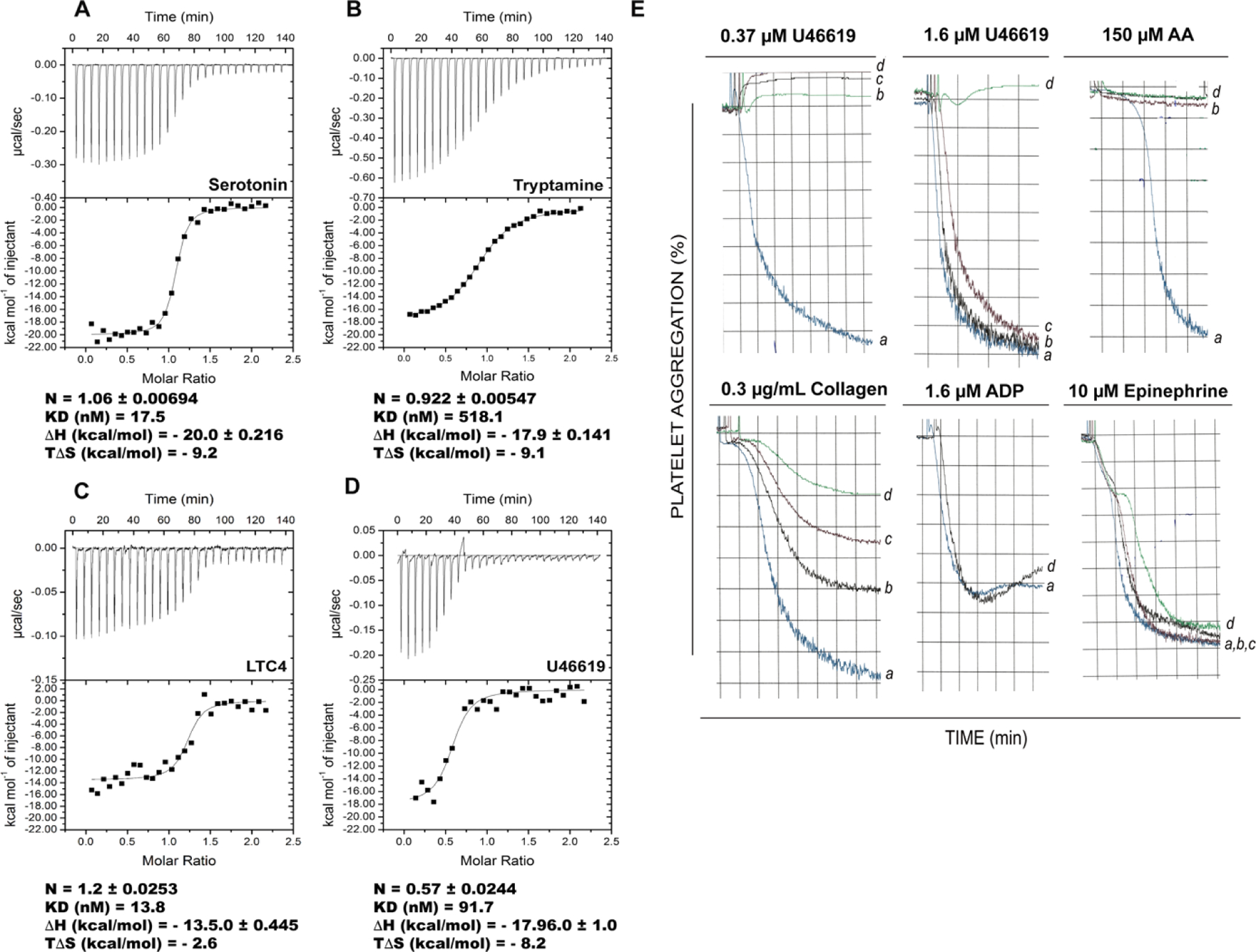
Binding of AnDar-D7L2 to (A) serotonin, (B) tryptamine, (C) LTC4 and (D) U46619 as measured by ITC. The calorimeter cell was filled with recombinant AnDar-D7L2, and experiments were performed at 30°C with successive 10 µL injections of ligand solution. Raw data (measured heats of each injection) are shown in the upper graph of each panel, while binding isotherms along with fitting using single binding site model are shown in lower panels. Binding to serotonin and to U46619 was investigated with 4μM protein and 40μM of ligand, binding to tryptamine was performed with 10 μM protein and 100 μM ligand, and binding to LTC4 was studied with 2 μM protein and 20 μM ligand. Thermodynamic parameters with standard errors were calculated based on each curve using MicroCal software package (Origin 7). Results obtained with other ligands are shown in Fig. S2. (E) The biological significance of AnDar-D7L2 ability to bind U46619 (a TXA2 analog) was further studied by investigating the effect of the recombinant protein on platelet-rich plasma aggregation assays. Platelet-rich plasma was incubated with different concentrations of AnDar-D7L2 for one minute, followed by addition of the respective agonist as shown in the top of each graph. Typical tracings are depicted for each protein concentration as indicated by letters: (a) 0 μM, (b) 0.6 μM, (c) 2 μM and (d) 6 μM. AA: arachidonic acid.
3.4. AnDar-D7L2 binds cysteinyl leukotrienes and thromboxane A2
Like other previously characterized D7L1/D7L2 proteins from culicine and anopheline mosquitoes, AnDar-D7L2, in addition to biogenic amines, binds the cysteinyl leukotrienes (Cys-LTs) LTC4 (Fig. 3C), LTD4 and LTE4 (Fig. S2 F and G) with high affinity as well as the thromboxane A2 (TXA2) analogue U46619 (Fig. 3D) but is unable to bind LTB4 (Fig. S2 H). This ability to bind cysteinyl leukotrienes and U46619 was observed previously in AnSt-D7L1(Alvarenga et al., 2010). Cysteinyl leukotrienes are eicosanoid mediators of inflammatory responses in the skin (Bisgaard et al., 1985; Camp et al., 1983; Soter et al., 1983) and other tissues, while TXA2 is a strong secondary agonist of collagen-mediated platelet activation (Francischetti, 2010; Moers et al., 2004; Nakahata, 2008). Both eicosanoid types also modulate smooth muscle contraction and regulate vascular tone (Boyce, 2005; Findlay et al., 1981; Nakahata, 2008). As would be expected from its thromboxane binding function, AnDar-D7L2 inhibits platelet activation and aggregation induced by collagen (at low concentrations), U46619 and the eicosanoid precursor arachidonic acid (AA) (Fig. 3E). Importantly, while TXA2 analogues were substituted for TXA2 in ITC experiments due to lability of natural TXA2, inhibition of platelet activation in the presence of collagen and arachidonic acid (AA) demonstrates the binding by AnDar-D7L2 of natural TXA2 that is produced and secreted by activated platelets (Fig. 3E). In this way AnDar-D7L2 behaves similarly not only to An. stephensi AnSt-D7L1 (Alvarenga et al., 2010) but also to Ae. aegypti, Ae. albopictus and Phlebotomus duboscqi (sand fly) D7L proteins previously described (Calvo et al., 2009; Jablonka et al., 2019; Martin-Martin et al., 2020b). AnDar-D7L2 did not inhibit ADP or epinephrine-induced platelet aggregation (Fig. 3E) consistent with its inability to bind these ligands in ITC experiments (Fig. S2 D and I).
3.5. The structure of AnDar-D7L2
We examined ligand-protein interactions of the N- and C-terminal domains by determining crystal structures of AnDar-D7L2 in the absence and presence of ligands (Fig. 4A and B, Table S2). The structures contain the two odorant-binding protein family domains seen in previously determined D7L structures that are arranged in a similar manner (Fig. 4A–C). The N-terminal domain contains seven α-helical elements designated A-G and is stabilized by two disulfide bonds (labeled DS1 and 2). It also contains the lipophilic channel (Fig. 4D) shown in previous studies with AnSt-D7L1 (Alvarenga et al., 2010) to make up the binding site for eicosanoid compounds (Fig. 4E). The C-terminal domain has a structure that is similar to those of AeD7L1 and D7r4 (Calvo et al., 2009; Mans et al., 2007) with eight distinct helical elements designated A2-H2. Unlike AnSt-D7L1 (Alvarenga et al., 2010) from An. stephensi, helix B2 is not unwound in the ligand-free form, positioning Cys 175 to form a disulfide bond with Cys 296 (DS4) of the terminal helix H2 and creating the large pocket that is suitable for binding serotonin (Fig. 4B and F) and other biogenic amines. In Ae. aegypti D7L1 the terminal helix H2 is unwound in the unliganded form and becomes ordered in the presence of ligands. It was proposed that this change acts as a structural switch that allows entry and high affinity binding of catecholamines in addition to serotonin (Calvo et al., 2009). This unwinding does not occur in the crystal of ligand-free AnDar-D7L2 (Fig. 4A), where H2 is positioned similarly to D7r4 (Fig. 2B) and ligand-bound AeD7L1. This would be consistent with the relatively low-affinity binding for catecholamines observed here.
Figure 4. AnDar-D7L2 structures and comparison with structures of Ae. aegypti AeD7L1, An. stephensi AnSt-D7L1 and An. gambiae D7r4.

Ribbon diagram of (A) AnDar-D7L2 crystallized in the absence of ligands, (B) AnDar-D7L2 co-crystallized with U46619 (white sticks labeled with U) and serotonin (green, labeled S), and (C) AeD7L1 (PDB: 3DYE) with norepinephrine (yellow sticks labeled as N). On panels A-C the N-terminal domain is colored in dark blue with helices labeled as A-G, while C-terminal domain is colored in cyan with helices labeled as A2-H2. Disulfide bonds are shown as yellow sticks and labeled as DS1–5. (D-E) The N-terminal eicosanoid binding pocket of (D) AnDar-D7L2 and (E) AnSt-D7L1from superimposed views, showing details of their interaction with U46619 (shown in white and indicated as U in both panels) and the amino acid residues lining their respective pockets are labeled and colored blue. (F) C-terminal binding pocket of the AnDar-D7L2-serotonin complex showing interactions with the ligand (represented in green, indicated as S) at the C-terminal domain. Residues lying the pocket and interacting with serotonin are labeled and their carbon atoms are colored cyan. (G) Binding interactions between D7r4 and serotonin, represented in magenta, labeled as S (PDB: 2QEH). Residues lining the pocket and interacting with serotonin are labeled and their carbon atoms are colored light grey. In all panels, ligands are represented as sticks and labeled as U: U46619, S: serotonin, N: norepinephrine. In all stick representations oxygen atoms are shown in red and nitrogen in blue. Hydrogen bonds are represented as dashed red lines.
The binding modes of TXA2 and biogenic amines were examined by crystallizing AnDar-D7L2 in the presence of both the TXA2 analog U46619 and serotonin (Fig. 4B). The ligands were considered to have separate binding sites based on structural homology with previously studied D7L proteins and by the fact that binding affinities for both ligands were not reduced in ITC experiments performed in the presence of saturating concentrations of the second ligand (Fig. S3). Overall, the structures showed that no major rearrangements occur on binding of the ligands, and electron density for both compounds was visible in the expected binding site for each; the N-terminal domain for U46619 and the C-terminal domain for serotonin (Fig. 4B). The ligand binding mode and binding site for U46619 are similar to those described for the same ligand in AnSt-D7L1 (Alvarenga et al., 2010). The amino acid residues in the pocket are highly conserved between the two proteins with Tyr 16, Trp 41, Val 57, Leu 61, Leu 58, Thr 54, Tyr 53, Trp 38, Leu 13 and Lys 153 (with numbering based on the AnDar-D7L2 structure) being identical between the two (many of these residues are seen in Fig. 4 D and E). Importantly, Tyr 53, which forms a hydrogen bond with the aliphatic hydroxyl group of U46619 (and presumably TXA2) and Lys 153, which forms a salt bridge with the eicosanoid carboxyl, are present. (Fig. 4 D). The similarity in the N-terminal binding pocket between AnSt-D7L1 and AnDar-D7L2, along with a high degree of binding pocket sequence conservation among D7L2 proteins from other Anopheles species (Fig. S1) suggests that D7L2 proteins throughout the genus would show similar eicosanoid binding properties.
The binding pocket of the C-terminal (Fig. 4F) domain is similar to that of D7r4 (Fig. 4G) (Mans et al., 2007) and AeD7L1 (Calvo et al., 2009) as is the binding mode of the serotonin ligand, but significant differences are present that may affect ligand selectivity and affinity. The binding site exists as a space surrounded by side chains of Arg 178, Tyr 180 and Phe 158 (Fig. 4F). This arrangement is conserved in AnDar-D7L2 and D7r4 with the exception that Phe 158 is replaced by Val 3 in D7r4, resulting in a less spacious pocket in AnDar-D7L2 (Fig. 4F and G). Additionally, His 35, positioned at the interior side of the pocket in D7r4, forms a hydrogen bond with the 5-hydroxyl group of serotonin. This residue is replaced by Met 191 in AnDar-D7L2 resulting in loss of a hydrogen bond. However, both proteins contain a glutamate residue at the margins of the pocket (position 162 in AnDar-D7L2 and position 7 in D7r4) that forms a hydrogen bond with the 5-hydroxyl (Fig. 4F, G). Both also contain an aspartate residue (position 261 in AnDar-D7L2 and position 111 in D7r4) at the apparent entry of the pocket that forms a salt bridge with the ionized amino group of serotonin (Fig. 4F, G). A hydrogen bond between the indole nitrogen atom and the hydroxyl group of Tyr 244 of AnDar-D7L2 is also present as it is in D7r4. The presence of the Met 191 side chain also tilts the indole nucleus of serotonin relative to its position in D7r4 (see views in Fig. 4F, G), but not enough to disrupt any of the existing hydrogen bond or salt bridge interactions. (Fig. 4F and G).
Together, the ITC (Fig. 3 A and B) and structural results (Fig. 4) show that, unlike AnSt-D7L1 and other D7L1 and D7L2 proteins from the subgenus Cellia, AnDar-D7L2 is capable of binding biogenic amines in its C-terminal domain, similarly to both Aedes aegypti D7L (Calvo et al., 2009; Martin-Martin et al., 2021) and to single domain D7S (D7r1-D7r4) from An. gambiae (Calvo et al., 2006). In addition, it shows the capability to bind eicosanoids at a site in its N-terminal domain (Fig. 3 C –E, Fig. S2 F and G). This bifunctional protein would act as an anti-hemostatic and anti-inflammatory molecule with distinct binding sites for serotonin and eicosanoids (Fig. 4 and Fig. S3).
3.6. D7L1 and D7L2 proteins from subgenus Anopheles
After establishing that AnDar-D7L2 is capable of binding both serotonin and eicosanoid compounds, we examined sequences from two members of the subgenus Anopheles, An. atroparvus and An. sinensis. Both species contain a tandem duplication at the D7L locus producing D7L1 and D7L2, rather than the single gene seen in An. darlingi and other members of the Nyssorhynchus subgenus which contain only D7L2 (Fig. 1 and Table S1). Examination of the C-terminal domains of these protein sequences (Fig. S1) shows conservation of the three disulfide bonds (DS3, DS4 and DS5) found in Ae. aegypti D7L1, AnDar-D7L2, and D7r4 as would be consistent with the presence of a functional biogenic amine binding pocket. Additionally, the sequences of An. atroparvus and An. sinensis D7L1 and L2 (Fig. S1 A and B) show the presence of the key ligand-interacting amino acids, Tyr 241 which forms a hydrogen bond with the indole nitrogen of serotonin and the two acidic residues Asp 258 and Glu 261 (numbered according to AnAtr-D7L1) which form salt bridge interactions with the ligand amino group. However, in both An. atroparvus D7L1 (AnAtr-D7L1) and An. sinensis D7L1 (AnSin-D7L1) the residue at position 159, which is normally a glutamate that forms a hydrogen bond with the serotonin hydroxyl group, is changed to alanine (Fig. S1A), making it unclear if the protein would be capable of binding serotonin or other biogenic amines. A structural model of AnAtr-D7L1, constructed using AlphaFold2, was consistent with the presence of an open pocket capable of binding biogenic amines but lacking the residues mentioned above that are responsible for hydrogen bonding of the serotonin hydroxyl group.
To resolve the question of whether a variant D7L with a large, normally structured biogenic amine binding pocket but missing potentially important hydrogen bonding groups would bind these ligands, we produced recombinant AnAtr-D7L1 and examined its ability to bind biogenic amines and eicosanoids by ITC (Fig. 5). Interestingly, despite the substitution of glutamate at position 159 and histidine at position 188 with alanine, the protein binds serotonin with high affinity (Kd ~ 3 nM), suggesting that hydrogen bonding of the 5-hydroxyl group can be dispensed with under some circumstances (Fig. 5A). This was confirmed by the observation that AnAtr-D7L1 binds tryptamine, which differs from serotonin only by the absence of the 5-hydroxyl group, with equally high affinity (Fig. 5B), while serotonin binds 30-fold more tightly than tryptamine in AnDar-D7L2 (Fig.3 A and B). This pattern of ligand selectivity would be consistent with hydrogen bonding of the 5-hydroxyl by Glu 162 in AnDar-D7L2 (Fig. 4F) and suggests that An. atroparvus D7L1 stabilizes serotonin by a mechanism not involving the 5-hydroxyl group. The structural model containing serotonin placed in the binding mode seen in the crystal structure of D7r4 shows that Trp 163 fills much of the binding pocket space left when the hydrogen bonding side chains discussed above are reduced in size to alanine in AnAtr-D7L1 (Fig. 5E). All other notable binding pocket residues are positioned nearly identically to other biogenic amine binding D7 forms. The AnAtr-D7L1 binding pocket is also unusually specific for serotonin and tryptamine, as no significant binding was observed for any other biogenic amine compound tested (Fig. S4 A–E).
Figure 5. Functional characterization of AnAtr-D7L1 by ITC and analysis of its structural model.

Binding of AnAtr-D7L1 to (A) serotonin, (B) tryptamine, (C) LTC4 and (D) U46619 was analyzed by ITC on a MicroCal VP-ITC instrument. The calorimeter cell was filled with recombinant AnAtr-D7L1 (4 μM), and experiments were performed at 30°C with successive 10 µL injections of ligand solution (40 μM in the syringe). Data were analyzed as in Fig. 3. Results obtained with other ligands are shown in Fig. S4. (E) Serotonin binding pocket structure of an AnAtr-D7L1 model generated using AlphaFold2 (residues labeled in black and represented with carbon atoms colored green) with the structure of An. gambiae D7r4 (residues labeled in blue and represented with carbon atoms in light grey) bound to serotonin shown in magenta (PDB: 2QEH). Oxygen atoms are colored red, nitrogen atoms blue and hydrogen bonds are represented as red dashed lines. (F) Superimposed view of the N-terminal binding site region around helices A and B from the AnAtr-D7L1 model (green) and AnDar-D7L2 (blue) bound to U46619, showing in detail Arg 11 in AnAtr-D7L1 in place of leucine, as well as Phe 50 in AnAtr-D7L1 in place of the Tyr 53 in AnDar-D7L2 shown to be important to stabilize U46619 in the binding pocket by forming a hydrogen bond (red dashed line) with its ω−5 hydroxyl.
In its N-terminal domain, AnAtr-D7L1 has some modifications of residues shown to be important for eicosanoid binding by some D7L, including AnDar-D7L2, as well as AnSte-D7L1 and Aedes aegypti D7L (Fig. S1A). Although Cys-LT binding is detectable, it is weak (Fig. 5C and Fig. S4 F and G) when compared to previously characterized eicosanoid-binding D7L such as AnSt-D7L1 (Alvarenga et al., 2010). This could be due to the presence of an unusual arginine residue at position 11 which may interfere with binding of the peptide fragment of Cys-LTs (Fig. 5F and Fig. S1 A). AnAtr-D7L1 does not bind TXA2 analog U46619 (Fig. 5D), consistent with the presence of phenylalanine rather than tyrosine at position 50 which is necessary for hydrogen bonding of the aliphatic hydroxyl group of TXA2. Despite having a well-formed binding pocket in its N-terminal domain, the affinity of AnAtr-D7L1 for cysteinyl leukotrienes was lower than other D7L forms, leading us to investigate its ability to bind other eicosanoids such as LTB4 and PGF2α, or 15-HETE. No binding was detected with any of these compounds leaving the existence of a high affinity eicosanoid ligand for this protein an open question (Fig. S4 H–J).
3.7. D7L3 a third D7L protein distinct from D7L1/D7L2.
The genome and salivary transcriptome of An. gambiae show the presence of a third D7L gene that is expressed at much lower levels than the D7L1/L2 homologs of AnSt-D7L1 and lies directly adjacent to the subcluster of D7S genes (Fig. 1). The Ae. aegypti D7L cluster does not contain an ortholog of this sequence, suggesting that D7L3 appeared after divergence of the anopheline and culicine mosquitoes (Fig. 1). Comparison of D7L3 sequences in the available genomes of Anopheles species show that it is conserved throughout the genus (Table S1 and Fig. S1C). Like D7L1/D7L2, D7L3 contains two OBP-like domains and based on the sequence criteria discussed above, the C-terminal domain was considered likely to bind biogenic amines, even in Cellia species (Fig. S1C) where the D7L1/D7L2 proteins do not bind these ligands. We produced recombinant An. gambiae D7L3 and found it to bind serotonin with high affinity (Kd = 22 nM) and ligand selectivity (Fig. 6A and Fig. S5A–I). It binds histamine and norepinephrine with low affinity (Kd = 1.53 and 2.53 μM, respectively) but not any other biogenic amine tested (Fig. S5A–E), including the serotonin derivative tryptamine (Fig. 6B). A molecular model constructed with AlphaFold2 shows a binding pocket in the C-terminal domain (Fig. 6E–G) that is almost identical to that of biogenic amine binding D7S protein D7r4 (PDB: 2QEH), which is consistent with the observation of strong serotonin binding. The α-helix H2 is present in the same position as in D7r4, AnDar-D7L2 and AeD7L1 and disulfide bond DS4 is present. Asp 280 and Glu 283 are in position to form hydrogen bonds with the amino group of serotonin, while Glu 173 and His 202 form hydrogen bonds with the hydroxyl group (Fig. 6G). Tyr 263 forms a hydrogen bond with the indole nitrogen and Tyr 190, Arg 188 and Phe 279 surround the ligand in the same manner as in D7r4. The C-terminal binding pocket of D7L3 most closely resembles that of the anopheline D7S proteins, while the D7L1/L2 proteins of Anopheles are more diverse in the structure of the C-terminal domain binding site but generally maintain the ability to bind biogenic amines.
Figure 6. Functional characterization of An. gambiae D7L3 by ITC and analysis of its structural model.

Binding of An. gambiae D7L3 to (A) serotonin, (B) tryptamine, (C) LTC4 and (D) U46619 was analyzed by ITC on a MicroCal VP-ITC instrument. The calorimeter cell was filled with recombinant D7L3 (4 μM), and experiments were performed at 30°C with successive 10 µL injections of ligand solution (40 μM in the syringe) with 20 seconds duration and spacing of 300 seconds between injections. Data were analyzed as in Fig. 3. Results obtained with other ligands are shown in Fig. S5. (E) Superimposed view of the N-terminal domain of the AlphaFold2 model of D7L3 (teal) with solved structures AnDar-D7L2 (blue) and AnSt-D7L1 (magenta, PDB: 3NHT) bound to U46619 (represented in sticks, with carbons in white and oxygens in red). The insertion loop present in D7L3 as indicated in the figure, occupies part of the N-terminal pocket, explaining why D7L3s are unable to bind eicosanoids. N-terminal helices A and B are labeled. (F) Ribbon diagram comparing the position of the helices (labeled A2-H2) of the C-terminal domain of modeled D7L3 (deep teal) with published structure of An. gambiae D7r4 (light grey) bound to serotonin (magenta sticks). Disulfide bonds are shown in yellow and labeled as DS1–5, corresponding to its order in the structure. (G) Detailed view of the interaction of An. gambiae D7r4 (light grey) with serotonin (PDB:2QEH) superimposed with the C-terminal region of the D7L3 AlphaFold2 model showing the putative C-terminal serotonin binding pocket (teal), demonstrating the conservation of key amino acids lining the binding pocket occupying practically the same positions in both proteins. Key residues present in the pocket are represented as sticks and labeled (position numbers based on D7L3 sequence), serotonin is represented in magenta, oxygen and nitrogen atoms are represented in red and blue, respectively. Hydrogen bonds between An. gambiae D7r4 and serotonin are represented as dashed red lines.
Sequence comparisons suggest that the N-terminal domain of D7L3 proteins is structurally different from the D7L1/D7L2 forms in regions involved with eicosanoid binding (Fig. S1C). Our molecular model suggests that a lengthening of the loop between helical elements A and B of the N-terminal domain results in occlusion of the N-terminal pocket at its entry (Fig. 6E). Additionally, a lysine residue conserved in eicosanoid binding D7 forms (including AnDar-D7L2 and AnSt-D7L1), which forms a salt bridge with the ligand carboxyl group is changed to proline (Pro 164). ITC experiments confirmed this prediction, showing that none of the compounds from our eicosanoid test panel, or the platelet agonist ADP, bound to this protein (Fig. 6C, D, Fig. S5 F–I).
3.8. D7S proteins: Biogenic amine binding and relationship of anopheline forms to orthologs from culicine mosquitoes
The D7S locus in Anopheles sp. is located near the D7L1/L2 genes and immediately adjacent to the D7L3 gene (Fig. 1). They are arranged as a cluster containing as many as six duplicate genes. As discussed above, D7S proteins in Anopheles are structurally similar to the C-terminal domains of AeD7L1 and AnDar-D7L2 and in a manner consistent with this structural similarity, bind biogenic amines. Structures of the D7S protein known as D7r4 from An. gambiae have been determined in complex with several biogenic amine ligands (Mans et al., 2007) and their ligand binding modes were also found to be similar to those in the C-terminal domains of biogenic amine binding D7L proteins. Duplication of D7S genes has apparently acted to increase the biogenic amine binding capacity of the anopheline saliva through gene dosage effects, although functional differentiation among the An. gambiae D7S proteins has also resulted in preferred binding to different biogenic amines by specific D7S forms (Calvo et al., 2006). In the subgenus Cellia, the D7L1/L2 proteins have lost the ability to bind biogenic amines (Alvarenga et al., 2010) and the D7L3 gene is expressed at low level, suggesting that in this subgenus D7S proteins are the primary salivary binders of serotonin and other biogenic amines.
We asked if biogenic amine binding is a unique feature of anopheline forms of D7S by comparing them with representatives of the apparently orthologous clusters in the culicine mosquitoes Ae. aegypti and Culex quinquefaciatus. Culicine D7S genes form a cluster linked to the D7L genes and are expressed in the salivary gland of females of Aedes (Arca et al., 2007; Ribeiro et al., 2007), Culex (Ribeiro et al., 2004) and Psorophora species (Chagas et al., 2013). They contain the same number of disulfide bonds as their anopheline counterparts (three), but amino acid sequence alignments show them to be shortened in the C-terminal region relative to anopheline D7S proteins (Fig. S6).
We produced recombinant D7S forms from Ae. aegypti (AeD7S1) and Culex quinquefasciatus (D7CQS1) and evaluated their capacity to bind biogenic amines. Neither AeD7S1 or D7CQ1 showed any detectable binding of serotonin (Fig. 7 A, B). We then crystallized both proteins and determined their structures by X-ray diffraction (Fig.7C, D, Table S2). While the D7S proteins from culicine mosquitoes share with anophelines most secondary structure elements (Fig.7C–E), they do not contain the distinctive biogenic amine binding pocket structure seen in D7r4 (Fig. 7E), AeD7L1 or AnDar-D7L2 (Fig. 4). In AeD7S1 (Fig. 7C), α-helices A2-G2 are positioned almost identically to An. gambiae D7r4 but the protein is truncated after helix G2 and does not contain an extended helix H2, which makes up one side of the biogenic amine binding pocket of An. gambiae D7r4 (Fig. 7E). Rather, helix G2 bends at its C-terminus allowing this region to form disulfide bond DS4 (numbered by homology with D7r4) between Cys 126 and Cys 20 of helix B2 resulting a filling of the binding pocket region with the side chains of Phe 120 and Phe 121 along with that of Phe 4 (Fig. S8A). D7CQ1 has similar structure to AeD7S1 and is also truncated C-terminal to helix G2 (Fig. 7D). In this case the side chains of Leu 34 and Tyr 46 fill the pocket along with an extension of the N-terminus that passes through the space occupied by the ligand in D7r4 (Fig. S8B). It appears that D7S proteins have either gained the ability to bind biogenic amines in anopheline mosquitoes or lost the ability in culicines. It has been previously noted that D7S proteins from Ae. aegypti and An. gambiae form distinct interspecific clades making clear orthologs in the two subfamilies difficult to identify (Ribeiro et al., 2007). It was suggested that gene conversion events have occurred in either anophelines or culicines leading to sequence divergence between the two groups. Based on structural and functional data presented here we extend this by suggesting that anopheline D7S forms have acquired a biogenic amine binding structure through gene conversion events involving D7L genes, possibly the highly conserved and directly adjacent D7L3 gene.
Figure 7. Structural and functional comparison of culicine and anopheline D7S proteins.

(A-B) Lack of serotonin binding by culicine D7S as measured by by ITC on a MicroCal VP-ITC instrument. The calorimeter cell was filled with 4 μM of recombinant (A) Ae. aegypti AeD7S1 or (B) Cu. quinquefasciatus D7CQS1, and experiments were performed at 30°C with successive 10 µL injections of serotonin solution (40 μM in the syringe) with 20 seconds duration and spacing of 300 seconds between injections. (C-F) Comparison of culicine and anopheline D7S structures. The crystal structures of recombinant (C) Ae. aegypti AeD7S1 and (D) Culex quinquefasciatus D7CQS1 were determined and compared with that of (E) An. gambiae D7r4 bound to serotonin. Unlike An. gambiae D7r4, and other anopheline D7S (r1-r4), culicine D7S do not have a well-formed biogenic amine binding pocket in accordance with their inability to bind serotonin observed on ITC experiments. (F) AlphaFold2 modeling of one of the An. darlingi shortened D7S (sequence AndarD7SS in Figure S6B), showing that similarly to culicine D7S, it lacks helix H and therefore a properly structured serotonin binding pocket. In all models helices are labeled A2-H2 and disulfide bonds are shown in yellow and labeled as DS followed by a number, corresponding to its order in the structure with respect to the numbers assigned to the corresponding DS in the D7L to facilitate comparison).
As a final note we observed that the D7S subclusters of species in the Cellia subgenus of Anopheles contain more genes encoding proteins with clear biogenic amine binding signatures than do members of the Nyssorhynchus and Anopheles subgenera (Fig. 1, Table S1 and Fig. S7). All Cellia species, except those belonging to series Neomyzomyia (that have lost D7r4), contain five D7S genes, with D7r1-D7r4 showing conservation of all the critical residues for serotonin binding discussed above Fig. S7. The D7r5 protein from An. gambiae is known not to bind biogenic amines (Calvo et al., 2006) and is expressed at low levels but is conserved in all Anopheles species (Table S1). The subgenus Nyssorhynchus represented by An. darlingi and An. albimanus has only one apparent biogenic amine binding D7S gene, along with D7r5 (Table S1) and 2–3 shortened D7S genes (Fig. 1, represented in yellow boxes) that have some features of the D7S genes from culicine mosquitoes. Modeling with AlphaFold2 (Jumper et al., 2021) indicates that these are missing helix H2 but maintain all three disulfide bonds found in biogenic amine binding D7S forms (Fig. S6B), resulting in an incompletely formed binding pocket that would not accommodate serotonin or other biogenic amines (Fig. 7F). Members of the subgenus Anopheles possess two D7S genes encoding proteins with features suggesting they are capable of binding biogenic amines in addition to D7r5 (Fig.1, Table S1 and Fig. S7). The fact that the Nyssorhynchus and Anopheles subgenera contain fewer putative biogenic amine-binding D7S genes than Cellia may be a reflection of the fact that the D7L1 and D7L2 proteins are capable of binding biogenic amines in the former two subgenera. The more extensive duplication in the Cellia may have occurred to compensate for the loss of biogenic amine binding activity in D7L1 and D7L2. We do not know why the D7L1 and L2 proteins from Cellia have lost the ability to bind serotonin in the C-terminal domain. Perhaps a new, unidentified function has been gained by these proteins that represents another step in the evolution of D7 function in mosquitoes. It is also possible that host immunity could play a role in increase the diversity since some salivary proteins can be immunogenic (Londono-Renteria et al., 2018; Malafronte Rdos et al., 2003; Oseno et al., 2022). Nevertheless, we believe that this is very unlikely since mosquitoes feed quickly and there is no evidence in the literature showing differential regulation of gene duplicate expression to avoid or escape immune response.
4. Conclusions
The salivary D7 proteins are present in all examined species of culicine and anopheline mosquitoes and throughout this taxonomic group they function to bind eicosanoid and biogenic amine compounds in host blood and tissues. A biogenic amine binding function is found in both D7L and D7S forms, with the D7L proteins binding these ligands only in the C-terminal domain. Salivary protein genes mutate more rapidly than conserved housekeeping genes and functional diversification appears to be coupled with extensive gene duplication. In the D7 genes, this rapid evolution has resulted in significant structural and functional differences between species (Andersen, 2010; Arca et al., 2017). Here we focused on D7 genes from the genus Anopheles and used structural and functional features of these proteins along with sequence information in an attempt to unravel the evolution of this group of proteins. The first characterized anopheline D7L protein, AnSt-D7L1 from An. stephensi, was found to have a modified C-terminal domain that does not bind serotonin or other biogenic amines (Alvarenga et al., 2010). We show here that this modification is restricted to the subgenus Cellia and that D7L1 and D7L2 proteins from the subgenera Nyssorhynchus and Anopheles are capable of binding serotonin in a manner similar to that described for Ae. aegypti D7L1 and 2 and the D7S proteins from An. gambiae (Calvo et al., 2006; Calvo et al., 2009; Mans et al., 2007; Martin-Martin et al., 2021). This indicates that loss of serotonin binding in D7L proteins is not a general feature of anopheline forms but only occurs in the subgenus Cellia. The ability of both anopheline and culicine D7L proteins to bind eicosanoids and serotonin strongly suggests that these functions were present in the common ancestor of these two subfamilies which diverged approximately 150 MYA. We also show here that the D7S proteins from two culicine species do not bind biogenic amines as opposed to the D7S proteins from An. gambiae and by inference from sequence characteristics, other anopheline species. It appears that the common ancestor of anophelines and culicines did not contain biogenic amine binding D7S proteins and that the anopheline forms gained this function after the splitting of the two groups. Anopheline species contain a second type of D7L protein, D7L3, that has no clear ortholog in culicine mosquitoes but is completely conserved in anophelines. Like D7L1 and D7L2 this protein has two OBP domains, while D7L3 N-terminal domain has lost the ability to bind eicosanoid compounds, modeling indicates that the C-terminal domain contains a biogenic amine binding pocket that is essentially identical to that of the D7S proteins, and whose functionality was confirmed by ITC. This similarity and the proximity of the D7L3 gene to the D7S genes leads us to suggest that anopheline D7S proteins may have gained biogenic amine binding capability through a gene conversion mechanism involving D7L3. The importance of high concentrations of biogenic amine binding proteins in the saliva blood feeders is suggested by high levels of expression of various biogenic amine binding proteins in the saliva of many different species of blood feeding insects and ticks. Different protein families have been recruited to perform this function in each taxonomic group, underscoring the selective pressure exerted to derive this function in unrelated types of proteins.
Supplementary Material
Highlights:
Gene duplication was pivotal for salivary proteins evolution and neofunctionalization.
Long form D7 proteins 1 and 2 (D7 L1/L2) expressed in subgenus Cellia cannot bind biogenic amines.
D7L1/L2 from subgenus Anopheles and Nyssorhynchus kept the ability to bind serotonin.
D7L3 forms are conserved and retained the ability to bind serotonin, even in Cellia.
Culicine and anopheline common ancestor’s D7 short could not bind biogenic amines.
Acknowledgements
The authors thank the staffs of the Southeast Regional Collaborative Access Team and the Structural Biology Center, Advanced Photon Source, Argonne National Laboratory for assistance with X-ray data collection. This work utilized the computational resource of the NIH HPC Biowulf cluster (https://hpc.nih.gov/).
Funding:
This work was funded by the intramural program of the NIAID, National Institutes of Health. P.H. Alvarenga was supported, in part, by a grant from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ. Use of the Advanced Photon Source SER-CAT beamlines was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. W-31-109-Eng-38. The funders had no role in the in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Some results shown in this report are derived from work performed at Argonne National Laboratory, Structural Biology Center (SBC) at the Advanced Photon Source. SBC-CAT is operated by UChicago Argonne, LLC, for the U.S. Department of Energy, Office of Biological and Environmental Research under contract DE-AC02-06CH11357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations:
Experiments using human blood were performed in accordance with Institutional guidelines and the agreement of the Ethical Committee of the Johns Hospital University School of Medicine.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary data files.
Structural data have been deposited at PDB (https://www.rcsb.org/) with accession numbers shown in the methods section.
Declaration of competing interest
The authors declare that they have no competing interests.
Aedes aegypti D7L1, was originally named AeD7 on its first characterization paper.
An. stephensi D7L1 (AnSt-D7L1) was originally named as L1, since it was the first D7L characterized in this species. Nevertheless posterior knowledge regarding other species genomes and phylogenetic analysis of different D7 protein family members, show that this protein can be rather classified as a D7L2 than L1.
The D7L2 shown in Anopheles stephensi represents the protein previously named AnSt-D7L1.
References
- Abdeladhim M, Kamhawi S, Valenzuela JG, 2014. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect Genet Evol 28, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH, 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Joshi G, Nagar DP, Sharma AK, Sukumaran D, Pant SC, Parida MM, Dash PK, 2016. Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression. Infect Genet Evol 40, 126–135. [DOI] [PubMed] [Google Scholar]
- Almagro Armenteros JJ, Tsirigos KD, Sonderby CK, Petersen TN, Winther O, Brunak S, von Heijne G, Nielsen H, 2019. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 37, 420–423. [DOI] [PubMed] [Google Scholar]
- Alvarenga PH, Francischetti IM, Calvo E, Sa-Nunes A, Ribeiro JM, Andersen JF, 2010. The function and three-dimensional structure of a thromboxane A2/cysteinyl leukotriene-binding protein from the saliva of a mosquito vector of the malaria parasite. PLoS Biol 8, e1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JF, 2010. Structure and mechanism in salivary proteins from blood-feeding arthropods. Toxicon 56, 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JF, Francischetti IM, Valenzuela JG, Schuck P, Ribeiro JM, 2003. Inhibition of hemostasis by a high affinity biogenic amine-binding protein from the saliva of a blood-feeding insect. J Biol Chem 278, 4611–4617. [DOI] [PubMed] [Google Scholar]
- Andersen JF, Gudderra NP, Francischetti IM, Ribeiro JM, 2005. The role of salivary lipocalins in blood feeding by Rhodnius prolixus. Arch Insect Biochem Physiol 58, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JF, Ribeiro JMC, 2017. Salivary Kratagonists: Scavengers of Host Physiological Effectors During Blood Feeding. Arthropod Vector: Controller of Disease Transmission, Vol 2: Vector Saliva-Host-Pathogen Interactions, 51–63.
- Arca B, Lombardo F, Francischetti IM, Pham VM, Mestres-Simon M, Andersen JF, Ribeiro JM, 2007. An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem Mol Biol 37, 107–127. [DOI] [PubMed] [Google Scholar]
- Arca B, Lombardo F, Lanfrancotti A, Spanos L, Veneri M, Louis C, Coluzzi M, 2002. A cluster of four D7-related genes is expressed in the salivary glands of the African malaria vector Anopheles gambiae. Insect Mol Biol 11, 47–55. [DOI] [PubMed] [Google Scholar]
- Arca B, Lombardo F, Struchiner CJ, Ribeiro JM, 2017. Anopheline salivary protein genes and gene families: an evolutionary overview after the whole genome sequence of sixteen Anopheles species. BMC Genomics 18, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arca B, Lombardo F, Valenzuela JG, Francischetti IM, Marinotti O, Coluzzi M, Ribeiro JM, 2005. An updated catalogue of salivary gland transcripts in the adult female mosquito, Anopheles gambiae. J Exp Biol 208, 3971–3986. [DOI] [PubMed] [Google Scholar]
- Arca B, Ribeiro JM, 2018. Saliva of hematophagous insects: a multifaceted toolkit. Curr Opin Insect Sci 29, 102–109. [DOI] [PubMed] [Google Scholar]
- Arca B, Struchiner CJ, Pham VM, Sferra G, Lombardo F, Pombi M, Ribeiro JM, 2014. Positive selection drives accelerated evolution of mosquito salivary genes associated with blood-feeding. Insect Mol Biol 23, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assumpcao TC, Alvarenga PH, Ribeiro JM, Andersen JF, Francischetti IM, 2010. Dipetalodipin, a novel multifunctional salivary lipocalin that inhibits platelet aggregation, vasoconstriction, and angiogenesis through unique binding specificity for TXA2, PGF2alpha, and 15(S)-HETE. J Biol Chem 285, 39001–39012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Lerche A, Kristensen JK, 1985. Leukotriene- and histamine-induced increases in vascular permeability and interstitial transport in the skin. J Invest Dermatol 84, 427–429. [DOI] [PubMed] [Google Scholar]
- Boyce JA, 2005. Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications. Chem Immunol Allergy 87, 59–79. [DOI] [PubMed] [Google Scholar]
- Calvo E, Mans BJ, Andersen JF, Ribeiro JM, 2006. Function and evolution of a mosquito salivary protein family. J Biol Chem 281, 1935–1942. [DOI] [PubMed] [Google Scholar]
- Calvo E, Mans BJ, Ribeiro JM, Andersen JF, 2009. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci U S A 106, 3728–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp RD, Coutts AA, Greaves MW, Kay AB, Walport MJ, 1983. Responses of human skin to intradermal injection of leukotrienes C4, D4 and B4. Br J Pharmacol 80, 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas AC, Calvo E, Rios-Velasquez CM, Pessoa FA, Medeiros JF, Ribeiro JM, 2013. A deep insight into the sialotranscriptome of the mosquito, Psorophora albipes. BMC Genomics 14, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DE, 2005. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol Haemost Thromb 34, 221–227. [DOI] [PubMed] [Google Scholar]
- Cowtan K, 2006. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr 62, 1002–1011. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K, 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Findlay SR, Lichtenstein LM, Siegel H, Triggle DJ, 1981. Mechanisms of contraction induced by partially purified slow reacting substance from human polymorphonuclear leukocytes and leukotriene D in guinea pig ileal smooth muscle. J Immunol 126, 1728–1730. [PubMed] [Google Scholar]
- Francischetti IM, 2010. Platelet aggregation inhibitors from hematophagous animals. Toxicon 56, 1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM, 2009. The role of saliva in tick feeding. Front Biosci (Landmark Ed) 14, 2051–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Valenzuela JG, Pham VM, Garfield MK, Ribeiro JM, 2002. Toward a catalog for the transcripts and proteins (sialome) from the salivary gland of the malaria vector Anopheles gambiae. J Exp Biol 205, 2429–2451. [DOI] [PubMed] [Google Scholar]
- Gillespie RD, Mbow ML, Titus RG, 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol 22, 319–331. [DOI] [PubMed] [Google Scholar]
- Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL, 2006. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol 36, 322–335. [DOI] [PubMed] [Google Scholar]
- Jablonka W, Kim IH, Alvarenga PH, Valenzuela JG, Ribeiro JMC, Andersen JF, 2019. Functional and structural similarities of D7 proteins in the independently-evolved salivary secretions of sand flies and mosquitoes. Sci Rep 9, 5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka W, Pham V, Nardone G, Gittis A, Silva-Cardoso L, Atella GC, Ribeiro JM, Andersen JF, 2016. Structure and Ligand-Binding Mechanism of a Cysteinyl Leukotriene-Binding Protein from a Blood-Feeding Disease Vector. ACS Chem Biol 11, 1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D, 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W, 2010. Xds. Acta Crystallogr D Biol Crystallogr 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhawi S, 2000. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect 2, 1765–1773. [DOI] [PubMed] [Google Scholar]
- Londono-Renteria BL, Shakeri H, Rozo-Lopez P, Conway MJ, Duggan N, Jaberi-Douraki M, Colpitts TM, 2018. Serosurvey of Human Antibodies Recognizing Aedes aegypti D7 Salivary Proteins in Colombia. Front Public Health 6, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Assumpcao TC, Li Y, Andersen JF, Ribeiro J, Francischetti IM, 2012. Triplatin, a platelet aggregation inhibitor from the salivary gland of the triatomine vector of Chagas disease, binds to TXA(2) but does not interact with glycoprotein PVI. Thromb Haemost 107, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafronte Rdos S, Calvo E, James AA, Marinotti O, 2003. The major salivary gland antigens of Culex quinquefasciatus are D7-related proteins. Insect Biochem Mol Biol 33, 63–71. [DOI] [PubMed] [Google Scholar]
- Mans BJ, 2011. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J Innate Immun 3, 41–51. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Calvo E, Ribeiro JM, Andersen JF, 2007. The crystal structure of D7r4, a salivary biogenic amine-binding protein from the malaria mosquito Anopheles gambiae. J Biol Chem 282, 36626–36633. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Featherston J, de Castro MH, Pienaar R, 2017. Gene Duplication and Protein Evolution in Tick-Host Interactions. Front Cell Infect Microbiol 7, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Ribeiro JM, 2008a. Function, mechanism and evolution of the moubatin-clade of soft tick lipocalins. Insect Biochem Mol Biol 38, 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Ribeiro JM, 2008b. A novel clade of cysteinyl leukotriene scavengers in soft ticks. Insect Biochem Mol Biol 38, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Ribeiro JM, Andersen JF, 2008. Structure, function, and evolution of biogenic amine-binding proteins in soft ticks. J Biol Chem 283, 18721–18733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin I, Kern O, Brooks S, Smith LB, Valenzuela-Leon PC, Bonilla B, Ackerman H, Calvo E, 2021. Biochemical characterization of AeD7L2 and its physiological relevance in blood feeding in the dengue mosquito vector, Aedes aegypti. FEBS J 288, 2014–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin I, Paige A, Valenzuela Leon PC, Gittis AG, Kern O, Bonilla B, Chagas AC, Ganesan S, Smith LB, Garboczi DN, Calvo E, 2020a. ADP binding by the Culex quinquefasciatus mosquito D7 salivary protein enhances blood feeding on mammals. Nat Commun 11, 2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin I, Smith LB, Chagas AC, Sa-Nunes A, Shrivastava G, Valenzuela-Leon PC, Calvo E, 2020b. Aedes albopictus D7 Salivary Protein Prevents Host Hemostasis and Inflammation. Biomolecules 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ, 2007. Phaser crystallographic software. J Appl Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moers A, Wettschureck N, Gruner S, Nieswandt B, Offermanns S, 2004. Unresponsiveness of platelets lacking both Galpha(q) and Galpha(13). Implications for collagen-induced platelet activation. J Biol Chem 279, 45354–45359. [DOI] [PubMed] [Google Scholar]
- Nakahata N, 2008. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther 118, 18–35. [DOI] [PubMed] [Google Scholar]
- Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, Assour LA, Basseri H, Berlin A, Birren BW, Blandin SA, Brockman AI, Burkot TR, Burt A, Chan CS, Chauve C, Chiu JC, Christensen M, Costantini C, Davidson VL, Deligianni E, Dottorini T, Dritsou V, Gabriel SB, Guelbeogo WM, Hall AB, Han MV, Hlaing T, Hughes DS, Jenkins AM, Jiang X, Jungreis I, Kakani EG, Kamali M, Kemppainen P, Kennedy RC, Kirmitzoglou IK, Koekemoer LL, Laban N, Langridge N, Lawniczak MK, Lirakis M, Lobo NF, Lowy E, MacCallum RM, Mao C, Maslen G, Mbogo C, McCarthy J, Michel K, Mitchell SN, Moore W, Murphy KA, Naumenko AN, Nolan T, Novoa EM, O’Loughlin S, Oringanje C, Oshaghi MA, Pakpour N, Papathanos PA, Peery AN, Povelones M, Prakash A, Price DP, Rajaraman A, Reimer LJ, Rinker DC, Rokas A, Russell TL, Sagnon N, Sharakhova MV, Shea T, Simao FA, Simard F, Slotman MA, Somboon P, Stegniy V, Struchiner CJ, Thomas GW, Tojo M, Topalis P, Tubio JM, Unger MF, Vontas J, Walton C, Wilding CS, Willis JH, Wu YC, Yan G, Zdobnov EM, Zhou X, Catteruccia F, Christophides GK, Collins FH, Cornman RS, Crisanti A, Donnelly MJ, Emrich SJ, Fontaine MC, Gelbart W, Hahn MW, Hansen IA, Howell PI, Kafatos FC, Kellis M, Lawson D, Louis C, Luckhart S, Muskavitch MA, Ribeiro JM, Riehle MA, Sharakhov IV, Tu Z, Zwiebel LJ, Besansky NJ, 2015. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science 347, 1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakanta G, Sultana H, Sonenshine DE, Andersen JF, 2018. Identification and characterization of a histamine-binding lipocalin-like molecule from the relapsing fever tick Ornithodoros turicata. Insect Mol Biol 27, 177–187. [DOI] [PubMed] [Google Scholar]
- Oseno B, Marura F, Ogwang R, Muturi M, Njunge J, Nkumama I, Mwakesi R, Mwai K, Rono MK, Mwakubambanya R, Osier F, Tuju J, 2022. Characterization of Anopheles gambiae D7 salivary proteins as markers of human-mosquito bite contact. Parasit Vectors 15, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W, 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276, 307–326. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, 1987. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol 32, 463–478. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, 1995. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis 4, 143–152. [PubMed] [Google Scholar]
- Ribeiro JM, Arca B, Lombardo F, Calvo E, Phan VM, Chandra PK, Wikel SK, 2007. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Charlab R, Pham VM, Garfield M, Valenzuela JG, 2004. An insight into the salivary transcriptome and proteome of the adult female mosquito Culex pipiens quinquefasciatus. Insect Biochem Mol Biol 34, 543–563. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Mans BJ, Arca B, 2010. An insight into the sialome of blood-feeding Nematocera. Insect Biochem Mol Biol 40, 767–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Walker FA, 1994. High affinity histamine-binding and antihistaminic activity of the salivary nitric oxide-carrying heme protein (nitrophorin) of Rhodnius prolixus. J Exp Med 180, 2251–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JMC, Arcà B, 2009. Chapter 2 From Sialomes to the Sialoverse, pp. 59–118.
- Rodriguez MH, Hernandez-Hernandez Fde L, 2004. Insect-malaria parasites interactions: the salivary gland. Insect Biochem Mol Biol 34, 615–624. [DOI] [PubMed] [Google Scholar]
- Schneider BS, Higgs S, 2008. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg 102, 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider TR, Sheldrick GM, 2002. Substructure solution with SHELXD. Acta Crystallogr D Biol Crystallogr 58, 1772–1779. [DOI] [PubMed] [Google Scholar]
- Sheldrick GM, 2002. Macromolecular phasing with SHELXE. Z Kristallogr 217, 644–650. [Google Scholar]
- Soter NA, Lewis RA, Corey EJ, Austen KF, 1983. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol 80, 115–119. [DOI] [PubMed] [Google Scholar]
- Titus RG, Ribeiro JM, 1988. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 239, 1306–1308. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, 2004. Exploring tick saliva: from biochemistry to ‘sialomes’ and functional genomics. Parasitology 129 Suppl, S83–94. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Pham VM, Garfield MK, Francischetti IM, Ribeiro JM, 2002. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem Mol Biol 32, 1101–1122. [DOI] [PubMed] [Google Scholar]
- Volfova V, Hostomska J, Cerny M, Votypka J, Volf P, 2008. Hyaluronidase of bloodsucking insects and its enhancing effect on leishmania infection in mice. PLoS Negl Trop Dis 2, e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chang BW, Mans BJ, Ribeiro JM, Andersen JF, 2013. Structure and ligand-binding properties of the biogenic amine-binding protein from the saliva of a blood-feeding insect vector of Trypanosoma cruzi. Acta Crystallogr D Biol Crystallogr 69, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Francischetti IM, Lai R, Ribeiro JM, Andersen JF, 2012. Structure of protein having inhibitory disintegrin and leukotriene scavenging functions contained in single domain. J Biol Chem 287, 10967–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Oliveira F, Chang BW, Collin N, Gomes R, Teixeira C, Reynoso D, My Pham V, Elnaiem DE, Kamhawi S, Ribeiro JM, Valenzuela JG, Andersen JF, 2011. Structure and function of a “yellow” protein from saliva of the sand fly Lutzomyia longipalpis that confers protective immunity against Leishmania major infection. J Biol Chem 286, 32383–32393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


