Abstract
Background & Aims:
While cholangiocarcinomas (CCAs) commonly express programmed cell death 1 (PD-1) and its ligand (PD-L1), they respond poorly to immune checkpoint inhibitors (ICIs). We aimed to determine whether stimulating antigen-presenting cells, including macrophages and dendritic cells, using a CD40 agonist could improve this response.
Methods:
We compared treatment responses in subcutaneous, orthotopic, and 2 plasmid-based murine intrahepatic CCA (iCCA) models. Mice were treated for 4 weeks with weekly IgG control, a CD40 agonistic antibody, anti-PD-1, or the combination of both (anti-CD40/PD-1). Flow cytometric (FACS) analysis of lymphocytes and myeloid cell populations (including activation status) was performed. We used dendritic cell knockout mice, and macrophage, CD4+ and CD8+ T cell depletion models to identify effector cells. Anti-CD40/PD-1 was combined with chemotherapy (gemcitabine/cisplatin) to test for improved therapeutic efficacy.
Results:
In all 4 models, anti-PD-1 alone was minimally efficacious. Mice exhibited a moderate response to CD40 agonist monotherapy. Combination anti-CD40/PD-1 therapy led to a significantly greater reduction in tumor burden. FACS demonstrated increased number and activation of CD4+ and CD8+ T cells, natural killer cells, and myeloid cells in tumor and non-tumor liver tissue of tumor-bearing mice treated with anti-CD40/PD-1. Depletion of macrophages, dendritic cells, CD4+ T cells, or CD8+ T cells abrogated treatment efficacy. Combining anti-CD40/PD-1 with gemcitabine/cisplatin resulted in a significant survival benefit compared to gemcitabine/cisplatin alone.
Conclusion:
CD40-mediated activation of macrophages and dendritic cells in iCCA significantly enhances response to anti-PD-1 therapy. This regimen may enhance the efficacy of first-line chemotherapy.
Lay summary:
Checkpoint inhibition, a common form of immune therapy, is generally ineffective for the treatment of cholangiocarcinoma. These tumors suppress the infiltration and function of surrounding immune cells. Stimulating immune cells such as macrophages and dendritic cells via the CD40 receptor activates downstream immune cells and enhances the response to checkpoint inhibitors.
Keywords: Cholangiocarcinoma, Liver cancer, Tumor-infiltrating lymphocyte, T cell, NK cell, Antigen-presenting cell, Macrophage, Dendritic cell, Immunotherapy, Immune checkpoint, CD40 agonist
Introduction
Cholangiocarcinoma (CCA), comprising both intrahepatic cholangiocarcinoma (iCCA) and extrahepatic CCA, represents the second most common primary liver malignancy accounting for approximately 10% of such cancers.1-3 CCA carries a dismal prognosis with a 5-year overall survival (OS) rate of 10% and a median OS of approximately 24 months. While surgical resection offers the only chance for a cure, patients typically present with advanced disease and fewer than 20% are deemed to have resectable tumors at the time of diagnosis. Surgery only offers long-term benefit to an even smaller subset of patients as recurrence is reported in up to 70% of those having undergone a complete resection. Therefore, nearly every patient presenting with CCA will require effective systemic therapy during their disease course.1,4 Current first-line therapy consists of multi-agent cytotoxic chemotherapy with gemcitabine and cisplatin (Gem/Cis), but the efficacy of this approach remains very limited.5,6 There are currently no other approved therapies for unresectable CCA. In addition to its poor prognosis, its incidence has recently been rising, further emphasizing the need for effective systemic therapies.1,7
Pre-clinical data on the role of immunotherapy for the treatment of CCA is scarce.8 Following some encouraging responses to immune checkpoint inhibitors (ICIs) in patients with hepatocellular carcinoma, similar approaches are currently being investigated for biliary tract cancers. Clinical trials involving small molecules and ICIs are currently underway.8-10 Studies examining the immune tumor microenvironment of patients with CCA suggest that this cancer can evade and even impair effective immunosurveillance. One mechanism by which this evasion occurs is through high expression of programmed cell death ligand 1 (PD-L1) on tumor cells and tumor-associated macrophages. A recent study reported that over 60% of patients with CCA had high amounts of PD-L1 expressed in their tumors and that high expression was associated with decreased OS.11,12 In contrast programmed cell death 1 (PD-1) expression does not appear to correlate with recurrence-free survival.13 An in vitro study examining human CCA samples demonstrated that treatment with anti-PD-1 and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) increased the cytotoxic potential of effector cells including natural killer (NK) cells and T cells.14 However, the success rate for treatment of CCA with ICIs remains disappointing.9,10,15,16 The immune suppressive microenvironment thought to be associated with CCA may contribute to the poor response to ICIs.
Few pre-clinical models of CCA have been designed to test novel immune-based treatment strategies and little remains known about the interplay between the microenvironment and immunotherapeutics in CCA. A study evaluating the different subtypes of CCA found that the immunogenic subtype was associated with better response to ICI suggesting that antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs) may contribute to T cell priming and activation.17 In CCA, the tumor microenvironment can impede effector T cell proliferation and activation.18 As demonstrated in several pre-clinical models, the crosstalk between APCs and effector cells is essential to overcoming the immune suppression fostered by such tumors.19-22
The interaction between CD40, a receptor on macrophages and DCs, and its ligand CD154 (CD40 Ligand or CD40L) drives activation and downstream priming of effector CD8+ T cells.23-26 Agonistic anti-CD40 combination therapies have shown promising results in pancreatic adenocarcinoma.25,27 We hypothesized that combining a CD40 agonist with a PD-1 antagonist could overcome the immunosuppressive barriers present in the tumor microenvironment. Using subcutaneous, orthotopic, and oncogene induced spontaneous iCCA models, we demonstrate that CD40 agonists enhance response to ICIs while treatment with anti-PD-1 alone did not provide meaningful anti-tumor benefits. When combined with Gem/Cis, this regimen offered a significant OS benefit compared to Gem/Cis alone. To our knowledge, this is the first study testing combination immune therapy for murine CCA.
Materials and methods
Mouse strains and reagents
C57BL/6 were purchased from The Charles River Laboratory. Baf3 knockout mice were purchased from Jackson Laboratory. SB1 cells were generated by the Gores laboratory (Mayo Clinic) and kindly provided by the Yarchoan laboratory (John’s Hopkins School of Medicine).28 SB1 was screened for mycoplasma contamination and confirmed to be CCA based on immunohistochemistry (IHC) (Fig. S1). For in vivo studies, the following antibodies were used: agonistic anti-CD40 (clone FGK4.5), anti-PD-1 (clone 29F.1A12), anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43) (BioxCell). Gemcitabine and cisplatin were obtained from the NIH veterinary pharmacy. All animals received humane care and experiments were conducted according to local institution guidelines and approved by the Animal Care and Use Committee of the National Institutes of Health, Bethesda, USA.
Subcutaneous tumor injection model
Subcutaneous injections were performed in 8-week-old C57BL/6 female mice. 1x106 SB1 CCA cells were suspended in 100 μl PBS and the solution was injected in the left flank. Mice were randomized prior to treatment initiation and were treated with either bi-weekly IgG control (alternating 10 ug/g and 5 ug/g) (days 3, 5, 10, 12, 17, 19, 24, 26 post tumor cell injection) (n = 10), weekly anti-PD-1 (10ug/g) (days 5, 12, 19, 26 post tumor cell injection) (n = 10), weekly anti-CD40 (5ug/g) (days 3, 10, 17, 24 post tumor cell injection) (n = 10), or the combination anti-CD40+anti-PD-1 (n = 10) as above. Tumor size was measured by caliper as greatest diameter. Mice were sacrificed when tumors reached 20 mm in diameter or tumor necrosis exceeded 50%.29 Mouse survival was calculated as time from inoculation to euthanasia. Measurements were performed by a blinded observer.
Intrahepatic tumor injection model
To induce liver tumors, 3 x 105 SB1 CCA cells mixed in 20 μl of a 50:50 solution of PBS and Matrigel were injected under the liver capsule into the left liver lobe of anesthetized recipient mice after subcostal laparotomy as described before.30,31 Mice were then treated with either bi-weekly IgG control (10μg/g) (days 3, 5, 10, 12, 17, 19, 24, 26 post orthotopic tumor cell injection) (n = 10), weekly anti-PD-1 (10μg/g) (days 5, 12, 19, 26 post orthotopic tumor cell injection) (n = 10), weekly anti-CD40 (5μg/g) (days 3, 10, 17, 24 post orthotopic tumor cell injection) (n = 10), or combination anti-CD40+anti-PD-1 (n = 10) with the treatment schedule as above. Mice were then euthanized at day 28. Mice were weighed and weights were compared between groups to evaluate treatment-associated adverse effects. Livers were weighed and compared between groups. Tumors were then carefully excised and weighed. Tumor to liver weight ratios were calculated and compared. Measurements were performed by a blinded observer. For the isolation of tumor-infiltrating lymphocytes (TILs), a different treatment regimen was used, as the aforementioned treatment regimen resulted in minimal tumor burden in the combination treatment group, making it impossible to isolate TILs. For this experiment, mice underwent orthotopic injection as above and were treated with either 2 doses of IgG2 control (days 11 and 13), 1 dose of CD40 agonist (day 11), 1 dose of anti-PD-1 (day 13), or both; TILs were harvested on day 15.
Statistical analysis
The sample sizes used for these animal studies were guided by a previous study in our laboratory in which similar or identical liver tumor models were used. For all models, randomization and blinding were performed. Statistical analysis was performed with GraphPad Prism 8 (GraphPad Software). Significance of the difference between groups was calculated by Student’s unpaired t test, one-way ANOVA (Tukey’s and Bonferroni’s multiple comparison test) or log-rank test. p<0.05 was considered as statistically significant.
For further details regarding the materials and methods used, please refer to the CTAT table and supplementary information.
Results
Murine iCCA has poor response to anti-PD-1 monotherapy
SB1 tumor cells, a murine iCCA tumor line,28 displayed the typical CCA growth pattern and immunohistopathological features, including cytokeratin (CK)-19, PD-1 and PD-L1 positivity on IHC (Fig. 1A,B), as well as protein overexpression of YAP, CK-7, and CK-17 (Fig. S1A-E). The therapeutic efficacy of anti-PD-1 monotherapy for iCCA was then tested in mice bearing subcutaneous SB1 tumors. Compared to control IgG, treatment with anti-PD-1 monotherapy had no effect on tumor growth (p = 0.2162) (Fig. 1C) or survival (p = 0.3454) (Fig. 1D). These results are consistent with the clinical observation that anti-PD-1 monotherapy is not effective in patients with CCA.9,10,15,16
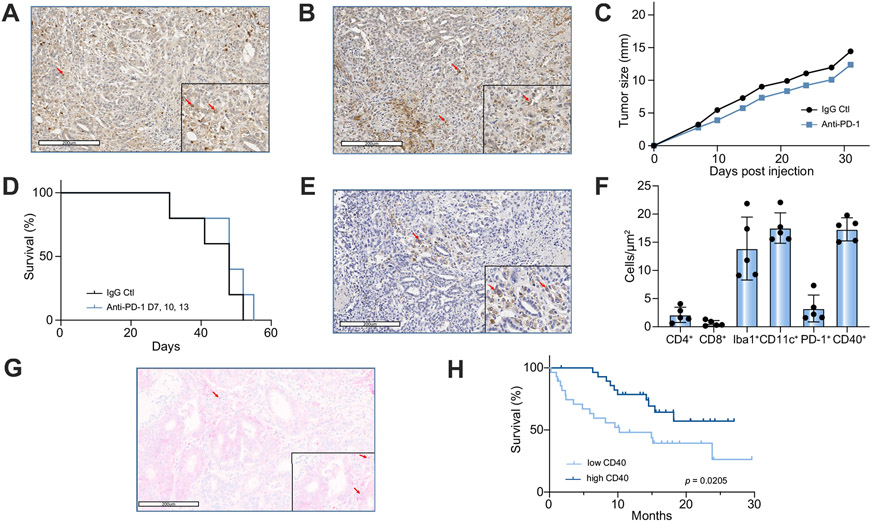
Fig. 1. PD-1 and CD40 in murine and human CCA.
IHC staining for (A) PD-1 and (B) PD-L1 (arrow) in tumor and adjacent liver of SB1 orthotopic CCA tumor-bearing mouse. (C) Tumor growth and (D) Kaplan-Meier curves for subcutaneous SB1 CCA tumor-bearing C57BL/6 mice treated with anti-PD-1 (n = 5 per group). IHC stain for CD40 (arrow) in (E) murine orthotopic CCA. (F) Halo quantification of IHC staining for CD4, CD8, Iba1 (macrophages), CD11c (DCs), PD-1, and CD40 in murine orthotopic CCA samples (n = 5). (G) IHC stain for CD40 (arrow) in human CCA. (H) Kaplan-Meier curves of patients with high (n = 29) vs. low (n = 28) expression of CD40 in their CCA tumor samples. Log-rank (Mantel-Cox) test. CCA, cholangiocarcinoma; IHC, immunohistochemistry; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Given that combining anti-PD-1 with other therapies presents an effective treatment option for several types of cancers, we queried whether this strategy applied to iCCA.25,32 Insights into the tumor microenvironment of iCCA can guide the design of new immunotherapeutic strategies. As such, we performed IHC analysis for various immune markers in SB1 tumors. As expected, there were few CD4+ T and CD8+ T cells surrounding or infiltrating the tumor (Fig. S1F,G). Interestingly, prominent tumor and peri-tumoral infiltration of CD11c+ DCs and Iba1+ macrophages was observed (Fig. S1H,I). Accompanying the presence of these APCs, a significant amount of CD40+ non-tumor cells were found in SB1 tumors (Fig. 1E+F). Using a tissue microarray, CD40+ was also detected in patient tumors (Fig. 1G). Interestingly, high tumor CD40 expression was associated with improved OS in a cohort of patients with CCA (n = 49) (p = 0.0205) (Fig. 1H) and multivariate analysis revealed that CD40 was an independent prognostic marker (p = 0.0152) (Table 1). The presence of CD40+ immune cells suggests that CD40-mediated activation of APCs can potentially enhance response to other immune therapeutics and may be used to treat iCCA.
Table 1.
Multivariate analysis for clinical parameters in iCCA cohort.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Hazard ratio* | p value** | Hazard ratio* | p value** | |
| Age | 0.98997 (0.9557–1.025) | 0.575 | 0.9794 (0.9421–1.018) | 0.2917 |
| Sex | ||||
| Female | 1.265e+07 (0–Inf) | 0.996 | 1.4802 (0.7140–3.069) | 0.2917 |
| Male | 8.148e+06 (0–Inf) | 0.996 | n.a. | n.a. |
| Albumin | 0.8299 (0.4837–1.424) | 0.499 | 0.5502 (0.2826–1.071) | 0.0788 |
| ALT | 0.995309 (0.9885–1.002) | 0.18 | 0.992 (0.9832–1.001) | 0.076 |
| Bilirubin | 0.98898 (0.9228–1.06) | 0.754 | 1.0072 (0.9430–1.076) | 0.8311 |
| INR | 0.7651 (0.3556–1.646) | 0.494 | 0.7979 (0.3207–1.985) | 0.6273 |
| CD40 low vs. high | 2.7052 (1.811–6.1961) | 0.0186* | 3.0275 (1.2377–7.405) | 0.0152* |
Hazard ratios were determined using Cox proportional regression.
p value was determined using the log-rank test.
ALT, alanine aminotransferase; INR, international normalized ratio.
Combined treatment with CD40 agonist and PD-1 antagonist effectively impairs murine iCCA growth
Next, we tested the effect of CD40 activation using an agonistic anti-CD40 antibody (FGK4.5) alone or in combination with anti-PD-1 in subcutaneous SB1 iCCA tumor model.23,25,33 Mice were treated with 4 doses of each agent between days 3 and 26 post tumor cell injection and tumors were monitored until mice reached the threshold for euthanasia (Fig. 2A). Anti-PD-1 monotherapy did not show any effect on tumor growth (p = 0.4495) or mouse survival (p = 0.3034) (Fig. 2B,C). Agonist anti-CD40 monotherapy resulted in a moderate reduction in SB1 tumor growth (p = 0.0007) (Fig. 2B). However, combination treatment with anti-CD40/PD-1 showed a significantly stronger anti-tumor effect than anti-CD40 alone (IgG Ctl vs. anti-CD40/PD-1 p<0.0001, and anti-CD40 vs. anti-CD40/PD-1 p = 0.0108) (Fig. 2B). Similarly, anti-CD40 agonist therapy improved survival (p<0.0001) but combination therapy provided an enhanced protection and resulted in long-term survivors (IgG Ctl vs. anti-CD40/PD-1 p<0.0001, and anti-CD40 vs. anti-CD40/PD-1 p = 0.00636) (Fig. 2C).
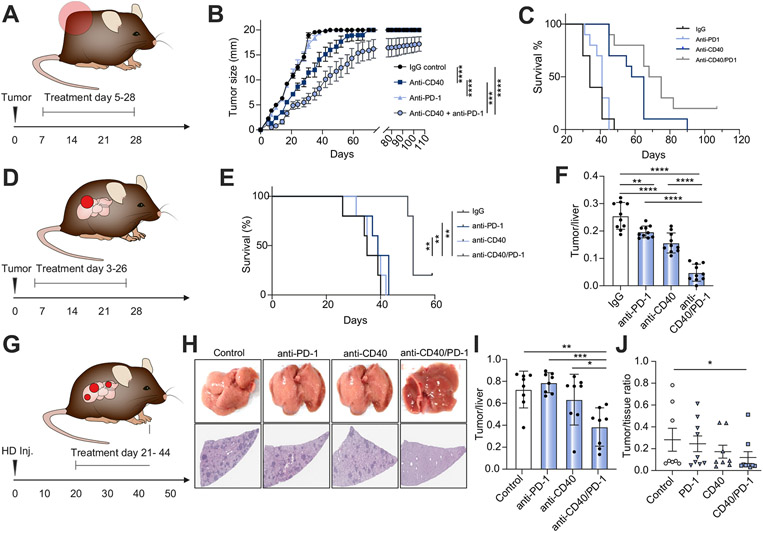
Fig. 2. Response to anti-PD-1, anti-CD40, and anti-CD40+anti-PD-1 (anti-CD40/PD-1) in 4 different intrahepatic CCA mouse models.
(A) Experimental set-up for subcutaneous tumor model (B) Flank tumor measurements over time (days) for subcutaneous SB1 CCA tumor-bearing C57BL/6 mice (n = 10 per group, mice with tumors >20 mm were euthanized, but continued to be reported as 20 mm) (C) corresponding survival analysis (n = 10 per group). (D) Experimental set-up for orthotopic tumor model using SB1 tumor cells. (E) Survival analysis (n = 5 per group, one animal in the combination group was censored at d59) and (F) analysis of tumor to liver ratios 28 days post tumor cell injection (n = 10 per group). (G) Experimental set-up for AKT-YAP and AKT-NOTCH induced tumor model (H) Representative livers (top) and H&E stains (bottom) of livers from YAP+AKT HD inj. CCA tumor-bearing C57BL/6 mice (n = 8 per group) (I) and analysis of tumor to liver ratios 49 days post plasmid injection (n = 8 per group). (J) Analysis of tumor to tissue ratios in AKT-NICD injected mice (n = 8-9 per group). (one-way ANOVA for F & I, student’s t test for J and Log-rank (Mantel-Cox) test for C&E. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). CCA, cholangiocarcinoma; HD, hydrodynamic; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
To better mimic the pathophysiology of human iCCA, SB1 tumor cells were orthotopically injected into mouse livers. Mice were treated with 4 doses of either CD40 agonist, anti-PD-1, or both between days 3 and 26 post tumor cell injection; mice were sacrificed on day 28. Anti-PD-1 monotherapy resulted in a small reduction in tumor burden (p = 0.0045) (Fig. 2D-F). Similar to the findings in subcutaneous tumors, CD40 agonist monotherapy inhibited SB1 tumor growth (p<0.0001), but a combination of anti-CD40/PD-1 offered a stronger anti-tumor effect than either monotherapy (p<0.0001) (Fig. 2E-F). In the same experimental setting, combination therapy with anti-PD-1 and anti-CD40 also significantly prolonged survival (p<0.01 compared to all other groups, Fig. 2E). To further confirm our finding, we delivered plasmids coding for AKT and YAP into mouse livers by hydrodynamic tail vein injection, a well-established murine iCCA model.34 Tumors were detectable 3 weeks post plasmid injection (Fig. S2B,C) and treatment was administered as above between days 21–44, following which mice were sacrificed on day 49 (Fig. 2G). Consistently, combination therapy of anti-CD40/PD-1 resulted in a significant reduction of tumor burden of primary iCCA compared to control (p = 0.0026) or monotherapy with anti-CD40 (p = 0.0363) or anti-PD-1 (p = 0.0004). Anti-PD-1 or anti-CD40 monotherapy did not offer any significant benefit (Fig. 2H-I, Fig. S2D). Finally, we decided to confirm the results of anti-CD40/PD-1 in a second genetically induced tumor model. Plasmids encoding for AKT and NICD (an activated form of NOTCH) were injected by hydrodynamic tail vein injection into mouse livers35 and mice were treated with anti-PD-1 monotherapy, anti-CD40 monotherapy of the combination of anti-CD40/PD-1. Again, combination therapy with anti-CD40/PD-1 provided the strongest anti-tumor effect when histological sections from treated livers were compared to mice, which had received IgG control (p<0.05, Fig. 2J). In summary, using 4 mouse CCA tumor models, our results consistently demonstrated that anti-PD-1 combined with CD40 agonist presents an effective treatment against iCCA.
Double therapy causes local immune cell infiltration, proliferation, and activation
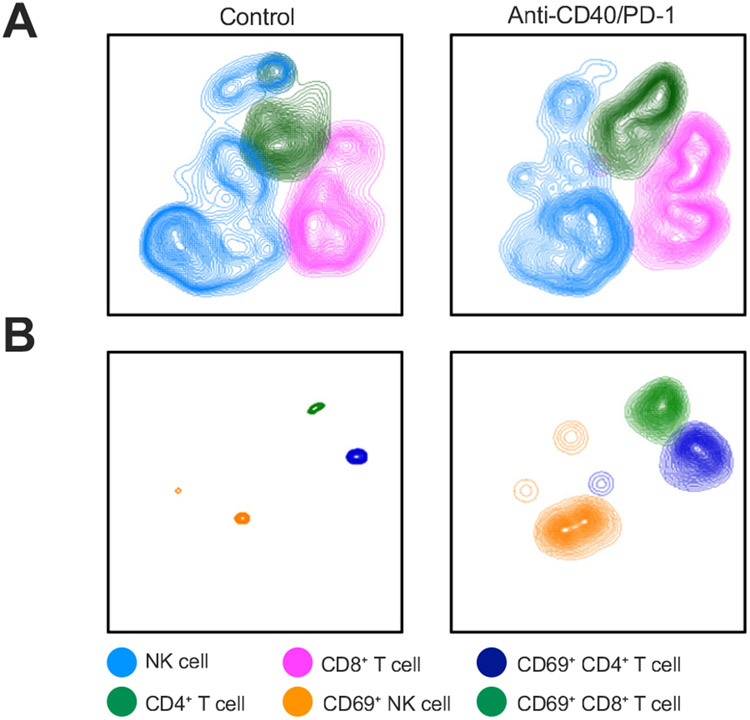
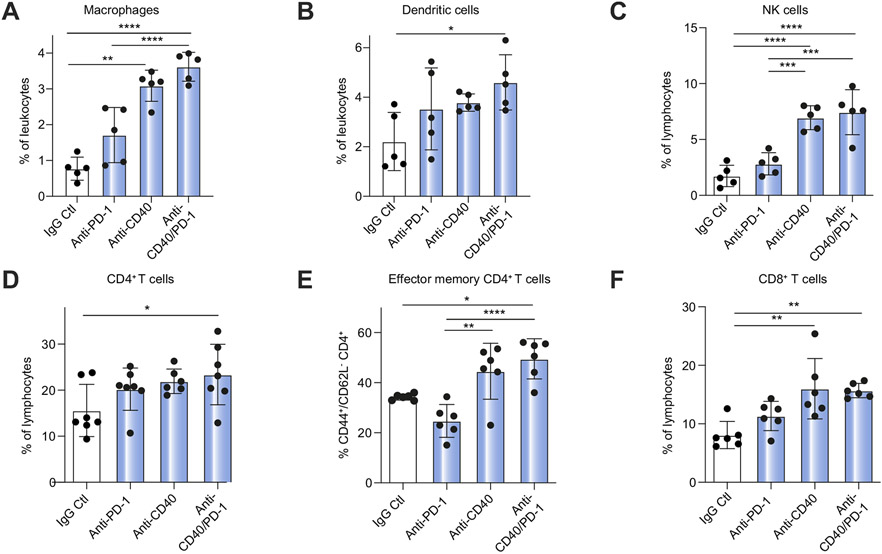
Next, we studied the effect of anti-PD-1 and/or anti-CD40 treatment on immune cells in iCCA to better understand the underlying immunological mechanism resulting in inhibition of tumor growth. For the examination of TILs, we performed flow cytometry analysis to determine the frequency and function of CD4+ and CD8+ T cells, NK cells, macrophages and DCs. t-distributed stochastic neighbor embedding analysis indicated a minor increase in CD4+, CD8+ and NK cell numbers (Fig. 3A) after combination therapy and more pronounced changes in CD4+, CD8+ and NK cell activation (Fig. 3B). Agonistic anti-CD40 monotherapy (single agent or in combination with anti-PD-1) showed the greatest increase in tumor-infiltrating macrophages, NK cells, CD44+CD62L+CD4+ effector memory and CD8+ T cells, with significant changes in DCs after anti-CD40/PD-1 treatment (Fig. 4A-F), which was not seen in immune cells of the spleen, a surrogate for systemic responses (Fig. S3).
Fig. 3. Flowcytometry analysis of immune cell populations in TILs from orthotopic SB1 CCA tumor-bearing mice treated with anti-PD-1, anti-CD40, or anti-CD40+anti-PD-1 (anti-CD40/PD-1).
(A) t-SNE plots comparing CD4+ T, CD8+ T, and NK (CD3−/NK1.1+) cell frequency in control vs. anti-CD40/PD-1. (B) t-SNE plots comparing frequency of CD4+ T, CD8+ T, and NK (CD3−/NK1.1+) cells positive for CD69 in control vs. anti-CD40/PD-1. (n = 4 for IgG control group and n = 5 per group for other groups). NK, natural killer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; TILs, tumor-infiltrating lymphocytes; t-SNE, t-distributed stochastic neighbor embedding.
Fig. 4. Flow cytometry analysis of immune cell populations among hepatic lymphocytes of tumor-bearing mice treated with anti-PD-1, anti-CD40, or anti-CD40/PD-1.
Frequency of (A) macrophages (CD11b+/F4/80+), (B) DCs (CD11C+), (C) NK cells, (D) CD4+ T cells, (E) effector memory (CD44+/CD62L−/CD4+) T cells, and (F) CD8+ T cells (n = 6 per group for CD4, effector memory, and CD8 cell analysis. n = 5 per group for macrophage, DC and NK analysis). (one-way ANOVA, *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). DC, dendritic cell; NK, natural killer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
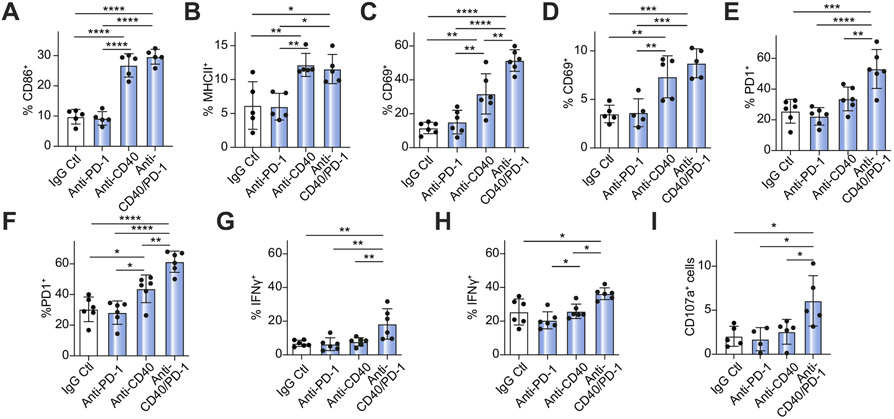
Next, activation markers (CD69, interferon (IFN)-γ, and PD-1) on lymphoid cells and myeloid cells (CD86 and MHCII) were evaluated. Anti-PD-1 monotherapy did not change the frequency of activated CD4+ and CD8+ T cells, NK cells, macrophages and DCs (Fig. 5A-H). On the other hand, CD40 activation and combination therapy resulted in a significant but equivalent increase in the frequency of CD86+ macrophages, MHCII+ DCs, and CD69+ NK cells (Fig. 5A-D). Interestingly, an increase in the frequency of CD69+CD8+ T cells, PD-1+CD4+ and CD8+ T cells as well as IFN-γ+ in CD4+ T and CD8+ T cells was seen after combination treatment with anti-CD40/PD-1 (Fig. 5C-H), which were not observed in spleen (data not shown). Tumor-specific cytotoxicity was tested after in vitro stimulation of CD8+ T cells from SB1-tumor-bearing mice using SB1 tumor cells and analysis of lysosomal-associated membrane protein-1 (CD107) surface expression. Only CD8+ T cells from anti-CD40/PD-1-treated mice revealed an increased CD107 signal, which was not seen with single agent treatment (Fig. 5I). Finally, changes in CD4+ T cell subsets (Th1, Th2, Th17, and Tregs) were examined, but no specific changes were seen after combination therapy with anti-CD40/PD-1 (Fig. S4).
Fig. 5. Flow cytometry analysis of immune cell activation among hepatic lymphocytes of tumor-bearing mice treated with anti-PD-1, anti-CD40, or anti-CD40/PD-1.
Frequency of (A) CD86+ macrophages (CD11b+/F4/80+), (B) MHCII+ DCs (CD11C+), (C) CD69+ CD8+ T cells (D) CD69+ NK (CD3−/NK1.1+) cells (E) PD-1+ CD8+ T cells (F) PD-1+ CD4+ T cells (G) IFN-γ+CD8+ T cells and (H) IFN-γ+CD4+ T cells (n = 5-7 animals per group) (I) CD107+CD8+ T cells after in vitro stimulation with tumor cells in response to anti-PD-1, anti-CD40 and anti-CD40/PD-1 treatment (n = 5 per group). (one-way ANOVA, *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). DC, dendritic cell; IFN, interferon; NK, natural killer; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
The efficacy of anti-CD40/PD-1 therapy in iCCA depends on DCs, macrophages, CD4+ T and CD8+ T cells
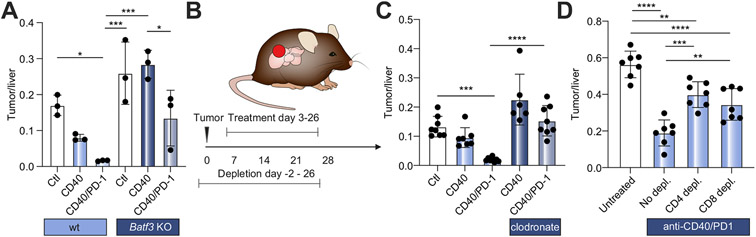
Next, we studied the effect of DCs and macrophages, using Batf3 knockout mice and clodronate liposome depletion, on the effectiveness of anti-CD40/PD-1 treatment using orthotopic SB1 tumor-bearing mice as described previously. In Batf3 knockout mice, the effect of anti-CD40 treatment was completely absent and markedly reduced after combination therapy with anti-CD40/PD-1 (Fig. 6A). Compared to control liposome, clodronate liposome completely abrogated the tumor reduction caused by combination therapy (p<0.001) (Fig. 6B,C). These results are consistent with our finding that anti-PD-1 monotherapy is ineffective against iCCA and that activating CD40 signaling in myeloid cells can boost the efficacy of T cell-dependent therapy. Next, antibody-mediated cell depletion of CD4+ T or CD8+ T cells was performed, and its impact on combination therapy in orthotopic SB1 tumor-bearing mice was tested. As expected, control IgG treatment had no effect on the anti-tumor function of anti-CD40/PD-1 therapy (Fig. 6D). In contrast, depleting either CD4+ T or CD8+ T cells significantly abrogated the efficacy of combination therapy and resulted in significantly larger iCCA tumors (p = 0.0001 and p = 0.0039, respectively) (Fig. 6D), suggesting that both CD4+ and CD8+ T cells were necessary for the anti-tumor efficacy of the treatment.
Fig. 6. Effects of dendritic cell, macrophage, CD4 T cell, and CD8 T cell depletion on effectiveness of combination anti-CD40/PD-1 treatment.
(A) SB1 tumor-bearing Batf3 KO and wild-type controls were treated with anti-CD40 or anti-CD40/PD-1 and tumor to liver ratios were determined 28 days post tumor cell injection (n = 3 per group). (B) Experimental set-up for depletion experiments shown in (C) and (D): Orthotopic SB1 tumor-bearing C57BL/6 mice received anti-CD40/PD-1 between day 3 and 26 and were sacrificed on day 28. Cell depletion was started 2 days prior to orthotopic tumor implantation. (C) Tumor to liver ratios for mice after depletion of macrophages (n = 6-8 animals per group) (D) Tumor to liver ratios for mice after depletion of CD4+ and CD8+ T cells (n = 7 per group) vs. IgG control (one-way ANOVA, *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). KO, knockout; PD-1, programmed cell death 1.
Addition of anti-CD40/PD-1 enhances response to chemotherapy in mice with advanced iCCA
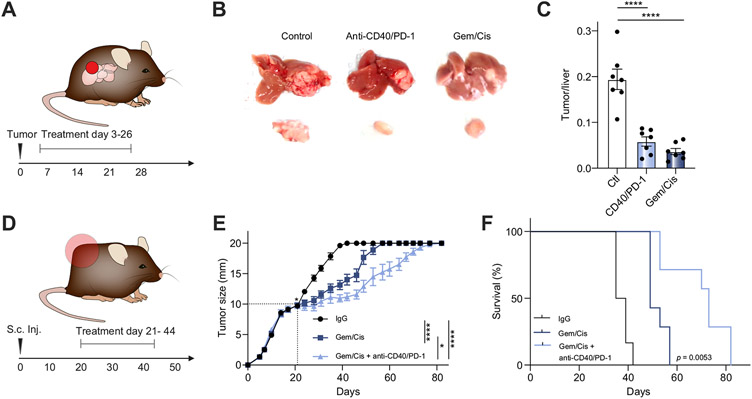
Chemotherapy with Gem/Cis remains the standard care for patients with CCA.5 We first tested whether anti-CD40/PD-1 combination therapy provides better protection than chemotherapy against iCCA. Significantly smaller SB1 orthotopic tumors were found in mice treated with either Gem/Cis or anti-CD40/PD-1 when compared to mice treated with IgG2 control (p<0.0001 for both) (Fig. 7A-C). However, no significant difference in tumor burden was found between the Gem/Cis- and anti-CD40/PD-1-treated mice (Fig. 7A-C). Next, we combined Gem/Cis chemotherapy with immunotherapy to treat mice with advanced iCCA tumors. SB1 subcutaneous tumor-bearing mice with tumors measuring 10 mm at the time of treatment initiation were used (Fig. 7D). Gem/Cis treatment was still able to reduce the tumor growth (p<0.0001) (Fig.7C) and improve OS significantly (p<0.0001) (Fig. 7E+F). However, combination with anti-CD40/PD-1 therapy showed significant improvements in tumor growth inhibition (p = 0.0108) (Fig. 7E) and OS (p = 0.0053) (Fig. 7F) compared to Gem/Cis alone.
Fig. 7. Efficacy of anti-CD40/PD-1 compared to and combined with standard of care chemotherapy (Gem/Cis).
(A) Experimental set-up for B+C. (B) Representative livers (top) and tumors (tumors) for orthotopic SB1 tumor-bearing C57BL/6 mice treated with weekly anti-CD40/PD-1 vs. Gem/Cis (n = 6 per group) and sacrificed 28 days post tumor cell injection. (C)Tumor to liver ratios for mice described in (A). (D) Experimental set-up for E+F (E) Tumor measurement over time for subcutaneous SB1 tumor-bearing mice treated once tumors ≥10 mm with Gem/Cis vs. Gem/Cis + anti-CD40/PD-1 (n = 7 animals per group, mice with tumors >20 mm were euthanized, but continued to be reported as 20 mm). (F) Kaplan-Meier curves comparing survival of mice described in (D). (one-way ANOVA, *p<0.05; ****p<0.0001). Gem/Cis, gemcitabine and cisplatin; PD-1, programmed cell death 1.
Anti-CD40/PD-1 therapy is well tolerated in mice
Tumor-free C57BL/6 mice treated with IgG control, anti-PD-1, anti-CD40, or anti-CD40/PD-1 did not display any significant difference in weight at the time of necropsy (Fig. S6A) and normal alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase levels were observed (Fig. S6B-D); histological analysis of livers, kidney and bone marrow revealed no abnormalities in mice treated with Gem/Cis plus anti-CD40/PD-1 (data not shown). These results suggest that this treatment appears to be well tolerated and does not appear to cause significant hepatotoxicity in mice.
Discussion
Our results are consistent with previous analyses demonstrating that checkpoint blockade confers minimal benefit in patients with CCA, despite positive tumor staining for PD-L1 and PD-1.9-11,15,16 IHC of our murine iCCA samples revealed a high number of macrophages (Iba1+) and DCs (CD11c+), as well as a relatively high expression of CD40. The presence of CD40+ immune cells was also observed in tumors from patients with iCCA. Importantly, high CD40 expression in human iCCA tumors was associated with improved survival. A CD40 signal is often necessary for the induction of effective anti-tumor immune responses, and treatment with a CD40 agonist provided significant benefit in pre-clinical models of breast and pancreatic cancer and an early phase clinical trial of pancreatic adenocarcinoma (PDAC).23,25,26,32,36,37 We hypothesized that priming of APCs by activating the CD40 signal in the tumor microenvironment can enhance downstream activation of effectors cells and sensitize CCA to checkpoint blockade. Indeed, combination treatment with a CD40 agonist and PD-1 antagonist resulted in a significantly lower tumor burden in all 4 of our murine iCCA models compared to the control or monotherapy groups. To our knowledge, this is the first study examining combination immunotherapy in murine models of iCCA and demonstrating the potential benefits of using a CD40 agonist for the treatment of primary liver malignancies.
Using flow cytometric analysis, we examined the changes in both infiltration and activation of myeloid, CD4+ T, CD8+ T, and NK cells in tumors and non-tumor liver tissue. In line with a recent study, our results demonstrated that checkpoint blockade was insufficient for effector cells to infiltrate and attack iCCA tumors.38 CD40 agonism appeared to be the principal driver of myeloid cell proliferation and activation as described before.33,39 However, optimal increases in the frequency of CD4+ T cells as well as activation of CD4+ T and CD8+ T cells could only be achieved with combination therapy. While the role of NK cells appears to be controversial,14,33 our CCA models demonstrate that anti-CD40/PD-1 drives increased frequency and activation of NK cells.
We also identified the immune cells mediating the anti-CCA function of the anti-CD40/PD-1 therapy. As expected, depleting APCs using chlodronate greatly abrogated treatment efficacy. In addition, anti-CD40/PD-1 only slightly reduced iCCA tumor growth in type 1 conventional DC deficient Batf3 knockout mice showing that both DCs and macrophages are needed for a therapeutic response. Further depletion studies showed that removing either CD4+ T and CD8+ T cells significantly impaired the tumor inhibition by anti-CD40/PD-1 therapy, suggesting that both CD4+ T and CD8+ T cells are critical downstream effector cells. Our finding on the importance of CD8+ T cells is in line with many reports that CD8+ T cells are necessary for effective anti-PD-1 therapy. In contrast, the role of CD4+ T cells in tumor immunotherapy is controversial. FOXP3+ CD4+ T regulatory cells are well known to inhibit various anti-tumor responses and depletion of CD4+ T cells has been reported to benefit tumor treatment by removing T regulatory cells.14,33 On the other hand, emerging evidence suggests that CD4+ T cells are also an important component of anti-tumor immunity. In our study, CD4+ T cell depletion abrogated treatment efficacy. This could be explained by the increased activation and proliferation of effector memory CD4+ T cells but not FOXP3+ CD4+ T cells in response to anti-CD40/PD-1. These findings may reflect organ-specific variations in the mechanism of action.33
Treatment with Gem/Cis offers limited benefits in patients with CCA. In our models, Gem/Cis and anti-CD40/PD-1 confer comparable outcomes, suggesting that neither provide sufficient translatable benefits.5 Studies examining CCA’s microenvironment describe tumors that foster immune suppression and hinder innate and adaptive immune surveillance.11,12,14,18,40-46 This is likely to contribute to poor responses to ICI.11,14 Our models’ minimal response to anti-PD-1 further illustrates the need for additional stimulus to enhance immune responses in iCCA. Several studies propose increasing tumor antigen presentation using vaccines or cytotoxic treatments to enhance the efficacy of immune-based therapies.33,47-50 Gem/Cis are cytotoxic agents known to provoke cellular damage and potentially increase tumor antigen presentation.51-54 Interestingly, we show that combining Gem/Cis with anti-CD40/PD-1 offers significant improvement in control of tumor growth and OS even when treatment is initiated at an advanced disease stage. While anti-CD40/PD-1 resulted in mild increases in activation and infiltration of macrophages and DCs, this treatment’s efficacy was dramatically increased when preceded by Gem/Cis. These agents may result in indirect priming of macrophages and DCs by increasing tumor antigen presentation, which, in turn, lead to better responses to anti-CD40. The addition of anti-PD-1 or anti-CTLA-4 may lift the inhibition exerted directly on NK and effector T cells, however, anti-CTLA-4 may also be associated with increased toxicity.55
Based on the increasing incidence and dismal prognosis associated with CCA, as well as the very limited success of current therapies, new and effective treatment options are urgently needed.1 The combination of cytotoxic and immunotherapeutic agents is approved for breast cancer and lung cancer and is currently being investigated for PDAC.36,56,57 Our findings suggest that this approach should also be investigated for CCA. The expression of CD40 on healthy tissue including hepatocytes raises some concerns regarding liver damage and therefore treatment tolerability.58 Strategies to minimize such risks are currently underway.59,60 Clinical evaluation of safety, tolerability, and efficacy of treatment with a CD40 agonist and anti-PD-1 following administration of cytotoxic chemotherapy is warranted.
In summary, the CCA tumor microenvironment impairs response to checkpoint blockade. Treatment with a CD40 agonist overcomes this inhibition and slows CCA tumor progression. Combining a CD40 agonist with anti-PD-1 checkpoint blockade offers significantly greater reduction in tumor burden than either monotherapy alone. This treatment primarily results in the localized proliferation and activation of myeloid cells, including macrophages and DCs, and lymphocytes, including CD4+ T, CD8+ T, and NK cells. This therapy depends on macrophages, CD4+ T, and CD8+ T cells for efficacy. Treatment combining cytotoxic chemotherapy followed by anti-CD40/PD-1 results in optimal tumor control and prolonged OS in murine models of advanced CCA.
Supplementary Material
Financial support
T.F.G. and X.W.W were supported by the Intramural Research Program of the NIH, NCI (ZIA BC 011345, ZO1 BC01087). Q.Z. received a training fellowship from the Cholangiocarcinoma foundation. T.L. and S.W. received support from the Deutsche Forschungsgemeinschaft (LO-1676/4-1, SFB/TR209 project B08, SFB/TR57 project Q1 and WA-4610/1-1).
Abbreviations
- CCA
cholangiocarcinoma
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- DCs
dendritic cells
- Gem/Cis
gemcitabine and cisplatin
- iCCA
intrahepatic CCA
- ICIs
immune checkpoint inhibitors
- IFN
interferon
- IHC
immunohistochemistry
- NK
natural killer
- OS
overall survival
- PD-1
programmed cell death 1
- PD-L1
programmed cell death ligand 1
- TIL
tumor-infiltrating lymphocytes
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2020.11.037.
Data availability statement
The authors confirmed that the data supporting the findings of this study are available within the article and/or supplementary materials.
References
- [1].Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010;2:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mertens JC, Rizvi S, Gores GJ. Targeting cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis 2018;1864:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New Engl J Med 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- [6].Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol 2019;5:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol 2020;10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Duffy AG, Makarova-Rusher OV, Greten TF. The case for immune-based approaches in biliary tract carcinoma. Hepatology 2016;64:1785–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim RD, Kim DW, Alese OB, Li D, Shah N, Schell MJ, et al. A phase II study of nivolumab in patients with advanced refractory biliary tract cancers (BTC). J Clin Oncol 2019;37. 4097–4097. [Google Scholar]
- [10].Gou M, Zhang Y, Si H, Dai G. Efficacy and safety of nivolumab for metastatic biliary tract cancer. Onco Targets Ther 2019;12:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gani F, Nagarajan N, Kim Y, Zhu Q, Luan L, Bhaijjee F, et al. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol 2016;23:2610–261. [DOI] [PubMed] [Google Scholar]
- [12].Fontugne J, Augustin J, Pujals A, Compagnon P, Rousseau B, Luciani A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget 2017;8:24644–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ghidini M, Cascione L, Carotenuto P, Lampis A, Trevisani F, Previdi MC, et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur J Cancer 2017;86:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhou G, Sprengers D, Mancham S, Erkens R Boor PPC, van Beek AA, et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol 2019;71:753–762. [DOI] [PubMed] [Google Scholar]
- [15].Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xie C, Duffy AG, Mabry-Hrones D, Wood B, Levy E, Krishnasamy V, et al. Tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer. Hepatology 2019;69:2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology 2020;72(3):965–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res 2016;22:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep 2017;20:558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med 2012;209:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res 2003;63:2150–2157. [PubMed] [Google Scholar]
- [22].Diskin B, Adam S, Cassini MF, Sanchez G, Liria M, Aykut B, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol 2020;21:442–454. [DOI] [PubMed] [Google Scholar]
- [23].Byrne KT, Vonderheide RH. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep 2016;15:2719–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL. IFNgamma and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov 2016;6:400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Angelou A, Antoniou E, Garmpis N, Damaskos C, Theocharis S, Margonis GA. The role of soluble CD40L ligand in human carcinogenesis. Anticancer Res 2018;38:3199–3201. [DOI] [PubMed] [Google Scholar]
- [27].O'Hara M, O'Reilly EM, Rosemarie M, Varadhachary G, Wainberg ZA, Ko A, et al. Abstract CT004: a Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Cancer Res 2019;79:CT004. [Google Scholar]
- [28].Rizvi S, Fischbach SR, Bronk SF, Hirsova P, Krishnan A, Dhanasekaran R, et al. YAP-associated chromosomal instability and cholangiocarcinoma in mice. Oncotarget 2018;9:5892–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].The Guide For The Care and Use of Laboratory Animals. National Academies Press; 2011. [PubMed] [Google Scholar]
- [30].Yu SJ, Ma C, Heinrich B, Brown ZJ, Sandhu M, Zhang Q, et al. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J Hepatol 2019;70:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brown ZJ, Heinrich B, Greten TF. Establishment of orthotopic liver tumors by surgical intrahepatic tumor injection in mice with underlying non-alcoholic fatty liver disease. Methods Protoc 2018;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Beatty GL, Li Y, Long KB. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev anticancer Ther 2017;17:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ma HS, Poudel B, Torres ER, Sidhom JW, Robinson TM, Christmas B, et al. A CD40 agonist and PD-1 antagonist antibody reprogram the microenvironment of nonimmunogenic tumors to allow T-cell-mediated anticancer activity. Cancer Immunol Res 2019;7:428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yamamoto M, Xin B, Watanabe K, Ooshio T, Fujii K, Chen X, et al. Oncogenic determination of a broad spectrum of phenotypes of hepatocyte-derived mouse liver tumors. Am J Pathol 2017;187:2711–2725. [DOI] [PubMed] [Google Scholar]
- [35].Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest 2012;122:2911–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O'Hara MHORE, Rosemarie M, Varadhachary G, Wainberg ZA, Ko A, Fisher GA Jr, et al. A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. In: AACR Annual Meeting 2019; 2019 March 31, 2019. Atlanta, GA, USA: AACR; 2019. [Google Scholar]
- [37].Ngiow SF, Young A, Blake SJ, Hill GR, Yagita H, Teng MW, et al. Agonistic CD40 mAb-driven IL12 reverses resistance to anti-PD1 in a T-cell-rich tumor. Cancer Res 2016;76:6266–6277. [DOI] [PubMed] [Google Scholar]
- [38].Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy CB, et al. Targeting tumor-associated macrophages and granulocytic-myeloid-derived suppressor cells augments pd-1 blockade in cholangiocarcinoma. J Clin Invest 2020;130(10):5380–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang JQ, Zeng S, Vitiello GA, Seifert AM, Medina BD, Beckman MJ, et al. Macrophages and CD8(+) T cells mediate the antitumor efficacy of combined CD40 ligation and imatinib therapy in gastrointestinal stromal tumors. Cancer Immunol Res 2018;6:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis primers 2015;1:15080. [DOI] [PubMed] [Google Scholar]
- [41].Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, et al. FAP promotes immuno-suppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res 2016;76:4124–4135. [DOI] [PubMed] [Google Scholar]
- [42].Raggi C, Invernizzi P, Andersen JB. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts. J Hepatol 2015;62:198–207. [DOI] [PubMed] [Google Scholar]
- [43].Okabe H, Beppu T, Ueda M, Hayashi H, Ishiko T, Masuda T, et al. Identification of CXCL5/ENA-78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer-associated fibroblasts. Int J Canc 2012;131:2234–2241. [DOI] [PubMed] [Google Scholar]
- [44].Chen Z, Guo P, Xie X, Yu H, Wang Y, Chen G. The role of tumour microenvironment: a new vision for cholangiocarcinoma. J Cell Mol Med 2019;23:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol 2017;12:153–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hogdall D, Lewinska M, Andersen JB. Desmoplastic tumor microenvironment and immunotherapy in cholangiocarcinoma. Trends Cancer 2018;4:239–255. [DOI] [PubMed] [Google Scholar]
- [47].Lu J, Liu X, Liao YP, Wang X, Ahmed A, Jiang W, et al. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano 2018;12:11041–11061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [48].Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. The Lancet Oncol 2015;16:e498–509. [DOI] [PubMed] [Google Scholar]
- [49].Shi G, Yang Q, Zhang Y, Jiang Q, Lin Y, Yang S, et al. Modulating the tumor microenvironment via oncolytic viruses and CSF-1R inhibition synergistically enhances anti-PD-1 immunotherapy. Mol Ther 2019;27:244–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016;44:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fu D, Wu J, Lai J, Liu Y, Zhou L, Chen L, et al. T cell recruitment triggered by optimal dose platinum compounds contributes to the therapeutic efficacy of sequential PD-1 blockade in a mouse model of colon cancer. Am J Cancer Res 2020;10:473–490. [PMC free article] [PubMed] [Google Scholar]
- [52].Hu J, Kinn J, Zirakzadeh AA, Sherif A, Norstedt G, Wikstrom AC, et al. The effects of chemotherapeutic drugs on human monocyte-derived dendritic cell differentiation and antigen presentation. Clin Exp Immunol 2013;172:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, et al. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res 2017;5:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sawasdee N, Thepmalee C, Sujjitjoon J, Yongpitakwattana P, Junking M, Poungvarin N, et al. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int Immunopharmacol 2020;78:106006. [DOI] [PubMed] [Google Scholar]
- [55].Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. The New Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- [57].Recchia F Maintenance immunotherapy in patients with metastatic breast cancer (MBC) who have a clinical benefit with chemotherapy. Long-term follow-up of a phase II study, 2018. Chicago: ASCO; 2018. Journal of Clinical Oncology; 2018. [Google Scholar]
- [58].Medina-Echeverz J, Ma C, Duffy AG, Eggert T, Hawk N, Kleiner DE, et al. Systemic agonistic anti-CD40 treatment of tumor-bearing mice modulates hepatic myeloid-suppressive cells and causes immune-mediated liver damage. Cancer Immunol Res 2015;3:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Totterman TH, et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol Res 2014;2:80–90. [DOI] [PubMed] [Google Scholar]
- [60].Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res Off J Am Assoc Canc Res 2011;17:2270–2280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirmed that the data supporting the findings of this study are available within the article and/or supplementary materials.