Abstract
Background
The Treatment as Prevention for Hepatitis C program started in 2016 in Iceland, offering treatment with direct-acting antivirals to hepatitis C virus (HCV)-infected individuals. Reinfections through injection drug use (IDU) can hamper elimination efforts. We determined reinfection rates of HCV among patients in the program.
Methods
Clinical data were gathered prospectively. The study cohort consisted of HCV-cured patients with an estimated sustained virologic response between 1 February 2016 and 20 November 2018, with follow-up until 20 November 2019. The observation period and time until reinfection was estimated using a single random point imputation method coupled with Monte Carlo simulation. The reinfection rates were expressed as reinfections per 100 person-years (PY).
Results
In total, 640 treatments of 614 patients (417 male; mean age, 44.3 years) resulted in cure, with 52 reinfections subsequently confirmed in 50 patients (37 male). Follow-up was 672.1 PY, with a median time to reinfection of 232 days. History of IDU was reported by 523 patients (84.8%) and recent IDU with 220 treatments (34.4%). Stimulants were the preferred injected drug in 85.5% of patients with a history of IDU. The reinfection rate was 7.7/100 PY. Using multivariate Cox proportional hazards models for interval-censored data, age (hazard ratio, 0.96 [95% confidence interval, .94–.99]) and recent IDU (2.91 [1.48–5.76]) were significantly associated with reinfection risk.
Conclusions
The reinfection rate is high in a setting of widespread stimulant use, particularly in young people with recent IDU. Regular follow-up is important among high-risk populations to diagnose reinfections early and reduce transmission.
Clinical Trials Registration
Keywords: hepatitis C, harm reduction, reinfection, injection drug use, treatment program
Rates of reinfection with hepatitis C virus after successful treatment with direct-acting antivirals are high in Iceland, particularly among young individuals with recent injection drug use. Emphasis on further follow-up of high-risk groups is essential.
INTRODUCTION
Infection with hepatitis C virus (HCV), which affects an estimated 70 million people globally, is a leading cause of cirrhosis and hepatocellular carcinoma [1]. HCV is usually transmitted through exposure to infected blood or blood products, with injection drug use (IDU) among people who inject drugs (PWID) and unsafe healthcare procedures being the main modes of transmission [2, 3]. In most high-income countries, IDU accounts for the majority of new and existing infections [4].
Direct-acting antivirals (DAAs) directed against HCV have revolutionized the treatment of hepatitis C, with rates of sustained virologic response (sustained virologic response at 12 weeks or later [SVR≥12]) commonly >95% [5, 6]. Furthermore, the safety of DAAs has been established in PWID and people receiving medication-assisted therapy for opioid use disorder [7, 8]. Treatment with DAAs reduces the risk of liver-related disease and death, most prominently among patients with advanced liver fibrosis [9, 10]. This benefit extends to PWID and other risk groups [11, 12]. Treatment may also lead to a subsequent decrease in HCV transmission, preventing both primary infections and reinfections [13].
In 2016 the World Health Organization (WHO) set the goal of eliminating HCV as a major health threat, including a 65% reduction in HCV-related deaths and 80% reduction in HCV incidence, by the year 2030 [2]. Because cure does not confer protective immunity, reinfection poses a major threat to elimination efforts [7, 8, 14]. Particular risk groups for reinfection include PWID and men who have sex with men (MSM), with reported reinfection rates varying from 1% to 8% per year [15, 16]. Indeed, reinfection rates seem to be extremely low among low-risk individuals [17, 18].
On 1 February 2016, Iceland initiated a nationwide HCV elimination program known as Treatment as Prevention for Hepatitis C (TraP Hep C), which aimed to maximize diagnosis and treatment access. Concomitantly, harm reduction was scaled up, including needle-syringe programs. Iceland is an ideal setting for the study of public health interventions, with national health insurance, low threshold for addiction treatment and central registries of reportable infectious diseases [8, 19]. Iceland is on track to meeting the HCV elimination targets by 2030, with reinfections among PWID the primary barrier [19–22].
Multiple studies on HCV reinfection have been published; however, none have been in the context of national treatment programs. We sought to determine the reinfection rate of HCV among patients cured within the nationwide TraP Hep C cohort and further elucidate risk factors for reinfection.
METHODS
Study Setting
Iceland is an island nation with a population of roughly 357 000, with a majority living in the capital region [23]. An estimated 800–1000 people had hepatitis C in the country by 2015, corresponding to a viremic population prevalence of 0.3% [24]. The vast majority of HCV-infected individuals in Iceland have history of IDU [25].
The TraP Hep C Program and Study Cohort
The TraP Hep C program has been described in detail elsewhere [8]. Briefly, from February 2016 all patients with chronic HCV infection were offered treatment with DAAs. Patients who dropped out before completing therapy or became reinfected were offered retreatment. Interviews were done at baseline, during treatment, and at SVR≥12.
In parallel, a monitoring study was initiated. An HCV RNA amplification by polymerase chain reaction (referred to hereafter as an HCV RNA measurement) was performed at the end of treatment and at week 12 or later after finishing treatment to confirm SVR≥12. Follow-up included sequential blood tests, particularly among PWID, for whom intervals of 6 months were encouraged.
The current study cohort included all patients cured of hepatitis C after treatment within the program with an estimated date of SVR≥12 between 1 February 2016 and 20 November 2018. The study period was chosen to ensure at least 1 year of follow-up.
Data Collection and Definitions
All results from HCV RNA measurements in Iceland, from 1 February 2016 to 20 November 2019, were obtained and connected to patient data. Patient-specific variables were registered prospectively at every treatment initiation in a baseline interview. Patients were said to have recent IDU if there was history of IDU within the last 6 months. Living situation at baseline was defined as either stable (living in one’s own property or with relatives or renting) or unstable (living in a halfway house or penitentiary, homeless, or with other living arrangements). A single reference virology laboratory serving the whole country performed all HCV RNA measurements. Genotyping was performed, if possible, for all suspected reinfections. For patients who had detectable HCV RNA within 12 weeks from the end of treatment, deep sequencing and phylogenetic analysis was performed to fully exclude virologic relapse. Next-generation sequencing was done on the NS5A open reading frame using the Miseq v2 sequencing kit with 300 cycles (Illumina). These services were provided by DDL Diagnostic Laboratory. In cases of doubt regarding whether a positive test signified reinfection or relapse, the cases were excluded from the study.
EOT virologic response (EOT) was defined as undetectable HCV RNA at the EOT. SVR≥12 was defined as undetectable HCV RNA ≥12 weeks after treatment. This distinction was made to allow for analysis of reinfections where SVR≥12 was not established. Reinfection was defined as the presence of detectable HCV RNA after SVR≥12, regardless of genotype, or after the EOT, either of the same genotype with exclusion of virologic relapse or of a different genotype.
Observation time within the primary study cohort (EOT cohort) started at the EOT and lasted until reinfection or the last undetectable HCV RNA (last negative test [LNT]), with observation time measured in person-years (PY). This observation period included all reinfections occurring after the EOT, without requiring SVR≥12. Time to reinfection was defined as the time from the EOT to reinfection. The exact timing of reinfection was unknown, but it was known to occur within an interval where the left end point was either the LNT (interval censored) or zero (left censored), and the right end point was the first positive test (FPT). In cases where reinfection was not observed, observation lasted until the LNT and the right limit of the resulting interval was infinity (ie, right censored). These concepts are further described by Bogaerts et al [26]. In cases where reinfection was treated within the study period, observation resumed after the end of retreatment. The number of HCV RNA measurements among patients in the program was also collated.
Primary Analysis
To estimate HCV reinfection rate, we used a single random point imputation method coupled with Monte Carlo simulation, as described by Vandormael et al [27]. This was done for both EOT and SVR≥12 cohorts, and stratified by the variables recent IDU, housing status, sex and age (stratified into the age groups 20–39, 40–59, and ≥60 years).
We plotted nonparametric survival curves stratified by recent IDU status, where the end point was reinfection and following the guideline of Bogaerts et al [26]. We then built a multivariate model that included 4 variables: age (mean centered), sex indicator, recent IDU indicator, and an unstable housing indicator. We implemented the Stata software solution stintcox (described in detail elsewhere [28]) to fit a Cox model to interval-censored data. Variables were chosen based on other studies on this topic [14, 18, 29, 30]. This approach does not account for recurrent events. Further description of these methods can be found in the Supplementary Materials (Supplementary Methods, primary analysis).
Secondary Analyses
The same analyses were done on the cohort with confirmed SVR≥12 (the SVR≥12 cohort), in which observation started at SVR≥12. Further analyses were done for both the EOT and SVR≥12 cohorts, with time of reinfection defined as the midpoint between the LNT and FPT. For further discussion of secondary analysis methods, see the Supplementary Materials (Supplementary Methods, secondary analysis).
Presentation of Results
Median values, interquartile ranges (IQRs), mean values, and standard deviation were used for descriptive statistics. Reinfection rate was presented along with 95% confidence intervals (CIs). The results of the multivariate Cox proportional hazards model were presented with hazard ratios (HRs) and 95% CIs. Two-sided P values of .05 were used as cutoff for statistical significance. Statistical analysis was performed using R (2020; R Foundation for Statistical Computing; https://www.R-project.org/) and Stata v17 (2021; StataCorp) software. Reporting of this study followed guidelines from the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) initiative [31]. The study was approved by the National Bioethics Committee of Iceland (no. 15-087).
RESULTS
Summary of Study Cohort
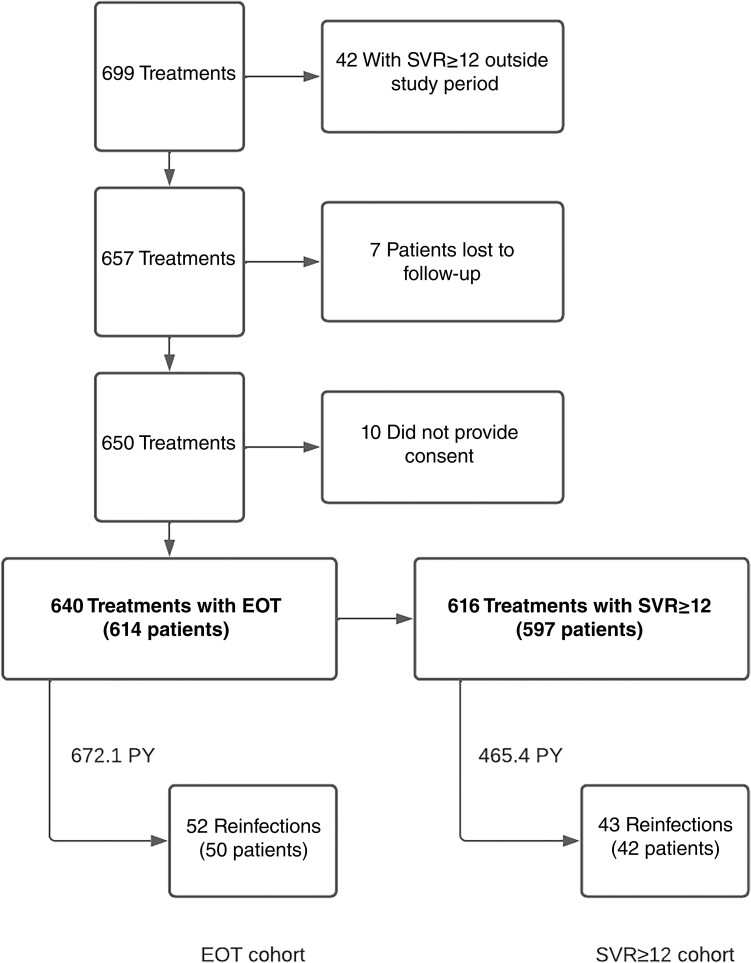
After data extraction and exclusion, the study included 640 HCV treatments in 614 patients within the EOT cohort (Figure 1). During 672.1 PY of follow-up (median observation time [IQR], 213 [91–688] days), 52 confirmed reinfections occurred in 50 patients. The median time to reinfection (IQR) was 232 (144–446) days.
Figure 1.
Data extraction and exclusion flowchart. Flowchart describing data extraction and exclusion along with the 2 study cohorts, with total number of treatments and patients, number of reinfections, and observation period. Abbreviations: EOT, end of treatment virologic response; PY, person-years; SVR≥12, sustained virologic response at ≥12 weeks.
The baseline characteristics of the study cohort are shown in Table 1. The mean age (standard deviation) was 44.3 (12.7) years; 67.9% of patients were male (n = 417). The duration of HCV infection at time of treatment initiation was available for 571 treatments, with a mean duration of 9 years (range, 0–27 years). History of IDU was affirmed by 84.8% of individuals (n = 523), with recent IDU reported in 34.4% at treatment initiation (n = 220). By self-report, stimulants were the preferred injected drug in 85.5% (n = 447) of patients with a history of IDU; of those, methylphenidate was preferred in 35.6%. During the study period, a median of 4 HCV RNA measurements (IQR, 3–5; range, 1–14) were performed per individual within the program, including the baseline measurement.
Table 1.
Baseline Characteristics of Study Cohort Before Initiation of Each Treatment
| Characteristic | No. (%) of Treatmentsa | |
|---|---|---|
| Female Patients | Male Patients | |
| No. of patients | 197 | 417 |
| No. of finished treatments | 206 | 434 |
| Age, mean, y | 42.9 | 45 |
| History of addiction treatmentb | 126 (64.0) | 380 (91.1) |
| History of IDUb | 156 (79.2) | 367 (88.0) |
| History of previous non-DAA treatmentb | 28 (14.2) | 49 (11.8) |
| HIV infectionb | 13 (6.6) | 26 (6.2) |
| Recent IDUc | 64 (31.1) | 156 (35.9) |
| MAT | 17 (8.3) | 43 (9.9) |
| Use of noninjected drugs | 36 (17.5) | 103 (23.7) |
| Cannabis | 21 (10.2) | 72 (16.6) |
| Opiates | 10 (4.9) | 13 (3.0) |
| Stimulants | 16 (7.8) | 38 (8.8) |
| Sedatives | 13 (6.3) | 17 (3.9) |
| Living situation at treatment initiation | ||
| Stable housingd | 166 (80.6) | 319 (73.5) |
| Halfway house | 23 (11.2) | 38 (8.8) |
| Homeless/streets | 10 (4.9) | 30 (6.9) |
| Penitentiary | 4 (1.9) | 37 (8.5) |
| Other living arrangements | 3 (1.5) | 10 (2.3) |
| Preferred intravenous druge | ||
| Methylphenidate | 61 (39.1) | 98 (26.7) |
| Other stimulants | 70 (44.9) | 218 (59.4) |
| Opiates | 19 (12.2) | 47 (12.8) |
| Other | 6 (3.8) | 4 (1.1) |
| Cirrhosis | 7 (3.4) | 38 (8.8) |
| Genotype | ||
| 3a | 124 (60.2) | 243 (56.0) |
| 1a | 63 (30.6) | 164 (37.8) |
| 1b | 16 (7.8) | 17 (3.9) |
| Other | 3 (1.5) | 10 (2.3) |
Abbreviations: DAA, direct-acting antiviral; HIV, human immunodeficiency virus; IDU, injection drug use; MAT, medication-assisted therapy for opioid use disorder.
Values represent no. (%) of treatments unless otherwise specified.
Values in these rows represent no. (%) of patients, not treatments.
Recent IDU was defined as IDU within the last 6 months, according to patient self-report during baseline interview.
Stable housing was defined as living in one’s own property or with relatives or renting.
Values for preferred intravenous drug represent no. (%) of all patients with a history of IDU.
Of the 52 reinfections, 59.6% (n = 31) were of the same genotype as the original infection, while 34.6% (n = 18) were of a different genotype (Supplementary Table 1). The reinfection genotype was unknown for 3 reinfections in 3 individuals, who all had confirmed SVR≥12 after treatment.
HCV Reinfection—Primary Analysis
In the EOT cohort, the mean reinfection rate was 7.7/100 PY (95% CI, 5.8–10.1). Table 2 shows univariate reinfection rates for recent IDU, housing status, sex, and age group.
Table 2.
Univariate Reinfection Ratesa
| Variable | Reinfections, No. | PY | Reinfections/100 PY (95% CI) |
|---|---|---|---|
| Sex | |||
| Male | 38 | 445.2 | 8.5 (6.0–11.7) |
| Female | 14 | 226.7 | 6.2 (3.4–10.4) |
| Recent IDU by self-reportb | |||
| Recent IDU | 39 | 245.7 | 15.9 (11.3–21.7) |
| No recent IDU | 13 | 426.4 | 3.0 (1.6–5.2) |
| Age group | |||
| 20–39 y | 34 | 267.4 | 12.7 (8.8–17.8) |
| 40–59 y | 16 | 310.4 | 5.2 (2.9–8.4) |
| ≥60 y | 2 | 94.4 | 2.1 (.3–7.7) |
| Living situation at treatment initiationc | |||
| Stable housing | 24 | 504.7 | 4.8 (3.0–7.0) |
| Unstable housing | 28 | 167.4 | 16.7 (11.1–24.2) |
Abbreviations: CI, confidence interval; IDU, injection drug use; PY, person-years.
Reinfection rates for the 4 explanatory variables examined in the primary analysis.
Recent IDU was defined as IDU within 6 months of the baseline interview.
Stable housing was defined as living in one’s own property or with relatives or renting; unstable housing, as living in a halfway house or penitentiary, homeless, or with other living arrangements.
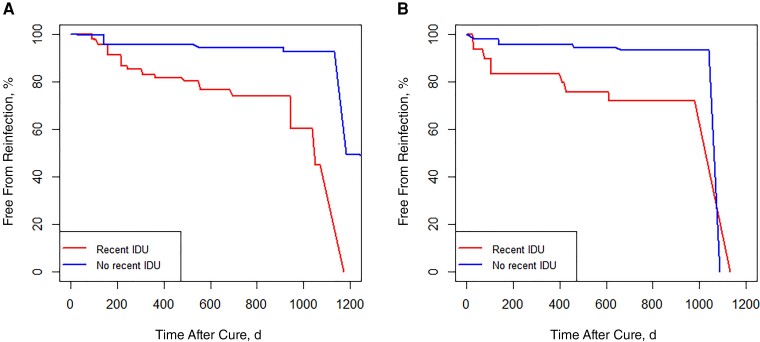
Nonparametric survival was lower among those with recent IDU than among those without recent IDU, using reinfection as the end point (Figure 2A).
Figure 2.
Nonparametric survival curves for interval-censored data with reinfection as end point. A, Survival curves for the end of treatment virologic response cohort. B, Survival curves for the sustained virologic response at ≥12 weeks cohort. Owing to the nature of the nonparametric maximum likelihood estimation, survival is defined as zero after the last Turnbull interval, so all of the survival curves eventually reach zero, regardless of the number of reinfections. Abbreviation: IDU, injection drug use.
In the Cox proportional hazards model for interval-censored data (Table 3), age (HR 0.96 [95% CI, .93–.99]) and recent IDU (2.91 [1.47–5.76]) were significantly associated with reinfection risk. Unstable housing was not significantly associated with reinfection risk but had a CI only slightly below 1.0 (HR, 1.81 [95% CI, .97–3.39]).
Table 3.
Multivariate Cox Proportional Hazards Model Resultsa
| Cohort and Variable | Adjusted HR (95% CI) | Standard Error | P-value |
|---|---|---|---|
| EOT cohort | |||
| Age | 0.96 (.93–.99) | 0.02 | .02 |
| Sex | 0.82 (.43–1.56) | 0.27 | .55 |
| Recent IDUb | 2.91 (1.47–5.76) | 1.01 | .002 |
| Unstable housingc | 1.81 (.97–3.39) | 0.58 | .06 |
| SVR≥12 cohort | |||
| Age | 0.96 (.93–1.00) | 0.02 | .04 |
| Sex | 0.77 (.36–1.65) | 0.30 | .50 |
| Recent IDUb | 2.84 (1.32–6.13) | 1.11 | .008 |
| Unstable housingc | 1.84 (.90–3.79) | 0.68 | .10 |
Abbreviations: CI, confidence interval; EOT, end of treatment virologic response; HR, hazard ratio; IDU, injection drug use; SVR≥12, sustained virologic response at ≥12 weeks.
Multivariate Cox proportional hazards models were fit to interval-censored data analysis.
Recent IDU defined as IDU within 6 months of the baseline interview.
Unstable housing was defined as living in a halfway house or penitentiary, homeless, or with other living arrangements.
Secondary Analyses
In the SVR≥12 cohort, there were a total of 616 treatments in 597 patients (Figure 1). During 465.4 PY of follow-up (median observation time [IQR], 16 [0–586] days), 43 confirmed reinfections occurred in 42 patients. The median time (IQR) to reinfection was 139 (61–361) days. The mean reinfection rate was thus 9.2/100 PY (95% CI, 6.7–12.4).
For the SVR≥12 cohort, nonparametric survival was lower among those with recent IDU than among those without recent IDU using reinfection as the end point (Figure 2B).
In the Cox proportional hazards model for interval-censored data in the SVR≥12 cohort (Table 3), age (HR, 0.96 [95% CI, .93–1.00]) and recent IDU (2.84 [1.32–6.13]) were significantly associated with reinfection risk.
Defining the time of reinfection as the midpoint between the LNT and FPT in the EOT cohort, preference for either stimulant or nonstimulant injected drugs was not significantly associated with reinfection risk. Self-reported number of injection days in the preceding month was not significantly associated with reinfection risk (see Supplementary Results).
DISCUSSION
In the current study we analyzed HCV reinfections within a prospective nationwide cohort of patients treated during the TraP Hep C program in Iceland. This enabled calculation of population-level reinfection rates owing to long-term follow-up and intensified screening efforts. A total of 52 reinfections in 50 patients occurred during the study period. Reinfection rates after treatment with DAAs in Iceland were high (overall, 7.7/100 PY), particularly among young individuals with recent IDU. Multivariate analyses for interval-censored data revealed higher reinfection risk in younger individuals and those with recent IDU.
Systematic reviews and meta-analyses have examined reinfection rates after HCV treatment. However, multiple limitations in the included studies (variable follow-up, limited populations, selective testing, and sparse genetic data) make interpretation and comparisons difficult. Simmons et al [32] presented a pooled estimate for reinfection of 1.91/100 PY (95% CI, 1.14–2.82) based on 14 studies of high-risk populations. Huang et al [18] calculated pooled reinfection rates based on group characteristics: low risk (16 studies; 0.01/100 PY), high risk (19 studies; 2.94/100 PY), and human immunodeficiency virus (HIV)/HCV coinfection (8 studies; 2.33/100 PY). Their calculated reinfection risk for PWID was 1.83/100 PY (95% CI, .97–2.92). Finally, Latham et al [33] focused on recently injecting PWID and those receiving medication-assisted therapy for opioid use disorder (26 studies), with pooled reinfection rates of 1.94/100 PY and 0.55/100 PY, respectively.
Other studies in recent years have added to the literature. Rossi et al [29] reported an overall reinfection rate of 1.44/100 PY, with higher reinfection rates among younger, male individuals with recent IDU. Cunningham et al [14] reported a reinfection rate of 3.1/100 PY with higher reinfection rates among those using intravenous stimulants. Valencia et al [34] reported 10 reinfections among 121 PWID with 101.1 PY of follow-up, yielding a reinfection rate of 9.8/100 PY, with higher rates associated with recent IDU (16.7/100 PY). Caven et al [35] calculated reinfection rates among PWID, with a reinfection rate of 7.17/100 PY. Marco et al [30] specifically studied individuals who were treated within a penitentiary setting; the subsequent reinfection rate was 2.9/100 PY and was particularly high among HIV-infected individuals (5.6/100 PY). All aforementioned single studies defined time of reinfection as the midpoint between SVR≥12 and confirmation of reinfection.
There were several important distinctions in the current study. The observation period began at the EOT since 9 reinfections occurred among individuals without established SVR≥12. Similar considerations were made by Martinello et al [36], who calculated a reinfection rate of 7.4/100 PY among PWID and HIV-positive MSM, including everyone who achieved an EOT. The current study’s primary analysis also uses specific methods to analyze interval-censored data. To increase comparability between studies, secondary analyses were performed on both the EOT and SVR≥12 cohorts, in which the midpoint between the LNT and FPT was defined as the time of reinfection.
Previous research has identified recent IDU, young age, and injected stimulants as risk factors for HCV reinfection [14, 29, 34, 37]. The robust association between younger age and higher reinfection risk is most likely multifactorial, possibly explained by larger social groups sharing injection paraphernalia or increased risky behavior, such as increased number of injections. This in turn may be attributed to recent debut of IDU, lack of knowledge, or different risk perceptions [38, 39]. However, older age has also been associated with increased reinfection risk [36]. The prevalence of HCV viremia among PWID remained stable at about 40% in Iceland before implementation of TraP Hep C [22]. This is not higher than for comparable populations in other Western countries and thus not a likely explanation for the high reinfection rates described here.
Injection use of stimulants has been particularly common among PWID in Iceland, with most preferring methylphenidate (used for treatment of attention-deficit hyperactivity disorder, sold as Ritalin among other formulations), followed by other stimulants. Comparatively, heroin use is rare; this may contribute to higher reinfection rates. Higher reinfection rates among those who inject stimulants may reflect increased risky behavior [40, 41]. Interestingly, higher self-reported numbers of injections within the last 30 days were not associated with reinfection risk. Although injection of stimulants was not associated with increased reinfection risk in this study compared with opiates, many reported mixed use of opiates and stimulants, making it difficult to tease these effects apart. Further work to delineate these important risk factors is needed [40]. Finally, although housing status was not significantly associated with risk of reinfection, given the effect size and bounds of the 95% CI, it can be classified as having a probable effect. Confirmation of effect requires further study.
The current study has several strengths. The prospective, nationwide, population-based, multidisciplinary study design of TraP Hep C provides real-world data that are representative of at-risk populations, such as PWID. The program structure allows for comprehensive follow-up, leading to fewer missed reinfections. Furthermore, starting follow-up at EOT instead of SVR≥12 allowed analysis of reinfections that would otherwise have been excluded. Harm reduction has been integral to the program from its inception; of note, Iceland exceeded target numbers of needles and syringes recommended by WHO before and during the program [22]. Results were available for all HCV RNA measurements performed in the country, which allowed analyses for interval-censored data based on important patient-derived variables. Furthermore, other secondary analyses were done in this study, and the results and their consistent effect sizes increase the robustness of the findings.
The main interpretive limitation of the study was the repeated testing during follow-ups with bias toward high-risk individuals to detect reinfection early. While this shortened diagnostic and treatment delay, it may lead to overestimation of reinfection rates. The analyses for interval-censored data do not account for recurrent events. However, secondary analyses were done to further address this. The data on risk behavior carry an inherent risk of recall bias and underreporting, partially reflected by a single case of reinfection where no risk behavior was reported after treatment. The study lacked information about sharing of injection paraphernalia and MSM activity, both important pathways to reinfection. Finally, with decreasing prevalence, the risk of HCV transmission may become more stochastic, complicating further risk attribution to certain epidemiologic factors.
The current study may offer both practical and analytical experience to other countries with national treatment programs, particularly if use of injected stimulants is common. With increasing availability of DAAs without restrictions, more challenging cases may emerge that require continued surveillance and retreatments. This may also be compounded by changing patient behavior and perceptions about the overall risk associated with this disease.
In conclusion, we demonstrate a high HCV reinfection rate after successful DAA treatment, particularly among young individuals and those with recent IDU. This emphasizes the importance of continued follow-up, harm reduction, and linkage to care despite early successes with the TraP Hep C program. There is also a need to further reduce behavior associated with reinfection, including sharing of injection paraphernalia, despite WHO targets of available needles and syringes having been achieved. Further mitigation efforts are needed to reduce HCV transmission. The importance of good access to healthcare for PWID is essential, including addiction treatment and intervention services. Continuous screening and prompt retreatment for HCV is needed at all levels of care.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jon M Johannesson, Department of Internal Medicine, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland.
Ragnheidur H Fridriksdottir, Department of Gastroenterology and Hepatology, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland.
Thorvardur J Löve, Faculty of Medicine, School of Health Sciences, University of Iceland, Reykjavik, Iceland; Department of Science, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland.
Valgerdur Runarsdottir, Vogur Hospital, SAA–National Center of Addiction Medicine, Reykjavik, Iceland.
Ingunn Hansdóttir, Vogur Hospital, SAA–National Center of Addiction Medicine, Reykjavik, Iceland.
Arthur Löve, Faculty of Medicine, School of Health Sciences, University of Iceland, Reykjavik, Iceland; Department of Clinical Microbiology, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland.
Marianna Thordardottir, Center for Health Security and Communicable Disease Control, Directorate of Health, Reykjavik, Iceland.
Ubaldo B Hernandez, Department of Science, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland.
Sigurdur Olafsson, Department of Gastroenterology and Hepatology, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland; Faculty of Medicine, School of Health Sciences, University of Iceland, Reykjavik, Iceland.
Magnus Gottfredsson, Faculty of Medicine, School of Health Sciences, University of Iceland, Reykjavik, Iceland; Department of Science, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland; Department of Infectious Diseases, Landspitali–The National University Hospital of Iceland, Reykjavik, Iceland.
Treatment as Prevention for Hepatitis C (TraP Hep C) group:
Einar Stefan Bjornsson, Ottar Mar Bergmann, Bryndis Sigurdardottir, Birgir Johannsson, Maria Heimisdottir, Thorarinn Tyrfingsson, Anna Tomasdottir, Bergthora Karlsdottir, Bjartey Ingibergsdottir, Hildigunnur Fridjonsdottir, Kristin Alexiusdottir, Thora Bjornsdottir, Bryndis Olafsdottir, and Asdis M Finnbogadottir
Notes
Acknowledgments. The authors thank all the participating patients and participating members of the Treatment as Prevention for Hepatitis C (TraP Hep C) team, Einar Stefan Bjornsson, Ottar Mar Bergmann, Bryndis Sigurdardottir, Birgir Johannsson, Maria Heimisdottir, and Thorarinn Tyrfingsson. They also thank the nurses Anna Tomasdottir, Bergthora Karlsdottir, Bjartey Ingibergsdottir, Hildigunnur Fridjonsdottir, Kristin Alexiusdottir, Thora Bjornsdottir, Bryndis Olafsdottir, and Asdis M Finnbogadottir for their vitally important contribution to the program.
Financial support. The Icelandic government provided special funding for the overall organization of the project, diagnostic tests, and other services related to the TraP Hep C program. Gilead Sciences provided direct-acting antivirals free of charge in an epidemiologic study setting and also provided financial support for a contract with PPD, a global contract research organization that provided services on clinical data management and trial monitoring for the TraP Hep C study.
References
- 1. Blach S, Zeuzem S, Manns M, et al. . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:161–76. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global hepatitis report, 2017. Available at:https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed 16 September 2021.
- 3. Havens PL, Anderson JR. Updated CDC recommendations for universal hepatitis C virus screening among adults and pregnant women. JAMA 2020; 323:2258. [DOI] [PubMed] [Google Scholar]
- 4. Nelson PK, Mathers BM, Cowie B, et al. . Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feld JJ, Jacobson IM, Hézode C, et al. . Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015; 373:2599–607. [DOI] [PubMed] [Google Scholar]
- 6. Pisaturo M, Russo A, Onorato L, Coppola N. Efficacy of 12-weeks velpatasvir plus sofosbuvir-based regimen in HCV-naive subjects with mild fibrosis: a meta-analysis. Acta Biomed 2019; 90:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graf C, Mücke MM, Dultz G, et al. . Efficacy of direct-acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta-analysis. Clin Infect Dis 2020; 70:2355–65. [DOI] [PubMed] [Google Scholar]
- 8. Olafsson S, Tyrfingsson T, Runarsdottir V, et al. . Treatment as Prevention for Hepatitis C (TraP Hep C)—a nationwide elimination programme in Iceland using direct-acting antiviral agents. J Intern Med 2018; 283:500–7. [DOI] [PubMed] [Google Scholar]
- 9. Carrat F, Fontaine H, Dorival C, et al. . Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019; 393:1453–64. [DOI] [PubMed] [Google Scholar]
- 10. Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2020; 71:44–55. [DOI] [PubMed] [Google Scholar]
- 11. Martinello M, Yee J, Bartlett SR, et al. . Moving towards hepatitis C microelimination among people living with human immunodeficiency virus in Australia: the CEASE study. Clin Infect Dis 2020; 71:1502–10. [DOI] [PubMed] [Google Scholar]
- 12. Martin NK, Vickerman P, Brew IF, et al. . Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis. Hepatology 2016; 63:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smit C, Boyd A, Rijnders BJA, et al. . HCV micro-elimination in individuals with HIV in the Netherlands 4 years after universal access to direct-acting antivirals: a retrospective cohort study. Lancet HIV 2021; 8:e96–105. [DOI] [PubMed] [Google Scholar]
- 14. Cunningham EB, Hajarizadeh B, Amin J, et al. . Reinfection following successful direct-acting antiviral therapy for hepatitis C infection among people who inject drugs. Clin Infect Dis 2021; 72:1392–400 [DOI] [PubMed] [Google Scholar]
- 15. Irvin R, Gamble T, Malone J, et al. . HPTN 078: high prevalence of HCV antibodies among urban U.S. men who have sex with men (MSM) independent of HIV status. Clin Infect Dis 2021; 73:e2205–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adu PA, Rossi C, Binka M, et al. . HCV reinfection rates after cure or spontaneous clearance among HIV-infected and uninfected men who have sex with men. Liver Int 2021; 41:482–93. [DOI] [PubMed] [Google Scholar]
- 17. Toyoda H, Yasuda S, Shiota S, Kumada T, Tanaka J. Lack of hepatitis C virus reinfection in lifetime of Japanese general population with previous hepatitis C virus (HCV) infection successfully treated with anti-HCV therapy. J Infect Chemother 2021; 27:1674–5. [DOI] [PubMed] [Google Scholar]
- 18. Huang P, Wang Y, Yue M, et al. . The risk of hepatitis C virus recurrence in hepatitis C virus-infected patients treated with direct-acting antivirals after achieving a sustained virological response: a comprehensive analysis. Liver Int 2021; 41:2341–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott N, Ólafsson S, Gottfreðsson M, et al. . Modelling the elimination of hepatitis C as a public health threat in Iceland: a goal attainable by 2020. J Hepatol 2018; 68:932–9. [DOI] [PubMed] [Google Scholar]
- 20. Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int 2020; 40:522–9. [DOI] [PubMed] [Google Scholar]
- 21. Gamkrelidze I, Pawlotsky JM, Lazarus JV, et al. . Progress towards hepatitis C virus elimination in high-income countries: an updated analysis. Liver Int 2021; 41:456–63. [DOI] [PubMed] [Google Scholar]
- 22. Olafsson S, Fridriksdottir RH, Love TJ, et al. . Cascade of care during the first 36 months of the Treatment as Prevention for Hepatitis C (TraP HepC) programme in Iceland: a population-based study. Lancet Gastroenterol Hepatol 2021; 6:628–37. [DOI] [PubMed] [Google Scholar]
- 23. Statistics Iceland . Available at:https://statice.is/. Accessed September 16 2021.
- 24. Liakina V, Hamid S, Tanaka J, et al. . Historical epidemiology of hepatitis C virus (HCV) in select countries—volume 3. J Viral Hepat 2015; 22(suppl 4):4–20. [DOI] [PubMed] [Google Scholar]
- 25. Fridriksson B, Bergmann OM, Olafsson S. Treatment of hepatitis C with peginterferon and ribavirin in Iceland from 2002–2012 [in Icelandic]. Laeknabladid 2017; 103:125–8. [DOI] [PubMed] [Google Scholar]
- 26. Bogaerts K, Komárek A, Lesaffre E. Survival analysis with interval-censored data: a practical approach with R, SAS, and BUGS. Boca Raton, FL: Chapman & Hall/CRC, 2020. [Google Scholar]
- 27. Vandormael A, Dobra A, Bärnighausen T, de Oliveira T, Tanser F. Incidence rate estimation, periodic testing and the limitations of the mid-point imputation approach. Int J Epidemiol 2018; 47:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. StataCorp . Stata 17 survival analysis reference manual. College Station, TX: Stata Press, 2021. [Google Scholar]
- 29. Rossi C, Butt ZA, Wong S, et al. . Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol 2018; 69:1007–14. [DOI] [PubMed] [Google Scholar]
- 30. Marco A, Guerrero RA, Vergara M, et al. . Reinfection in a large cohort of prison inmates with sustained virological response after treatment of chronic hepatitis C in Catalonia (Spain), 2002–2016. Int J Drug Policy 2019; 72:189–94. [DOI] [PubMed] [Google Scholar]
- 31. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147:573–7. [DOI] [PubMed] [Google Scholar]
- 32. Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis 2016; 62:683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Latham NH, Doyle JS, Palmer AY, et al. . Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int 2019; 39:2244–60. [DOI] [PubMed] [Google Scholar]
- 34. Valencia J, Alvaro-Meca A, Troya J, et al. . High rates of early HCV reinfection after DAA treatment in people with recent drug use attended at mobile harm reduction units. Int J Drug Policy 2019; 72:181–8. [DOI] [PubMed] [Google Scholar]
- 35. Caven M, Baiano CX, Robinson EM, et al. Hepatitis C reinfection by treatment pathway among people who inject drugs in Tayside, Scotland. J Viral Hepat 2021; 28:1744–750. [DOI] [PubMed] [Google Scholar]
- 36. Martinello M, Grebely J, Petoumenos K, et al. . HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat 2017; 24:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hajarizadeh B, Cunningham EB, Valerio H, et al. . Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: a meta-analysis. J Hepatol 2020; 72:643–57. [DOI] [PubMed] [Google Scholar]
- 38. Bonem EM, Ellsworth PC, Gonzalez R. Age differences in risk: perceptions, intentions and domains. J Behav Dec Making 2015; 28:317–30. [Google Scholar]
- 39. Reniers RLEP, Murphy L, Lin A, Bartolomé SP, Wood SJ. Risk perception and risk-taking behaviour during adolescence: the influence of personality and gender. PLoS One 2016; 11:e0153842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tavitian-Exley I, Vickerman P, Bastos FI, Boily MC. Influence of different drugs on HIV risk in people who inject: systematic review and meta-analysis. Addiction 2015; 110:572–84. [DOI] [PubMed] [Google Scholar]
- 41. Bjarnadottir GD, Magnusson A, Rafnar BO, et al. . Intravenous use of prescription psychostimulants; a comparison of the pattern and subjective experience between different methylphenidate preparations, amphetamine and cocaine. Eur Addict Res 2016; 22:259–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.