Abstract
Background
Microbial translocation is a known characteristic of pulmonary tuberculosis (PTB). Whether microbial translocation is also a biomarker of recurrence in PTB is not known.
Methods
We examined the presence of microbial translocation in a cohort of newly diagnosed, sputum smear, and culture positive individuals with drug-sensitive PTB. Participants were followed up for a year following the end of anti-tuberculosis treatment. They were classified as cases (in the event of recurrence, n = 30) and compared to age and gender matched controls (in the event of successful, recurrence free cure; n = 51). Plasma samples were used to measure the circulating microbial translocation markers. All the enrolled study participants were treatment naïve, HIV negative and with or without diabetes mellitus.
Results
Baseline levels of lipopolysaccharide (LPS) (P = .0002), sCD14 (P = .0191), and LPS-binding protein (LBP) (P < .0001) were significantly higher in recurrence than controls and were associated with increased risk for recurrence, whereas intestinal fatty acid binding protein (I-FABP) and Endocab showed no association. Receiver operating characteristic (ROC) curve analysis demonstrated the utility of these individual microbial markers in discriminating recurrence from cure with high sensitivity, specificity, and area under the curve (AUC).
Conclusions
Recurrence following microbiological cure in PTB is characterized by heightened baseline microbial translocation. These markers can be used as a rapid prognostic tool for predicting recurrence in PTB.
Keywords: tuberculosis, recurrent tuberculosis, microbial translocation
Findings from our cohort revealed that microbial translocation markers (MTM) are a good prognostic tool for predicting recurrence in pulmonary tuberculosis (PTB) and in addition these MTMs showed high sensitivity and specificity in differentiating recurrent from cured TB patients.
Microbial translocation (with resultant endotoxemia) typically results from intestinal dysbiosis and increased intestinal permeability leading to translocation of microbial products into the circulation [1, 2]. Microbial translocation has also been described as a prominent feature of infection with Mycobacterium tuberculosis in humans [3, 4]. Systemic immune activation due to microbial translocation is a significant feature of infection with M. tuberculosis in humans [3, 5]. Immune markers of innate immune activation comprise circulating microbial products, such as lipopolysaccharide (LPS), LPS-binding protein (LBP), sCD14, and core (EndoCAb) [2]. Published studies on these markers demonstrated elevation of these markers in several infections, including human immunodeficiency virus (HIV) [6], hepatitis [7], and parasitic infections [8, 9].
Tuberculosis (TB) treatment monitoring is important for making clinical decisions regarding cessation of treatment and the host immune biomarkers may be important predictors of TB treatment recurrence [10]. Recurrent tuberculosis following successful anti-TB treatment is a major challenge to TB control approaches. This TB recurrence may be mainly due to either reactivation of the same strain, that is, relapse, or reinfection with a new strain, which in turn adds greatly to the burden of TB worldwide [11, 12]. Therefore, biomarkers that predict successful cure at the initial treatment phase and biomarkers that predict recurrence free cure at the completion of anti-TB treatment could immensely advance the clinical prognosis [10].
Our group has recently published that plasma chemokines [13], as well as matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) [14] can be used as unique immune biomarkers for predicting unfavorable treatment outcomes such as failure, recurrence (relapse), and death in individuals with pulmonary TB. We postulated that TB treatment recurrence would be driven by enhanced systemic inflammation at baseline. To test this assumption, we examined the baseline levels of microbial translocation markers in a nested case-control study of TB recurrence versus cure in a cohort of PTB individuals in Chennai, India. Our findings reveal that baseline microbial translocation markers are predictors of TB recurrence.
METHODS
Ethics Statement
This study was approved by the Ethical Committees of the Prof. M. Viswanathan Diabetes Research Center (ECR/51/INST/TN/2013/MVDRC/01) and NIRT (NIRT-INo:2014004). Written informed consent was obtained from all participants. All the methods were performed in accordance with the relevant institutional ethical committee guidelines.
Study Population
Participants were enrolled from the Effect of Diabetes on Tuberculosis Severity (EDOTS) study, a prospective cohort study conducted in Chennai, India [15]. The inclusion criteria were new smear and culture positive adults between 20 and 75 years of age. All the blood samples were collected before initiation of anti-TB treatment. The exclusion criteria were previous TB history or treatment, drug resistant TB, more than 1 week of TB treatment currently, pregnancy or lactation, HIV positivity, or on immunosuppression. The diagnosis of pulmonary TB was established by positive sputum culture on solid media with compatible chest X-ray. Drug resistance testing was done using solid cultures in Lowenstein-Jensen media. Drug susceptibility testing was performed for all the first-line drugs: isoniazid, rifamycin, pyrazinamide, and ethambutol. Anti-TB treatment (ATT) was managed by government clinics in Chennai according to National Tuberculosis Elimination Program standards, which is based on the Directly Observed Treatment Short Course (DOTS) therapy. Participants were followed up monthly through the 6-month course of treatment and every 3 months thereafter until 1 year after treatment completion. During course of follow-up, sputum sample was collected every month until 6 months for sputum culture and chest X-ray was performed at months 6, 12, and 18. Biochemical parameters such as liver function test and kidney function test were performed at baseline and month 2. Hematological parameters were measured at baseline, month 2, 6, and 18. Lymph node involvement was continuously monitored throughout the study for all the recurrence free cured individuals was shown to be normal until month 18. We conducted a nested case-control study with microbiological TB treatment recurrence (n = 30) matched to microbiological cure (n = 51). Cure was defined as negative sputum cultures at months 5 and 6 of treatment. Adverse treatment outcomes included recurrent TB within 12 months after initial cure [14]. There was a total of 30 recurrences. Case-control matching was carried out on the basis of age, gender, body mass index (BMI), and diabetic status. Patients who were on antibiotic treatment prior to TB treatment were not included in the study. Peripheral blood was collected in heparinized tubes. Following centrifugation, plasma was collected and stored at −80°C until further analysis.
Microbial Translocation Markers
Plasma samples may contain endotoxin inhibiting compounds and, therefore, to inactivate plasma proteins, plasma samples were heated to 75°C for 5 minutes. LPS levels were measured using a limulus amebocyte lysate assay (Cell Sciences Hycult Biotech, Canton, Massachusetts, USA) according to the manufacturer’s protocol. Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure plasma levels of lipid-binding protein (LBP), endotoxin core antibodies immunoglobulin G (IgG) (EndoCAb), intestinal fatty acid binding protein (iFABP), (all Cell Sciences Hycult Biotech), and sCD14 (R&D Systems, Minneapolis, Minnesota, USA).
Statistical Analysis
Geometric means (GM) were used for measurements of central tendency. Differences between TB recurrence cases and cured control groups were analyzed using the Mann-Whitney test. Receiver operator characteristic (ROC) curves were designed to test the power of each biomarker. Analyses were performed using MedCalc version 20.019. P-values < .05 were considered statistically significant. Classification and regression trees (CART) model were employed to identify the cutoff for the biomarkers which separate the TB recurrence and cured treatments. The analysis was done using R (A Language and Environment for Statistical Computing) software version 4.1.2.
RESULTS
Study Population
The demographics of the study population are shown in Table 1. The median age was 43 (interquartile range [IQR] 25–65) years for treatment recurrence and 42 (IQR 25–73) years for cured individuals. There were no significant differences in gender, BMI, diabetic status, cough duration (in days), lipid profile, alcoholism, education level, or socioeconomic status (Table 1). There were no differences in culture grades and cavities, but the smear grade was significantly different between the study groups. There were also no differences in the duration of delay in starting anti-TB treatment between the study groups.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Study Characteristics Table | |||
|---|---|---|---|
| Variables | TB Recurrent Cases (n = 30) | TB Cured Controls (n = 51) | P-value |
| DM status | |||
| Pre-DM | 0 (0) | 1 (1.96) | .53 |
| Previously known type 2 DM | 9 (30) | 13 (25.49) | |
| Newly diagnosed type 2 DM | 9 (30) | 10 (19.61) | |
| No type 2 DM | 12 (40) | 27 (52.94) | |
| Age, median (IQR) | 43.5 (25–65) | 42 (25–73) | .81 |
| Sex | |||
| Female | 4 (13.33) | 8 (15.69) | .77 |
| Male | 26 (86.67) | 43 (84.31) | |
| BMI, median (IQR) | 16.93 (12.82–25.11) | 17.35 (12.74–26.14) | .39 |
| Cough | |||
| Yes | 30 (100) | 51 (100) | NA |
| No | 0 (0) | 0 (0) | |
| Cough duration, median (IQR) days | 4 (1–20) | 5 (2–28) | .39 |
| Smoking | |||
| No, never | 13 (43.33) | 32 (62.75) | .09 |
| Past/current | 17 (56.67) | 19 (37.25) | |
| Chest X-Ray score, median (IQR) | 35 (0–90) | 35 (0–110) | .8 |
| Alcohol | |||
| No, never | 6 (20) | 20 (39.22) | .07 |
| Past/current | 24 (80) | 31 (60.78) | |
| Cavity | |||
| Yes | 9 (30) | 16 (31.37) | .89 |
| No | 21 (70) | 35 (68.63) | |
| Smear results | |||
| ≥1 | 13 (43.33) | 36 (70.59) | .03 |
| ≥2 | 16 (53.34) | 13 (25.49) | |
| ≥3 | 1 (3.33) | 2 (3.92) | |
| Culture results | |||
| ≥1 | 9 (30) | 24 (47.06) | .13 |
| ≥2 | 4 (13.33) | 10 (19.61) | |
| ≥3 | 17 (56.67) | 17 (33.33) | |
Values were presented as n (%) and median (first to third quartile); Fisher exact test, and Mann-Whitney test were used to check the significance.
Abbreviations: BMI, body mass index; DM, diabetes mellitus; IQR, interquartile range; NA, not applicable.
Recurrence in PTB Is Characterized by Increased Microbial Translocation Markers
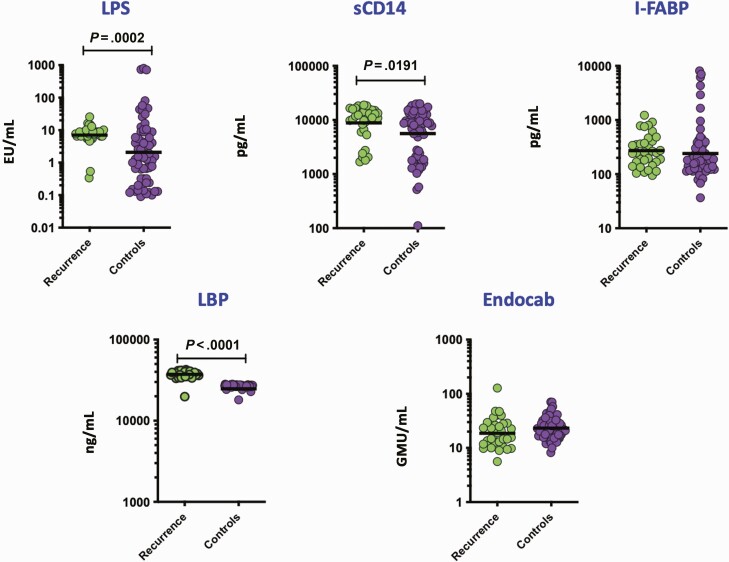
To assess baseline microbial translocation in recurrent and cured individuals, we measured the levels of microbial translocation markers at baseline (pre-treatment). As shown in Figure 1, the levels of LPS (GM of 7.06 EU/mL in cases versus 2.08 EU/mL in controls), sCD14 (GM of 8850 pg/mL in cases versus 5591 pg/mL in controls) and LBP (GM of 37424 ng/ml in cases versus 24 821 ng/mL in controls) were significantly higher in cases compared to controls. However, no statistical differences were seen among the other microbial translocation markers such as iFAB and Endocab. Thus, recurrence in PTB is associated with increased baseline levels of microbial translocation markers.
Figure 1.
Elevated baseline plasma levels of microbial translocation markers in cases. The baseline plasma levels of microbial translocation markers, LPS, sCD14, I-FABP, LBP, and Endocab, were measured in TB recurrence cases (n = 30) and TB recurrence cured controls (n = 51). The data are represented as scatter plots with each circle representing a single individual. P-values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons. Abbreviations: I-FABP, intestinal fatty acid binding protein; LBP, LPS-binding protein; LPS, lipopolysaccharide; TB, tuberculosis.
Biomarkers Discriminating TB Recurrence From Cure
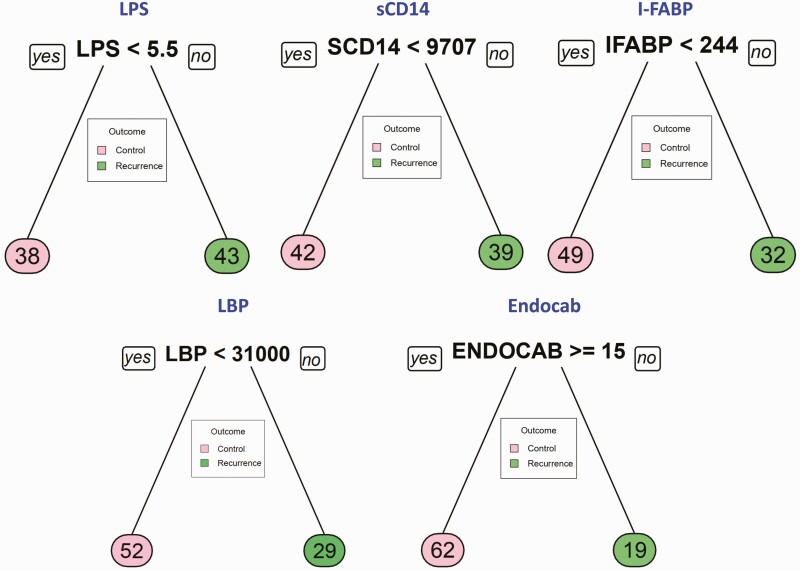
Classification and regression trees (CART) models were employed to identify the cutoff for the biomarkers, which separate recurrence from cure. As input for tree construction, we used data on all the markers and selected the most relevant biomarker that classifies the group more accurately (Figure 2). Briefly, the data set formed a parent node, which contains the whole population. The best peak to separate the data set was selected. As shown in Figure 2, LPS with a cutoff value of 5.5 EU/mL with AUC:0.74, sCD14 with a cutoff value of < 9707 pg/mL with AUC:0.64 and LBP with a cutoff value of < 31 000 ng/mL with AUC:0.97, was able distinguish recurrence versus cure. However, other microbial translocation markers such as I-FABP with a cutoff value of 244 pg/mL with AUC:0.61 and Endocab with a cutoff value of 15 GMU/mL with AUC:0.62, were statistically unable to distinguish recurrence from cure. This CART analysis was able to demonstrate that LPS, sCD14, and LBP (especially LBP), act as sensitive prognostic biomarkers for prediction of TB recurrence.
Figure 2.
Identification of biomarkers showing the strongest associations with recurrent TB disease. CART model analysis shows the microbial translocation markers LPS, sCD14, I-FABP, LBP, and Endocab that exhibited the highest accuracy in discriminating TB recurrence from cure. Abbreviations: I-FABP, intestinal fatty acid binding protein; LBP, LPS-binding protein; LPS, lipopolysaccharide; TB, tuberculosis.
Recurrence in PTB Is Marked by a Signature of Microbial Translocation Markers
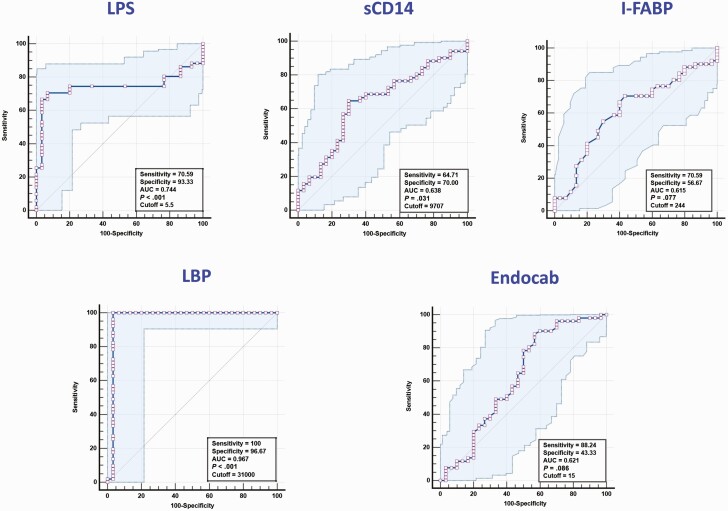
To determine if we could utilize microbial translocation markers as individual biomarkers for prediction of recurrence versus cure, we performed ROC analysis on microbial translocation markers. As shown in Figure 3, ROC analysis of LPS (sensitivity 70%, specificity 93%, and AUC = 0.744), sCD14 (sensitivity 65%, specificity 70%, and AUC = 0.638) and LBP (sensitivity 100%, specificity 97%, and AUC = 0.967) showed significantly high AUC with sensitivity and specificity, especially for LBP. However, other microbial translocation markers such as I-FABP (sensitivity 70%, specificity 56%, and AUC = 0.615) and Endocab (sensitivity 88%, specificity 44%, and AUC = 0.621) did not exhibit significant discrimination between the groups. Taken together, these data strongly suggest that at least 2 of the biomarkers evaluated prior to treatment were predictive of TB recurrence in this cohort.
Figure 3.
ROC analysis to estimate the discriminatory power of microbial translocation markers in TB recurrence. ROC analysis to estimate the sensitivity, specificity, and AUC was performed using LPS, sCD14, I-FABP, LBP, and Endocab to estimate the capacity of these markers to distinguish individuals with recurrence vs cure. Abbreviations: AUC, area under the curve; I-FABP, intestinal fatty acid binding protein; LBP, LPS-binding protein; LPS, lipopolysaccharide; ROC, receiver operating characteristic; TB, tuberculosis.
DISCUSSION
TB recurrence after end of anti-TB treatment is an occurrence that adds substantially to the burden of tuberculosis worldwide [11]. In TB endemic countries, reinfection is the main reason for the observed recurrence rates [16]. In addition, other possible explanations are due to a coexistent high frequency of other infectious diseases like HIV that has been determined to aggravate recurrence rates due to reinfection. Equally, a high relapse rate may also signify unsuccessful anti-TB treatment rather than recent TB transmission within the community [17]. Shortening the duration of chemotherapy would greatly strengthen the TB elimination program, but the lack of correlates of risk for unfavorable treatment outcomes remains a roadblock [18, 19].
A number of published studies have revealed the spectrum of immune biomarkers of TB disease [10, 20]; however, there are limited reports on biomarkers of recurrent TB. Microbiology-based diagnosis have limitations in case of both smear microscopy and culture, which depend on sputum samples and which are not easily available in all populations (eg, pediatric TB or extrapulmonary TB) [21–23]. Use of smear microscopy is also not capable of discerning viable from nonviable TB, which in turn results in poor sensitivity and specificity for outcome prediction [24]. Another limitation with TB culture is the limited accessibility in primary care settings and the delay in time to results which constrain its clinical use [25]. For example, the rate of recurrence is highly variable and has been estimated to range from 4.9% to 47% [26]. This variability is related to differences in regional epidemiology of recurrence and differences in the definitions used by the TB control programs. Therefore, there is an urgent demand for understanding the correlates of risk that are allied with recurrence-free cure to identify those persons who need a complete six-month course of anti-TB treatment and those who can complete shorter courses of treatment. Recognition of individuals at high risk of treatment recurrence before treatment initiation could help in escalating treatment regimens for such individuals including using higher doses of drugs, adding additional drugs or host-directed therapies [13, 14, 18, 19].
Our study examined whether microbial translocation markers could differentiate recurrence cases vs TB free cure. Our findings report that microbial translocation markers such as LPS, sCD14, and LBP are associated with increased risk of TB recurrence and are unique correlates of risk that could serve as predictors of unfavorable treatment at baseline before initiation of anti-TB treatment. Microbial translocation of bacterial products results in enhanced levels of LPS in the circulation without overt bacteremia [2]. During chronic infectious diseases, the pro-inflammatory response may disrupt the mucosal barrier integrity resulting in the translocation of gastrointestinal bacteria, leading to elevated levels of circulating LPS [27]. A recent published study has shown elevated circulating LPS in TB patients with advanced pulmonary involvement [27]. sCD14 is 1 of the main elements of the innate immune system and it functions as a coreceptor for bacterial lipopolysaccharide and occurs in membrane-bound and soluble forms [28]. Few published studies have reported that sCD14 systemic levels were notably elevated in individual TB patients and TB patients with HIV coinfection in comparison to normal healthy donors [5]. Our group also reported that sCD14 circulating levels were significantly increased in pulmonary TB with coexisting diabetes mellitus in comparison to pulmonary TB patients alone and healthy controls indicating that heightened immune activation is seen in active TB disease. LBP is an acute-phase reactant that facilitates immune responses triggered by microbial products [29]. One other study on TB-HIV coinfection has reported that LBP is associated with increased risk of TB recurrence in HIV-infected individuals indicating that translocation of microbial products are associated with chronic immune activation [30]. LBP is an acute phase serum protein that regulates the LPS-induced immune response and also enhances the inflammatory response to LPS [31]. Our study findings indicate that LPS, sCD14, and LBP have the potential to serve as good prognostic biomarkers for TB treatment monitoring, which in turn would help with clinical decision making by identifying patients who respond favorably to anti-TB treatment. Hence, these microbial immune markers act as unique correlates of risk that could function as predictors of adverse treatment outcomes at baseline. iFABP may also suggest disruption in epithelial integrity associated with chronic intestinal infections [9]. Occurrence of anti-LPS core antibodies, EndoCAb, is also used as an alternate measure of circulating LPS. In our cohort, we did not observe any significant alteration in iFABP and EndoCAb circulating levels in TB recurrence versus cure.
Our study delivers encouraging results to unravel the effect of unique non-sputum-based prognostic disease biomarkers in TB patients. Thus, our study offers the novelty of being one of the first studies to be performed in a HIV-uninfected population in India. Future validation of these findings in other cohorts and other populations would then provide an incentive to translate the results to a simple point-of-care rapid diagnostic test for treatment shortening trials and other studies. Moreover, our study also adds to the growing understanding of the pathogenesis of host responses that govern treatment recurrence in TB.
Notes
Author contributions. Designed the study (S. B., H. K., N. P. K.); conducted experiments (N. P. K., K. M.); acquired data (N. P. K., K. M., S. S.); analyzed data (N. P. K., K. M., S. F. H., C. P.); contributed reagents and also revised subsequent drafts of the manuscript (H. K., S. B.); responsible for the enrollment of participants and also contributed to acquisition and interpretation of clinical data (V. V., S. H.); wrote the article (S. B. N. P. K.). All authors read and approved the final article.
Acknowledgments. The authors thank the staff of Department of Clinical Research and the Department of Bacteriology, NIRT, for valuable assistance in bacterial cultures and radiology and the staff of MVDRC, RNTCP, and Chennai corporation for valuable assistance in recruiting the patients for this study. Data in this article were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium.
Ethics approval . Ethics approval was obtained from the ethical committees of participating research institute and hospitals. Patient consent for publication was obtained.
Data and materials availability. All the reported data are available within the article.
Financial support. This project has been funded in whole or in part with Federal funds from the Government of India’s (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the US National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR), and distributed in part by CRDF Global (grant number USB1-31149-XX-13). This work is also funded by CRDF Global RePORT India Consortium Supplementary Funding (grant number OISE-17-62911-1). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the ICMR, the NIH, or CRDF Global. This work was also funded in part by the Division of Intramural Research, NIAID, NIH.
Contributor Information
Nathella Pavan Kumar, National Institute for Research in Tuberculosis, Chennai, India.
Kadar Moideen, National Institutes of Health-NIRT- International Center for Excellence in Research, Chennai, India.
Vijay Viswanathan, Prof. M. Viswanathan Diabetes Research Center, Chennai, India.
Shanmugam Sivakumar, National Institute for Research in Tuberculosis, Chennai, India.
Shaik Fayaz Ahamed, National Institute for Research in Tuberculosis, Chennai, India.
C Ponnuraja, National Institute for Research in Tuberculosis, Chennai, India.
Syed Hissar, National Institute for Research in Tuberculosis, Chennai, India.
Hardy Kornfeld, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Subash Babu, National Institute for Research in Tuberculosis, Chennai, India; Laboratory of Parasitic Diseases (LPD), of National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, Maryland, USA.
References
- 1. Sandler NG, Douek DC.. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol 2012; 10:655–66. [DOI] [PubMed] [Google Scholar]
- 2. Brenchley JM, Douek DC.. Microbial translocation across the GI tract. Annu Rev Immunol 2012; 30:149–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hertoghe T, Wajja A, Ntambi L, et al. . T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB). Clin Exp Immunol 2000; 122:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goletti D, Weissman D, Jackson RW, et al. . Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J Immunol 1996; 157:1271–8. [PubMed] [Google Scholar]
- 5. Toossi Z, Funderburg NT, Sirdeshmuk S, et al. . Systemic immune activation and microbial translocation in dual HIV/tuberculosis-infected subjects. J Infect Dis 2013; 207:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenchley JM, Price DA, Schacker TW, et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 7. Sandler NG, Koh C, Roque A, et al. . Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141:1220–30, 1230.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anuradha R, George PJ, Pavan Kumar N, et al. . Circulating microbial products and acute phase proteins as markers of pathogenesis in lymphatic filarial disease. PLoS Pathog 2012; 8:e1002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. George PJ, Anuradha R, Kumar NP, Kumaraswami V, Nutman TB, Babu S.. Evidence of microbial translocation associated with perturbations in T cell and antigen-presenting cell homeostasis in hookworm infections. PLoS NeglTrop Dis 2012; 6:e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yong YK, Tan HY, Saeidi A, et al. . Immune biomarkers for diagnosis and treatment monitoring of tuberculosis: current developments and future prospects. Front Microbiol 2019; 10:2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guerra-Assuncao JA, Houben RM, Crampin AC, et al. . Recurrence due to relapse or reinfection with Mycobacterium tuberculosis: a whole-genome sequencing approach in a large, population-based cohort with a high HIV infection prevalence and active follow-up. J Infect Dis 2015; 211:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zong Z, Huo F, Shi J, et al. . Relapse versus reinfection of recurrent tuberculosis patients in a national tuberculosis specialized hospital in Beijing, China. Front Microbiol 2018; 9:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar NP, Moideen K, Nancy A, et al. . Plasma chemokines are baseline predictors of unfavorable treatment outcomes in pulmonary tuberculosis. Clin Infect Dis 2021; 73:e3419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar NP, Moideen K, Nancy A, et al. . Association of plasma matrix metalloproteinase and tissue inhibitors of matrix metalloproteinase levels with adverse treatment outcomes among patients with pulmonary tuberculosis. JAMA Netw Open 2020; 3:e2027754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kornfeld H, West K, Kane K, et al. . High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) study. Chest 2016; 149:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Rie A, Warren R, Richardson M, et al. . Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med 1999; 341:1174–9. [DOI] [PubMed] [Google Scholar]
- 17. McIvor A, Koornhof H, Kana BD.. Relapse, re-infection and mixed infections in tuberculosis disease. Pathog Dis 2017; 75. [DOI] [PubMed] [Google Scholar]
- 18. Goletti D, Lindestam Arlehamn CS, Scriba TJ, et al. . Can we predict tuberculosis cure? What tools are available? Eur Respir J 2018; 52:1801089. [DOI] [PubMed] [Google Scholar]
- 19. Rockwood N, du Bruyn E, Morris T, Wilkinson RJ.. Assessment of treatment response in tuberculosis. Expert Rev Respir Med 2016; 10:643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A.. Immunological biomarkers of tuberculosis. Nat Rev Immunol 2011; 11:343–54. [DOI] [PubMed] [Google Scholar]
- 21. Marais BJ, Schaaf HS.. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am 2010; 24:727–49. [DOI] [PubMed] [Google Scholar]
- 22. Purohit M, Mustafa T.. Laboratory diagnosis of extra-pulmonary tuberculosis (EPTB) in resource-constrained setting: state of the art, challenges and the need. J Clin Diagn Res 2015; 9:EE01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peter JG, Theron G, Singh N, Singh A, Dheda K.. Sputum induction to aid diagnosis of smear-negative or sputum-scarce tuberculosis in adults in HIV-endemic settings. Eur Respir J 2014; 43:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horne DJ, Royce SE, Gooze L, et al. . Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010; 10:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhat PG, Kumar AM, Naik B, et al. . Intensified tuberculosis case finding among malnourished children in nutritional rehabilitation centres of Karnataka, India: missed opportunities. PLoS One 2013; 8:e84255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mirsaeidi M, Sadikot RT.. Patients at high risk of tuberculosis recurrence. Int J Mycobacteriol 2018; 7:1–6. [DOI] [PubMed] [Google Scholar]
- 27. Gallucci G, Santucci N, Diaz A, et al. . Increased levels of circulating LPS during tuberculosis prevails in patients with advanced pulmonary involvement. PLoS One 2021; 16:e0257214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ranoa DRE, Kelley SL, Tapping RI.. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J Biol Chem 2013; 288:9729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ha EK, Kim JH, Yon DK, et al. . Association of serum lipopolysaccharide-binding protein level with sensitization to food allergens in children. Sci Rep 2021; 11:2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pillay K, Lewis L, Rambaran S, et al. . Plasma biomarkers of risk of tuberculosis recurrence in HIV co-infected patients from South Africa. Front Immunol 2021; 12:631094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goovaerts O, Jennes W, Massinga-Loembe M, et al. . LPS-binding protein and IL-6 mark paradoxical tuberculosis immune reconstitution inflammatory syndrome in HIV patients. PLoS One 2013; 8:e81856. [DOI] [PMC free article] [PubMed] [Google Scholar]