Abstract
Lipoproteins in the cell membranes of both Mycoplasma salivarium and Mycoplasma fermentans were demonstrated to trigger the transcription of intercellular adhesion molecule-1 mRNA in normal fibroblasts isolated from human gingival tissue and to induce its cell surface expression by a mechanism distinct from that of Escherichia coli lipopolysaccharide. The lipid moiety of the lipoproteins was suggested to play a key role in the expression of the activity.
Mycoplasmas are the smallest self-replicating microorganisms and lack cell wall. The genus Mycoplasma can be divided into fermentative and nonfermentative species which utilize glucose and arginine as main energy sources, respectively. Some Mycoplasma species are the causative agents of some infectious diseases such as primary atypical pneumonia and nongonococcal urethritis (13), and have been implicated as possible causes of human joint diseases (26, 36) and a possible cofactor in AIDS pathogenesis (15).
Mycoplasma salivarium, a nonfermentative mycoplasma, is one of the human oral microbial flora and inhabits gingival sulci and dental plaques (8, 14). The organism is isolated from the periodontal pockets of periodontally diseased subjects at a significantly higher rate than from the gingival sulci of healthy subjects (8, 14). Antibody response to the organism is significantly higher in diseased subjects than in healthy subjects (14, 35). M. salivarium induces interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-6 in monocytes/macrophages (17) and IL-6 and IL-8 in human gingival fibroblasts (27). On the basis of these findings, M. salivarium is suspected to play an etiological role in some cases of oral infections, including periodontal diseases.
Periodontal diseases are recognized as an inflammatory disorder caused by microbial plaque and the host response to its accumulation (28). Secretion of IL-1β, TNF-α, IL-6, and IL-8 is an important step in the inflammatory and immune responses. Local induction of cell adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) is one of the key mechanisms in focusing and potentiating inflammatory and immunological response (5). Oral gram-negative bacteria, suspected to be pathogens in periodontal diseases, are known to induce proinflammatory cytokines such as IL-1, IL-6, and IL-8 in human gingival fibroblasts (31, 34) and to upregulate the expression of ICAM-1 in gingival fibroblasts (10).
Therefore, we were very much interested in knowing whether M. salivarium induced ICAM-1 expression in gingival fibroblasts. For comparative study, Mycoplasma fermentans, a fermentative mycoplasma, was used, because the organism has recently been detected at a high rate in human saliva (1). In this study, we demonstrated that M. salivarium and M. fermentans triggered transcriptional activation of ICAM-1 mRNA in gingival fibroblasts and induced its surface expression on the cells.
Escherichia coli lipopolysaccharide (LPS) was obtained from Difco Laboratories (Detroit, Mich.), proteinase K was obtained from Takara Shuzo Co., Ltd. (Shiga, Japan), and endoglucosidases H and D (EC 3.2.1.96) were obtained from Seikagaku Kogyo Co., Ltd. (Tokyo, Japan). Monoclonal antibody (HA58) to ICAM-1 used for cell enzyme-linked immunosorbent assay (Cell-ELISA) was obtained from PharMingen (San Diego, Calif.); monoclonal antibody (BBIG-I1) to human ICAM-1 used for immunostaining from R and D Systems Europe Ltd. (Oxon, United Kingdom); peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) was obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, Pa.); and VECTOR-ABC and VECTOR-VIP kits were obtained from Vector Laboratories, Inc. (Burlingame, Calif.).
All of the other chemicals were obtained from commercial sources and were of analytical or reagent grade.
M. salivarium ATCC 23064 and M. fermentans ATCC 19989 were grown in PPLO broth (Difco Laboratories) supplemented with 10% (vol/vol) horse serum (GIBCO Life Technologies, Inc., Grand Island, N.Y.), 1% (wt/vol) yeast extract (Difco), 1% (wt/vol) l-arginine-hydrochloride (for M. salivarium) or 1% (wt/vol) d-glucose (for M. fermentans), 0.002% (wt/vol) phenol red, and penicillin G (1,000 U/ml). When pH rose or fell 1 U, the cells were harvested by centrifuging the cultures at 15,000 × g for 15 min, washed three times with sterile phosphate-buffered saline (PBS), and suspended in PBS.
Cell membrane (CM) fractions of M. salivarium and M. fermentans were prepared according to the method described previously (27). Proteins were determined by the method of Dully and Grieve (4).
M. salivarium cells were treated with Triton X-114 to extract membrane lipoproteins according to the method described previously (27). Lipoproteins from the Triton X-114 phase were precipitated by methanol and used for stimulation after being suspended in sterile PBS by light sonication.
Gin-1 cells (a normal human gingival fibroblast cell line, ATCC CRL-1292) with passage 4 obtained from American Type Culture Collection (Rockville, Md.) were cultured in Dulbecco’s modified Eagle’s medium (DME medium; GIBCO Laboratories, Grand Island, N.Y.) containing 10% (vol/vol) fetal bovine serum, penicillin G (100 U/ml), and streptomycin (100 μg/ml) and passaged by trypsinization. Gin-1 cells between passages 6 and 10 were used in this study.
Human gingival tissue adhering to third molars was obtained from 18- to 35-year-old individuals. Immediately after extraction, molars were immersed in Isodine (povidone iodine; Meijiseika, Co., Ltd., Japan) for 30 s and washed three times with PBS. Gingival tissue and periodontal ligaments were detached and then sliced by a scalpel. The slices were cultured in DME medium in plastic culture dishes. After a confluent monolayer of the migrating cells had formed, the cells were passaged by trypsinization. After the fifth passage, the cells were homogenous fibroblasts with a spindle shape. In this study, fibroblasts from periodontal ligament (PDL-F) or gingiva (Gin-F) between passages 6 and 8 were used.
Gin-1, PDL-F, and Gin-F cells were cultured in DME medium, with the medium changed every 3 days for 7 to 10 days until the cells reached confluency. The single-cell suspensions (4 × 104/200 μl) prepared by trypsinization were seeded in wells of a 6-well tissue culture plate (well diameter, 35 mm). After incubation for 2 days at 37°C in an atmosphere of 5% CO2, the cells were stimulated at 37°C for 6 h. The culture plate was then centrifuged at 800 × g for 10 min. The culture supernatants were discarded, and the monolayers were washed three times with PBS. Total RNA was prepared from the monolayers by using a RNeasy kit (Qiagen Inc., Chatsworth, Calif.) according to the manufacturer’s instructions.
In order to detect the expression of mRNA of β-actin, ICAM-1, or vascular cell adhesion molecule 1 (VCAM-1), reverse transcription (RT)-PCR was done according to the method described previously (27). The nucleotide sequences of oligonucleotide primers for β-actin, ICAM-1, and VCAM-1 were described previously (6, 11). The PCR products were separated on 2% gel of NuSieve 3:1 agarose (FMC, Rockland, Maine) in 0.5× Tris-borate-EDTA (TBE) buffer (25) containing ethidium bromide (5 μg/ml). The specificity of primers for β-actin, ICAM-1, or VCAM-1 was confirmed by Southern hybridization, with a probe coding for internal sequence.
Cell-ELISA was carried out according to the method of Hayashi et al. (10). Briefly, 104 Gin-1 cells were seeded into 96-well flat-bottomed microplate. After Gin-1 cells reached confluency, the cells were stimulated. The cells were fixed with 3% (wt/vol) paraformaldehyde in PBS supplemented with 8% (wt/vol) saccharose. Nonspecific binding was blocked by the addition of PBS containing 10% (vol/vol) horse serum. The cells were reacted with anti-ICAM-1 monoclonal antibody (HA58) and then with peroxidase-conjugated goat anti-mouse IgG antibody. Peroxidase activity was measured by the addition of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) peroxidase substrate and stopped by the addition of an equal volume of 2% (wt/vol) sodium dodecyl sulfate. The optical density at 405 nm was measured by using a microplate reader.
Glass coverslips were put into each well (well diameter, 3.5 cm) of a 6-well plate. Gin-1 cells were grown on the coverslips up to approximately 60% confluency. Then, medium was removed. The cells were washed three times with PBS and followed by the addition of CM (80 μg of protein) of M. salivarium in 2 ml of DME (−) medium. After incubation at 37°C for 6 h, the culture supernatants were discarded. After being washed three times with PBS, the Gin-1 cells on coverslips were dried and then fixed with 20% (vol/vol) acetone in PBS supplemented with 10% (wt/vol) saccharose for 10 min at 4°C. After fixation, the coverslips were immersed in PBS for 1 h at 4°C. Then, Gin-1 cells were reacted with monoclonal antibodies to human ICAM-1 (BBIG-I1) diluted 1:350 with PBS containing 1% (vol/vol) horse serum for 12 h at 4°C and then reacted with biotinylated horse anti-mouse IgG with the VECTOR-ABC kit for 0.5 h at room temperature. After each reaction, the coverslips were washed with PBS for 0.5 h at 4°C. The reaction products were visualized with the VECTOR-VIP kit.
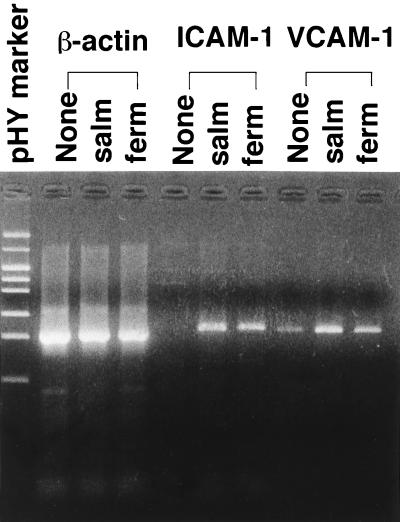
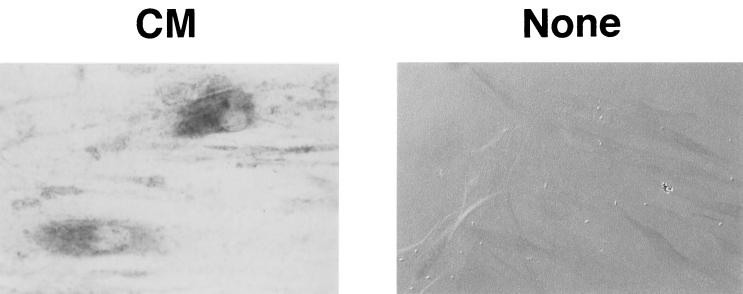
Gin-1 cells were incubated at 37°C for 6 h with CM of M. salivarium or M. fermentans and then examined for the expression of mRNAs of ICAM-1 and VCAM-1 by RT-PCR. CM of both mycoplasmas triggered the transcription of ICAM-1 mRNA in Gin-1 cells and upregulated the expression level of VCAM-1 mRNA (Fig. 1). ICAM-1 was expressed on the surfaces of Gin-1 cells stimulated with M. salivarium CM but not on the surfaces of unstimulated cells (Fig. 2).
FIG. 1.
Analysis of expression of mRNAs of β-actin, ICAM-1, and VCAM-1 in Gin-1 cells stimulated with CM of M. salivarium (salm) or M. fermentans (ferm). The confluent monolayers of Gin-1 cells in 3.5-cm-diameter culture dishes were incubated at 37°C for 6 h in the absence (None) or the presence of CM of M. salivarium (salm) or M. fermentans (ferm) at a protein concentration of 40 μg/ml. The RNAs were prepared from Gin-1 cells and analyzed by RT-PCR.
FIG. 2.
Immunostaining of ICAM-1 expressed on the cell surface of Gin-1 cells stimulated with cell membranes of M. salivarium. Gin-1 cells were incubated at 37°C for 6 h in the presence (CM) or the absence (None) of cell membranes of M. salivarium. The cells were fixed in PBS containing 20% (vol/vol) acetone and 10% (wt/vol) saccharose. The fixed cells were reacted with monoclonal antibodies to ICAM-1 (BBIG-I1) and with VECTOR-ABC and -VIP kits. The stimulated cells were stained (CM), but the nonstimulated cells were not stained and were therefore visualized by differential interference microscopy (None). Magnification, ×300.
Thus, it was found that the ICAM-1 mRNA transcripted in response to M. salivarium CM was translated into protein molecules and then expressed on the surface of the cells.
The cells of M. salivarium or M. fermentans were treated with Triton X-114 to extract lipoproteins, because mycoplasmal lipoproteins and lipopeptides have been shown to be responsible for induction of cytokine production by monocytes/macrophages (18, 19, 24). In both mycoplasmas, approximately 40% of the activity to induce the cell surface expression of ICAM-1 was recovered in the Triton X-114 phase and another 40% was recovered in the insoluble phase (Table 1). Thus, approximately 80% of the activity was found to be associated with the cell membranes, and approximately 50% of the activity of the cell membranes was recovered in the lipoproteins obtained by Triton X-114 phase separation. The specific activity of Triton X-114 phase was almost 10-fold higher than that of the aqueous phase and 30% (M. salivarium) or 80% (M. fermentans) higher than that of the insoluble phase (Table 1). Thus, the active entities responsible for induction of the cell surface expression of ICAM-1 seem to be lipoproteins associated with cell membranes in both mycoplasmas.
TABLE 1.
Triton X-114 phase fractionation of M. salivarium and M. fermentans cells and ICAM-1-inducing activity of each fraction
| Cells and phase | Total amt of protein (mg) | Mean ± SD of ICAM-1-inducing activitya
|
|
|---|---|---|---|
| Sp act (A405/mg of protein) | Total activity | ||
| M. salivarium | |||
| Aqueous | 1.300 | 0.55 ± 0.34 | 0.72 ± 0.04 (28) |
| Triton X-114 | 0.173 | 5.32 ± 0.28 | 0.92 ± 0.05 (37) |
| Insoluble | 0.207 | 4.19 ± 0.42 | 0.87 ± 0.09 (35) |
| M. fermentans | |||
| Aqueous | 0.540 | 0.86 ± 0.16 | 0.46 ± 0.09 (20) |
| Triton X-114 | 0.113 | 8.37 ± 0.37 | 0.95 ± 0.04 (41) |
| Insoluble | 0.185 | 4.74 ± 0.30 | 0.88 ± 0.06 (39) |
ICAM-1-inducing activity was measured by Cell-ELISA. A405 was expressed as absorbance at 405 nm of each well − mean absorbance at 405 nm of three wells of control medium. Data, expressed as means ± standard deviations from triplicate wells, are representative of three separate experiments. Values in the parentheses show distribution of total activity, calculated as the mean of total activity/sum of the means of total activity.
In order to characterize the active entities, lipoproteins obtained from M. salivarium (Lpsal) and M. fermentans (Lpfer) were treated with various types of enzymes. The activity of Lpsal or Lpfer to induce the surface expression of ICAM-1 was not affected by proteinase K (Table 2), although protein components of Lpsal or Lpfer were mostly digested by the enzyme. Endoglucosidases D and H had no effect on the activity of Lpsal or Lpfer. Lipoprotein lipase abrogated the activity of Lpsal or Lpfer (Table 2). Gin-1 cells treated with lipoprotein lipase in the absence of Lpsal or Lpfer did not express ICAM-1. These results suggest that the lipid moiety, but not the protein moiety, plays a key role in the expression of the activity of Lpsal or Lpfer.
TABLE 2.
Effect of enzymes on ICAM-1-inducing activity of LPsal and LPfer
| Treatment | Mean ± SD of ICAM-1-inducing activitya(A405)
|
|
|---|---|---|
| LPsal | LPfer | |
| None | 0.99 ± 0.07 (100) | 1.08 ± 0.04 (100) |
| Proteinase K | 1.17 ± 0.09 (118) | 1.16 ± 0.04 (107) |
| Endoglucosidase H | 1.07 ± 0.06 (108) | 1.11 ± 0.03 (103) |
| Endoglucosidase D | 1.05 ± 0.06 (106) | 1.14 ± 0.03 (115) |
| Lipoprotein lipase | 0.08 ± 0.01 (8) | 0 |
Two hundred microliters of Lpsal (173 μg of protein/ml of PBS) or Lpfer (113 μg of protein/ml of PBS) was treated at 37°C for 2 h with 0.2 μg of each enzyme, and then 20 μl of the reaction mixture was added to the monolayer of Gin-1 cells grown in 200 μl of DME medium in each well of a 96-well flat-bottomed microplate. After a 6-h incubation, ICAM-1 expression was measured by Cell-ELISA. Enzyme and buffer controls were run at the same time. Absorbance at 405 nm of enzyme or buffer control was the same as that for the control medium. Therefore, A405 was expressed as the absorbance at 405 nm of each well − the mean absorbance at 405 nm of three medium control wells). Data, expressed as means ± standard deviations from triplicate wells, are representative of three separate experiments. Values in the parentheses are 100 × (mean A405 of a specimen treated with enzyme/mean A405 of a specimen left untreated).
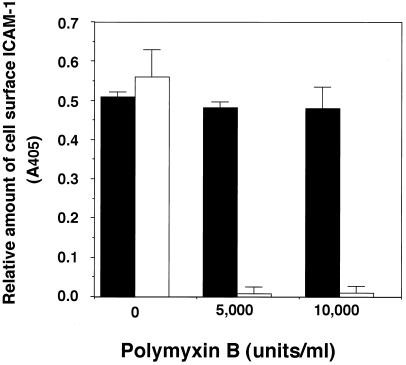
Gin-1 cells were incubated with Lpsal or E. coli LPS in the absence or the presence of polymyxin B. Polymyxin B had no effect on the activity of Lpsal to induce cell surface expression of ICAM-1 but abrogated the activity of E. coli LPS (Fig. 3). Thus, Lpsal was shown to induce the cell surface expression of ICAM-1 on Gin-1 cells by a mechanism distinct from that of E. coli LPS.
FIG. 3.
Effect of polymyxin B on cell surface expression of ICAM-1 on Gin-1 cells stimulated with Lpsal or E. coli LPS. Gin-1 cells were grown in DME medium until they reached confluency. The confluent monolayers of Gin-1 cells were preincubated for 37°C for 1 h with polymyxin B (5,000 or 10,000 U/ml) in the presence of Lpsal (50 μg of protein/ml [■]) or E. coli LPS (1 μg/ml [□]) and then examined for cell surface expression of ICAM-1 on Gin-1 cells by Cell-ELISA.
We incubated Gin-F and PDL-F with Lpsal to determine whether or not the transcription of ICAM-1 mRNA was triggered. As shown in Fig. 4, Lpsal triggered the transcription of ICAM-1 mRNA in Gin-F and PDL-F cells as well as Gin-1 cells.
FIG. 4.
Analysis of expression of mRNAs of β-actin and ICAM-1 in Gin-F and PDL-F as well as Gin-1 cells stimulated with Lpsal. The confluent monolayers of Gin-F and PDL-F as well as Gin-1 cells in 3.5-cm-diameter culture dishes were incubated at 37°C for 6 h in the absence (None) or the presence of Lpsal at a protein concentration of 60 μg/ml. The expression of mRNAs of β-actin or ICAM-1 was analyzed by RT-PCR.
Chronic periodontal diseases are characterized by dense infiltrations of lymphocytes and macrophages in the connective tissue (22). It is becoming clear that lymphocytes and gingival fibroblasts are capable of influencing each other. Cell adhesion molecules are involved in the infiltration of activated leukocytes in inflammatory sites. Among these molecules, ICAM-1, but not VCAM-1, is known to be induced on gingival fibroblasts in response to cytokines such as IL-1 and TNF-α and LPS from oral gram-negative bacteria (10, 32). Gingival fibroblasts may be involved in the regulation of gingival inflammation in cell-to-cell interaction with immunocompetent cells via cytokine production and the cell surface expression of adhesion molecules. It is indeed confirmed in vivo by immunohistological analysis that the expression of ICAM-1 on fibroblasts in adult periodontitis tissues was greater than that in normal gingiva (9). Thus, there is accumulated evidence for the importance of ICAM-1 in periodontal diseases (2, 9, 10, 33).
In this study, we demonstrated that M. salivarium and M. fermentans triggered transcriptional activation of ICAM-1 mRNA of Gin-1 cells and induced its expression on the cells and upregulated the expression of VCAM-1 mRNA. ICAM-1 is a member of the Ig superfamily of recognition molecules (30) and has two independent binding sites for the leukocyte function-associated antigen (LFA-1, CD 11a/CD18) (16) and Mac 1 (CD 11b/CD 18) (3), which are expressed on leukocytes (29). VCAM-1, a member of the Ig superfamily, is induced by cytokines such as IL-1 and TNF-α on vascular endothelial cells and binds lymphocytes (23). The very late antigen-4 (VLA-4), the counter-receptor of VCAM-1 (7), is known to play a key role in binding of lymphocytes to human gingival fibroblasts as well as to vascular endothelial cells (20, 21).
M. salivarium is one of the oral microbial flora and preferentially inhabits gingival sulci (8, 14). M. fermentans was detected in human saliva at a high rate (approximately 50%) by a PCR-based assay (1). We are interested in etiological roles of these mycoplasmas in oral diseases. Both mycoplasmas induce IL-1β and TNF-α in monocytes/macrophages (17), which are capable of inducing ICAM-1 expression on gingival fibroblasts and the production of IL-6 and IL-8 in gingival fibroblasts (27).
Taken together, it is speculated that M. salivarium and M. fermentans play an etiological role in periodontal diseases by facilitating infiltration, accumulation, or retention of inflammatory cells in gingival connective tissue.
M. fermentans has also been implicated as a causative agent for human joint diseases (26, 36). Judging from the finding that M. fermentans induced ICAM-1 expression on gingival fibroblasts, it is considered that the organism may induce ICAM-1 expression on synovial fibroblasts as well, by which infiltration, accumulation, or retention of inflammatory cells in joint tissue may be facilitated.
Several reports that fractions containing lipoproteins or enriched with lipoproteins from mycoplasmas show macrophage stimulatory activity (12, 18, 19). The active entity of M. fermentans capable of inducing the production of IL-1β, TNF-α, and IL-6 by macrophages/monocytes has been identified as a lipoprotein (MDHM) containing S-(2,3-dihydroxypropyl)cystein by Mühlradt et al. (18, 19). MDHM is resistant to lipase and proteinase K (18). Kostyal et al. (12) find that a 48-kDa membrane protein (48 KMP) of M. fermentans induces TNF-α by human monocytes. The 48 KMP treated with proteinase K lost the activity completely, but the lipase-treated sample retained some of the activity. Lipoprotein lipase abrogated the ICAM-1-inducing activity of Lpsal and Lpfer, whereas proteinase K had no effect. Therefore, it is speculated that active entities of Lpsal and Lpfer responsible for the induction of ICAM-1 in gingival fibroblasts might be substances different from MDHM and 48 KMP of M. fermentans.
Further studies are in progress in our laboratories to characterize the active entities of Lpsal and Lpfer.
ACKNOWLEDGMENTS
This work was partially supported by grants-in-aid for scientific research (B) (09470389) and (C) (10671762), which were provided by the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Chingbingyong M I, Huges C V. Detection of Mycoplasma fermentans in human saliva with polymerase chain reaction-based assay. Arch Oral Biol. 1997;41:311–314. doi: 10.1016/0003-9969(96)84556-0. [DOI] [PubMed] [Google Scholar]
- 2.Crawford J M. Distribution of ICAM-1, LFA-3 and HLA-DR in healthy and diseased gingival tissues. J Periodontal Res. 1992;27:291–298. doi: 10.1111/j.1600-0765.1992.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 3.Diamond M S, Staunton D E, Marlin S D, Springer T A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 4.Dully J R, Grieve P A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975;64:136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- 5.Dustin M L, Rothlein R, Bhan A K, Dinarello C A, Springer T A. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 6.Ebnet K, Brown K D, Siebenlist U K, Simon M M, Shaw S. Borrelia burgdorferi activates nuclear factor-kB and is a potent inducer of chemokine molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–3292. [PubMed] [Google Scholar]
- 7.Elices M J, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler M E, Lobb R R. VCAM-1 on activated endothelium interacts with leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 8.Engel L D, Kenny G E. Mycoplasma salivarium in human gingival sulci. J Periodontal Res. 1970;5:163–171. doi: 10.1111/j.1600-0765.1970.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 9.Gemmell E, Walsh L J, Savage N W, Seymour G J. Adhesion molecule expression in chronic inflammatory periodontal disease tissue. J Periodontal Res. 1994;29:46–53. doi: 10.1111/j.1600-0765.1994.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi J, Saito I, Ishikawa I, Miyasaka N. Effects of cytokines and periodontopathic bacteria on the leukocyte function-associated antigen 1/intercellular adhesion molecule 1 pathway in gingival fibroblasts in adult periodontitis. Infect Immun. 1994;62:5205–5212. doi: 10.1128/iai.62.12.5205-5212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirk A D, Bollinger R R, Finn O J. Rapid, comprehensive analysis of human cytokine mRNA and its application to the study of acute renal allograft rejection. Human Immunol. 1995;43:113–128. doi: 10.1016/0198-8859(94)00158-m. [DOI] [PubMed] [Google Scholar]
- 12.Kostyal D A, Butler G H, Beezhold D H. A 48-kilodalton Mycoplasma fermentans membrane protein induces cytokine secretion by human monocytes. Infect Immun. 1994;62:3793–3800. doi: 10.1128/iai.62.9.3793-3800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause D C, Taylor-Robinson D. Mycoplasmas which infect humans. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 417–444. [Google Scholar]
- 14.Kumagai K, Iwabuchi T, Hinuma Y, Yuri K, Ishida N. Incidence, species, and significance of Mycoplasma species in the mouth. J Infect Dis. 1971;23:16–21. doi: 10.1093/infdis/123.1.16. [DOI] [PubMed] [Google Scholar]
- 15.Lo S-C. Mycoplasmas and AIDS. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 525–545. [Google Scholar]
- 16.Marlin S D, Springer T A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 17.McGarrity G J, Kotani H, Butler G H. Mycoplasmas and tissue culture cells. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 445–454. [Google Scholar]
- 18.Mühlradt P F, Frisch M. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophage to release nitric oxide, tumor necrosis factor, and interleukin-6. Infect Immun. 1994;62:3801–3807. doi: 10.1128/iai.62.9.3801-3807.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mühlradt P F, Meyer H, Jansen R. Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry. 1996;35:7781–7786. doi: 10.1021/bi9602831. [DOI] [PubMed] [Google Scholar]
- 20.Murakami S, Saho T, Shimabukuro Y, Isoda R, Miki Y, Okada H. Very late antigen integrins are involved in the adhesive interaction of lymphoid cells to human gingival fibroblasts. Immunology. 1993;79:425–433. [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami S, Shimabukuro Y, Saho T, Isoda R, Kameyama K, Yamashita K, Okada H. Evidence of a role of VLA integrins in lymphocyte-human gingival fibroblast adherence. J Periodontal Res. 1993;28:494–496. doi: 10.1111/j.1600-0765.1993.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 22.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365–374. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborn L, Catherine H, Richard T, Cornelia V, Stefan L, Gloria C-R, Roy L. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 24.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induce proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643. doi: 10.1128/iai.64.2.637-643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Schaeverbeke T, Gilroy C B, Bebear C, Dehais J, Taylor-Robinson D. Mycoplasma fermentans in joints of patients with rheumatoid arthritis and other joint disorders. Lancet. 1996;347:1418. doi: 10.1016/s0140-6736(96)91065-x. [DOI] [PubMed] [Google Scholar]
- 27.Shibata K-I, Hasebe A, Sasaki T, Watanabe T. Mycoplasma salivarium induces interleukin-6 and interleukin-8 in human gingival fibroblasts. FEMS Immunol Med Microbiol. 1998;19:275–283. doi: 10.1111/j.1574-695X.1997.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 28.Slots J, Genco R J. Microbial pathogenicity: black-pigmented Bacteroides species, Capnocytophaga species and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 29.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–433. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 30.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 31.Takada H, Mihara J, Morisaki I, Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi K, Takigawa M, Takashiba S, Hagai A, Miyamoto M, Kurihara H, Murayama Y. Role of cytokine in the induction of adhesion molecules on cultured human gingival fibroblasts. J Periodontol Res. 1994;65:230–235. doi: 10.1902/jop.1994.65.3.230. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi Y, Sakurai K, Ike I, Yoshie H, Kawasaki K, Hara K. ICAM-1-expressing pocket epithelium, LFA-1-expressing T cells in gingival tissue and gingival crevicular fluid as features characterizing inflammatory cell invasion and exudation in adult periodontitis. J Periodontal Res. 1995;30:426–435. doi: 10.1111/j.1600-0765.1995.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 34.Tamura M, Tokuda M, Nagaoka S, Takada H. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 expression in human gingival fibroblast cultures. Infect Immun. 1992;60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T, Matsuura M, Seto K. Enumeration, isolation and species identification of mycoplasmas in saliva sampled from the normal and pathological human oral cavity and antibody response to an oral mycoplasma (Mycoplasma salivarium) J Clin Microbiol. 1986;23:1034–1038. doi: 10.1128/jcm.23.6.1034-1038.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams M H, Brostoff J, Roitt I M. Possible role of Mycoplasma fermentans in pathogenesis of rheumatoid arthritis. Lancet. 1970;ii:277–280. doi: 10.1016/s0140-6736(70)91328-0. [DOI] [PubMed] [Google Scholar]