Abstract
Background
Identifying factors that determine the frequency of latently infected CD4+ T cells on antiretroviral therapy (ART) may inform strategies for human immunodeficiency virus (HIV) cure. We investigated the role of CD4+ count at ART initiation for HIV persistence on ART.
Methods
Among participants of the Strategic Timing of Antiretroviral Treatment Study, we enrolled people with HIV (PWH) who initiated ART with CD4+ T-cell counts of 500–599, 600–799, or ≥ 800 cells/mm3. After 36–44 months on ART, the levels of total HIV-DNA, cell-associated unspliced HIV-RNA (CA-US HIV-RNA), and two-long terminal repeat HIV-DNA in CD4+ T cells were quantified and plasma HIV-RNA was measured by single-copy assay. We measured T-cell expression of Human Leucocyte Antigen-DR Isotype (HLA-DR), programmed death-1, and phosphorylated signal transducer and activator of transcription-5 (pSTAT5). Virological and immunological measures were compared across CD4+ strata.
Results
We enrolled 146 PWH, 36 in the 500–599, 60 in the 600–799, and 50 in the ≥ 800 CD4 strata. After 36–44 months of ART, total HIV-DNA, plasma HIV-RNA, and HLA-DR expression were significantly lower in PWH with CD4+ T-cell count ≥ 800 cells/mm3 at ART initiation compared with 600–799 or 500–599 cells/mm3. The median level of HIV-DNA after 36–44 months of ART was lower by 75% in participants initiating ART with ≥ 800 vs 500–599 cells/mm3 (median [interquartile range]: 16.3 [7.0–117.6] vs 68.4 [13.7–213.1] copies/million cells, respectively). Higher pSTAT5 expression significantly correlated with lower levels of HIV-DNA and CA-US HIV-RNA. Virological measures were significantly lower in females.
Conclusions
Initiating ART with a CD4+ count ≥ 800 cells/mm3 compared with 600–799 or 500–599 cells/mm3 was associated with achieving a substantially smaller HIV reservoir on ART.
Keywords: HIV, HIV reservoir, antiretroviral therapy, HIV cure
In people with human immunodeficiency virus, initiating antiretroviral therapy with CD4+ counts ≥ 800 cells/mm3versus 600–799 or 500–599 cells/mm3was associated with achieving a substantially smaller HIV reservoir on ART. Higher phosphorylated signal transducer and activator of transcription-5 expression correlated with a smaller HIV reservoir independent of CD4+ count.
Despite long-term virological suppression with antiretroviral therapy (ART), human immunodeficiency virus (HIV) persists in long-lived and proliferating CD4+ T cells [1]. Because latently infected cells constitute the main barrier to a cure, identifying factors that determine their frequency may provide insights into HIV cure strategies. Initiating ART early (eg, during seroconversion, within 6 or 12 months of infection) is associated with a lower frequency of latently infected CD4+ T cells (ie, lower HIV reservoir size) [2–11], faster decay of cell-associated HIV-DNA [12], better CD4+ and CD8+ T-cell recovery [13–15], and better preserved B- and T-cell function [2, 9, 16, 17]. However, the capacity to preserve higher levels of CD4+ counts, regardless of duration of untreated infection, may also be an important factor in restricting or reducing the latent HIV reservoir. For example, ART initiation at higher CD4+ T-cell counts has been associated with a lower frequency of CD4+ T cells containing HIV-DNA in people with HIV (PWH), regardless of duration of untreated infection [4, 18, 19]. Also, exceptional CD4+ T-cell recovery on ART, defined as achieving a CD4+ T-cell count of ≥ 1000 cells/mm3, has been associated with a smaller HIV reservoir [20].
Thus, PWH who have not yet received ART, have the capacity to preserve CD4+ T-cell counts to levels typically observed in otherwise healthy HIV-seronegative persons, may constitute an elite immunological subgroup. Mean CD4+ T-cell counts ranged from 771 to 1109 cells/mm3 in HIV-negative individuals across 25 studies [15]. It is unknown whether maintaining a CD4+ T-cell count within this normal range despite HIV infection, and regardless of duration of untreated infection, is associated with a lower reservoir size on ART.
Biological sex may also affect HIV persistence on ART. Women comprise approximately 50% of the 38 million PWH worldwide [21]. Studies have demonstrated differences between men and women in the dynamics of the HIV reservoir [22]; however, females are greatly underrepresented in HIV persistence studies [23]. Adult females have greater longevity and are more immunocompetent with stronger innate and adaptive immune responses compared with men [24, 25]. Additionally, a recent study demonstrates that across all age ranges, females have a greater capacity to preserve a marker of immunologic resilience that associates with resistance to immunodeficiency syndrome (AIDS) and COVID-19 (ie, higher CD4+ counts and a relatively lower degree of CD8+ T-cell expansion [26]). Hence, this female-biased capacity may associate with a lower HIV reservoir size in females versus males.
The Strategic Timing of Antiretroviral Treatment (START) Study was a randomized clinical trial in which PWH with > 500 CD4 cells/mm3 were randomized to initiate ART immediately, or defer ART until CD4+ T-cell counts decreased to < 350 cells/mm3 [27]. We enrolled START study participants randomized to the immediate ART arm to test the hypothesis that preservation of CD4+ T-cell counts ≥ 800 versus 500–599 or 600–799 cells/mm3 before ART initiation associates with a lower frequency of latently infected CD4+ T cells on suppressive ART.
METHODS
Study Design and Participants
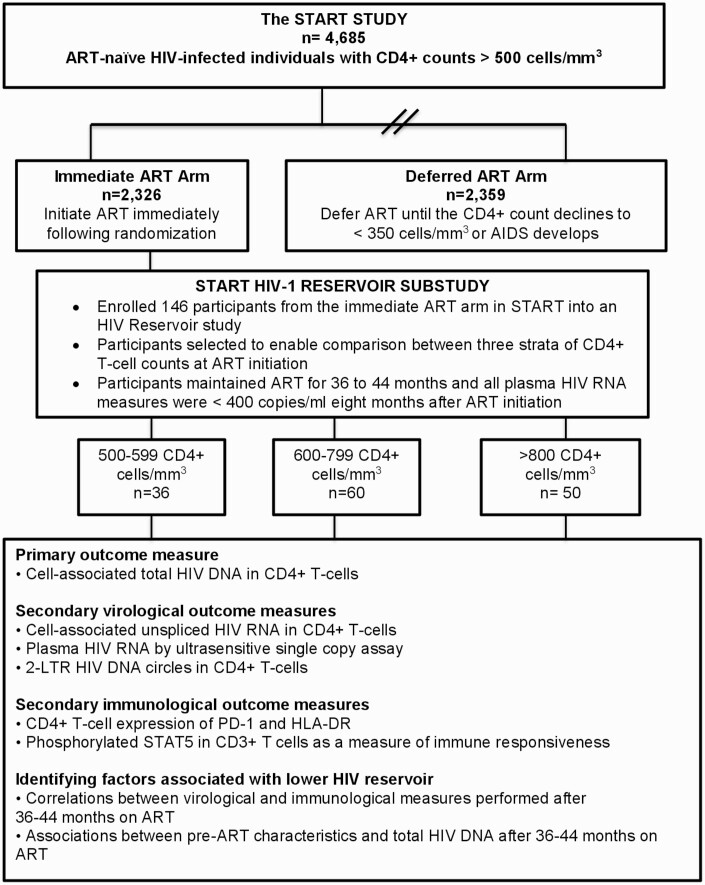
Details of the START study are described elsewhere [27]. We enrolled a subset of START study participants randomized to the immediate ART arm into the HIV reservoir study; participants were eligible if they had received ART for 36–44 months without interruption > 2 weeks, and if all plasma HIV-RNA levels obtained 8 months after initiating ART were < 400 copies/mL (Figure 1). Study participants were categorized according to whether their CD4+ count at ART initiation was 500–599, 600–799, or ≥ 800 cells/mm3. We selected 800 cells/mm3 as a threshold because it approximated the lower bounds of the interquartile range of median CD4+ counts in healthy HIV-seronegative persons [15]. Virological and immunological analyses were performed in peripheral blood mononuclear cells collected after 36–44 months of ART (Figure 1). Study participants were enrolled at 8 sites in Peru, South Africa, and Uganda; sites were chosen based on their rapid early enrollment into the main START study.
Figure 1.
Consort diagram for study enrollment. 2-LTR, 2-long terminal repeat; ART, antiretroviral therapy; HLA-DR, Human Leucocyte Antigen-DR Isotype; PD-1, programmed death-1; pSTAT5, phosphorylated signal transducer and activator of transcription-5.
The study was approved by the Alfred Hospital Research and Ethics Committee and the Institutional Review Boards/Ethics Committees at the recruiting sites and was conducted in accordance with the principles of the Declaration of Helsinki (1996). Each participant provided written informed consent before any study procedures.
Outcomes
The primary/secondary measures and associations are outlined in Figure 1. The primary outcome measure was the level of total HIV-DNA in peripheral blood CD4+ T cells after 36–44 months of ART [28]. Secondary virological outcome measures were the level of cell-associated unspliced HIV-RNA (CA-US HIV-RNA) and 2-long terminal repeat (2-LTR) HIV-DNA in CD4+ T cells and plasma HIV-RNA measured by an ultrasensitive assay [29]. Secondary immunological outcome measures were (1) CD4+ T-cell expression of the activation marker Human Leucocyte Antigen-DR Isotype (HLA-DR) and the exhaustion marker programmed death-1 (PD-1) [28] and (2) as a measure of T-cell responsiveness, the proportion of CD3+ T cells expressing phosphorylated signal transducer and activator of transcription-5 (pSTAT5) without or following ex vivo stimulation with interleukin-2 (IL-2). See Supplementary Methods for more details.
START Study Data
Baseline START study data collected were age, sex, race, estimated (self-reported) duration of HIV infection, CD4+ T-cell count, CD4+ T-cell nadir, plasma HIV-RNA, CD4+ T-cell percentage, CD4:CD8 ratio, co-infection with hepatitis B or C, ART regimen, and current smoking, medical history including cardiovascular disease, hypertension, and diabetes. We also accessed START study data on plasma levels of IL-6, high-sensitivity C-reactive protein and D-dimer at ART initiation.
Statistical Analyses
Based on prior work [30], we assumed a standard deviation for the level of total HIV-DNA of 0.65 log10 per million CD4+ T cells. Using this estimate, and after inflating our calculations by 20%, we estimated that 150 participants would provide 80% statistical power at a 5% significance level to detect a difference in total HIV-DNA of at least 0.4 log10 per million CD4+ T cells between participants commencing ART at CD4+ T-cell count ≥ 800 compared with 500–599 or 600–799 cells/mm3.
We compared virological and immunological measures across the CD4+ strata using Kruskal-Wallis equality of populations rank test and the Dunn multiple pairwise comparison test. To analyze associations of immunological measures or clinical characteristics at ART initiation with HIV reservoir size, we applied a generalized negative binomial regression model with all replicate data used in the analysis as previously described [30]. We assessed covariates for collinearity using the variance inflation factor together with Akaike’s Information Criterion. We performed both univariate and multivariable analyses using stepwise regression in the multivariate model.
RESULTS
Study Participants
We enrolled 146 study participants, 36 in the 500–599, 60 in the 600–799, and 50 in the ≥ 800 CD4+ T-cells/mm3 strata. Of these, 59 (40%) were males and 87 (60%) were females (Table 1). The median age was significantly different across the CD4+ strata, oldest in the ≥ 800 followed by the 600–799 cells/mm3 stratum. Congruently, the median CD4:CD8 ratio was significantly different across the 3 CD4+ T-cell strata, highest in the ≥ 800, and then decreasing to the lowest in the 500–599 cells/mm3 stratum. However, other parameters were evenly distributed across the CD4+ strata (Table 1). Plasma levels of IL-6, D-dimer, and high-sensitivity C-reactive protein at ART initiation did not differ across the CD4+ T-cell strata (Supplementary Figure S1). Age is associated with CD4+ lymphopenia [26]; thus, the older age of individuals within the ≥ 800 cells/mm3 stratum may reflect a length-time bias wherein START study participants with ≥ 800 cells/mm3 stratum had preserved higher CD4+ counts a longer time before HIV diagnosis.
Table 1.
Clinical Characteristics at ART Initiation
| Variable | Overall (n = 146) | Strata of CD4+ Count (cells/mm3) at ART Initiation | P-Value | ||
|---|---|---|---|---|---|
| 500–599 (n = 36) | 600–799 (n = 60) | ≥800 (n = 50) | |||
| Age, median (IQR), y | 39.5 (34, 48) | 36.5 (30.0, 41.5) | 40 (35.0, 47.5) | 44.5 (36.0, 50.0) | .021 |
| Sex, n (%) | .452 | ||||
| Male | 59 (40.4) | 17 (47.2) | 25 (41.7) | 17 (34.0) | |
| Female | 87 (59.6) | 19 (52.8) | 35 (58.3) | 33 (66.0) | |
| Race, n (%) | .188 | ||||
| Black | 124 (84.9) | 28 (77.8) | 53 (88.3) | 43 (86.0) | |
| Hispanic/Latino | 20 (13.7) | 8 (22.2) | 5 (8.3) | 7 (14.0) | |
| Other | 2 (1.37) | 0 | 2 (3.3) | 0 | |
| Country where participant enrolled, n (%) | .372 | ||||
| Peru | 20 (13.7) | 8 (22.2) | 5 (8.3) | 7 (14.0) | |
| South Africa | 57 (39.0) | 11 (30.6) | 25 (41.7) | 21 (42.0) | |
| Uganda | 69 (47.3) | 17 (47.2) | 30 (50.0) | 22 (44.0) | |
| Estimated (self-reported) duration of HIV infection before ART initiation, median (IQR), y | 2.0 (0.4, 5.4) | 1.7 (0.4, 4.3) | 1.5 (0.4, 5.5) | 2.8 (0.5, 7.1) | .502 |
| Time on ART at time of sampling for HIV reservoir analyses, median (IQR), m | 38.2 (36.3, 41.7) | 38.6 (36.4, 41.8) | 39.2 (36.5, 41.8) | 37.1 (36.3, 41.3) | .379 |
| CD4+ count at ART initiation (cells/mm3), median (IQR) | 710.3 (604.0, 854.5) | 564.3 (533, 577) | 678 (641, 733) | 932 (852.5, 1081.5) | N/A |
| Recorded nadir CD4+ count before ART (cells/mm3), median (IQR) | 630 (530, 781) | 519 (491.5, 536.5) | 612.5 (541, 663.5) | 837 (762, 972) | N/A |
| CD4:CD8 T-cell ratio at ART initiation, median (IQR) | 0.8 (0.6, 1.0) | 0.6 (0.4, 0.8) | 0.7 (0.6, 0.9) | 0.9 (0.7, 1.2) | .000 |
| Plasma HIV RNA at ART initiation (log10 copies/mL), median (IQR) | 3.9 (3.1, 4.7) | 4.4 (3.7, 4.9) | 3.8 (3.0, 4.7) | 3.9 (3.0, 4.5) | .505 |
| Current smoking, n (%) | 19 (13.0) | 6 (16.7) | 9 (15.0) | 4 (8.0) | .403 |
| Positive cardiovascular disease, n (%)a | 0 | 0 | 0 | 0 | N/A |
| Positive diabetes, n (%)b | 3 (2.1) | 0 | 2 (3.3) | 1 (2.0) | .787 |
| Positive hypertension, n (%)c | 21 (14.4) | 2 (5.56) | 12 (20.0) | 7 (14.0) | .143 |
| Positive hepatitis B, n (%) | 6 (4.1) | 1 (2.8) | 3 (5.0) | 2 (4.0) | 1.000 |
| Positive hepatitis C, n (%) | 2 (1.4) | 0 (0.0) | 1 (1.7) | 1 (2.0) | 1.000 |
| ART regimen prescribed, n (%) | .450 | ||||
| NRTI + PI | 9 (6.2) | 1 (2.8) | 5 (8.3) | 3 (6.0) | |
| NRTI + NNRTI | 136 (93.2) | 34 (94.4) | 55 (91.7) | 47 (94.0) | |
| NRTI only (protocol deviation) | 1 (0.70) | 1 (2.8) | 0 (0.0) | 0 (0.0) | |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; N/A: not applicable; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Acute myocardial infarction, stroke, or coronary revascularization.
Diabetes mellitus diagnosis or receiving antidiabetic medication (insulin, metformin, sulfonylureas, thiazolidinediones or biguanides, or other) or 8-hour fasting glucose ≥ 126 mg/dL.
Systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or receiving blood pressure medication (beta blockers, diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, calcium channel antagonists, other).
Primary and Secondary Virological Outcome Measures
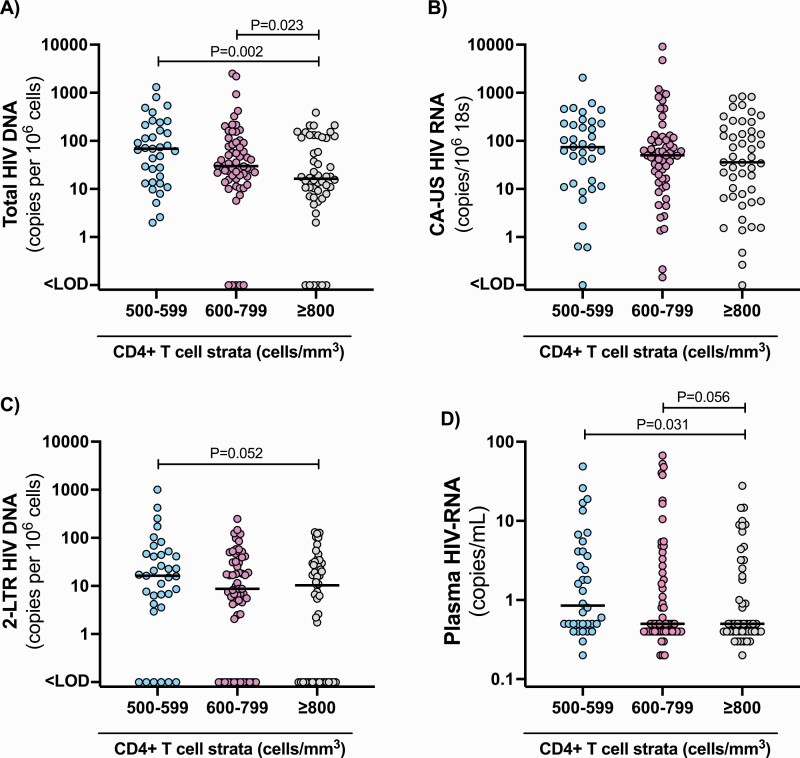
We quantified the level of total HIV-DNA in peripheral blood CD4+ T cells as a proxy for the frequency of infected cells, acknowledging that this measurement includes unintegrated, integrated, defective, and intact virus [31, 32]. The frequency of cells containing HIV-DNA was significantly lower in participants initiating ART with CD4+ ≥ 800 compared with either 600–799 (P = .023) or 500–599 (P = .002) cells/mm3 (Figure 2A). Median (interquartile range) levels of total HIV-DNA in persons initiating ART with 500–599, 600–799, and ≥ 800 cells/mm3 were 68.4 (13.7–213.1), 30.0 (17.1–91.9), and 16.3 (7.0–117.6) copies/million cells, respectively. Hence, the median level of HIV-DNA in the ≥ 800 stratum was less than 25% of that in the 500–599 cells/mm3 stratum.
Figure 2.
Levels of cell-associated and plasma HIV across strata of CD4+ T-cell count at ART initiation. The frequency of total HIV-DNA (A), CA-US HIV-RNA (B), and 2-LTR circles (C) in CD4+ T cells, and the level of plasma HIV-RNA measured by single-copy assay (D) within each stratum of CD4+ T-cell count as indicated. 2-LTR: 2-long terminal repeat; CA-US HIV-RNA, cell-associated unspliced HIV-RNA. Each symbol represents a different participant and the horizontal black line the median value.
We quantified CA-US HIV-RNA as a measure of persistent HIV transcription on ART and 2-LTR circles as a measure of recently infected cells. Although these measures were slightly lower in the ≥ 800 stratum, differences were not statistically significant (P = .55 for CA-US HIV-RNA and P = .27 for 2-LTR using Kruskal-Wallis test; Figure 2B and 2C). Analysis of residual viremia on ART (quantified by an ultrasensitive assay with lower limit of detection of 1 copy per milliliter) revealed that plasma HIV-RNA was significantly lower in participants initiating ART with ≥ 800 versus 500–599 (P = .031) and trending lower versus the 600–799 cells/mm3 stratum (P = .056) (Figure 2D). Collectively, these analyses showed that of those participants randomized to immediate ART, those initiating ART at ≥ 800 cells/mm3 had a much lower frequency of latently infected CD4+ T cells and a lower level of residual viremia after 36–44 months on ART.
We did not detect an association between participants’ highest CD4+ T-cell counts reported after 36–44 months of ART, and total HIV-DNA and did not find a significant association overall, or across the CD4 + cell strata (Supplementary Table S8).
Secondary Immunological Outcome Measures
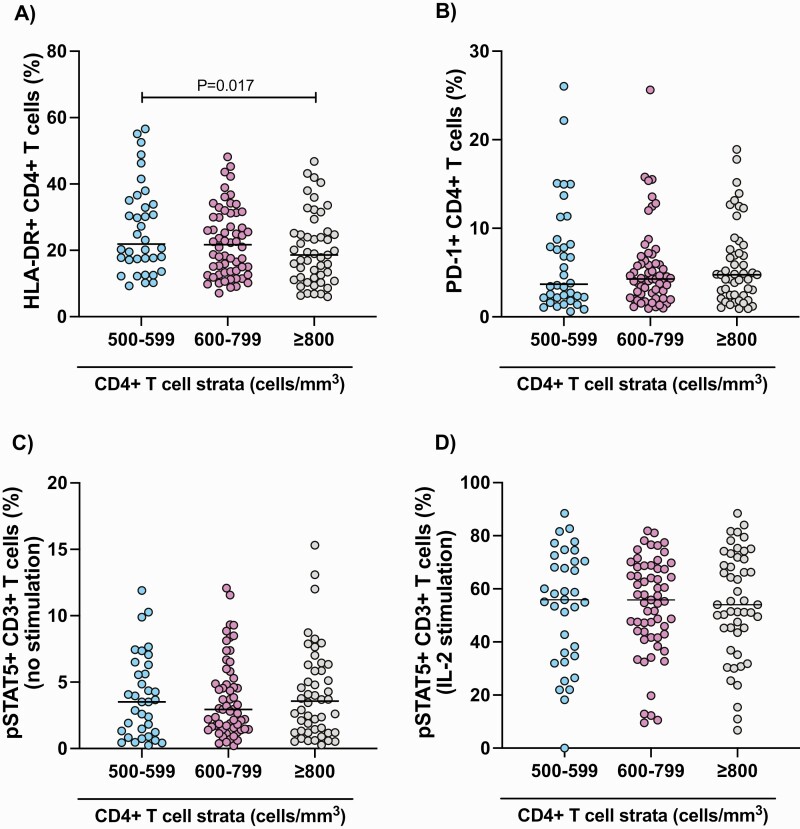
We found that CD4+ T-cell expression of HLA-DR was significantly lower in PWH initiating ART with CD4+ T-cell counts ≥ 800 compared with 500–599 cells/mm3 (Figure 3A). However, expression of programmed death-1 or pSTAT5 on T cells did not differ by CD4+ strata (Figure 3B–D).
Figure 3.
T-cell expression of HLA-DR, PD-1, and pSTAT5 across strata of CD4+ T-cell count at ART initiation. The proportion of CD4+ T cells expressing HLA-DR (A) and PD-1 (B), and the proportion of CD3+ T cells expressing phosphorylated STAT5 without (C) or following ex vivo stimulation with IL-2 (D) measured by flow cytometry. IL, interleukin; PD-1, programmed death-1; STAT5, phosphorylated signal transducer and activator of transcription-5.
Correlations Between Immunological and Virological Outcome Measures
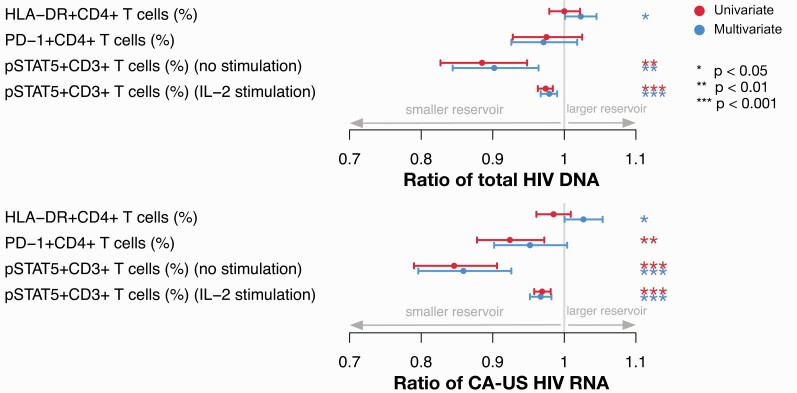
In multivariate analyses, higher levels of CD4+ T-cell expression of HLA-DR after 36–44 months of ART were associated with higher levels of both total HIV-DNA and CA-US HIV-RNA (Figure 4; Supplementary Tables S1 and S3). At the same timepoint, although expression levels of pSTAT5 were similar across CD4 strata, there was a highly significant association between pSTAT5 levels and measures of HIV persistence. Higher expression of pSTAT5, with or without ex vivo IL-2 stimulation, was correlated with lower levels of HIV-DNA and CA-US HIV-RNA in both uni- and multivariate analyses, with the latter including adjustment for CD4+ count at ART initiation (Figure 4; Supplementary Tables S1–S4). Together, these results suggest that T-cell activation was modestly associated with a larger HIV reservoir and that higher pSTAT5 expression correlated with a lower frequency of infected cells as well as lower HIV transcriptional activity, independent of CD4+ count at ART initiation.
Figure 4.
Multivariate and univariate analyses of associations between immune activation/exhaustion parameters and total HIV-DNA and CA-US HIV-RNA in CD4+ T cells. Fold-change in total HIV-DNA and CA-US HIV-RNA in CD4+ T cells for each unit increase in the indicated parameters for all participants. IL, interleukin; PD-1, programmed death-1; pSTAT5, phosphorylated signal transducer and activator of transcription-5.
Associations of Clinical Characteristics at ART Initiation With HIV Reservoir Size on ART
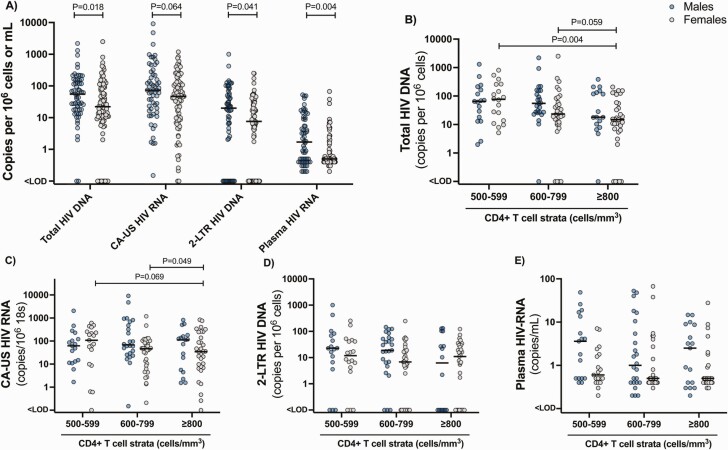
Older age associated with a lower level of total HIV-DNA (Table 2), which may relate to the length-time bias discussed earlier (Table 1). Female sex showed a strong association with lower total HIV-DNA (ratio 0.565; 95% confidence interval, .350–0.912) compared with male sex (Table 2). Correspondingly, we found significantly lower levels of total HIV-DNA, CA-US HIV-RNA, 2-LTR HIV-DNA, and plasma HIV-RNA in females compared with males (Figure 5A). To examine whether the lower frequency of total HIV-DNA in the ≥ 800 cells/mm3 stratum related to a higher proportion of females, we performed sensitivity analyses stratified by sex. In the analyses restricted to females, initiating ART with ≥ 800 cells/mm3 associated with lower frequency of HIV-DNA (Figure 5B). In contrast, in males, the slightly lower median levels of total HIV-DNA in the 500–599 and 600–799 CD4 cells/mm3 strata compared with the ≥ 800 cells/mm3 stratum, no longer reached statistical significance, possibly because of loss of statistical power (Figure 5B). For females, there was also a consistent trend toward a lower level of CA-US HIV-RNA in the ≥ 800 cells/mm3 stratum compared with the 500–599 and 600–799 cells/mm3 strata (Figure 5C), whereas no differences across CD4+ strata for either sex was found for 2-LTR HIV-DNA and plasma HIV-RNA (Figure 5D and 5E).
Table 2.
Multivariate Analysis of Associations Between Total HIV-DNA in CD4+ T Cells and Clinical Characteristics at ART Initiation
| Variable | Overall (N = 146) | |||
|---|---|---|---|---|
| Ratio | 95% CI | P-Value | ||
| CD4+ count at ART initiation (cells/mm3) | 0.998 | .997 | 1.000 | .009 |
| Age, y | 0.973 | .951 | .995 | .015 |
| Sex (referent, male) | ||||
| Female | 0.565 | .350 | .912 | .019 |
| Current smoking | - | - | - | .201 |
| Country where participant enrolled (referent, Peru) | ||||
| South Africa | 0.705 | .330 | 1.507 | .367 |
| Uganda | 2.491 | 1.151 | 5.392 | .021 |
| Estimated (self-reported) duration of HIV infection before ART initiation, y | - | - | - | .631 |
| Time on ART at time of sampling for HIV reservoir analyses, m | - | - | - | .942 |
| CD4:CD8 ratio at ART initiation | - | - | - | .420 |
| Plasma HIV RNA at ART initiation (log10 copies/mL) | 1.189 | 1.000 | 1.413 | .050 |
| Positive hypertensiona | - | - | - | .427 |
| Positive hepatitis Bb | 0.224 | .068 | .738 | .014 |
| ART regimen (referent, PI/NRTI) | ||||
| NRTI/NNRTI | - | - | - | .333 |
| Plasma IL-6 (pg/mL) at ART initiation | 0.842 | .676 | 1.047 | .122 |
| Plasma d-dimer (µg/mL) at ART initiation | 0.753 | .568 | .998 | .048 |
| Plasma hs-CRP (µg/m) at ART initiation | 1.005 | .985 | 1.025 | .645 |
Adjusted for: age, sex, country, viral load, hepatitis B.
Hyphen (-) indicates that variables with P-values >.20 were removed from model by stepwise regression; Abbreviations: ART, antiretroviral therapy; CI, confidence interval; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or receiving blood pressure medication (beta blockers, diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, calcium channel antagonists, other).
Hepatitis B measured as being hepatitis B surface antigen positive.
Figure 5.
Levels of cell-associated and plasma HIV in males versus females across strata of CD4+ T-cell count at ART initiation. Comparison of virological measures between males and females for the entire cohort (A) including the frequency of total HIV-DNA (B), CA-US HIV-RNA (C), 2-LTR circles (D), and plasma HIV-RNA measured by single-copy assay (E) for males and females within each stratum of CD4+ T-cell counts. Statistical comparisons were done for males versus females across the entire cohort for each virological measure (A) and for males and females separately to compare each measure across the CD4 strata (B–E). Only statistically significant results are shown. 2-LTR, 2-long terminal repeat; CA-US HIV-RNA, cell-associated unspliced HIV-RNA.
Because of the older age of study participants in strata with higher CD4+ counts (Table 1), we examined whether age confounded the main finding of lower total HIV-DNA in the ≥ 800 CD4+ T cells/mm3 stratum. In a multivariate analysis adjusted for age, sex, enrollment country, plasma viral load at ART initiation and hepatitis B, the CD4+ T-cell count at ART initiation remained significantly associated with total HIV-DNA after 36–44 months on ART (Table 2).
The pre-ART CD4:CD8 ratio was significantly associated with total HIV-DNA in univariate analysis (0.37; 95% confidence interval, .216–.639). However, given the higher correlation between CD4+ counts and CD4:CD8 ratio values (collinearity), the association of the ratio with HIV-DNA was no longer significant in the multivariate analysis (Table 2). There was no association with type 2 diabetes or hypertension, whereas enrollment at Ugandan compared with Peruvian sites was associated with higher total HIV-DNA (Table 2; Supplementary Figure S2). Similar observations were observed in univariate analyses (Supplementary Figure S2 and Supplementary Table S5). Hepatitis B surface antigen positivity was significantly associated with a lower total HIV-DNA, but the significance of this association is unclear because only 6 study participants were seropositive.
Exploratory Outcomes
We analyzed the association between CA-US HIV-RNA and clinical characteristics at ART initiation. Being hepatitis B surface antigen positive and longer time on ART at time of sample collection were both significantly associated with a lower level of CA-US HIV-RNA (Supplementary Figure S2 and Supplementary Tables S6 and S7), whereas being on a nonnucleoside reverse transcriptase inhibitor-based regimen compared with a protease inhibitor-based regimen was associated with a higher level of CA-US HIV-RNA. Current smoking afforded a nearly 3-fold change in CA-US HIV-RNA. These exploratory analyses suggested that factors associated with the frequency of infected cells were distinct from those associated with transcriptional activity of the reservoir.
DISCUSSION
Among participants randomized to the immediate arm of the START study, we found that levels of total HIV-DNA and plasma HIV-RNA were significantly lower in participants who initiated ART with CD4+ T-cell count ≥ 800 compared with either 600–799 or 500–599 cells/mm3. The median level of HIV-DNA assessed after 36–44 months on ART was lower by 75% in participants initiating ART with ≥ 800 versus 500–599 cells/mm3. In multivariate analyses, the association of total HIV-DNA with CD4+ T-cell count at ART initiation remained statistically significant after controlling for potential confounders including age and sex. Notably, HIV persistence on ART was greater in males than females. Finally, we found that higher CD4+ T-cell expression of HLA-DR was associated with a higher frequency of infected CD4+ T cells, whereas higher pSTAT5 expression correlated with a lower frequency of cells containing HIV-DNA and US HIV-RNA. Collectively, these findings suggest that PWH with the elite capacity to preserve CD4+ T cells ≥ 800/mm3 before commencing ART manifest a substantially lower HIV reservoir on suppressive ART.
Others have found a lower frequency of latently infected cells following early ART initiation [2–11]. However, this is the first study to directly address the role of the CD4+ T-cell count at ART initiation using prespecified CD4 strata regardless of duration of untreated infection. Because the study was conducted exclusively among PWH who initiated ART with CD4+ T-cell counts > 500 cells/mm3, we were uniquely positioned to address nuanced effects of higher CD4+ T-cell counts on the HIV reservoir under current treatment guidelines.
The larger proportion of females in the study facilitated the identification of differences in measures of HIV persistence on ART between sexes. This is aligned with cross-sectional studies in PWH on ART [22, 33–35] and may relate to the stronger innate and adaptive immune responses in adult females [24, 25] and the potential role of estrogen in HIV persistence through its effect on the HIV LTR whereupon it inhibits HIV transcription [36].
The transcription factor STAT5 is activated through phosphorylation, responding to drivers of T-cell proliferation, in particular IL-7 and IL-2 [37], and plays a key role in in shaping the CD4+ T-cell immune response [38]. Thus, pSTAT5 levels serve as a proxy for the functionality/responsiveness of T cells. STAT5 activity is involved in driving tumor-specific [39] and cytomegalovirus (CMV)-specific [40] T-cell polyfunctionality and cytokine production, thus emphasizing the potential role of STAT5 in the generation of a potent immune response. Hence, the significant associations between pSTAT5 and HIV reservoir size underscore the importance of reconstituted immunologic health in reducing the frequency of infected cells that is independent of CD4+ T-cell count at ART initiation.
It is plausible that the association we observed between higher levels of pSTAT5 and a lower HIV reservoir size could be explained by greater homeostatic proliferation of CD4+ T cells. However, our finding that levels of pSTAT5 were not significantly different between the 3 CD4+ cell strata and that there was no significant difference either overall, or between the CD4+ cell strata in highest levels of CD4+ cells gained during 36–44 months of ART makes this a less likely explanation for our findings.
Several limitations of our study require consideration. First, factors other than CD4+ T-cell count at ART initiation have the potential to confound our findings. We addressed this by performing stepwise regression in multivariate analyses but recognize there may be additional unrecognized confounders. Second, because samples were not collected for HIV reservoir analyses at the time of ART initiation, we were unable to longitudinally track levels of cell-associated HIV-DNA, RNA, or pSTAT5. Third, we did not analyze the HIV subtypes in study participants, which would have varied between countries. However, adjustment for country of enrollment may mitigate potential confounding because of this factor. Fourth, because of limitations in cell numbers, we were unable to analyze HIV-specific T-cell function, quantify the frequency of cells containing intact HIV provirus, or measure the frequency of cells with inducible replication competent virus. It has been estimated, using near full-genome sequencing of HIV provirus, that only approximately 2.5% of HIV provirus is intact [41]. If enhanced immune-mediated elimination of virus-expressing cells among PWH with CD4+ T-cell counts ≥ 800/mm3 at ART initiation is the main mechanism leading to a lower HIV reservoir in this stratum after 36–44 months of ART, this may primarily be directed against cells containing intact HIV. It would therefore have been of great interest to compare levels of intact HIV DNA across the three strata, but unfortunately this was not feasible in the present study. Fifth, we analyzed pSTAT5 in total CD3+ T cells; hence, it is uncertain whether this association reflected pSTAT5 levels in CD8+ or CD4+ T cells, or both. Our data revealed no difference between pre-ART CD4 strata in pSTAT5 after 36–44 months of ART but do not rule out that such differences might have been present at ART initiation. Finally, CMV antibody tests were not available for study participants. Hence, we were unable to determine potential associations between CMV infection and HIV persistence. CMV antibody positivity rates are very high in PWH [42]; hence, these high rates across CD4+ strata would have likely precluded the power to detect a significant association.
In conclusion, we found that initiating ART with a CD4+ count ≥ 800 compared with 600–799 or 500–599 cells/mm3 was associated with a significantly lower level of total HIV-DNA, plasma HIV-RNA, and T-cell activation after 36–44 months of suppressive ART. Higher pSTAT5 expression correlated with a lower level of HIV-DNA independent of CD4+ T-cell count at ART initiation. Additionally, we observed that reservoir sizes were lower in females, which we suggest is related to the impact of estrogen, which represses HIV reactivation, and because of an enhanced innate immune response in females compared with males, in response to similar levels of HIV RNA. Taken together, these findings suggest that PWH who are able to preserve CD4+ T cells ≥ 800 cells/mm3 before ART initiation, especially females, have a smaller reservoir on ART. Interventional cure studies in this subgroup could potentially have a favorable outcome.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. E. J. W. and S. R. L. conceived the study. E. J. W., S. R. L., S. K. A., and J. N. designed the study. E. J. W., L. L., M. J. V., and F. H. oversaw all aspects of the clinical study including study protocol, ethics submission, and management of study participants. C. C. contributed to the design of the laboratory protocol. E. J. W, S. R. L., A. R., J. C., M. J. V., F. H., S. P., R. M., S. B. F., J. K., J. L., P. P., H. M., E. K., P. A. M., C. G., A. L. R., and R. W. coordinated all sample collection, planned sample analyses, and coordinated all data generation. A. R. and J. C. performed polymerase chain reaction analyses of CA-US HIV-RNA, 2-LTR, HIV-DNA, and all flow cytometry analyses. S. P. and K. F. oversaw and performed analyses of plasma HIV-RNA. L. K. and K. P. performed all statistical analysis. E. W., S. R. L., T. A. R., J. N., and S. K. A. performed data analysis and interpretation. T. A. R. drafted the manuscript. All authors reviewed and provided input to the manuscript and approved the final version.
Acknowledgments. The authors acknowledge the study participants and all human and animal participants of previous HIV cure studies. The authors also acknowledge the excellent work of other researchers that we have not been able to cite in this publication. This manuscript is dedicated to Dr. Fred Gordin.
Financial Support. The study was funded by grants from Gilead Sciences Inc. (grant number CO-US-232-1804), Merck (grant number 51859), The Australian National Health and Medical Research Council (program grant APP149990, National Health Medical Research Council practitioner fellowship APP 1135851, and early career fellowship APP 1092160) and The Australian Centre for HIV and Hepatitis Virology Research. This work was also supported by the Delaney AIDS Research Enterprise (DARE) to Find a Cure (1U19AI096109 and 1UM1AI126611-01). The INSIGHT Washington International Coordinating Center provided in-kind effort and supplemental support to participating sites.
Contributor Information
Thomas A Rasmussen, Department of Infectious Diseases, The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia; Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark.
Sunil K Ahuja, Department of Medicine, University of Texas Health Science Center, San Antonio, Texas, USA.
Locadiah Kuwanda, The Kirby Institute, University of New South Wales, Sydney, Australia.
Michael J Vjecha, Institute for Clinical Research, Inc., Veterans Affairs Medical Center, Washington D.C., USA.
Fleur Hudson, MRC Clinical Trials Unit at UCL, London UK Uganda Virus Research Institute/MRC, London, United Kingdom; LSHTM Uganda Research Unit, HIV Intervention Programme, Entebbe, Uganda.
Luxshimi Lal, Burnet Institute, Melbourne, Australia.
Ajantha Rhodes, Department of Infectious Diseases, The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia.
Judy Chang, Department of Infectious Diseases, The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia.
Sarah Palmer, Centre for Virus Research, The Westmead Institute for Medical Research, The University of Sydney, Sydney, Australia.
Paula Auberson-Munderi, UNAIDS, HIV Prevention, Geneva, Switzerland.
Henry Mugerwa, Joint Clinical Research Centre, Entebbe, Uganda.
Robin Wood, The Desmond Tutu HIV Centre, Institute for Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Sharlaa Badal-Faesen, Clinical HIV Research Unit, Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa.
Sandy Pillay, Enhancing Care Foundation, Department of Research and Post-graduate Support, Durban University of Technology, Durban, South Africa.
Rosie Mngqibisa, Enhancing Care Foundation, Department of Research and Post-graduate Support, Durban University of Technology, Durban, South Africa.
Alberto LaRosa, Asociación Civil Impacta Salud y Educación, Lima, Perú.
Jose Hildago, Via Libre, Lima, Perú.
Kathy Petoumenos, The Kirby Institute, University of New South Wales, Sydney, Australia.
Chris Chiu, Department of Infectious Diseases, The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia.
Joseph Lutaakome, LSHTM Uganda Research Unit, HIV Intervention Programme, Entebbe, Uganda; Uganda Virus Research Institute/MRC, Entebbe, Uganda.
Jonathan Kitonsa, LSHTM Uganda Research Unit, HIV Intervention Programme, Entebbe, Uganda; Uganda Virus Research Institute/MRC, Entebbe, Uganda.
Esther Kabaswaga, Joint Clinical Research Centre, Entebbe, Uganda.
Pietro Pala, Immunova Limited, London, United Kingdom.
Carmela Ganoza, Asociación Civil Impacta Salud y Educación, Lima, Perú; Universidad Peruana Cayetano Heredia, Lima, Perú.
Katie Fisher, Centre for Virus Research, The Westmead Institute for Medical Research, The University of Sydney, Sydney, Australia.
Christina Chang, The Kirby Institute, University of New South Wales, Sydney, Australia; Centre for the AIDS Programme of Research in South Africa, Durban, South Africa; Central Clinical School, Monash University, Infectious Diseases, Melbourne, Australia; Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Australia.
Sharon R Lewin, Department of Infectious Diseases, The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia; Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Australia; Victorian Infectious Diseases Service, Royal Melbourne Hospital at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia.
Edwina J Wright, Department of Infectious Diseases, The University of Melbourne at The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia; Burnet Institute, Melbourne, Australia; Central Clinical School, Monash University, Infectious Diseases, Melbourne, Australia; Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Australia.
References
- 1. Sneller MC, Huiting ED, Clarridge KE, et al. . Kinetics of plasma HIV rebound in the era of modern antiretroviral therapy. J Infect Dis 2020; 222:1655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crowell TA, Fletcher JL, Sereti I, et al. . Initiation of antiretroviral therapy before detection of colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J Int AIDS Soc 2016; 19:21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananworanich J, Chomont N, Eller LA, et al. . HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine 2016; 11:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hocqueloux L, Avettand-Fènoël V, Jacquot S, et al. . Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 2013; 68:1169–78. [DOI] [PubMed] [Google Scholar]
- 5. Chéret A, Bacchus-Souffan C, Avettand-Fenoël V, et al. . Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother 2015; 70:2108–20. [DOI] [PubMed] [Google Scholar]
- 6. Buzon MJ, Martin-Gayo E, Pereyra F, et al. . Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol 2014; 88:10056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ngo-Giang-Huong N, Deveau C, Da Silva I, et al. . Proviral HIV-1 DNA in subjects followed since primary HIV-1 infection who suppress plasma viral load after one year of highly active antiretroviral therapy. AIDS 2001; 15:665–73. [DOI] [PubMed] [Google Scholar]
- 8. Strain MC, Little SJ, Daar ES, et al. . Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 2005; 191:1410–8. [DOI] [PubMed] [Google Scholar]
- 9. Jain V, Hartogensis W, Bacchetti P, et al. . Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013; 208:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pires A, Hardy G, Gazzard B, Gotch F, Imami N.. Initiation of antiretroviral therapy during recent HIV-1 infection results in lower residual viral reservoirs. J Acquir Immune Defic Syndr 2004; 36:783–90. [DOI] [PubMed] [Google Scholar]
- 11. Bachmann N, von Siebenthal C, Vongrad V, et al. . Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun 2019; 10:3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laanani M, Ghosn J, Essat A, et al. . Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 2015; 60:1715–21. [DOI] [PubMed] [Google Scholar]
- 13. Cao W, Mehraj V, Trottier B, et al. . Early initiation rather than prolonged duration of antiretroviral therapy in HIV infection contributes to the normalization of CD8 T-cell counts. Clin Infect Dis 2016; 62:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okulicz JF, Le TD, Agan BK, et al. . Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med 2015; 175:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le T, Wright EJ, Smith DM, et al. . Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kök A, Hocqueloux L, Hocini H, et al. . Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal Immunol 2015; 8:127–40. [DOI] [PubMed] [Google Scholar]
- 17. Planchais C, Hocqueloux L, Ibanez C, et al. . Early antiretroviral therapy preserves functional follicular helper T and HIV-specific B cells in the gut mucosa of HIV-1-infected individuals. J Immunol 2018; 200:3519–29. [DOI] [PubMed] [Google Scholar]
- 18. Boulassel MR, Chomont N, Pai NP, Gilmore N, Sékaly RP, Routy JP.. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol 2012; 53:29–32. [DOI] [PubMed] [Google Scholar]
- 19. Chomont N, El-Far M, Ancuta P, et al. . HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodríguez-Castañón JM, McNaugthon A, Cárdenas-Ochoa A, et al. . Exceptional T CD4+ recovery post-ART is linked to a lower HIV reservoir with a specific immune differentiation pattern. AIDS Res Hum Retroviruses 2021; 38:11–21. [DOI] [PubMed] [Google Scholar]
- 21. UNAIDS. UNAIDS 2020 global report, 2020. [Google Scholar]
- 22. Scully EP, Gandhi M, Johnston R, et al. . Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J Infect Dis 2019; 219:1084–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari SA.. Systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr 2016; 71:181–8. [DOI] [PubMed] [Google Scholar]
- 24. Klein SL, Flanagan KL.. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 25. Austad SN, Fischer KE.. Sex differences in lifespan. Cell Metab 2016; 23:1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee GC, Restrepo MI, Harper N, et al. . Immunologic resilience and COVID-19 survival advantage. J Allergy Clin Immunol 2021; 148:1176–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lundgren JD, Babiker AG, Gordin F, et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasmussen TA, McMahon JH, Chang JJ, et al. . The effect of antiretroviral intensification with dolutegravir on residual virus replication in HIV-infected individuals: a randomised, placebo-controlled, double-blind trial. Lancet HIV 2018; 5:e221–30. [DOI] [PubMed] [Google Scholar]
- 29. Bakkour S, Deng X, Bacchetti P, et al. . Replicate Aptima assay for quantifying residual plasma viremia in individuals on ART. Conference on Retroviruses and Opportunistic Infections (CROI). Seattle, 2019. [Google Scholar]
- 30. Elliott JH, McMahon JH, Chang CC, et al. . Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2015; 2:e520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simonetti FR, White JA, Tumiotto C, et al. . Intact proviral DNA assay analysis of large cohorts of people with HIV provides a benchmark for the frequency and composition of persistent proviral DNA. Proc Natl Acad Sci USA 2020; 117:18692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdel-Mohsen M, Richman D, Siliciano RF, et al. . Recommendations for measuring HIV reservoir size in cure-directed clinical trials. Nat Med 2020; 26:1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cuzin L, Pugliese P, Sauné K, et al. . Levels of intracellular HIV-DNA in patients with suppressive antiretroviral therapy. AIDS 2015; 29:1665–71. [DOI] [PubMed] [Google Scholar]
- 34. Fourati S, Flandre P, Calin R, et al. . Factors associated with a low HIV reservoir in patients with prolonged suppressive antiretroviral therapy. J Antimicrob Chemother 2014; 69:753–6. [DOI] [PubMed] [Google Scholar]
- 35. Prodger JL, Capoferri AA, Yu K, et al. . Reduced HIV-1 latent reservoir outgrowth and distinct immune correlates among women in Rakai, Uganda. JCI Insight 2020; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Das B, Dobrowolski C, Luttge B, et al. . Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci USA 2018; 115:E7795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rani A, Murphy JJ.. STAT5 in cancer and immunity. J Interferon Cytokine Res 2016; 36:226–37. [DOI] [PubMed] [Google Scholar]
- 38. Owen DL, Farrar MA.. STAT5 and CD4 (+) T cell immunity. F1000Res 2017; 6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ding ZC, Shi H, Aboelella NS, et al. . Persistent STAT5 activation reprograms the epigenetic landscape in CD4(+) T cells to drive polyfunctionality and antitumor immunity. Sci Immunol 2020; 5:eaba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Healy ZR, Weinhold KJ, Murdoch DM.. Transcriptional profiling of CD8+ CMV-specific T cell functional subsets obtained using a modified method for isolating high-quality RNA from fixed and permeabilized cells. Front Immunol 2020; 11:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bruner KM, Wang Z, Simonetti FR, et al. . A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamori D, Joachim A, Mizinduko M, et al. . Seroprevalence of human herpesvirus infections in newly diagnosed HIV-infected key populations in Dar es Salaam, Tanzania. Int J Microbiol 2021; 10.1155/2021/4608549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.