ABSTRACT.
The Center for the Study of Complex Malaria in India (CSCMi) is one of 10 International Centers of Excellence in Malaria Research funded by the National Institutes of Health since 2010. The Center combines innovative research with capacity building and technology transfer to undertake studies with clinical and translational impact that will move malaria control in India toward the ultimate goal of malaria elimination/eradication. A key element of each research site in the four states of India (Tamil Nadu, Gujarat, Odisha, and Meghalaya) has been undertaking community- and clinic-based epidemiology projects to characterize the burden of malaria in the region. Demographic and clinical data and samples collected during these studies have been used in downstream projects on, for example, the widespread use of mosquito repellants, the population genomics of Plasmodium vivax, and the serological responses to P. vivax and Plasmodium falciparum antigens that reflect past or present exposure. A focus has been studying the pathogenesis of severe malaria caused by P. falciparum through magnetic resonance imaging of cerebral malaria patients. Here we provide a snapshot of some of the basic and applied research the CSCMi has undertaken over the past 12 years and indicate the further research and/or clinical and translational impact these studies have had.
INTRODUCTION TO THE OVERALL THEME AND GOALS OF THE INDIA ICEMR
The Center for the Study of Complex Malaria in India (CSCMi), one of 10 International Centers of Excellence in Malaria Research (ICEMRs) located in malaria-endemic regions of the world, was launched in 2010.1 Unlike countries in the African subcontinent where Plasmodium falciparum is the dominant species and is transmitted by the major vector Anopheles gambiae, malaria in India can be caused by at least four parasite species (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale), reflect coinfections of two or more species or genotypes, and can be transmitted by at least six Anopheles species, including An. stephensi, An. fluviatilis, An. culicifacies, An. minimus, An. baimaii, and An. annularis. The overall goal of CSCMi is to address major gaps in our understanding of such “complex malaria” and to elucidate how it affects the epidemiology, transmission, drug resistance, parasite population genomics, and pathogenesis of malaria in India. Key questions that we have attempted to answer include the following: What is the eco-epidemiology and transmission of complex malaria at our field sites and how is it changing? What is the role of environmental conditions in determining malaria transmission intensity in different eco-epidemiological contexts? And how genetically diverse is P. vivax and how does this compare with P. falciparum?
Twelve years later our research questions remain highly relevant. According to the WHO, India has had the largest absolute reductions in malaria cases in Southeast Asia, from 19.7 million in 2000 to 5.6 million in 2019, although it still contributed 86% of malaria cases in the region that year.2 The decrease in burden has changed the landscape of epidemiology and transmission in India, which in turn may influence the clinical presentation of malaria due to reduced exposure and a reduction in the resulting immunity.3 In the case of P. falciparum, a drop in incidence may be accompanied by a rise in the proportion of acute cases,4 and studies investigating the pathogenesis of severe malaria are thus crucial to inform new adjunctive therapies and improve clinical outcomes. In addition, in P. falciparum/P. vivax coendemic areas of the world, where the burden of the former has been reduced by control programs, a rise in the proportion of malaria caused by the latter has been reported.5 Vivax malaria has often been considered benign, but reports demonstrating severe complications arising from P. vivax infection challenge this assumption.6 Further studies are warranted to evaluate the extent of vivax epidemiology, and malaria-associated delayed morbidity and indirect mortality.7
The multidisciplinary partnership between Indian and international institutions under the umbrella of the CSCMi has advanced both basic and translational research, from updating epidemiology in malaria endemic states with distinct ecological and ethnicity profiles, to generating the first whole genome sequences of Indian malaria parasites and deciphering molecular mechanisms leading to the development of cerebral malaria. The dynamic and adaptive design of the CSCMi has enabled rapid responses to new challenges and allowed the development of spinoff projects such as a study of the etiology of nonmalaria febrile illness (e.g., Dengue, chikungunya, and scrub typhus) in a hospital setting in Rourkela, Odisha.8 Capacity building and technology transfer through access to international collaborations and training workshops are other essential components of the CSCMi, through which we have introduced state-of-the-art research platforms and facilities in endemic areas (for example, novel neuroimaging protocols, micro-electroencephalogram devices, next generation sequencing platforms, and high-throughput immunoassays).
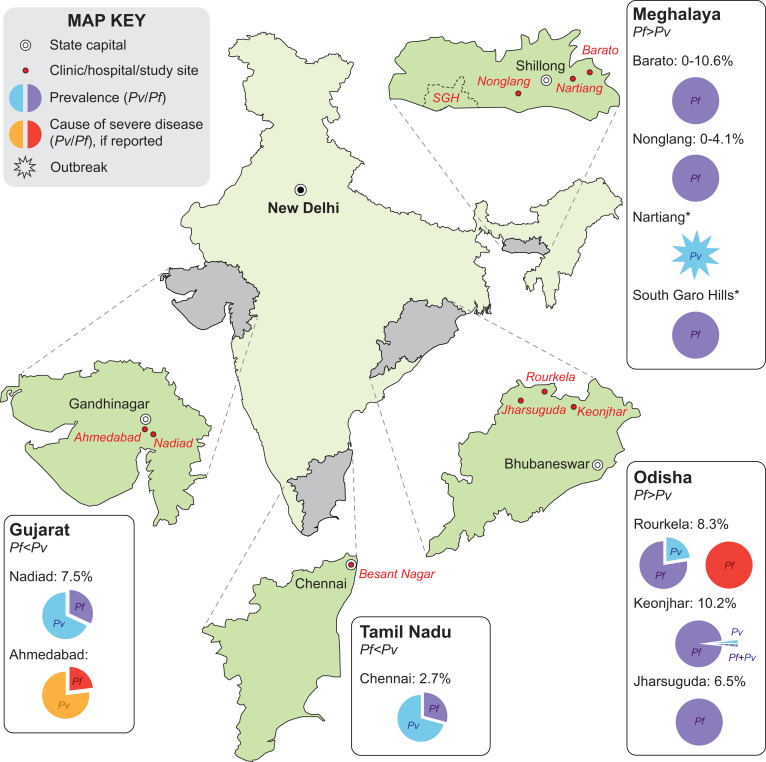
Here we outline some of the malaria research in India that has been undertaken by the Center over the past 12 years. The initial research theme of the Center from 2010 to 2017 centered on the study of complex malaria with respect to eco-epidemiological profiles, transmission and vector capabilities, and potential impact on drug resistance using genomic technologies, at three field sites in the east (Odisha with predominantly P. falciparum in forest/riparian ecology), in the south (the urban city of Chennai in Tamil Nadu, predominantly P. vivax), and in the west (the town and surrounding villages of Nadiad in Gujarat State with both P. vivax and P. falciparum) (Figure 1); see Das et al.1 for more details. A subsequent Special Project and Immunology Supplement award enabled the Center to expand its studies to the pathogenetic mechanisms involved in severe and cerebral falciparum malaria using magnetic resonance imaging technology at the Odisha field site. An Administrative Core, Genomics Core, and a Data Management, Biostatistics and Bioinformatics Core supported the research projects. From 2017 to date, studies have continued on severe and cerebral falciparum malaria at our Odisha site, while expanding to studies on the eco-epidemiology of vivax and falciparum malaria in new field sites of the northeastern state of Meghalaya. A second supplement award has enabled the Center to expand into 1) ongoing sociobehavioral and social network–based observational studies in the tribal villages of Meghalaya to determine what types of malaria-preventive interventions reduce malaria through good coverage and use and to identify demand and/or supply side barriers to malaria control measures; and 2) to undertake a randomized clinical trial evaluating the effectiveness of “malaria camps” implemented by the government of Odisha as part of India’s pivot to malaria elimination.9 Both these studies leverage the baseline data, infrastructure, and capacity established by the India ICEMR during the past 12 years. In what follows, we summarize the major scientific findings of several of these research projects and describe their clinical impact and translational value where apparent and/or further research is suggested (summarized in Table 1). Due to space limitations, we cannot describe each publication in detail, and the reader is referred to the original publication for further information.
Figure 1.
Malaria epidemiology at the Center for the Study of Complex Malaria in India sites in India, 2012–2021. Baseline epidemiology of Plasmodium falciparum (Pf) and Plasmodium vivax (Pv) was assessed in four states highlighted in grey through surveys undertaken at clinics/hospitals and in the community. Data are shown for: Gujarat (Ahmedabad57 and Nadiad10), Tamil Nadu (Chennai10), Meghalaya (Nonglang and Barato12,13), and Odisha (Rourkela and surrounding villages,10,17,20,45–47,49,53 and Jharsuguda and Keonjhar districts9). Pie charts in the inserts show Plasmodium species prevalence and species associated with severe disease for regions surveyed in each state; final data from two sites (1) Nartiang (P. vivax outbreaks monitored 2019–2021) and (2) South Garo Hills (a P. falciparum endemic district monitored 2020–2021, abbreviated SGH) are pending and indicated with an asterisk (*).
Table 1.
Summary of the clinical impact and translational aspects of some of the research projects undertaken by the Center
| Research area, reference | Research finding | Clinical impact and/or translational aspect and/or further research suggested |
|---|---|---|
| Epidemiology | ||
| Use of mosquito repellents17 | Mosquito repellants (vaporizers, coils, creams) widely used in India, influenced by socioeconomic status, and not always associated with less malaria | Further clinical testing and evaluation of safety necessary for an evidence-based public health recommendation about how to choose and use repellents |
| Male behavior a risk factor for malaria18 | Repellents used by ∼30% of 234 households in Sundargarh district, Odisha, but insecticide creams not used at all | Promotion of repellent cream use by at-risk groups could be explored in addition to mass screen or treat programs |
| Malaria symptoms and asymptomatic infections16 | Many differences in complaints and symptoms between our sites, and factors associated with asymptomatic Plasmodium infections |
|
| Reactive case detection20 | RCD in areas of low malaria transmission and/or Plasmodium vivax is a labor-intensive strategy, and its benefit is not clear | Further studies are needed to assess how RCD can be optimized or to determine alternatives where interventions are targeted to family members, hotspots, or using serological markers (SeroTAT) |
| Transmission | ||
| Anopheles stephensi breeding habitat29 | Wells and overhead tanks are major breeding sources of the urban vector An. stephensi in Chennai | Overhead tanks as potential vector breeding sites could be targeted for intensified vector intervention measures |
| Zoophilic vectors24 | Shift in vector species toward increased zoophilic behavior in recent years; modeling of regions dominated by zoophilic vectors indicate existing vector control tactics will be insufficient to achieve elimination | Cattle sheds could be targeted by control methods to focus on zoophilic behavior of Anopheles mosquitoes |
| Genome-wide studies | ||
| P. falciparum and P. vivax whole genome sequences38 | P. vivax exhibits twice as much genetic diversity than P. falciparum, suggesting a more stable and older association of this species with humans and suggests an increased capacity for functional variation in the global P. vivax population | P. vivax will be the more difficult species to eliminate. |
| P. vivax population genomics39 | Analysis of 182 clinical isolate genomes from 11 countries identified signals of natural selection suggesting that P. vivax is evolving in response to antimalarial drugs, adapting to regional differences in the human host and mosquito vector | P. vivax has the more variable epidemiology, requiring greater sampling of P. vivax in different endemic regions to capture the standing genetic variation |
| Antibody responses to genome-wide Plasmodium antigens22 | 515 P. vivax and 500 P. falciparum antigens assayed with 353 plasma samples identified most immunogenic antigens of both species and P. falciparum antigens associated with asymptomatic infections | Range of immune responses characterized in different endemic settings in India argues for targeted surveillance approaches tailored to the diverse epidemiology |
| Pathogenesis | ||
| Clinical characterization of CM45 | Posterior reversible encephalopathy syndrome is associated with a reversible nature of the blood–brain barrier and is often associated with nonfatal CM | Evaluation of compounds aimed at reversing vasogenic edema is needed to provide potential new adjunct therapies |
| CM pathology53 | Fatal CM is associated with severe brain swelling in children and with global brain hypoxia in adults | Adjunctive treatment highly likely to differ between the two age groups based on the predominance of cytotoxic edema in adults; focus should be made on decreasing brain hypoxia in adults |
| CM pathogenesis60 | Endothelial protein C receptor-binding PfEMP-1 variants induce endothelial cell swelling and disrupt the blood-brain barrier in CM | Parasite ingress into brain endothelium is a contributing factor to the pathology of human CM |
| CM diagnosis/prognosis46 | hsa-miR-3158-3p represents a promising biomarker candidate for CM prognosis across age groups | hsa-miR-3158-3p may be considered instead of neuroimaging to diagnose and monitor disease progression |
| CM pathogenesis48 | CM-associated brain swelling has common determinants in both African and Indian populations | Adjunct therapies targeting brain swelling in CM patients may be effective in both children and adults, through restoration of normal function of the cytoprotective APC-EPCR signaling pathway |
| Clinical definition of CM52 | Brain changes are frequent in P. falciparum infection, irrespective of the presence of coma | Spectrum of “cerebral” malaria is wider than initially thought; development of neurological sequelae in both uncomplicated malaria and severe noncerebral malaria groups must be evaluated |
CM = cerebral malaria; RCD = reactive cased detection.
DETERMINING BASELINE EPIDEMIOLOGY IN FOUR STATES OF INDIA
Our initial projects elucidated the baseline epidemiology of regions in four states of India (Gujarat, Meghalaya, Odisha, and Tamil Nadu) that differ by climate, ecology, and ethnic make-up (Figure 1), through census, cross-sectional, cohort, and clinic-based studies. We use polymerase chain reaction (PCR)-based methods as the gold standard for parasite detection, a strategy few other studies in India have used. At each visit, subjects who are enrolled after giving informed consent complete an extensive clinical questionnaire and supply a finger-prick blood sample for a rapid diagnostic test (RDT), microscopy, species-specific PCRs, and serology studies. If they are RDT positive, a vacutainer of venous blood is taken for downstream subprojects. Because these baseline epidemiology studies are observational only, patients diagnosed with malaria parasites by RDT or microscopy are treated by the malaria control authorities as per the National Vector Borne Disease Control Program malaria treatment guidelines. Here we describe some of our findings.
Plasmodium infections in India are increasingly asymptomatic and submicroscopic.
A major revelation of our cross-sectional studies in Gujarat, Odisha, and Tamil Nadu during 2012–2015 has been the number of asymptomatic and submicroscopic infections. For example, 71% of infections in cross-sectional surveys in Chennai, Tamil Nadu, were asymptomatic, and 71% of infections were submicroscopic.10 Our more recent surveys in the northeastern state of Meghalaya in 2018–2019, where malaria prevalence has decreased dramatically over the past 5 years,11 present a similar picture, with 97% of all infections being asymptomatic and all submicroscopic,12,13 concordant with other studies in the northeast.14 As transmission declines across India, Plasmodium infections are increasingly associated with few symptoms and low parasite biomass, making diagnosis challenging and underscoring the need for more sensitive diagnostic tests at the point of care. Recently, it was proposed that facilities created for COVID-19 diagnosis—including PCR assays in field-friendly formats deployed across the country—can easily be coopted and harnessed for malaria diagnosis.15 Indeed, our own ongoing studies include implementation of molecular field assays in community health centers as part of a randomized clinical trial to evaluate Odisha State malaria camps. We also undertook an analysis of complaints and symptoms of 3,031 participants in our studies in Gujarat, Odisha, and Tamil Nadu, together with factors associated with asymptomatic Plasmodium infections. We found that addition of the symptoms “headache,” “aches,” and “chills” to “fever” improved the case definition of symptomatic malaria. Our findings indicate that malaria and asymptomatic infections differ by region in India, indicating that eliminating malaria will require localized approaches.16
Mosquito repellents are commonly used in India but need more evaluation.
Our household census data in Gujarat, Odisha, and Tamil Nadu showed that the use of mosquito repellents such as coils, vaporizers, and mats was common and influenced by education level and socioeconomic status, but was not consistently associated with a reduction in malaria in our cross-sectional and clinic data.17 The market for repellents in India is considerable, but it is not clear if the different types of products are worth the investment, so more clinical testing and safety evaluation of these methods is warranted for an evidence-based recommendation of repellents and their role in elimination programs. In our follow-up study in Odisha that showed repellents used by ∼30% of households, insect repellant creams were not used at all,18 despite the finding that creams containing DEET have so far provided the longest protection in laboratory studies.19 The promotion of repellent cream use by at-risk groups could be further explored in addition to mass screen-and-treat programs in high-risk villages.
Testing novel surveillance tools as malaria transmission in India wanes.
We have piloted novel strategies for malaria surveillance and elimination at several of our sites. In 2014, our reactive case detection (RCD) studies at two urban areas with low prevalence of mainly P. vivax used PCR to detect Plasmodium parasites in 131 contacts of 20 index cases (Nadiad, Gujarat) and 868 contacts of 18 index cases (Chennai, Tamil Nadu), a strategy that identified only four new infections at the latter site. RCD thus proved to be a labor-intensive strategy that was not useful at our vivax endemic sites.20 Further studies are needed to assess whether RCD can be used in such settings—for example, by using serological markers for detecting recent P. vivax infection that could indicate hypnozoite carriers to be targeted for treatment with antihypnozoite drugs (a strategy called SeroTAT).21 We have also undertaken pilot studies of serological responses to P. vivax and P. falciparum at all four of our sites in India, using either ∼1,000 Plasmodium antigens spotted on microarray chips22 (the same microarrays as used by several other ICEMRs, allowing for comparison of these pilot studies between sites), or a high-throughput bead-based assay with 17 Plasmodium antigens that reflect past or present exposure.13 Our studies in Gujarat, Odisha, and Tamil Nadu identified the most immunogenic Plasmodium antigens and P. falciparum antigens associated with asymptomatic infections.22 In Meghalaya, analysis of serological exposure markers at two sites found that responses increased with age, providing further evidence of a decrease in transmission in this area.13 We continue to use the bead-based assay with a refined panel of Plasmodium antigens to classify exposure as either recent or long term in studies investigating a recent P. vivax outbreak in Jaintia Hills, Meghalaya, and as part of the malaria camps effectiveness trial mentioned earlier.9 Seroepidemiology is a promising approach both for determining the changing epidemiology of a region and for targeted malaria control and elimination interventions.23
TRANSMISSION AND VECTOR STUDIES
Our transmission and vector studies in Tamil Nadu, Odisha, and Meghalaya have included surveillance projects of Anopheles species in the same villages as our epidemiology studies, quantifying the role of environmental conditions in determining malaria transmission intensity, and identifying novel mutations associated with insecticide resistance that key Indian Anopheles vectors have developed.
Anopheles surveillance.
Given the diversity of Anopheles mosquitoes in India, an important component of the CSCMi has been capturing Anopheles adults using CDC light traps and aspirators, accompanied by morphological and molecular classification, salivary gland and midgut dissections for malaria parasite detection, and blood meal analysis. One surveillance study in 15 villages in Odisha during 2012–2013 confirmed An. culicifacies and An. fluviatilis as the major vectors in this area, with greater densities found in cattle sheds compared with human dwellings.24 We found a shift from the strongly anthropophilic An. fluviatilis S-type to the more zoophilic T-type with a preference of cattle sheds over human dwellings (as described in other studies)25; such a shift in zoophilic behavior could be a response to the intensified use of indoor residual spraying (IRS) and long-lasting insecticide-treated nets (LLINs) in the area. Blood meal analysis indicated possible frequent switching of feeding between humans and animals, and our modeling studies suggested that such a zoophilic cycle was unlikely to be affected by scaling up conventional tools such as IRS or LLINs. Alternatively, redirecting control efforts toward the zoophilic cycle could put the elimination threshold within reach. A year-long survey of cattle sheds and human dwellings in Chennai identified An. stephensi as greatly preferring cattle sheds to human dwellings for both resting and feeding, and those found in human dwellings greatly preferring thatched structures.26 We also reported the first detection of malaria parasites in Anopheles subpictus, not previously considered a human malaria vector in this city.26 In Meghalaya, our surveillance studies during 2018–2019 identified ∼13 Anopheles species, some of which are known malaria vectors, including Anopheles jeyporiensis.12 Absence of An. baimaii and An. minimus corroborated recent reports from northeast India that these forest-associated mosquito species are in decline.27 Further studies are needed to determine which species are now contributing to malaria transmission and to characterize their biological attributes relevant to vector control, including biting-time, host-preference, larval ecology, and seasonal abundance.
Environmental conditions determining malaria transmission.
We monitored temperatures in a range of indoor and outdoor resting habitats of mosquitos in two urban slum sites at our Chennai field site because standard estimates of environmental temperature derived from the local weather stations do not provide realistic measures of temperatures within actual transmission environments, and even small differences in mean temperatures or diurnal temperature ranges could lead to large variations in mosquito and/or parasite life history traits that determine transmission intensity.28 A year-long weekly study of the available clear/clean water mosquito breeding habitats, such as wells, cement cisterns, plastic barrels, and overhead tanks (OHT) found that OHTs were the predominant breeding habitat for the local malaria vector An. stephensi, leading us to recommend directing intervention efforts to that habitat.29 The presence of fluoride correlated with An. stephensi immature density, an indicator/predictor of vector breeding.30 Cattle sheds are the preferred resting place of An. stephensi, and dawn is the optimal time to collect and estimate its densities.31
Molecular mechanisms of insecticide resistance in Indian Anopheles.
Our initial studies involved detection of “knockdown resistance” (kdr) in Indian mosquito species due to mutations in the voltage-gated sodium channel gene that confers resistance against DDT and the pyrethroid group of insecticides. We reported two alternate point mutations leading to the same amino acid substitution (L1014F) in An. subpictus and developed a new assay that detected the mutations at high frequency (82%) in the Indian study area.32 We also mapped the distribution of two kdr mutations, L1014F and L101S, in An. stephensi33 as well as An. culicifacies33 in different Indian populations. In another study, we studied the evolution of the DDT-resistance mechanism in An. stephensi under laboratory conditions and identified tandem duplication of a genomic segment of the GSTe (epsilon class of glutathione S-transferases) gene array encoding GSTe2 and GSTe4, leading to increased transcription of these two genes. The duplication event also led to the diversification of these two genes, resulting in two paralogues of GSTe2 and three paralogues of GSTe4 being present in a single mosquito.34
POPULATION GENOMICS AND ANTIMALARIAL DRUG RESISTANCE STUDIES
We have exploited advances in next generation sequencing (NGS) technology35 to study antimalarial drug resistance and population genomics of P. falciparum and P. vivax in India.
An NGS facility in India and studies on antimalarial drug resistance.
We set up an NGS facility at the ICMR/National Institute for Malaria Research in Delhi36 and used it to develop an amplicon sequencing method for high-throughput deep sequencing of six full-length P. falciparum drug resistance genes (Pfmdr1, Pfcrt, PfDHFR, PfDHPS, PfK13, Pfmrp1).37 We identified known and novel mutations of these genes in patient samples collected from our epidemiology studies in Tamil Nadu, Gujarat and Odisha, for example, in PfK13 associated with artemisinin resistance, as well as heterozygous loci containing allelic variants, indicative of mixtures of resistant and sensitive clones in the same individual. Any marker gene can be swapped into the panel, making this “amplicon-seq” method potentially suitable for studies of, for example, P. vivax drug resistance or the number of Plasmodium genotypes in an infection (“complexity of infection”).
The first P. falciparum and P. vivax reference genomes from India.
One of our major achievements has been to generate the first P. vivax and P. falciparum reference genomes from India.38 Our population genomic analysis of six P. vivax strains from India, Latin America, North Korea, and Mauritania with six comparator P. falciparum lines revealed twice as much genetic diversity in P. vivax, suggesting a more stable and older association of this species with humans than for P. falciparum. In addition, the greater genetic diversity and gene family variability that we found suggests an increased capacity for functional variation in the global P. vivax population. We concluded that P. vivax malaria will likely be the more difficult parasite to eliminate.38
P. vivax population genomics across ICEMRs.
We generated whole genome sequences of ∼180 global P. vivax clinical isolates from five of the ICEMRs,39 identifying diversity hotspots in the genome and the first P. vivax selective sweeps of two drug resistance-associated genes PvDHR and PvDHPS. Other signals of natural selection that we identified suggest that P. vivax is adapting to regional differences in the human host and mosquito vector. The several Indian P. vivax genomes from our Chennai site showed significant admixture with isolates from Africa and support the hypothesis that contemporary African and South Asian P. vivax populations are genetically similar, suggesting that the latter may have genetically mingled with European (“New World”) lineages during the colonial era.
PATHOGENESIS OF SEVERE MALARIA
India is endemic for P. vivax and P. falciparum, which has allowed us to investigate severe malaria caused by both species in different eco-epidemiological settings.
Severe falciparum malaria.
Severe falciparum malaria is a complex disease with a wide spectrum of manifestations40 and poorly understood pathogenesis. It affects sub-Saharan children and Southeast Asian adults but with different clinical presentations41: whereas African children predominantly develop life-threatening anemia, metabolic acidosis, and cerebral malaria (CM), severe malaria in Southeast Asian adults is characterized by a multiorgan system involvement,3,42 including brain (CM), kidneys (acute kidney injury), liver (jaundice), and lungs (acute respiratory distress syndrome).
Cerebral malaria.
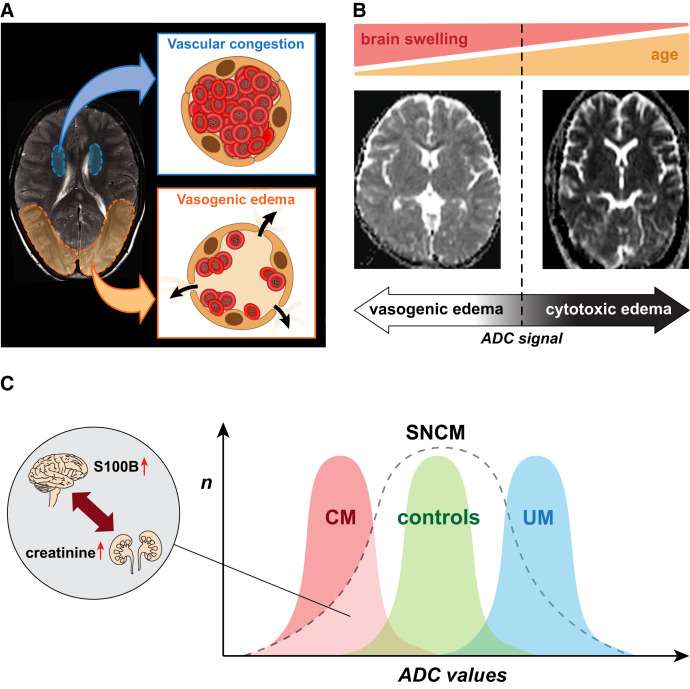
Approaches such as in vitro and in vivo models and autopsy studies have been used to unravel the mechanisms leading to the development of CM, but have provided limited answers and are inherently flawed.3 The recent use of neuroimaging has circumvented these obstacles and enabled imaging of pediatric and adult patients at both the acute stage of CM and also at follow-up for survivors.43,44 Our magnetic resonance imaging (MRI) project in the P. falciparum-endemic setting of Odisha, where both pediatric and adult CM cases occur, leveraged the availability of a 1.5-Tesla MRI scanner at Ispat General Hospital, Rourkela, allowing imaging comparisons between different age groups within the same cohort. We first analyzed brain changes in CM survivors and observed two distinct patterns of cerebral swelling in both adult and pediatric patients, often developing simultaneously in distinct parts of the brain. The occurrence of posterior vasogenic edema caused by an impairment of the blood–brain barrier that reversed upon treatment was indicative of posterior reversible encephalopathy syndrome, which we demonstrated for the first time in the context of CM (Figure 2A).45 Several patients in our cohort had concurrent cytotoxic edema in their basal nuclei with perfusion parameters indicative of vascular engorgement, likely to be caused by the sequestration of P. falciparum–infected erythrocytes (Figure 2A). Our results support the hypothesis that both endothelial dysfunction and microvascular obstruction make independent contributions to the pathogenesis of CM, providing opportunities for novel therapeutic interventions.45
Figure 2.
Summary of magnetic resonance imaging findings in India. (A) Schematic representation of the two main mechanisms leading to brain swelling in nonfatal Indian cerebral malaria (CM) patients—namely, reversible vasogenic edema with posterior predominance (redolent of posterior reversible encephalopathy syndrome), and vascular congestion in the basal ganglia (likely caused by the sequestration of P. falciparum–infected erythrocytes). (B) The cause of death in fatal CM differs with age: in children, severe brain swelling is associated with globally increased apparent diffusion coefficient (ADC) signal, indicative of diffuse vasogenic edema (left). In contrast, fatal CM in adults is associated with a profound, global hypoxia evidenced by the decreased ADC signal and mild or no brain swelling (right). (C) ADC value ranges in uncomplicated malaria (UM), healthy control, and CM cases overlap, and severe noncerebral malaria (SNCM) patients display a wide spectrum of ADC values. Extremes values in SNCM cases are similar to those seen in UM and CM; in the latter case, brain involvement is supported by elevated plasma S100B, a biomarker of brain injury, despite no WHO-defined coma. Low ADC values are strongly correlated with elevated plasma levels of creatinine in our cohort, indicative of acute kidney injury. Collectively, our findings suggest a brain–kidney pathogenic crosstalk in SNCM.
In a second study, we investigated cerebral changes associated with fatality in both adults and children of the same cohort. We confirmed previous findings showing that severe brain swelling was the main feature associated with death in pediatric CM.44 Our results demonstrated for the first time that this swelling decreased with age and that fatal adult CM is associated with severe hypoxic injury (Figure 2B), suggesting that different adjunct therapies need to be considered according to the patient age.53 However, detecting these brain changes upon admission is not feasible in most clinics in endemic settings. Our team therefore assessed potential biomarker candidates associated with specific neuroimaging features in CM. We found that plasma levels of hsa-miR-3158-3p, a microRNA involved in hypoxia-related processes, are significantly higher in fatal CM compared with nonfatal cases and are associated with MRI features of fatal CM for both children and adults.46
Lastly, we leveraged the presence of an MRI scanner at a different ICEMR site in Blantyre, Malawi,47 to perform the first comparative blood and brain MRI profiling of its kind between our Indian cohort of pediatric and adult CM and a Malawian cohort of pediatric CM. We identified common determinants of brain swelling across the two study sites—namely, high parasite biomass determined by plasma PfHRP2 levels and elevated endothelial protein C receptor (EPCR)-binding var transcripts.48 These findings confirm the role of parasite biomass as a pivotal pathogenic mechanism in CM and further suggest that restoring the cytoprotective activated EPCR signaling pathway abrogated by sequestered parasitized erythrocytes may represent a promising therapeutic avenue for patients with CM.49,50
Severe noncerebral malaria.
The focus of the CSCMi MRI project was recently broadened to characterize brain changes in severe, noncerebral malaria patients (SNCM), a group first recruited to serve as control. Indeed, two earlier studies reported that mild to moderate brain swelling43 and/or parenchymal changes51 on MRI were common in severe malaria patients with or without CM. Using the same imaging protocol from our previous studies, we found that both uncomplicated malaria and SNCM patients show a wide pattern of cerebral changes compared with healthy controls, suggesting that the brain is frequently affected in P. falciparum infection.52 We showed that some SNCM patients had patterns of brain changes similar to CM despite the absence of coma, and these were strongly associated with the presence of acute kidney injury. Our findings not only indicate a kidney–brain pathogenic crosstalk but also that severe malaria leads to a spectrum of neurological findings, where CM is only defined by the presence of coma. This highlights the need to revise the existing definition of “cerebral” malaria because severity syndromes are overlapping (Figure 2C) and the current Glasgow Coma Score cutoff is not optimal to identify all cases with acute brain alterations. Indeed, although not technically defined as having cerebral involvement, SNCM patients can develop brain change patterns like those observed in CM. The short- and long-term impact on neurological and psychological functions have never been assessed in this patient group, and these are now warranted so that strategies to identify patients at risk and help their recovery and rehabilitation can be developed.
The CSCMi MRI project benefited greatly from the long duration of the funding, which allowed nurturing of pivotal international collaborations, ultimately facilitating reproducibility and validation of imaging results. It has also led to the development of a range of research and clinical tools available to the malaria community, including the design of hypotheses-driven imaging protocols and quantitative methods to assess brain changes in falciparum malaria infection, and to the establishment of plasma biomarkers that strongly correlate with MRI findings and can therefore be used for diagnostic and prognostic approaches. These include lipocalin-2,53 hsa-miR-3158-3p,46 and S100 calcium-binding protein B (S100B)52; an assessment of their use for case management at the point of care is currently under way. Lastly, the project generated clinical datasets and reports that can be used to inform novel adjunctive therapies in the future.
Severe vivax malaria.
Evidence of P. vivax–associated morbidity and mortality has accumulated over the past decade,54 including delayed morbidity and indirect mortality,55 challenging the historic dogma of a benign illness. It is therefore important to evaluate rigorously the spectrum of disease caused by P. vivax in India, both at the acute and follow-up stages. Our first study in this area enrolled PCR-confirmed severe malaria patients at the Civil Hospital in Ahmedabad, Gujarat, followed over 12 months to compare morbidity and mortality between severe vivax and severe falciparum malaria.56 In this setting, severe vivax was found to be more frequent than severe falciparum malaria, jaundice was the most common complication of severe vivax malaria, and adults were predominantly affected rather than children, potentially indicating an age shift in antimalarial immunity. Although this may be a result of the recent decrease in transmission across India, further studies using serological evaluation of exposure markers to monitor the impact of elimination programs are warranted, as well as follow-up studies combining clinical characterization of severe vivax malaria with relapse and indirect mortality assessments.57,58
SUMMARY AND CONCLUSIONS
Our research and discoveries during the 12 years of the CSCMi highlight what can be achieved with continuous, long-term, stable funding and underscore the importance of undertaking basic and translational research on this important disease. A key factor has been the adaptability of the Center in the face of the changing epidemiology of malaria in India, making it responsive to new developments and questions as they emerge. For example, our studies on severe vivax malaria in Ahmedabad, Gujarat, were precipitated by a notable increase in number of cases in this area in 2016–2017, reported to us by clinicians at BJMC Civil Hospital in Gujarat. A central component of the CSCMi is interaction with malaria research, policy, and control counterparts in India to ensure that the research is relevant, significant, and strategic and that it provides opportunities for training, capacity building, and transfer of new technology. Our efforts and interactions in this area are described in the accompanying paper.59
ACKNOWLEDGMENTS
The authors thank the several director generals of the Indian Council of Medical Research and the directors of the ICMR-NIMR who have provided their support and encouragement for India–United States research collaborations over the years. We also thank past and current members of the CSCMi team including the scientists at ICMR-NIMR in Delhi and the field stations, at SAIL-IGH and at ICMR-NIRTH, who played highly valuable and key roles in the formulation, direction, and operation of the research. Finally, we are indebted to the state Program Officers, the Regional Offices of Health and Family Welfare officials, and the community where the studies were conducted.
REFERENCES
- 1. Das A et al. 2012. Malaria in India: the center for the study of complex malaria in India. Acta Trop 121: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO , 2021. World Malaria Report 2021. Geneva, Switzerland: World Health Organization, 322. [Google Scholar]
- 3. Wassmer SC Taylor TE Rathod PK Mishra SK Mohanty S Arevalo-Herrera M Duraisingh MT Smith JD , 2015. Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am J Trop Med Hyg 93: 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fowkes FJ Boeuf P Beeson JG , 2016. Immunity to malaria in an era of declining malaria transmission. Parasitology 143: 139–153. [DOI] [PubMed] [Google Scholar]
- 5. Price RN Commons RJ Battle KE Thriemer K Mendis K , 2020. Plasmodium vivax in the era of the shrinking P. falciparum map. Trends Parasitol 36: 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kojom Foko LP Arya A Sharma A Singh V , 2021. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect 82: 231–246. [DOI] [PubMed] [Google Scholar]
- 7. Phyo AP Dahal P Mayxay M Ashley EA , 2022. Clinical impact of vivax malaria: a collection review. PLoS Med 19: e1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rao PN et al. 2019. Dengue, chikungunya, and scrub typhus are important etiologies of non-malarial febrile illness in Rourkela, Odisha, India. BMC Infect Dis 19: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ompad DC et al. 2021. The effectiveness of malaria camps as part of the Durgama Anchalare Malaria Nirakaran (DAMaN) program in Odisha, India: study protocol for a cluster-assigned quasi-experimental study. Glob Health Action 14: 1886458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Eijk AM et al. 2019. The burden of submicroscopic and asymptomatic malaria in India revealed from epidemiology studies at three varied transmission sites in India. Sci Rep 9: 17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kessler A van Eijk AM Jamir L Walton C Carlton JM Albert S , 2018. Malaria in Meghalaya: a systematic literature review and analysis of data from the National Vector-Borne Disease Control Programme. Malar J 17: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kessler A et al. 2021. Spatial and temporal village-level prevalence of Plasmodium infection and associated risk factors in two districts of Meghalaya, India. Malar J 20: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarkar R Kessler A Mawkhlieng B Sullivan SA Wilson ML Carlton JM Albert S , 2021. Household and individual level risk factors associated with declining malaria incidence in Meghalaya, India: implications for malaria elimination in low-endemic settings. Malar J 20: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhowmick IP et al. 2021. Dry post wintertime mass surveillance unearths a huge burden of P. vivax, and mixed infection with P. vivax P. falciparum, a threat to malaria elimination, in Dhalai, Tripura, India. Pathogens 10: 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rahi M Sharma R Saroha P Chaturvedi R Bharti PK Sharma A , 2022. Polymerase chain reaction-based malaria diagnosis can be increasingly adopted during current phase of malaria elimination in India. Am J Trop Med Hyg 106: 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Eijk AM Mannan AS Sullivan SA Carlton JM , 2020. Defining symptoms of malaria in India in an era of asymptomatic infections. Malar J 19: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Eijk AM et al. 2016. The use of mosquito repellents at three sites in India with declining malaria transmission: surveys in the community and clinic. Parasit Vectors 9: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Eijk AM Choubey S Barla P Haque MA Nandini P Acharya S Sullivan SA Mohanty S Satpathi S Carlton JM , 2020. Malaria in Sundargarh district, Odisha, India: Epidemiological and behavioral aspects from surveys. Acta Trop 211: 105647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fradin MS Day JF , 2002. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med 347: 13–18. [DOI] [PubMed] [Google Scholar]
- 20. van Eijk AM et al. 2016. What is the value of reactive case detection in malaria control? A case-study in India and a systematic review. Malar J 15: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Longley RJ et al. 2020. Development and validation of serological markers for detecting recent Plasmodium vivax infection. Nat Med 26: 741–749. [DOI] [PubMed] [Google Scholar]
- 22. Uplekar S et al. 2017. Characterizing antibody responses to Plasmodium vivax and Plasmodium falciparum antigens in India using genome-scale protein microarrays. PLoS Negl Trop Dis 11: e0005323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tayipto Y Liu Z Mueller I Longley RJ , 2022. Serology for Plasmodium vivax surveillance: a novel approach to accelerate towards elimination. Parasitol Int 87: 102492. [DOI] [PubMed] [Google Scholar]
- 24. Waite JL Swain S Lynch PA Sharma SK Haque MA Montgomery J Thomas MB , 2017. Increasing the potential for malaria elimination by targeting zoophilic vectors. Sci Rep 7: 40551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rath A Prusty MR Das M Mahapatra N Tripathy H Hazra RK , 2015. A shift in resting habitat and feeding behavior of Anopheles fluviatilis sibling species in the Keonjhar district of Odisha, India. Trans R Soc Trop Med Hyg 109: 730–737. [DOI] [PubMed] [Google Scholar]
- 26. Thomas S Ravishankaran S Justin NA Asokan A Mathai MT Valecha N Montgomery J Thomas MB Eapen A , 2017. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar J 16: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yadav K Dhiman S Rabha B Goswami D Saikia PK Veer V , 2017. Disappearance of Anopheles minimus and Anopheles dirus from certain malaria endemic areas of Assam, India. J Arthropod Borne Dis 11: 27–35. [PMC free article] [PubMed] [Google Scholar]
- 28. Cator LJ Thomas S Paaijmans KP Ravishankaran S Justin JA Mathai MT Read AF Thomas MB Eapen A , 2013. Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malar J 12: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas S Ravishankaran S Justin JA Asokan A Mathai MT Valecha N Thomas MB Eapen A , 2016. Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai, India. Malar J 15: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas S Ravishankaran S Johnson Amala Justin NA Asokan A Maria Jusler Kalsingh T Mathai MT Valecha N Eapen A , 2016. Does fluoride influence oviposition of Anopheles stephensi in stored water habitats in an urban setting? Malar J 15: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravishankaran S Asokan A Justin NJA Thomas S Mathai MT Eapen A , 2021. Are dawn collections of Anopheles stephensi a better method to estimate the resting vector density? A study from Chennai, India. Am J Trop Med Hyg 105: 507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh OP Dykes CL Sharma G Das MK , 2015. L1014F-kdr mutation in Indian Anopheles subpictus (Diptera: Culicidae) arising from two alternative transversions in the voltage-gated sodium channel and a single PIRA-PCR for their detection. J Med Entomol 52: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dykes CL Das MK Eapen A Batra CP Ghosh SK Vijayan VA Mishra S Singh OP , 2016. Knockdown resistance (kdr) mutations in Indian Anopheles stephensi (Diptera: Culicidae) Populations. J Med Entomol 53: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dykes CL , 2017. Mechanisms of insecticide resistance in Indian malaria vector Anopheles stephensi. PhD dissertation, University of Liverpool, Liverpool, United Kingdom.
- 35. Carlton JM Sullivan SA Le Roch KG , 2013. Plasmodium genomics and the art of sequencing malaria parasite genomes. Carlton JM, Perkins S, Deitsch K, eds. Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Norfolk, United Kingdom: Caister Academic Press. [Google Scholar]
- 36. Carlton JM et al. 2015. Population genetics, evolutionary genomics, and genome-wide studies of malaria: a view across the International Centers of Excellence for Malaria Research. Am J Trop Med Hyg 93: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rao PN et al. 2016. A method for amplicon deep sequencing of drug resistance genes in Plasmodium falciparum clinical isolates from India. J Clin Microbiol 54: 1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neafsey DE et al. 2012. The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet 44: 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hupalo DN et al. 2016. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat Genet 48: 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller LH Baruch DI Marsh K Doumbo OK , 2002. The pathogenic basis of malaria. Nature 415: 673–679. [DOI] [PubMed] [Google Scholar]
- 41. WHO , 2014. Severe malaria. Trop Med Int Health 19 (Suppl 1): 7–131. [DOI] [PubMed] [Google Scholar]
- 42. Beales PF et al. 2000. Severe falciparum malaria. Trans R Soc Trop Med Hyg 94 (Suppl 1): S1–S90. [PubMed] [Google Scholar]
- 43. Mohanty S Mishra SK Patnaik R Dutt AK Pradhan S Das B Patnaik J Mohanty AK Lee SJ Dondorp AM , 2011. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis 53: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seydel KB et al. 2015. Brain swelling and death in children with cerebral malaria. N Engl J Med 372: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mohanty S et al. 2017. Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. MSphere 2: e00193–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta H Sahu PK Pattnaik R Mohanty A Majhi M Mohanty AK Pirpamer L Hoffmann A Mohanty S Wassmer SC , 2021. Plasma levels of hsa-miR-3158-3p microRNA on admission correlate with MRI findings and predict outcome in cerebral malaria. Clin Transl Med 11: e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kavinya T, 2009. Malawi’s new magnetic resonance imaging (MRI) centre. Malawi Med J 21: 5, 11. [PMC free article] [PubMed] [Google Scholar]
- 48. Sahu PK et al. 2021. Determinants of brain swelling in pediatric and adult cerebral malaria. JCI Insight 6: e145823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bernabeu M Smith JD , 2017. EPCR and malaria severity: the center of a perfect storm. Trends Parasitol 33: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Storm J Wu Y Davies J Moxon CA Craig AG , 2020. Testing the effect of PAR1 inhibitors on Plasmodium falciparum-induced loss of endothelial cell barrier function. Wellcome Open Res 5: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maude RJ et al. 2014. Magnetic resonance imaging of the brain in adults with severe falciparum malaria. Malar J 13: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohanty S et al. 2021. Evidence of brain alterations in noncerebral falciparum malaria. Clin Infect Dis. Available at: 10.1093/cid/ciab907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sahu PK et al. 2021. Brain magnetic resonance imaging reveals different courses of disease in pediatric and adult cerebral malaria. Clin Infect Dis 73: e2387–e2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baird JK , 2013. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev 26: 36–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dini S Douglas NM Poespoprodjo JR Kenangalem E Sugiarto P Plumb ID Price RN Simpson JA , 2020. The risk of morbidity and mortality following recurrent malaria in Papua, Indonesia: a retrospective cohort study. BMC Med 18: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anvikar AR et al. 2020. Clinical and epidemiological characterization of severe Plasmodium vivax malaria in Gujarat, India. Virulence 11: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Douglas NM et al. 2014. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua, Indonesia. BMC Med 12: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patriani D et al. 2019. Early and late mortality after malaria in young children in Papua, Indonesia. BMC Infect Dis 19: 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carlton JM Eapen A Kessler A Anvikar AR Singh OP Albert S Sahu PK Hoffmann A Mohanty S Wassmer SC , 2022. Advances in basic and translational research as part of the Center for the Study of Complex Malaria in India. Am J Trop Med Hyg (accepted as part of this supplement). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adams Y et al. 2021. Plasmodium falciparum erythrocyte membrane protein 1 variants induce cell swelling and disrupt the blood-brain barrier in cerebral malaria. J Exp Med 218: e20201266. [DOI] [PMC free article] [PubMed] [Google Scholar]