ABSTRACT.
The Program for Resistance, Immunology, Surveillance, and Modeling of Malaria (PRISM) has been conducting malaria research in Uganda since 2010 to improve the understanding of the disease and measure the impact of population-level control interventions in the country. Here, we will summarize key research findings from a series of studies addressing routine health facility-based surveillance, comprehensive cohort studies, studies of the molecular epidemiology, and transmission of malaria, evaluation of antimalarial drug efficacy, and resistance across the country, and assessments of insecticide resistance. Among our key findings are the following. First, we found that in historically high transmission areas of Uganda, a combination of universal distribution of long-lasting insecticidal-treated nets (LLINs) and sustained indoor residual spraying (IRS) of insecticides lowered the malaria burden greatly, but marked resurgences occurred if IRS was discontinued. Second, submicroscopic infections are common and key drivers of malaria transmission, especially in school-age children (5–15 years). Third, markers of drug resistance have changed over time, with new concerning emergence of markers predicting resistance to artemisinin antimalarials. Fourth, insecticide resistance monitoring has demonstrated high levels of resistance to pyrethroids, appreciable impact of the synergist piperonyl butoxide to pyrethroid susceptibility, emerging resistance to carbamates, and complete susceptibility of malaria vectors to organophosphates, which could have important implications for vector control interventions. Overall, PRISM has yielded a wealth of information informing researchers and policy-makers on the malaria burden and opportunities for improved malaria control and eventual elimination in Uganda. Continued studies concerning all the types of surveillance discussed above are ongoing.
INTRODUCTION
Uganda is emblematic of other high malaria burden countries in Sub-Saharan Africa. Malaria is endemic in over 95% of the country and in the remaining highland areas, transmission is unstable and epidemic-prone.1 Malaria is the leading cause of morbidity and mortality in Uganda, accounting for 30–50% of outpatient visits and 15–20% of hospital admissions.2 Like many other African countries, malaria control efforts in Uganda have focused on long-lasting insecticide-treated nets (LLINs), indoor residual spraying (IRS) of insecticide, and effective case management with artemisinin-based combination therapies (ACTs). The country has conducted three mass LLIN distribution campaigns (2010–2011, 2013–2014, and 2020–2021) leading to an increase in the proportion of households owning at least one LLIN from 47% in 2009 to 83% in 2018.1,3 Uganda’s IRS program was reinitiated in 2006, after a gap of 40 years, and is currently being implemented in 14 of 112 districts. Uganda adopted artemether-lumefantrine (AL) as its first-line therapy in 2004, and AL is provided free of charge at public health facilities. Despite roll out of effective control intervention, the burden of malaria remains high in Uganda. The country currently ranks third in terms of number of malaria cases and number of malaria deaths globally.4
The Program for Resistance, Immunology, Surveillance, and Modeling of Malaria in Uganda (PRISM) was established in 2010 and represents the East African region of the International Center of Excellence for Malaria Research (ICEMR) network with a focus on Uganda. Uganda is emblematic of the challenges faced by high-burden countries, where routine surveillance systems have limited ability to assess trends in the burden of malaria or to monitor the impact of control interventions. Through PRISM, researchers have implemented a comprehensive malaria surveillance program including enhanced health facility-based surveillance and detailed longitudinal cohort studies in areas with differing transmission intensities. Complementary laboratory-based studies have included surveillance for markers of antimalarial drug and insecticide resistance, serologic measures of malaria exposure, highly sensitive molecular assays for the detection of asexual- and sexual-stage parasites, and membrane feeding assays to assess human to mosquito transmission. These studies have greatly improved our understanding of the epidemiology of malaria in Uganda and of the impact of control interventions. In recent years, the program has expanded its malaria surveillance work and the scope of longitudinal studies to address more fundamental questions about interactions among the parasite, mosquito vector, and human host. Here, we report a summary of key research findings from our PRISM projects and some related studies from our group, which together offer a comprehensive understanding of malaria in Uganda, one of the highest malaria burden countries in the world.
Health facility-based malaria surveillance.
Malaria surveillance, which encompasses monitoring and evaluation of malaria control efforts, is essential to guide program planning and management. Malaria surveillance in Uganda is mainly dependent on passive case detection at health facilities as part of a country’s routine health management information system (HMIS). There are several strengths in conducting surveillance at health facilities: available data provide direct measures of morbidity, are collected continuously over time, and cover a wide geographic area.5 However, as is the case in many African countries, HMIS data in Uganda is provided as aggregate numbers from public facilities that are often inadequate for monitoring disease trends. Health management information system data also often has incomplete reporting, poor accuracy, and limited diagnostic testing.
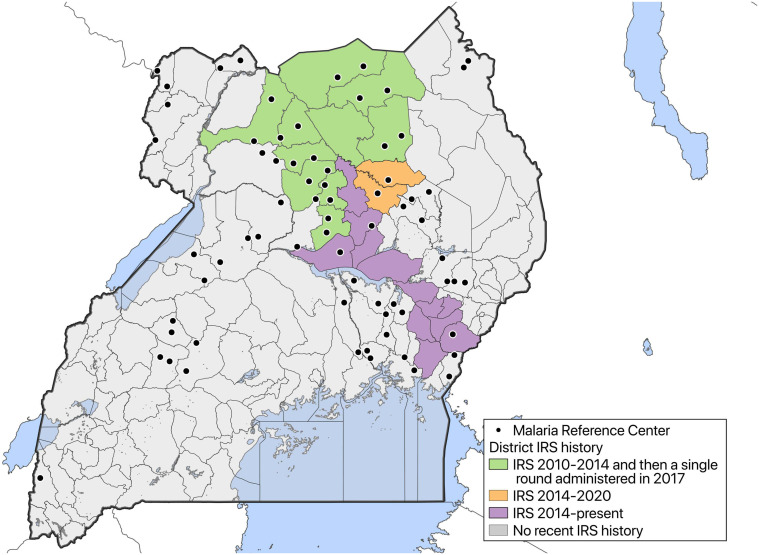
In 2006, the Uganda Malaria Surveillance Program (UMSP) was established to collect high-quality malaria surveillance data at six health facilities, known as Malaria Reference Centers (MRCs), in collaboration with the Uganda National Malaria Control Division (NMCD).6 From 2014 to 2020, this surveillance network was gradually expanded to include 70 MRCs located in 38 districts across the country, with support from PRISM (Figure 1). At the participating facilities, individual-level data for all outpatients that present to these MRCs are collected using standardized registers provided by the Ministry of Health and entered on site into an electronic database. These data include patient demographics (age, sex, and village of residence), results of laboratory tests, diagnoses given, and treatments prescribed. Uganda Malaria Surveillance Program conducts site support supervision, mentorship of health workers in malaria diagnosis and laboratory testing, and training to ensure the collection of high-quality malaria surveillance data. This enhanced malaria surveillance using data routinely collected from health facilities provides a powerful tool to monitor trends in malaria burden across time and space.
Figure 1.
Map of Uganda with locations of sites where health facility-based malaria surveillance is being conducted. This figure appears in color at www.ajtmh.org.
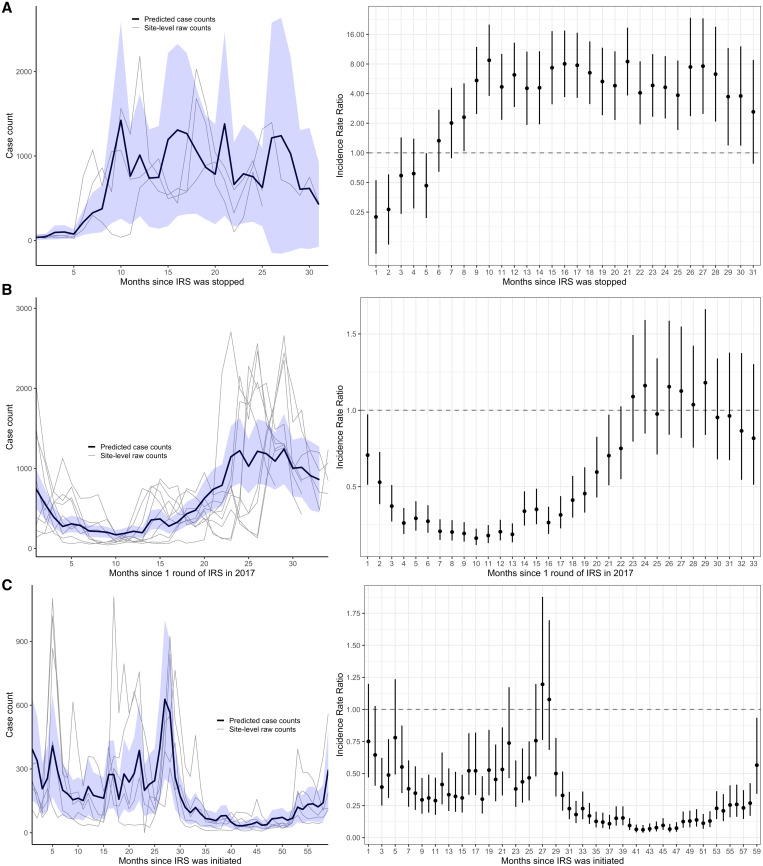
In 2020, we used data from this enhanced malaria surveillance system to assess the impact of starting and stopping IRS of insecticide on malaria burden in Northern and Eastern Uganda.7 Stopping IRS at three sites (Figure 2A) resulted in a 5-fold increase in malaria incidence within 10 months relative to the period before IRS was stopped (adjusted IRRc = c5.24, 95% CI 3.67–7.50); restarting IRS at nine sites (Figure 2B) led to an over 5-fold decrease in malaria incidence within 8 months (adjusted IRRc = c0.17, 95% CI 0.15–0.20). At five sites, where IRS was initiated and sustained over 5 years (Figure 2C), malaria incidence had dropped by 85% in the fourth and fifth years of sustained use relative to the pre-IRS period (adjusted IRRc = c0.15, 95% CI 0.12–0.18).
Figure 2.
(A) Adjusted Incidence rate ratio (IRR) and predicted case counts from multilevel negative binomial model assessing the impact of withdrawing IRS after 5 years of sustained use, (B) restarting IRS with a single round, and (C) initiating and sustaining IRS. Incidence rate ratios were adjusted for time-varying variables that impact malaria burden and malaria case detection at the health facility including monthly rainfall at the health facility lagged by 1 month, indicator variables for month of the year (to adjust for seasonal effects), the proportion of tests that were RDTs in that month (vs. microscopy), and the number of individuals who attended the health facility but were not suspected of having malaria in that month (to adjust for potential changes in care-seeking behaviors over time). The blue shaded regions represent the 95% confidence interval (CI) around the mean predicted case counts across sites from the adjusted regression model. Gray lines represent observed monthly case counts from individual sites. Vertical bars represent the 95% CI around adjusted IRR. This figure appears in color at www.ajtmh.org.
Since 2018, emphasis has been placed on collecting data on village of residence for all patients presenting to the MRCs. These data—along with the identification of target areas around the MRCs and enumeration surveys to estimate the populations of these target areas—have allowed for the estimation of malaria incidence within these target areas. Currently, data from our enhanced malaria surveillance network are being used as a platform for a cluster randomized trial evaluating the effect of two different types of newer generation LLINs on malaria incidence. A total of 64 clusters (MRC target areas) covering 32 high-burden districts have been randomized in a 1:1 ratio in blocks of two by district to receive one of each LLIN type: Permanet 3.0® LLINs containing deltamethrin and piperonyl butoxide (PBO) or Royal Guard® LLINs containing alpha-cypermethrin and pyriproxyfen. LLINs were distributed during Uganda’s 2020–2021 universal coverage campaign. By using malaria incidence measured within the target areas as the primary outcome for this trial, we will monitor temporal trends in malaria morbidity, and compare absolute disease burden in each trial arm.
In summary, data from our enhanced malaria surveillance provides an efficient means to quantify the impact of malaria control interventions at the population-level using observational study designs (i.e., “before and after” comparisons) or cluster randomized trials through coordination with implementing partners. This approach is more cost-effective and timely compared with the Demographic and Health Surveys that are conducted every 5 years and yet provides more comprehensive information beyond what is provided by the routine HMIS.
Comprehensive cohort studies.
Comprehensive cohort studies, including clinical and entomological assessments, provide a powerful design for generating longitudinal data on measures of malaria transmission, infection, and disease. We have conducted comprehensive malaria surveillance in cohorts from Tororo District, Uganda, an area with historically high transmission intensity that saw a dramatic decline in the burden of malaria following the implementation of intensive malaria control interventions (EIR = 562 infective bites/person/year in 2006 and 310 infective bites/person/year in 2011).8,9 Prior to 2013, malaria control in Tororo was limited to the distribution of LLINs through antenatal care services, promotion of intermittent preventive treatment during pregnancy, and malaria case management with AL. In November 2013, universal distribution of free LLINs was conducted as part of a national campaign, and a similar campaign was repeated in May 2017. Indoor residual spraying with the carbamate bendiocarb was first initiated in December 2014–January 2015, with additional rounds administered in June–July 2015 and November–December 2015. In June–July 2016, the insecticide delivered through IRS was changed to the organophosphate pirimiphos-methyl (Actellic), which was repeatedly delivered in June–July 2017, June–July 2018, and March–April 2019.
Two consecutive cohort studies (referred to as the “PRISM” cohorts) were conducted in randomly selected households from Nagongera subcounty, Tororo District.9,10 In PRISM 1, all children aged 0.5–10 years of age were enrolled from 100 houses and followed from October 2011 to September 2017. In PRISM 2, all household members were enrolled from 80 houses and followed from October 2017 to October 2019. All cohort members were given access to an LLIN at enrollment and followed for all their healthcare needs in a dedicated study clinic open 7 days/week. Routine visits were conducted every 1–3 months and included the collection of blood for assessment of parasitemia by microscopy and molecular methods (loop-mediated isothermal amplification [LAMP] in PRISM 1 and quantitative PCR [qPCR] in PRISM 2). Malaria was defined as a recent fever (defined as tympanic temperature > 38.0°C or history of fever in the previous 24 hours) and a positive thick blood smear by light microscopy and managed according to national guidelines. The cohorts were dynamic such that all newly eligible members from participating households were enrolled. In all cohort households, mosquitoes were collected using CDC light traps, monthly in PRISM 1 and every 2 weeks in PRISM 2. Entomological assessments included quantifying the number of female Anopheles and the detection of sporozoites using ELISA.
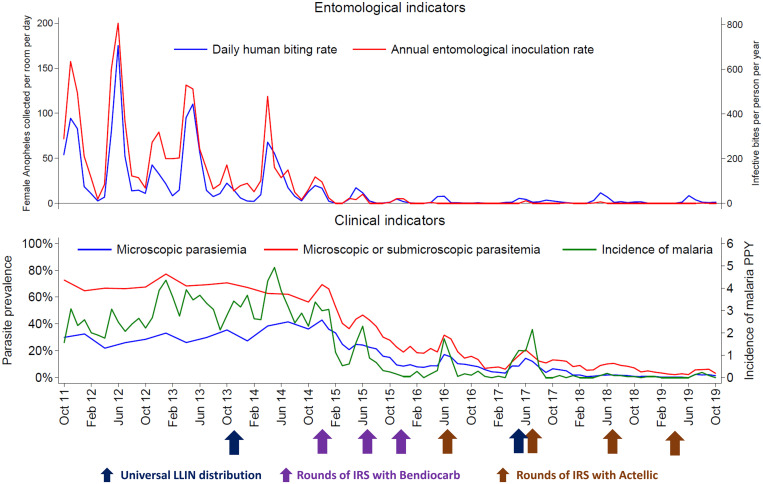
Following the implementation of IRS, measures of transmission, infection, and disease reduced dramatically (Figure 3). Pre-IRS, the daily anopheline human biting rate (HBR) was 34.3 and the annual entomological inoculation rate (EIR) was 238 infective bites per person per year (PPY). During the last 2 years of follow-up (corresponding to the fourth and fifth years following the initiation of IRS: October 2017–October 2019), the daily HBR was 2.1 and the annual EIR was 0.43, corresponding to an over 15-fold decrease in anopheline biting and over 500-fold decrease in transmission intensity. Clinical indicators of malaria were considered only in children 0.5–10 years of age, as this age group was evaluated in both cohorts. Pre-IRS, the prevalence of microscopic parasitemia was 31.8%, the prevalence of microscopic or submicroscopic parasitemia was 67.5%, and the incidence of malaria was 2.96 episodes PPY. During the last 2 years of follow-up, the prevalence of microscopic parasitemia was 1.8%, the prevalence of microscopic or submicroscopic parasitemia was 6.8%, and the incidence of malaria was 0.05 episodes PPY, corresponding to a 10-fold reduction in the prevalence of any parasitemia and over 50-fold reduction in the incidence of malaria. Thus, great progress was made in decreasing the malaria burden, although a substantial reservoir of asymptomatic (largely submicroscopic) infections remained. These cohort studies also highlighted the effectiveness of providing prompt and effective antimalarial treatment. Over 3,000 episodes of malaria were diagnosed in both cohorts combined, but only four cases met criteria for severe malaria, and no malaria deaths occurred. Highly effective malaria control was also associated with other health benefits. Following the implementation of IRS mean hemoglobin levels increased significantly in children, with the overall prevalence of anemia reducing from to 3.4%.11 Over the 8-year observation period, care-seeking rates declined by 75% and the incidence of clinic visits at which antibiotics were prescribed to children declined by 70%.
Figure 3.
Temporal trends in entomological and clinical indicators. Clinical indicators limited to children 0.5–10 years of age. Measures of parasite prevalence based on active surveillance at the time of all enrollment and routine clinic visits. Incidence of malaria based on passive surveillance. This figure appears in color at www.ajtmh.org.
In summary, our comprehensive cohort studies quantified dramatic reductions in malaria and nonmalaria indicators in an historically high-burden setting following the implementation of sustained malaria control interventions including access to prompt and effective antimalarial therapy, universal LLIN distribution, and IRS. However, despite these reductions, a substantial reservoir of asymptomatic infections remained, primarily among school-aged children and adults.10 In addition, nonadherence to LLINs (defined as participant not using a bed net the night prior to the survey as reported by the adult respondent during the household visits) after transmission declined was associated with an increased risk of malaria, highlighting the importance of LLIN use even in the setting of sustained IRS.12 Given the relatively high cost of IRS and the risk of resurgence following the withdrawal of IRS,7 further research is needed to facilitate effective IRS exit strategies. Furthermore, interventions in addition to those now widely available will likely be needed to achieve elimination in historically high-burden settings.
Molecular epidemiology.
Conventional point-of-care malaria diagnostics, such as microscopy and rapid diagnostic tests (RDTs), do not have the sensitivity required to detect low-density infections, often referred to as submicroscopic or subpatent infections. More sensitive molecular techniques, such as LAMP (limit of detection 1–5 parasites/µL) or ultrasensitive varATS qPCR (limit of detection 0.03–0.15 parasites/µL) can be used to detect these infections through active surveillance.13 Multiple studies have shown that submicroscopic infections are common across various transmission settings and are more frequent in adults than in children due to naturally acquired antimalarial immunity.14,15 Therefore, we have integrated these highly sensitive molecular assays as routine evaluations in our cohort studies to further characterize the burden and dynamics of asymptomatic infections.
In PRISM 1 prior to IRS, measured parasite prevalence was approximately twice as high in children and 8-fold higher in adults with results based on LAMP compared with microscopy alone,16 providing additional evidence that submicroscopic infections are common in high-transmission settings and that they become more frequent with increasing age. As transmission declined throughout the PRISM studies due to repeated rounds of IRS, the proportion of submicroscopic infections increased in all age groups; by the end of PRISM 2 in 2019, submicroscopic infections accounted for > 75% of infections in children (ages 0–15) and > 90% of infections in adults > 15 years of age10,16,17 These findings provided longitudinal data to support conclusions from previous cross-sectional studies that indicate an increase in the proportion of submicroscopic infections relative to microscopically detectable infections in settings, where malaria control efforts have been successful.14,18 Submicroscopic infections may also be clinically relevant; some studies suggest that low-density, chronic infections are associated with anemia, altered cognitive function, and/or systemic bacterial infection.16,19,20 In PRISM 1, children with submicroscopic parasitemia had an increased risk of fever and nonfebrile illness compared with children without parasitemia, suggesting that submicroscopic infections may have clinically relevant consequences for children.21
Our experience with the use of highly sensitive molecular diagnostics shows that these techniques are critical to fully characterize the parasite reservoir, given that microscopic infections represent only the “tip of the iceberg,” and that the relative proportion of submicroscopic infections increases with decreasing transmission intensity. Furthermore, these techniques can be used to identify asymptomatic individuals who provide persistent reservoirs of transmission in lower transmission settings, to identify infected populations who, although asymptomatic, may benefit clinically from treatment, and to evaluate the results of interventions.
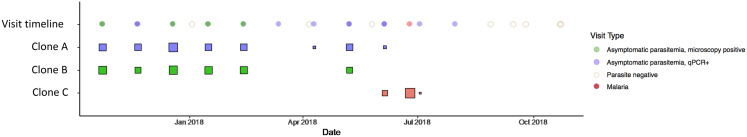
In addition to characterizing the parasite reservoir, highly sensitive molecular diagnostics can provide insight into longitudinal dynamics of infections. In PRISM 1 and 2, some individuals had low-density infections detected sequentially over numerous months. To further characterize the dynamics of these chronic infections, it is necessary to genotype parasite DNA to distinguish among individual clones (strains) as individuals in high-transmission settings are often coinfected with overlapping infections of multiple clones. Using genetic data, it is possible to follow distinct clones over time, which allows for assessment of the molecular force of infection (mFOI), or the incidence of genetically distinct parasite clones acquired over time, and estimation of the duration of infections.22 In the PRISM 2 cohort, we genotyped all samples with a parasite density ≥0.1 parasites/µL using amplicon deep-sequencing of the apical membrane antigen-1 (AMA-1) gene, a highly diverse region of the genome, allowing us to distinguish most parasite clones from each other (45 unique haplotypes detected).23,24 Infections with identical AMA-1 haplotypes were defined as genetically identical (from the same clone), which allowed differentiation of persistent from new infections (Figure 4). By using frequent sampling, ultrasensitive qPCR, and AMA-1 amplicon deep-sequencing, it was possible to accurately detect the onset of new infections and to estimate infection durations. This allowed us to estimate the clearance of asymptomatic Plasmodium falciparum infections and to investigate whether there were sex-based differences in clearance of these infections, given that we observed higher prevalence of asymptomatic infection in males compared with females. Analyzing the data by clone, where each unique clone was counted as an infection and each clone’s disappearance as a clearance event, we found that females cleared their asymptomatic infections nearly twice as quickly as males, even after adjusting for age, baseline infection status, and parasite density (adjusted hazard ratio for clearance of infection without antimalarial use = 1.82, 95% CI 1.20–2.75).23 There was no evidence for a sex-based difference in exposure to infection through behaviors or as measured by mFOI. Although previous studies have reported a higher prevalence of malaria infection in males compared with females, these differences were often ascribed to differences in exposure. However, in PRISM 2, the observed male bias in parasite prevalence was best explained by slower clearance of infections in males.
Figure 4.
Top row shows the visit timeline of a participant along with parasitemia status. Apical membrane antigen-1 (AMA-1) genotyping reveals that this individual had a polyclonal infection with clones A and B that persisted until the participant was infected with a new clone that caused symptoms. After treatment of malaria, all parasite clones cleared. Given the oscillations in parasite density that occurred during the period of observation, which included some parasite negative visits, and the polyclonal nature of the infection, AMA-1 genotyping was essential to differentiate between chronic and new infections. This figure appears in color at www.ajtmh.org.
As described previously, amplicon deep-sequencing provides information vital to understanding the host response to malaria infection and better characterizes the dynamics of polyclonal asymptomatic infections compared with molecular methods that cannot differentiate among parasite clones. Because amplicon sequencing data can be used to estimate the mFOI, it can also be used to better understand changes in transmission in the setting of malaria control interventions. We plan to use amplicon deep-sequencing to assess changes in the mFOI and clinical antimalarial immunity in the setting of repeated rounds of IRS during longitudinal evaluation of the PRISM 1 and 2 cohorts.
Assessing the human infectious reservoir for malaria.
Understanding who in the human population sustains transmission to mosquitoes is of great relevance for malaria control and elimination efforts.25 The increased use of molecular malaria diagnostics has established that submicroscopic infections are common in nearly all malaria endemic settings15 and that they may produce gametocytes at densities sufficient for transmission to mosquitoes.26 To examine infectivity to mosquitoes, an insectary with functioning mosquito membrane feeding facilities was established at our study site in Nagongera subcounty, Tororo District.27 In the PRISM 2 cohort, running from October 2017 to October 2019, gametocyte density and sex ratio were examined by molecular markers with mosquito feeding assays using a colony of An. gambiae s.s. mosquitoes.28 Key goals in these assessments were to determine: 1) the contribution to transmission of infections in relation to their detectability by different diagnostics (microscopy, molecular qPCR); 2) the contribution of different age groups to transmission; and 3) the duration of parasite carriage in relation to onward transmission potential.17
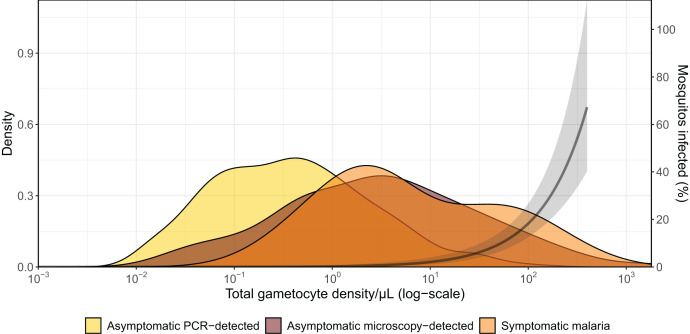
Gametocyte prevalence among symptomatic malaria infections in the PRISM 2 cohort was 28.9%, considerably lower than that among asymptomatically infected individuals (67.6%; P = 0.033); this result suggests that in a cohort that is treated promptly whenever symptomatic malaria is diagnosed, most transmission will be from asymptomatic individuals. Mosquito feeding assays were successfully performed on both symptomatic and asymptomatic parasite carriers. At least one infected mosquito was observed in 7.2% of feeding experiments, with 1.2% of all examined mosquitoes becoming infected. The likelihood that a mosquito became infected rose rapidly when gametocyte densities exceeded 10 gametocytes/µL (assessed by molecular methods), corroborating findings from other African settings (Figure 5).29
Figure 5.
Gametocyte density distributions stratified by cohort participant infection and disease status. Relationship between gametocyte density and proportion of mosquitoes infected from membrane feeding assays. This figure appears in color at www.ajtmh.org.
Gametocyte density distributions differed among populations, and densities were highest overall in asymptomatic microscopy-detected malaria infections, and lower in clinical malaria cases and asymptomatic submicroscopic infections (Figure 5). After adjusting for gametocyte density, mosquito infection rates were lower for symptomatic malaria cases, suggesting that in symptomatic infections, gametocytes may be less mature or serum factors associated with inflammation may reduce gametocyte viability.30 When concurrently considering their prevalence in the population and their infectivity to mosquitoes, asymptomatic microscopy-positive individuals comprised 83.8% of the infectious reservoir, with asymptomatic submicroscopic infections responsible for 15.6% and symptomatic infections for only 0.6% of transmission. These results are broadly in line with direct assessments of the human infectious reservoir in Ethiopia31 and an indirect assessment from Western Kenya based on household-caught mosquitoes.32 In our study setting, children aged 5–15 years were responsible for 58.7% of the infected mosquitoes; individuals 16 years or older contributed the least to transmission among all age groups examined (15.6%). These population estimates may help in targeting malaria interventions to those demographics most important for malaria transmission,33,34 but they do not do justice to considerable heterogeneity in the infectivity of individual gametocyte carriers. Remarkably, four children (0.8% of the total population) were responsible for 62.6% of all infected mosquitoes. Some of the high-transmission infections in these children were chronic but others of short infection duration.
In summary, our findings suggest that passive detection of symptomatic infections or community mass screening and treatment based on conventional diagnostics might be insufficient to reduce the infectious reservoir and prevent onward transmission in settings of declining transmission.
Antimalarial drug efficacy and resistance.
The treatment of malaria is challenged by resistance of P. falciparum to multiple drugs. Resistance to the older agents chloroquine and sulfadoxine-pyrimethamine (SP) has been common in Uganda for many years, and first-line therapy changed to the ACT AL in 2004. Sulfadoxine-pyrimethamine remains the standard-of-care for intermittent preventive treatment of malaria in pregnancy.
We have systematically studied antimalarial drug efficacy and resistance in Uganda for the last two decades, with therapeutic efficacy studies, characterization of genetic markers of resistance, and measurement of ex vivo drug susceptibilities of cultured isolates. Our results have demonstrated changing susceptibilities to key treatment regimens over time.
Evaluation of the therapeutic efficacy of chloroquine in 1998–1999 suggested poor efficacy,35,36 although early studies lacked genotyping to definitively assign outcomes. Subsequent trials demonstrated improved efficacy of SP, compared with chloroquine,36 and good efficacy for amodiaquine plus SP;37–41 although this combination was never widely established as a standard treatment, it is now widely used as seasonal malaria chemoprevention in parts of West and Central Africa.42
Trials of ACTs showed excellent antimalarial efficacies for artesunate-SP,38,43 artesunate-amodiaquine,41,44–47 AL,44,45,47–51 and dihydroartemisinin-piperaquine.48–51 After treatment with all tested ACTs, recrudescences were very uncommon, but new infections were common in areas of high-transmission intensity. Compared with other ACTs, treatment with dihydroartemisinin-piperaquine was followed by fewer recurrent infections, consistent with the long half-life of piperaquine,49 and the combination has been shown to be highly efficacious for chemoprevention in Ugandan children52,53 and pregnant women.54,55
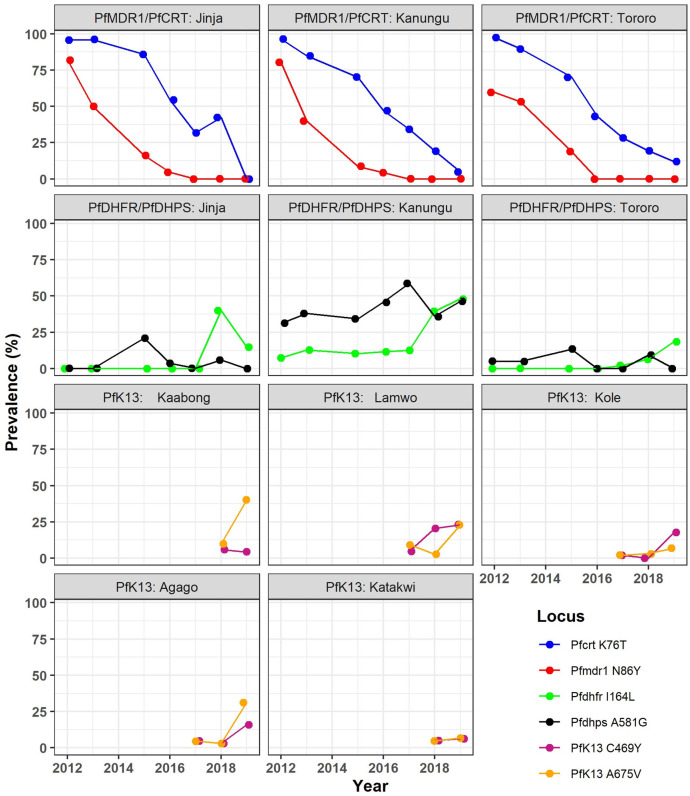
Considering molecular markers of resistance (Figure 6), serial studies in Tororo, in Eastern Uganda, showed steady declines over the last two decades in the prevalences of the aminoquinoline resistance markers pfcrt 76T and pfmdr1 86Y.56–60 Surveillance at sites across Uganda showed similar prevalences.56,57,60,61 Changes in resistance mediators over time are likely due to selective pressures of antimalarials, with evidence for opposite pressures on aminoquinoline resistance markers of regimens containing amodiaquine62,63 or piperaquine64 compared with lumefantrine.65 More recently described polymorphisms associated with resistance to piperaquine (other mutations in pfcrt and plasmepsin gene amplification) or lumefantrine (pfmdr1 amplification) have shown very low prevalence in Uganda.66,67 Overall, the large majority of P. falciparum parasites now circulating in Uganda appear to be sensitive to available aminoquinolines and lumefantrine.
Figure 6.
Prevalence of key drug resistance associated genetic polymorphisms at health centers in selected districts in Uganda. Prevalences are shown for the indicated years and districts. Jinja, Kanungu, and Tororo are in Central, Southwestern, and Eastern Uganda, respectively. The five districts for which PfK13 data are shown are all in Northern Uganda. This figure appears in color at www.ajtmh.org.
Considering SP, five mutations that mediate increasing levels of resistance showed > 90% prevalence in parasites collected across Uganda as early as 2002.60,61 Prevalences of two additional mutations that mediate high-level resistance to the components of SP, pfdhfr 164L, and pfdhps 581G, were very low through ∼2012, but have since increased, especially in Western Uganda, with prevalences near or exceeding 40% at multiple sites in 2018–2019.56,57 Thus, the antimalarial activity of SP, which has been compromised for many years, is now likely diminished even further.
Of recent concern is potential emergence of resistance to ACTs in Africa.68 About 20 PfK13 propeller domain mutations are validated or candidate markers of delayed parasite clearance after treatment with artemisinins, generally referred to as artemisinin resistance.69 Consistent with studies across Africa,70 older studies from Uganda showed very low prevalence of validated/candidate PfK13 mutations.60,71–73 In contrast, recent surveillance has shown emergence of parasites with either of two PfK13 candidate resistance mutations, 469Y and 675V,57,74 with prevalences increasing to 20–30% in multiple districts of Northern Uganda in 2018–2019.56 Another validated PfK13 mutation, 561H, was seen at similar prevalences in Central Rwanda.75–77 Recent studies have shown the Ugandan78 and Rwandan77 mutations to be associated with delayed parasite clearance after treatment with artemisinins. Thus, evidence is now strong that artemisinin resistance has emerged in East Africa, and specifically in Northern Uganda. However, drug efficacy studies have not recently been performed in relevant regions of Uganda, and in Rwanda, PfK13 561H was not clearly associated with AL treatment failure. Thus, although emergence of resistance-mediating PfK13 mutations is very concerning, impacts on antimalarial treatment efficacy remain uncertain.
Ex vivo analyses of cultured parasites have shown results consistent with molecular studies of resistance markers. Isolates collected in Kampala in 2006–2008 demonstrated chloroquine susceptibilities suggesting high-level resistance.79 More recently, with the reversion to wild type sequences described above, > 90% of isolates collected in Eastern Uganda had IC50s < 100 nM, consistent with chloroquine sensitivity, although a minority had high IC50s.66,67,80 Susceptibilities to the key ACT partner drugs lumefantrine, amodiaquine, piperaquine, mefloquine, and pyronaridine were all generally excellent.66,67,80 In older studies, the ring survival assay (RSA), a laboratory marker for artemisinin resistance, showed sensitive isolates in Kampala72 and Tororo.66,67 However, parasites from Northern Uganda with PfK13 675V were recently shown to have abnormal RSAs,78 and additional genotype–phenotype association studies are underway.
In summary, surveillance over the last two decades has demonstrated remarkable changes in drug resistance profiles of Ugandan malaria parasites. Recent results suggest a return to chloroquine sensitivity for most, but not all circulating parasites; major limitations in the antimalarial chemopreventive activity of SP; and worrisome emergence of artemisinin resistance in Northern Uganda. There is an urgent need for continued surveillance and clinical trials to determine whether current regimens for the treatment and prevention of malaria in Uganda will need to be replaced.
Insecticide resistance.
The dramatic scale-up of malaria vector control interventions across Africa, including LLINs and IRS, has been associated with an estimated 40% decrease in the incidence of disease between 2000 and 2015.81 In tandem with global gains, expanded coverage of LLINs and IRS in Uganda has been associated with declines in the prevalence of malaria in children under 5, from 19% in 2014 to 9% in 2018.82 Although a variety of insecticides are deployed through IRS, all widely available LLINs are treated with pyrethroids, and reports of increasing resistance to this class of insecticides are highly concerning.83 Pyrethroid resistance is commonly mediated through two main mechanisms including “knock down resistance” caused by target-site mutations in the receptor for pyrethroids (kdr),84,85 and metabolic resistance mediated by cytochrome p450 enzymes.86 To combat pyrethroid resistance, dual active ingredient LLINs have been developed, which combine pyrethroid insecticides with other agents, including PBO, a synergist;87 chlorfenapyr, a proinsecticide;88 and pyriproxyfen, an insect growth regulator.89
In our PRISM projects, we have conducted entomologic surveillance, monitored for insecticide resistance, and assessed the impact of vector control interventions on Anopheles mosquitoes.90 In Nagongera, Tororo district, near collapse of An. gambiae s.s. and An. funestus populations was observed following universal distribution of LLINs plus multiple rounds of IRS, with the more behaviorally resilient An. arabiensis becoming the predominant vector species.90 In Tororo, these interventions were also associated with declines in the indoor HBR and sporozoite infections, and increased outdoor biting.91 Routine monitoring of insecticide resistance to pyrethroids revealed high levels of resistance to both class I (permethrin) and class II (deltamethrin) pyrethroids,83 and to carbamates (bendiocarb).92 However, no resistance to the organophosphate pirimiphos-methyl, which is routinely used for IRS by the NMCD, was observed. We recorded evidence of contemporary gene flow between sympatric An. gambiae s.s and An. arabiensis,93 which is of particular concern, as it may result in the transfer of adaptively important genetic traits such as insecticide resistance between these two species.
Through our PRISM projects, we have established foundations and collaborations that have been leveraged to conduct additional research on insecticide resistance,94 and contributed to mapping of insecticide resistance in Africa.95 Improved molecular analysis tools and increased surveillance for genetic markers of insecticide resistance has been propelled by availability of the An. gambiae genome;96 PRISM contributed > 100 samples toward its development.96 The An. gambiae genome has enabled exploration of gene targets with putative association with insecticide resistance. The PRISM projects used this platform to aid the discovery of two novel variants (Cyp4j5 and Coeaeld),97 which are associated with resistance to pyrethroids. These markers appear to be widely spread in Uganda, approaching fixation in some areas.94 Overall, our insecticide resistance monitoring has demonstrated high levels of resistance to pyrethroids, appreciable impact of the synergist PBO to pyrethroid susceptibility, emerging resistance to carbamates, and complete susceptibility of malaria vectors to organophosphates, underscoring the importance of this class to current insecticide resistance management strategies.
CONCLUSION
Utilizing comprehensive malaria surveillance studies, we demonstrated reductions in measures of transmission, infection, and disease following roll out of effective malaria control interventions. Similar trends were seen and conclusions drawn from data collected from both enhanced health facility-based surveillance and comprehensive cohort studies, highlighting our understanding that both surveillance methods are effective tools for quantifying the impacts of population-level malaria control inventions. We also demonstrated that although younger children are at greatest risk of malaria, school-aged children are the main reservoir of infection and drivers of transmission. This finding is particularly true in settings, where recent malaria control efforts have been successful. Thus, with the goal of reducing human to mosquito transmission, school-aged children may be an ideal group to target for control interventions. We have shown that surveillance of drug and insecticide resistance is critical for informing policy, as evidenced by recent emergence of markers predicting resistance to artemisinin antimalarials and expanding vector resistance to multiple classes of commonly used insecticides. With a recent emphasis on the high burden to high impact approach in countries such as Uganda, our ICEMR program is committed to providing valuable data to support an evidence-based approach to reducing the burden of malaria, eventually moving toward elimination.
ACKNOWLEDGMENTS
We thank the study teams and the Infectious Diseases Research Collaboration (IDRC) for administrative and technical support. We are grateful to the study participants who participated in the various studies and their families.
REFERENCES
- 1. Uganda Ministry of Health , 2020. Uganda Malaria Indicator Survey 2018–19. Available at: https://dhsprogram.com/pubs/pdf/MIS34/MIS34.pdf. Accessed May 10, 2021.
- 2. Ministry of Health Overview of Malaria in Uganda (2014–2020). Available at: https://www.health.go.ug/programs/national-malaria-control-program/. Accessed July 29, 2021.
- 3.Uganda Ministry of Health, 2010. The Uganda Malaria Indicator Survey 2009. Available at: https://dhsprogram.com/pubs/pdf/MIS6/MIS6.pdf. Accessed February 8, 2022.
- 4. World Health Organization , 2021. World Malaria Report 2021. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed February 8, 2022.
- 5.World Health Organization, 2018. Malaria Surveillance, Monitoring & Evaluation: A Reference Manual. Available at: https://apps.who.int/iris/bitstream/handle/10665/272284/9789241565578-eng.pdf.
- 6. Sserwanga A. et al. , 2011. Improved malaria case management through the implementation of a health facility-based sentinel site surveillance system in Uganda. PLOS ONE 6: e16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Namuganga JF. et al. , 2021. The impact of stopping and starting indoor residual spraying on malaria burden in Uganda. Nat Commun 12: 2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D’Alessandro U, Coosemans M, 2006. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 75: 219–225. [PubMed] [Google Scholar]
- 9. Kamya MR. et al. , 2015. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 92: 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nankabirwa JI. et al. , 2020. Malaria transmission, infection, and disease following sustained indoor residual spraying of insecticide in Tororo, Uganda. Am J Trop Med Hyg 103: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zinszer K. et al. , 2020. The impact of multiple rounds of indoor residual spraying on malaria incidence and hemoglobin levels in a high-transmission setting. J Infect Dis 221: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rek J. et al. , 2020. Non-adherence to long-lasting insecticide treated bednet use following successful malaria control in Tororo, Uganda. PLOS ONE 15: e0243303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katrak S. et al. , 2017. Performance of loop-mediated isothermal amplification for the identification of submicroscopic Plasmodium falciparum infection in Uganda. Am J Trop Med Hyg 97: 1777–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 15. Slater HC. et al. , 2019. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun 10: 1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nankabirwa JI. et al. , 2019. Persistent parasitemia despite dramatic reduction in malaria incidence after 3 rounds of indoor residual spraying in Tororo, Uganda. J Infect Dis 219: 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andolina C. et al. , 2021. Sources of persistent malaria transmission in a setting with effective malaria control in Eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis 21: 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ, 2012. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3: 1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, O’Meara W, Price RN, Riley EM, 2016. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLOS Med 13: e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rek J. et al. , 2016. Characterizing microscopic and submicroscopic malaria parasitaemia at three sites with varied transmission intensity in Uganda. Malar J 15: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katrak S, Nayebare P, Rek J, Arinaitwe E, Nankabirwa JI, Kamya M, Dorsey G, Rosenthal PJ, Greenhouse B, 2018. Clinical consequences of submicroscopic malaria parasitaemia in Uganda. Malar J 17: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koepfli C, Mueller I, 2017. Malaria epidemiology at the clone level. Trends Parasitol 33: 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Briggs J. et al. , 2020. Sex-based differences in clearance of chronic Plasmodium falciparum infection. eLife 9: e59872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller RH, Hathaway NJ, Kharabora O, Mwandagalirwa K, Tshefu A, Meshnick SR, Taylor SM, Juliano JJ, Stewart VA, Bailey JA, 2017. A deep sequencing approach to estimate Plasmodium falciparum complexity of infection (COI) and explore apical membrane antigen 1 diversity. Malar J 16: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabinovich RN. et al. , 2017. malERA: an updated research agenda for malaria elimination and eradication. PLOS Med 14: e1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goncalves BP. et al. , 2017. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musiime AK. et al. , 2019. Is that a real oocyst? Insectary establishment and identification of Plasmodium falciparum oocysts in midguts of Anopheles mosquitoes fed on infected human blood in Tororo, Uganda. Malar J 18: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meerstein-Kessel L. et al. , 2018. A multiplex assay for the sensitive detection and quantification of male and female Plasmodium falciparum gametocytes. Malar J 17: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradley J. et al. , 2018. Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. eLife 7: e34463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barry A. et al. , 2021. Higher gametocyte production and mosquito infectivity in chronic compared to incident Plasmodium falciparum infections. Nat Commun 12: 2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tadesse FG. et al. , 2018. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis 66: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 32. Sumner KM, Freedman E, Abel L, Obala A, Pence BW, Wesolowski A, Meshnick SR, Prudhomme-O’Meara W, Taylor SM, 2021. Genotyping cognate Plasmodium falciparum in humans and mosquitoes to estimate onward transmission of asymptomatic infections. Nat Commun 12: 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohee LM. et al. , 2021. School-based screening and treatment may reduce P. falciparum transmission. Sci Rep 11: 6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Staedke SG. et al. , 2018. Assessment of community-level effects of intermittent preventive treatment for malaria in schoolchildren in Jinja, Uganda (START-IPT trial): a cluster-randomised trial. Lancet Glob Health 6: e668–e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dorsey G, Kamya MR, Ndeezi G, Babirye JN, Phares CR, Olson JE, Katabira ET, Rosenthal PJ, 2000. Predictors of chloroquine treatment failure in children and adults with falciparum malaria in Kampala, Uganda. Am J Trop Med Hyg 62: 686–692. [DOI] [PubMed] [Google Scholar]
- 36. Kamya MR, Dorsey G, Gasasira A, Ndeezi G, Babirye JN, Staedke SG, Rosenthal PJ, 2001. The comparative efficacy of chloroquine and sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in Kampala, Uganda. Trans R Soc Trop Med Hyg 95: 50–55. [DOI] [PubMed] [Google Scholar]
- 37. Bakyaita N. et al. , 2005. Sulfadoxine-pyrimethamine plus chloroquine or amodiaquine for uncomplicated falciparum malaria: a randomized, multisite trial to guide national policy in Uganda. Am J Trop Med Hyg 72: 573–580. [PubMed] [Google Scholar]
- 38. Dorsey G, Njama D, Kamya MR, Cattamanchi A, Kyabayinze D, Staedke SG, Gasasira A, Rosenthal PJ, 2002. Sulfadoxine/pyrimethamine alone or with amodiaquine or artesunate for treatment of uncomplicated malaria: a longitudinal randomised trial. Lancet 360: 2031–2038. [DOI] [PubMed] [Google Scholar]
- 39. Gasasira AF, Dorsey G, Nzarubara B, Staedke SG, Nassali A, Rosenthal PJ, Kamya MR, 2003. Comparative efficacy of aminoquinoline-antifolate combinations for the treatment of uncomplicated falciparum malaria in Kampala, Uganda. Am J Trop Med Hyg 68: 127–132. [PubMed] [Google Scholar]
- 40. Staedke SG, Kamya MR, Dorsey G, Gasasira A, Ndeezi G, Charlebois ED, Rosenthal PJ, 2001. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial. Lancet 358: 368–374. [DOI] [PubMed] [Google Scholar]
- 41. Staedke SG, Mpimbaza A, Kamya MR, Nzarubara BK, Dorsey G, Rosenthal PJ, 2004. Combination treatments for uncomplicated falciparum malaria in Kampala, Uganda: randomised clinical trial. Lancet 364: 1950–1957. [DOI] [PubMed] [Google Scholar]
- 42. Cairns ME. et al. , 2020. Evaluation of seasonal malaria chemoprevention in two areas of intense seasonal malaria transmission: secondary analysis of a household-randomised, placebo-controlled trial in Hounde District, Burkina Faso and Bougouni District, Mali. PLOS Med 17: e1003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dorsey G, Vlahos J, Kamya MR, Staedke SG, Rosenthal PJ, 2003. Prevention of increasing rates of treatment failure by combining sulfadoxine-pyrimethamine with artesunate or amodiaquine for the sequential treatment of malaria. J Infect Dis 188: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 44. Bukirwa H. et al. , 2006. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLOS Clin Trials 1: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Dokomajilar C, Kamya MR, Rosenthal PJ, 2007. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA 297: 2210–2219. [DOI] [PubMed] [Google Scholar]
- 46. Yeka A. et al. , 2005. Artemisinin versus nonartemisinin combination therapy for uncomplicated malaria: randomized clinical trials from four sites in Uganda. PLOS Med 2: e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeka A. et al. , 2016. Artesunate/amodiaquine versus artemether/lumefantrine for the treatment of uncomplicated malaria in Uganda: a randomized trial. J Infect Dis 213: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arinaitwe E. et al. , 2009. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis 49: 1629–1637. [DOI] [PubMed] [Google Scholar]
- 49. Kamya MR. et al. , 2007. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLOS Clin Trials 2: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yeka A, Dorsey G, Kamya MR, Talisuna A, Lugemwa M, Rwakimari JB, Staedke SG, Rosenthal PJ, Wabwire-Mangen F, Bukirwa H, 2008. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLOS ONE 3: e2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yeka A. et al. , 2018. Comparative efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria in Ugandan children. J Infect Dis 219: 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bigira V. et al. , 2014. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLOS Med 11: e1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, Brooker SJ, Staedke SG, Kamya MR, 2014. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 58: 1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kajubi R. et al. , 2019. Monthly sulfadoxine-pyrimethamine versus dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a randomized controlled trial. Lancet 393: 1428–1439. [DOI] [PubMed] [Google Scholar]
- 55. Kakuru A. et al. , 2016. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 374: 928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Asua V. et al. , 2021. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis 223: 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Asua V, Vinden J, Conrad MD, Legac J, Kigozi SP, Kamya MR, Dorsey G, Nsobya SL, Rosenthal PJ, 2019. Changing molecular markers of antimalarial drug sensitivity across Uganda. Antimicrob Agents Chemother 63: e01818–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dorsey G, Kamya MR, Singh A, Rosenthal PJ, 2001. Polymorphisms in the Plasmodium falciparum pfcrt and pfmdr-1 genes and clinical response to chloroquine in Kampala, Uganda. J Infect Dis 183: 1417–1420. [DOI] [PubMed] [Google Scholar]
- 59. Mbogo GW. et al. , 2014. Temporal changes in prevalence of molecular markers mediating antimalarial drug resistance in a high malaria transmission setting in Uganda. Am J Trop Med Hyg 91: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tumwebaze P. et al. , 2017. Changing antimalarial drug resistance patterns identified by surveillance at three sites in Uganda. J Infect Dis 215: 631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Francis D, Nsobya SL, Talisuna A, Yeka A, Kamya MR, Machekano R, Dokomajilar C, Rosenthal PJ, Dorsey G, 2006. Geographic differences in antimalarial drug efficacy in Uganda are explained by differences in endemicity and not by known molecular markers of drug resistance. J Infect Dis 193: 978–986. [DOI] [PubMed] [Google Scholar]
- 62. Nawaz F, Nsobya SL, Kiggundu M, Joloba M, Rosenthal PJ, 2009. Selection of parasites with diminished drug susceptibility by amodiaquine-containing antimalarial regimens in Uganda. J Infect Dis 200: 1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ, 2007. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob Agents Chemother 51: 3023–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taylor AR, Flegg JA, Holmes CC, Guerin PJ, Sibley CH, Conrad MD, Dorsey G, Rosenthal PJ, 2017. Artemether-lumefantrine and dihydroartemisinin-piperaquine exert inverse selective pressure on Plasmodium falciparum drug sensitivity-associated haplotypes in Uganda. Open Forum Infect Dis 4: ofw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dokomajilar C, Lankoande ZM, Dorsey G, Zongo I, Ouedraogo JB, Rosenthal PJ, 2006. Roles of specific Plasmodium falciparum mutations in resistance to amodiaquine and sulfadoxine-pyrimethamine in Burkina Faso. Am J Trop Med Hyg 75: 162–165. [PubMed] [Google Scholar]
- 66. Rasmussen SA, Ceja FG, Conrad MD, Tumwebaze PK, Byaruhanga O, Katairo T, Nsobya SL, Rosenthal PJ, Cooper RA, 2017. Changing antimalarial drug sensitivities in Uganda. Antimicrob Agents Chemother 61: e01516–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tumwebaze PK. et al. , 2021. Drug susceptibility of Plasmodium falciparum in Eastern Uganda: a longitudinal phenotypic and genotypic study. Lancet Microbe 2: e441–e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rosenthal PJ, 2021. Are artemisinin-based combination therapies for malaria beginning to fail in Africa? Am J Trop Med Hyg 105: 857–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Group WKG-PS , 2019. Association of mutations in the Plasmodium falciparum Kelch13 gene (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments-a WWARN individual patient data meta-analysis. BMC Med 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Menard D. et al. , 2016. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 374: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Conrad MD, Bigira V, Kapisi J, Muhindo M, Kamya MR, Havlir DV, Dorsey G, Rosenthal PJ, 2014. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PLOS ONE 9: e105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cooper RA, Conrad MD, Watson QD, Huezo SJ, Ninsiima H, Tumwebaze P, Nsobya SL, Rosenthal PJ, 2015. Lack of artemisinin resistance in Plasmodium falciparum in Uganda based on parasitological and molecular assays. Antimicrob Agents Chemother 59: 5061–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Taylor SM. et al. , 2015. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in Sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis 211: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ikeda M. et al. , 2018. Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis 24: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bergmann C. et al. , 2021. Increase in kelch 13 polymorphisms in Plasmodium falciparum, Southern Rwanda. Emerg Infect Dis 27: 294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Uwimana A. et al. , 2020. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 26: 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Uwimana A. et al. , 2021. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 21: 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Balikagala B. et al. , 2021. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 385: 1163–1171. [DOI] [PubMed] [Google Scholar]
- 79. Nsobya SL, Kiggundu M, Nanyunja S, Joloba M, Greenhouse B, Rosenthal PJ, 2010. In vitro sensitivities of Plasmodium falciparum to different antimalarial drugs in Uganda. Antimicrob Agents Chemother 54: 1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tumwebaze P. et al. , 2015. Impact of antimalarial treatment and chemoprevention on the drug sensitivity of malaria parasites isolated from Ugandan children. Antimicrob Agents Chemother 59: 3018–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle K, Moyes C, Henry A, Eckhoff P, 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Uganda National Malaria Control Division (NMCD) UBoSUaI , 2019. 2018–2019 Uganda Malaria Indicator Survey Atlas of Key Indicators. Kampala, Uganda, and Rockville, MD: NMCD, UBOS, and ICF. [Google Scholar]
- 83. Katureebe A, Zinszer K, Arinaitwe E, Rek J, Kakande E, Charland K, Kigozi R, Kilama M, Nankabirwa J, Yeka A, 2016. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLOS Med 13: e1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Martinez‐Torres D, Chandre F, Williamson M, Darriet F, Bergé JB, Devonshire AL, Guillet P, Pasteur N, Pauron D, 1998. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae ss. Insect Mol Biol 7: 179–184. [DOI] [PubMed] [Google Scholar]
- 85. Ranson H, Jensen B, Vulule J, Wang X, Hemingway J, Collins F, 2000. Identification of a point mutation in the voltage‐gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol 9: 491–497. [DOI] [PubMed] [Google Scholar]
- 86. Ranson H, N’guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V, 2011. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27: 91–98. [DOI] [PubMed] [Google Scholar]
- 87. Staedke SG, Kamya MR, Dorsey G, Maiteki-Sebuguzi C, Gonahasa S, Yeka A, Lynd A, Opigo J, Hemingway J, Donnelly MJ, 2019. LLIN Evaluation in Uganda Project (LLINEUP)–impact of long-lasting insecticidal nets with, and without, piperonyl butoxide on malaria indicators in Uganda: study protocol for a cluster-randomised trial. Trials 20: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. N’Guessan R, Odjo A, Ngufor C, Malone D, Rowland M, 2016. A chlorfenapyr mixture net interceptor® G2 shows high efficacy and wash durability against resistant mosquitoes in West Africa. PLOS ONE 11: e0165925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koffi AA, Alou LPA, Djenontin A, Kabran J-PK, Dosso Y, Kone A, Moiroux N, Pennetier C, 2015. Efficacy of Olyset® Duo, a permethrin and pyriproxyfen mixture net against wild pyrethroid-resistant Anopheles gambiae ss from Côte d’Ivoire: an experimental hut trial. Parasite 22: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mawejje HD, Kilama M, Kigozi SP, Musiime AK, Kamya M, Lines J, Lindsay SW, Smith D, Dorsey G, Donnelly MJ, 2021. Impact of seasonality and malaria control interventions on Anopheles density and species composition from three areas of Uganda with differing malaria endemicity. Malar J 20: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, Kamya MR, Conrad MD, Dorsey G, Akol AM, 2019. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar J 18: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Isaacs AT, Mawejje HD, Tomlinson S, Rigden DJ, Donnelly MJ, 2018. Genome-wide transcriptional analyses in Anopheles mosquitoes reveal an unexpected association between salivary gland gene expression and insecticide resistance. BMC Genomics 19: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weetman D, Steen K, Rippon EJ, Mawejje HD, Donnelly MJ, Wilding CS, 2014. Contemporary gene flow between wild An. gambiae ss and An. arabiensis . Parasit Vectors 7: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lynd A, Gonahasa S, Staedke SG, Oruni A, Maiteki-Sebuguzi C, Dorsey G, Opigo J, Yeka A, Katureebe A, Kyohere M, 2019. LLIN Evaluation in Uganda Project (LLINEUP): a cross-sectional survey of species diversity and insecticide resistance in 48 districts of Uganda. Parasit Vectors 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Quiñones ML, Norris DE, Conn JE, Moreno M, Burkot TR, Bugoro H, Keven JB, Cooper R, Yan G, Rosas A, 2015. Insecticide resistance in areas under investigation by the International Centers of Excellence for Malaria Research: a challenge for malaria control and elimination. Am J Trop Med Hyg 93: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Consortium AgG, Consortium A, 2017. Genetic diversity of the African malaria vector Anopheles gambiae . Nature 552: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Weetman D, Wilding CS, Neafsey DE, Müller P, Ochomo E, Isaacs AT, Steen K, Rippon EJ, Morgan JC, Mawejje HD, 2018. Candidate-gene based GWAS identifies reproducible DNA markers for metabolic pyrethroid resistance from standing genetic variation in East African Anopheles gambiae . Sci Rep 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]