Abstract
Introduction
Incorporation of a plant-based diet was effective in both induction and short-term relapse prevention in Crohn’s disease. Ten-year long-term relapse-free rates in Crohn’s disease are around 10% to 23%.
Objective
We investigated whether infliximab and plant-based diet as first-line therapy enhance the long-term relapse-free rate in patients with Crohn’s disease.
Methods
This single-group, prospective study was performed in tertiary hospitals in Japan. Remission was induced in 24 consecutive newly diagnosed adult patients with Crohn’s disease during hospitalization via 3 standard infliximab infusions together with a plant-based diet. Patients were instructed to continue the diet after discharge. Scheduled maintenance infliximab infusion was not used. The primary endpoint was relapse, which was defined as the appearance of symptoms resulting in the alteration of therapeutic modality. The secondary endpoints were C-reactive protein level, plant-based diet score, and surgery.
Results
The median follow-up period was 8.6 years. Thirteen cases were relapse-free. The relapse-free rate evaluated by Kaplan-Meier survival analysis at 1, 2, 3, and 4 years was 79%, 66%, 57%, and 52%, respectively. There was no further reduction afterward up to 10 years. The relapse-free rate with normal C-reactive protein levels at 1 to 2 and 3 to 10 years was 57% and 52%, respectively. The plant-based diet score at 20 months and 5 years was significantly higher relative to baseline (p < 0.0001). Surgical rates at 5 and 10 years were 12% and 19%, respectively.
Conclusions
Infliximab and plant-based diet as first-line therapy created an unprecedented relapse-free course in nearly half of patients with Crohn’s disease.
Introduction
The incidence of inflammatory bowel disease (IBD), a collective term for Crohn’s disease (CD) and ulcerative colitis (UC), has been increasing over time and expanding to different regions around the world, indicating that IBD is a global disease. 1 The natural history of most cases of CD is characterized by a chronic progressive disabling course. 2 Resumption of meals after suppression of inflammation by total parenteral nutrition or exclusive enteral nutrition always provokes inflammation, ie, an increase in C-reactive protein (CRP) concentration. Repeated flare-ups result in intestinal obstruction/penetration, necessitating surgical interventions. In a population-based cohort study conducted from 1955 to 1989 in Sweden, the cumulative rate of surgery (intestinal resection) was 44%, 61%, and 71% at 1, 5, and 10 years after diagnosis. 3 In other words, the majority of patients (3 of 4 patients) underwent surgery. A French study showed that 85.2% of patients with CD were categorized as disabling within the first 5 years of the disease. 4 On the other hand, there are long-term (10 years) relapse-free patients, and they constituted around 10% to 23% of a population-based cohort. 5–9 Decades of advancement in therapy, including biologics, have substantially improved prognosis in patients with CD, resulting in decreased mortality, lower surgical rate, less hospitalization, and increased quality of life. 10 However, there have been no reports on long-term relapse-free rates in the biologic era, 9 and the nature of the chronic progressive disabling course of CD has been unchanged. It is obvious that further improvement is eagerly sought.
IBD is a polygenic disease thought to be triggered by environmental factors; however, without recognition of key environmental factors, a radical strategy against IBD cannot be established. We regard a Westernized diet as the most ubiquitous environmental factor underling IBD 11–13 . Namely, IBD is a lifestyle disease mediated mainly by a Westernized diet. Consequently, if a suitable diet is identified and patients stick to the diet, we believe that the majority of patients with IBD could be free from relapse without medication. 11 We developed a lacto-ovo-semi-vegetarian diet, a kind of plant-based diet (PBD), to counter the Westernized diet, hoping for an increase in beneficial microbiota in the gut. 11 We began by replacing a traditional low-residue diet or omnivorous (Westernized) diet with a PBD for inpatients with IBD in 2003. 11 We reported the relapse-preventive effect of the PBD in patients with CD. 11 In patients with UC, we achieved a low relapse rate compared with the current standard treatment. 14,15 We now have confidence that a PBD without medication can induce remission in a subset of patients with mild UC. 14,16,17 Therefore, we provide a PBD first for mild UC. Medication is administered later if induction is not attained solely by the PBD. Focusing on diet, we reported cases with new onset of IBD after a dietary change to an unhealthy one. 16–18
In patients with CD, we anticipated that relapse would be prevented by the PBD. 11 Thus, we were concerned with a swift, safe, and reliable induction of remission. 11 This is attainable by infliximab. 19 Therefore, we designed a protocol for CD treatment: induction with infliximab, followed by maintenance remission by a PBD. 11 Considering that most patients with CD exhibit a chronic progressive disabling course 2–4,10 and that there is no way to identify patients who will exhibit a long-term relapse-free course, 20 all new patients diagnosed as having CD, even mild cases, were given infliximab and a PBD as the first-line (IPF) therapy. 21 This therapy induced remission in all 44 patients with CD (24 newly diagnosed adults, 11 newly diagnosed children, and 9 relapsed adults) without any primary nonresponders. 21–24 Preliminary follow-up studies indicated that the relapse rate was lower in newly diagnosed adult patients with CD as compared with relapsed adults and that of newly diagnosed children. 21 Our results are consistent with the current knowledge that early use of biologics is associated with a better prognosis. 10,25,26 The concept of a window of opportunity 27 —ie, early commencement of therapy before irreversible damage occurs—encouraged us to provide a potent best therapy for patients newly diagnosed with CD.
In this study, we investigated long-term relapse-free rates without biologic therapy in newly diagnosed adult patients with CD who achieved remission with IPF therapy. We hypothesized that these modalities could enhance the long-term relapse-free rate.
Methods
Design and settings
We designed a single-group, prospective, nonrandomized, open-label, uncontrolled trial, which was conducted in Akita City in northern Japan (study nos. University Hospital Medical Information Network [UMIN] UMIN000019061 and UMIN000020335; registration: www.umin.ac.jp). Both Nakadori General Hospital and Akita City Hospital are tertiary care hospitals in Akita City. Mitsuro Chiba, the first author, worked for the former between 2003 and 2012 and has been working for the latter since 2013.
The protocol was approved by the ethical committees of Nakadori General Hospital and Akita City Hospital (protocol nos. 19-2003, 17-2014, 15-2015). MC obtained informed consent from all study participants.
Patients
The use of infliximab for CD treatment was approved in 2002 in Japan. In our hospital, infliximab was first used for CD in 2003. Only newly diagnosed adult patients with CD who achieved remission with IPF therapy 21 were included in this study. Twenty-three of the 24 newly diagnosed adult patients with CD in this follow-up study were the same patients as those in the previous paper. 21 Relapsed cases and children aged 18 years and younger were excluded.
IPF therapy during hospitalization
The protocol comprised standard induction therapy with infliximab combined with a PBD, as previously described. 21 Briefly, metronidazole 750 mg/d was given after admission. Then, infliximab (5 mg/kg; Remicade, Janssen Biotech, Horsham, PA, USA) was infused at weeks 0, 2, and 6. Azathioprine was not coadministered. The PBD was initiated on the same day as the infusion. The PBD comprised a lacto-ovo-vegetarian diet, with fish once a week and meat once every 2 wk. 11 The rates of protein, fat, and carbohydrate in total calories were 16.1 ± 0.5%, 18.6 ± 1.4%, and 66.1 ± 1.6%, respectively. The PBD contained 32.4 ± 2.1 g of dietary fiber/2000 kcal (soluble dietary fiber, 6.8 ± 0.7 g; insoluble dietary fiber, 23.3 ± 1.6 g). 11 Calories were gradually increased to a maximum of about 30 kcal/kg standard body weight. After about 1 month, the metronidazole was switched to mesalamine or sulfasalazine. After the third infusion of infliximab, patients were discharged. Patients who could not be admitted for the entire induction phase were discharged after the second infliximab infusion and readmitted for the third infusion. 21
Patients were provided with educational material on lifestyle diseases, healthy lifestyle habits (ie, no smoking, regular physical activity, moderate or no use of alcohol, regularity of meals, and not eating between meals), 28 the pathogenesis of IBD, and information on the PBD. 14 Patients were provided with answers to any questions that they had. A registered dietitian also visited the patients and talked to them about the PBD and helped them get used to it. At the end of the hospitalization, a qualified dietitian gave dietary guidance to the patients and to the person who prepared the patient’s meals. 11 Patients were advised to continue with the PBD after discharge. Patients who were smokers before their admission were encouraged to continue nonsmoking after discharge.
Follow-up studies
Patients who achieved remission with IPF therapy were followed up as long as possible. Medication was mesalamine or sulfasalazine. The medication was stopped if the patient was in remission and the patient so desired. Periodic or episodic maintenance therapy with biologics was not administered. Patients visited the IBD Outpatient Department (responsible doctor: Mitsuro Chiba) every 8 weeks. In relapse-free patients with normal CRP levels for several years, visits became less frequent (2 to 4 times a year), depending on the inclination of the patient. Blood samples for measurement of CRP, complete blood counts, albumin, transaminase, and so on were taken at each visit.
Food-frequency questionnaire and PBD score
A questionnaire of dietary habits and lifestyle behaviors before onset of the disease was obtained immediately after admission, as described in a previous report. 29 The questionnaire was repeated during short-term (≤ 2 years) and/or long-term ( > 2 years) follow-up. They were designated as baseline PBD score (PBDS), short-term PBDS, and long-term PBDS, respectively. 14,15
A PBDS for Japanese patients with IBD was calculated from the questionnaire. The method for how the PBDS was calculated has been described previously. 29 Briefly, 8 items considered to be preventive factors for IBD (ie, vegetables, fruits, pulses, potatoes, rice, miso soup, green tea, and plain yogurt) contributed to a positive score (PBDS+), whereas 8 items considered to be IBD risk factors (ie, meat, minced or processed meat, cheese/butter/margarine, sweets, soft drinks/sugar-sweetened beverages, alcohol, bread, and fish) contributed to a negative score (PBDS–). Scores of 5, 3, and 1 were given according to the frequency of consumption: every day, 3 to 5 times/wk, and 1 to 2 times/wk, respectively. The PBDS was calculated as the sum of the positive and negative scores, and it ranged between –40 to +40, with a higher PBDS indicating greater adherence to the PBD. The PBDS of the PBD during hospitalization was 35. 29
Assessment of the efficacy of IPF therapy
The primary endpoint was clinical relapse. In this study, we followed the definition of relapse defined by Solberg et al. 7 Namely, relapse was defined as the appearance of symptoms of CD resulting in the alteration of therapeutic modality. 7 Duration of remission was calculated as the number of days between discharge and the appearance of symptoms of relapse.
The secondary endpoints were surgery, normalization of CRP concentration, 30,31 the change in PBDS, and mucosal healing. Mucosal healing was defined as the absence of active findings of CD, such as ulcer, 32 aphthoid lesions, edema, redness, and bleeding. 21 In this study, remission means clinical remission unless otherwise specified. Remission was classified into 2 groups: remission with normal CRP levels, and remission with elevated CRP levels. An abrupt CRP increase due to a common cold, or some other infection rather than inflammation associated with Crohn’s disease, was ignored.
Safety evaluations
Safety assessments included an examination and blood tests at scheduled visits.
Statistical analysis
Demographic parameters are expressed as mean (standard deviation [SD]) and/or median (interquartile range [IQR]), as appropriate. The frequency of categorical variables in relapse-free cases and relapsed cases was assessed with the χ2 method. The age, disease duration, Crohn’s disease activity index (CDAI) score, CRP concentration, and follow-up period in relapse-free cases and relapsed cases were analyzed through the Student t-test and the Wilcoxon rank sum test, respectively, depending on whether scores showed standard normal distribution. Chronological changes in PBDS+, PBDS−, and scores in identical patients were compared using the paired t-test or the Wilcoxon signed-rank test. Kaplan-Meier survival analysis was used to calculate the cumulative proportion of patients who were relapse-free, relapse-free with normal CRP levels, and surgery-free. Patients who stopped the treatment for any reason other than relapse were considered censored at the time of their last observation. A p value of 0.05 or less was considered to indicate a statistically significant difference. Statistical analysis was performed using JMP 8 software (SAS Institute Inc., Cary, NC, USA).
Results
Patients
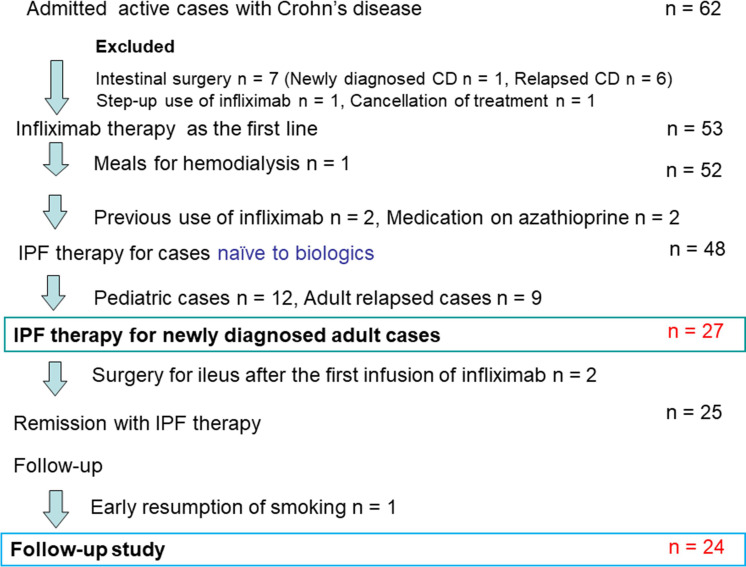
Between August 2003 and December 2017, 62 patients with active CD were admitted. Seven of the 62 patients were indicated for and underwent surgical treatment: One patient was a newly diagnosed case, and 6 patients were relapsed cases (Figure 1). Another 7 cases were excluded due to previous use of infliximab, step-up use of infliximab, and so on. IPF therapy was administered in 48 infliximab-naïve cases (Figure 1). Adult relapsed cases and children ages 18 years and younger were excluded. Twenty-seven newly diagnosed adult cases received IPF therapy. Two of the 27 cases underwent surgery due to intestinal obstruction 2 weeks after the first infusion. 24 The remaining 25 cases achieved remission. One of the 25 cases was excluded from the follow-up study due to early resumption of smoking, which is a well-known risk factor for CD, including flare. 33 The remaining 24 cases were included in the follow-up study (Figure 1).
Figure 1:
Enrollment of inpatients with active Crohn’s disease (CD) for first-line therapy with infliximab and a plant-based diet (IPF). One newly diagnosed patient with CD who underwent intestinal surgery before medical therapy was excluded from this study.
The demographic characteristics of the 24 patients are presented in Table 1. The median age, disease duration from onset of symptoms to IPF therapy, CDAI score, and CRP (reference range ≤ 0.14 mg/dL since 2017 by Japanese Committee for Clinical Laboratory Standards; ≤ 0.3 mg/dL until 2016) were 27.5 years old, 5.0 months, 265, and 2.5 mg/dL, respectively. The most frequent location and behavior 34 were ileocolonic type (n = 15) and B1 (nonstricturing, nonpenetrating) (n = 17), respectively. Perianal fistula(s) draining pus and/or anal tag(s) were present in 16 cases. 34 Five cases were current smokers, who accepted the doctor’s advice and stopped smoking after admission (Table 1). Five patients had a CDAI score < 150; 4, 150–220 (mild–moderate); 8, 220–450 (moderate–severe); and 7, > 450 (severe/fulminant) (Table 1). 35 There were no statistical differences in sex, age, disease duration, disease type, and follow-up duration between relapse-free cases and relapsed cases (Table 1). The median follow-up period was 8.6 years. Individual data for the 24 cases are provided in Supplemental Tables 1–3.
Table 1:
Demographic and clinical characteristics of the patients
| Case information | Total | Relapse-free cases | Relapsed cases | p Value |
|---|---|---|---|---|
| Number of cases | 24 | 13 | 11 | |
| Men/women | 15/9 | 8/5 | 7/4 | 0.9157 a |
| Age (y) | ||||
| Range | 19–65 | 19–65 | 19–44 | |
| Mean ± SD | 31.4 ± 13.9 | 34.2 ± 17.4 | 28.0 ± 7.7 | 0.9305 b |
| Median (IQR) | 27.5 (21.0–36.8) | 28.0 (19.5–52.5) | 27.0 (21.0–32.0) | |
| Disease duration (mo) | ||||
| Range | 1–60 | 1–60 | 1–39 | |
| Mean ± SD | 11.0 ± 14.8 | 10.3 ± 16.5 | 11.9 ± 13.3 | 0.5202 b |
| Median (IQR) | 5.0 (2.0–12.8) | 3.0 (2.0–12.0) | 8.0 (2.0–13.0) | |
| Location | 0.4083 a | |||
| L1 (ileal) | 1 | 1 | 0 | |
| L2 (colonic) | 8 | 5 | 3 | |
| L3 (ileocolonic) | 15 | 7 | 8 | |
| L4 (isolated upper lesions) | 0 | 0 | 0 | |
| Behavior | 0.4757 a | |||
| B1 (nonstricturing, nonpenetrating) | 17 | 10 | 7 | |
| B2 (stricturing) | 7 | 3 | 4 | |
| B3 (penetrating) | 0 | 0 | 0 | |
| Perianal disease modifier | 16 | 9 | 7 | |
| Anal fistula | 11 | 7 | 4 | |
| Anal skin tag | 8 | 4 | 4 | |
| Current smoker | 5 | 5 | 0 | 0.0071 a |
| Before induction treatment | ||||
| CDAI score | ||||
| Range | 52–834 | 52–566 | 95–834 | |
| Mean ± SD | 340 ± 218 | 242 ± 145 | 456 ± 239 | 0.0132 c |
| Median (IQR) | 265 (177–547) | 202 (146–293) | 346 (281–661) | |
| < 150 | 5 | 4 | 1 | |
| 150–220 (mild–moderate) | 4 | 3 | 1 | |
| 220–450 (moderate–severe) | 8 | 4 | 4 | |
| > 450 (severe/fulminant) | 7 | 2 | 5 | |
| CRP concentration (mg/dL) | ||||
| Mean ± SD | 5.3 ± 6.0 | 2.8 ± 3.8 | 8.1 ± 7.0 | 0.0127 b |
| Median (IQR) | 2.5 (0.8–7.1) | 1.0 (0.6–4.5) | 5.7 (2.2–12.8) | |
| After induction treatment (before follow-up studies) | ||||
| CDAI score | ||||
| Range | 2–98 | 2–98 | 28–90 | |
| Mean ± SD | 53 ± 26 | 46 ± 27 | 62 ± 23 | 0.1400 c |
| Median (IQR) | 53 (35–79) | 47 (31–61) | 64 (39–84) | |
| CRP concentration (mg/dL) | ||||
| Mean ± SD | 0.13 ± 0.20 | 0.09 ± 0.15 | 0.19 ± 0.24 | 0.1012 b |
| Median (IQR) | 0.1 (0–0.10) | 0 (0–0.10) | 0.10 (0.04–0.30) | |
| Mucosal healing | 10 | 4 | 6 | 0.2376 a |
| Follow-up period (d) | ||||
| Range | 163–5475 | 523–5320 | 163–5475 | |
| Mean ± SD | 2996 ± 1525 | 3096 ± 1589 | 2878 ± 1514 | 0.7362 c |
| Median (IQR) | 3121 (1676–4221) | 3201 (1697–4636) | 3041 (1675–4023) |
Comparison between relapse-free cases and relapsed cases through either χ2 test, Wilcoxon rank sum test, or Student t-test.
χ2 test.
Wilcoxon rank sum test.
Student t-test.
CDAI, Crohn’s disease activity index;CRP, C-reactive protein; IQR, interquartile range; SD, standard deviation.
Table 2:
Change over time in plant-based diet score
| Time frame | N | Follow-up period (mo) | PBDS+ | p Value | PBDS– | p Value | PBDS | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||||
| Baseline | 24 | 21.4 (8.9) | 22.0 (15.0–27.0) | 13.2 (5.1) | 12.5 (9.0–16.8) | 8.2 (8.3) | 6.5 (2.3–14.0) | |||||
| Follow-up | ||||||||||||
| Short-term | 23 | 17.8 (7.3) | 20.0 (13.0–24.0) | 33.0 (5.1) | 34.0 (29.0–36.0) | < 0.0001 | 2.4 (2.6) | 1.0 (0–4.0000) | < 0.0001 a | 30.6 (6.3) | 31.0 (27.0–36.0) | < 0.0001 |
| Baseline | 23 | 21.7 (9.0) | 23.0 (15.0–27.0) | 13.3 (5.2) | 13.0 (9.0–17.0) | 8.4 (8.4) | 7.0 (2.0–14.0) | |||||
| Long-term | 21 | 68.0 (35.7) | 60.0 (39.5–85.5) | 29.9 (7.3) | 30.0 (25.0–36.0) | 0.0014 | 5.7 (6.9) | 4.0 (1.0–6.0) | 0.0003 a | 24.2 (12.2) | 29.0 (17.5–34.0) | < 0.0001 |
| Baseline | 21 | 22.5 (8.7) | 26.0 (16.0–28.0) | 13.3 (5.4) | 13.0 (8.5–18.0) | 9.2 (8.3) | 7.0 (3.5–15.5) | |||||
p value obtained with the Wilcoxon signed-rank test.
IQR, interquartile range; SD, standard deviation.
Table 3:
Literature review of relapse-free rate and surgical rate in adult patients with Crohn’s disease
| Reference | Country | Study participants and Y(s) | Number of cases | Cumulative relapse-free rate (y [%]) | Cumulative surgical rate (y [%]) | ||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 5 | 10 | ||||
| Munkholm et al 5 | Denmark | PBIC 1962–1987 | 373 | 22 | 12 | n.d. | n.d. |
| Wolters et al 6 | European countries, Israel | PBIC 1991–1993 | 358 | 31 | 23 | 21 | 29 |
| Solberg et al 7 | Norway | PBIC 1990–1994 | 237 | 15 | 10 | 27 | 38 |
| Nguyen et al 36 | Canada | 2001–2008 | 1119 | 18 | n.a. | ||
| Frolkis et al 37 | Meta-analysis | 1990– | 3,239, a 3,253 b | 28 | 39 | ||
| 2000– | 1193 | 24 | n.a. | ||||
| Rungoe et al 38 | Denmark | 1995–2002 | 3718 | 27 | 31 | ||
| 2003–2011 | 5552 | 20 | 23 | ||||
| Niewiadomski et al 39 | Australia | PBIC 2007–2013 | 146 | 26 | n.a. | ||
| Kim et al 40 | Korea | PBIC 2006–2012 | 11,267 | 9 | n.a. | ||
| Okada et al 41 | Japan | 1973–1988 | 58 | 29 | 46 | ||
| Present study | Japan | 2003–2017 | 26 | 52 | 52 | 12 | 19 |
Case number for surgical rate at 5 y.
Case number for surgical rate at 10 y.
n.a., not available; n.d., not described; PBIC, population-based inception cohort.
Efficacy
Primary endpoint: Relapse-free rate
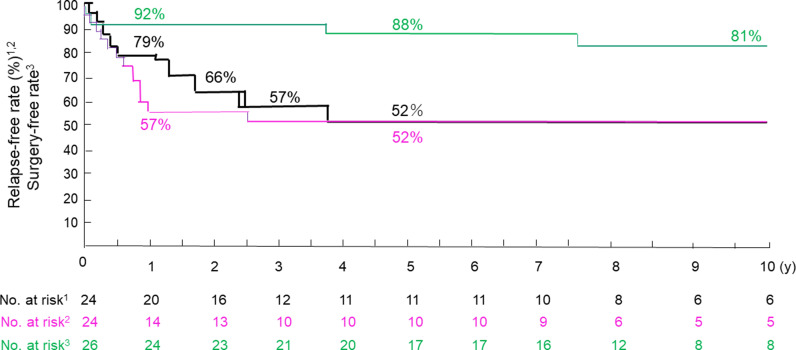
Thirteen patients were relapse-free, and 11 patients relapsed during the follow-up period. The relapse-free rates at 1, 2, 3, and 4 years of follow-up were 79%, 66%, 57%, and 52%, respectively. There was no further reduction afterward up to 10 years (Figure 2). Mean time to relapse was 2 years, 9 months (33 months). The relapse-free rates in patients with normal CRP levels at 1 to 2 and at 3 to 10 years of follow-up were 57% and 52%, respectively (Figure 2). Mean time to relapse with normal CRP was 1 y, 7 months (19 months).
Figure 2:
Life table estimate of relapse-free, relapse-free with normal C-reactive protein, and surgery-free cases in the follow-up period after infliximab and plant-based diet as first-line (IPF) therapy for active Crohn’s disease. Black Line = relapse-free, red line = relapse-free with normal C-reactive protein, green line = surgery-free.
Secondary endpoints
Between relapse-free cases and relapsed cases, there were significant differences in mean (SD) baseline CDAI score and CRP concentration before IPF therapy. Those values—242 (145) and 2.8 (3.8) mg/dL, respectively—in relapse-free cases were significantly lower than those—456 (239) and 8.1 (7.0) mg/dL, respectively—in relapsed cases (p = 0.0132 and p = 0.0127, respectively) (Table 1). Their values after induction treatment were also lower in relapse-free cases than those in relapsed cases, but their differences were not significant (Table 1).
CRP levels became abnormal in 15 of 24 cases (Supplemental Table 2). It occurred within 1 year in the majority of such cases (11 of 15 cases) (Supplemental Table 2). The time from elevated CRP to relapse in relapsed cases varied from 0 to 831 d, with a median of 176 (IQR 54–520) d (Supplemental Table 2). There was no case in which patients with a normal CRP level relapsed.
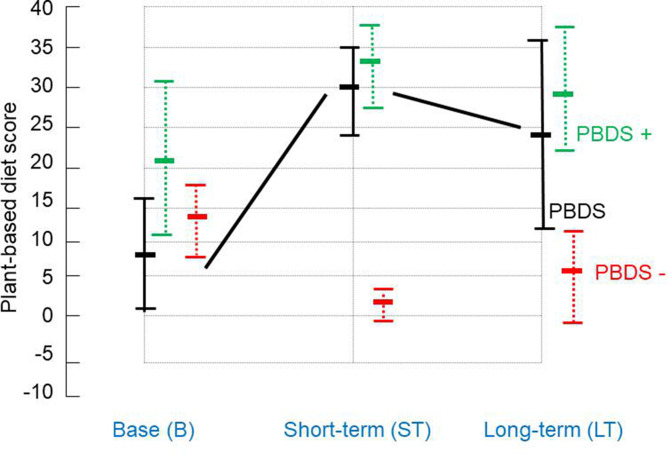
Mean (SD) baseline PBDS+, PBDS−, and PBDS were 21.4 (8.9), 13.2 (5.1), and 8.2 (8.3), respectively (Table 2). For 23 patients, at the median follow-up period of 20 months, respective scores were 33.0 (5.1), 2.4 (2.6), and 30.6 (6.3). These 3 values were significantly better than those at baseline (p < 0.0001) (Table 2 and Figure 3). In 21 patients, at the median follow-up period of 5 years, the respective scores were 29.9 (7.3), 5.7 (6.9), and 24.2 (12.2). These 3 values were significantly better than those at baseline (p = 0.0014, p = 0.0003, and p < 0.0001, respectively) (Table 2 and Figure 3).
Figure 3:
Change over time in plant-based diet score (PBDS) (mean ± SD). PBDS+ (green dotted line) at baseline, in short-term, and in long-term was 21.4 (8.9), 33.0 (5.1), and 29.9 (7.3), respectively. PBDS– (red dotted line) for those was 13.2 (5.1), 2.4 (2.6), and 5.7 (6.9), respectively. PBDS (black line) for those was 8.2 (8.3), 30.6 (6.3), and 24.2 (12.2), respectively (Table 2). SD = standard deviation.
There was no significant difference in mean short-term PBDS between relapse-free cases and relapsed cases: 29.4 (6.2) in the former (n = 13), and 32.1 (6.3) in the latter (n = 10) (Supplemental Table 3).
Twenty-seven cases received IPF therapy (Figure 1). Two cases developed intestinal obstruction within 10 d after the first infliximab infusion during the induction phase. 24 Two patients underwent surgery due to intestinal obstruction at 3.7 and 7.6 years into follow-up, respectively (Supplemental Table 2). The cumulative surgical rate at 1, 5, and 10 years was 8%, 12%, and 19%, respectively (Table 3), and the surgery-free rate was 92%, 88%, and 81%, respectively (Figure 2).
Ileocolonoscopy was performed in 8 of 13 relapse-free patients. Mucosal healing was observed in 4 patients, but tiny lesion(s) were observed in 3 patients. Active ulcers were found in 1 patient with persistent elevated CRP levels (Supplemental Table 2).
Other health problems occurred during long-term follow-up after a few to several years of a relapse-free period. There were no obese patients at discharge, but 5 patients subsequently became obese (body mass index ≥ 25) (Supplemental Table 2). New disease(s) occurred in some patients (eg, breast cancer, hypertension, hyperuricemia) (Supplemental Table 3). Resumption of smoking and alcohol occurred in a few patients (Supplemental Table 3). In 1 patient, long-term PBDS dropped to –11, which was far below of their baseline score of 7 (Supplemental Table 3).
Safety
No patients experienced a serious adverse effect due to IPF therapy, as reported in a previous paper. 21
Discussion
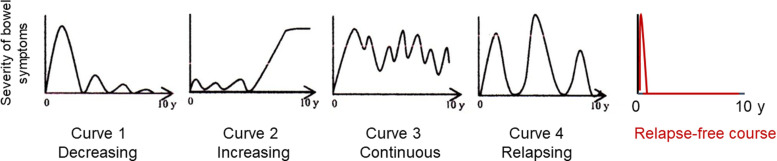
The present study showed a long-term relapse-free course in nearly half of newly diagnosed adult patients with CD (52%) who received IPF therapy. Solberg et al 7 described 4 clinical courses in 191 patients with CD in Norway: 1) decreasing severity of relapses, 2) increasing severity of relapses, 3) chronic continuous symptoms, and 4) chronic relapsing symptoms. They were designated as curves 1, 2, 3, and 4, respectively (Figure 4). 7 Recently, Wintjens et al 42 similarly depicted 6 clinical courses in 432 patients in the Netherlands. Neither of the studies described a relapse-free course. IPF therapy created an unprecedented clinical course: a relapse-free course accounting for approximately half of the patients with CD (Figure 4). This indicates that there is much room for improvement in the current modality and much room for ameliorating the clinical course of CD. Reflecting the relapse-free course, the cumulative surgical rate at 5 and 10 years was 12% and 19%, respectively. Table 3 shows representative surgery rates for patients with CD in the pre- and post-biologic eras, before 2000 and afterward, respectively. Our surgical rates were lower than those reported, except for a Korean study, which was 9% at 5 y (Table 3). 6,7,36–41 Reported Japanese surgical rates at 5 and 10 y in the pre-biotic era were 29% and 46%, respectively. 41
Figure 4:
Graphic clinical course in Crohn’s disease (CD). Solberg et al. 7 presented 4 graphic clinical courses of CD: Curve 1 (decrease in the severity of bowel symptoms), curve 2 (increase in the severity of bowel symptoms), curve 3 (chronic continuous bowel symptoms), and curve 4 (chronic relapsing bowel symptoms) (with permission from Elsevier). In the present study, a relapse-free course was attained in nearly half of the patients with CD treated with infliximab and a plant-based diet as first-line (IPF) therapy.
In practice, there are patients who experience softening stool but no disturbance in daily activity. Consequently, there are no changes in therapy that would be categorized as remission by most definitions. 7 In these patients, CRP levels are consistently elevated beyond the reference range. Therefore, clinical remission can be classified into 2 groups: remissions with either normal or elevated CRP levels. Patients with the latter are candidates for future relapse. 30,31 In this study, maintaining a normal CRP level was reconfirmed for sustained remission. CRP levels became abnormal within 1 year in most relapsed cases (Figure 2 and Supplemental Table 2). Therefore, several scheduled maintenance infliximab infusions to ascertain that normal CRP levels are consistently maintained may further increase the relapse-free rate in patients with unstable CRP levels.
The prevailing consensus is to limit early use of biologics in patients with signs of a poor prognosis. Considering the disabling course of most patients with CD 2–4 and considering the great benefit of the long-term relapse-free course in nearly half of the patients treated with IPF therapy, the first-line use of infliximab for all newly diagnosed patients with CD can be justified.
The incidence of diet-related obesity and chronic diseases, including coronary heart disease, stroke, and diabetes mellitus, has been steadily increasing and is a global health concern. 43 Global current consumption consists of an excess of unhealthy foods such as red meat, sugar, and refined grains and a shortage of healthy foods such as vegetables, fruits, legumes, whole grains, and nuts. 43 This means that the current omnivorous (Westernized) diet is problematic and should be corrected. Replacing our omnivorous Westernized diet with a prudent (the healthy reference) diet has been recommended for decades. 43–46 Unfortunately, people have not necessarily followed the advice. There are many barriers to popularization of the healthy reference diet, including preferences for palatable diets, urbanization, availability of cheap unhealthy foods, and lack of education on nutrition and lifestyle medicine in medical schools. 46,47
Case–control studies and a cohort study in diet in IBD disclosed more or less similar findings in the general population as described above: vegetables, fruits, and dietary fiber as preventive factors, and meat, animal protein, sweets, fast foods, and cola drinks as risk factors. 11,48,49 A diet moderating meat and animal protein and increasing daily consumption of vegetables and fruits is categorized as a PBD. PBDs are recommended to the public as healthy diets to prevent common chronic diseases. 43,44,50
Recent research is unraveling the interplay between diet, gut microbiota, microbial metabolites, and health/disease. 51–55 The diseases extend beyond the confines of the gut (eg, IBD) to various chronic diseases (eg, obesity, diabetes, coronary artery disease, stroke, rheumatoid arthritis, cancer, psychiatric diseases). We have coevolved with gut microbiota to exist in a symbiotic relationship. Westernized diets (high in fat, animal protein, and sugar and low in dietary fiber) proportionately decrease Firmicutes and increase Bacteroidetes at the phylum level, with Bacteroides dominating at the species level. In contrast, PBDs (low in fat, animal protein, and sugar and high in dietary fiber) largely induce the opposite changes. They proportionately increase Firmicutes and decrease Bacteroidetes at the phylum level, with Prevotella species dominating at the species level. In total, Westernized diets tend to cause gut dysbiosis (reduced microbial diversity), whereas PBDs increase microbial diversity (symbiosis). Firmicutes include Faecalibacterium and Roseburia, which produce short-chain fatty acids by fermentation of dietary fiber. Short-chain fatty acids (butyrate, acetate, and propionate) are deeply involved in the regulation of host defense mechanisms. 56 Diverse beneficial effects of butyrate are well described. 56,57 Decreased production of short-chain fatty acids by Westernized diets as compared with PBDs has been observed. Altogether, Westernized diets are pro-inflammatory and PBDs are anti-inflammatory. 51–59 The above phenomena associated with Westernized diets are also observed in IBD. 60 These observations indicate that Westernized diets increase susceptibility to not only IBD but also other chronic diseases. They also indicate that refraining from Westernized diets and replacing them with prudent diets is critically needed in IBD treatment.
Approximately 30% of patients with IBD are known to be primary nonresponders to infliximab or adalimumab. 61 Recently, butyrate-producing bacteria and butyrate level were reported to be related to outcomes of induction therapy and maintenance therapy. 62–64 For example, low levels of butyrate in fecal samples were found in primary nonresponders to biologics compared with responders. 64 Butyrate is produced by bacterial fermentation of dietary fiber. A decrease of butyrate-producing bacteria has been consistently reported in patients with IBD. 60 Our IPF therapy for CD treatment, containing a sufficient quantity of dietary fiber, did not result in any primary nonresponders to infliximab in 46 consecutive cases. 21–23
In our previous study, the mean baseline PBDS in patients with CD (n = 70) and UC (n = 159) was 8.2 and 10.9, respectively. 29 PBDS in the short-term (≤ 2 y) in patients with UC after discharge from educational hospitalization, 14 from conventional hospitalization, 15 and from hospitalization for severe UC 65 were significantly increased (p < 0.0001 in these 3 studies). Although sustained dietary modification is desired, the majority of patients tended to lose their determination once they had been in remission for a few to several years. However, they still consumed more of the recommended foods and less of the discouraged foods compared with baseline. In this study, the mean PBDS (24.2) was significantly higher compared with its baseline (9.2) even at a median follow-up period of 5 years (p < 0.0001) (Figure 3, Table 2). We used mesalamine or sulfasalazine in the follow-up phase. Considering that the relapse-preventive effect of mesalamine or sulfasalazine is absent or modest at most in patients with CD, 66 it is reasonable to conclude that the PBD was the sustainer for the relapse-free condition.
It is obvious that the majority of diseases we face are chronic diseases (lifestyle diseases) that develop due to an unhealthy lifestyle. 67,68 Therefore, incorporation of a comprehensive healthy lifestyle in medicine based on pathophysiology, namely, lifestyle medicine, is fundamental for prevention and treatment of the chronic diseases. 68 Making changes in lifestyle, including dietary habits, is not easy. 69–71 Substantial adherence to a PBD compared with the baseline even 5 years later in this study was remarkable. This was probably achieved because of patients’ acceptance of CD as a lifestyle disease. Patients thereby accepted the PBD as a lifestyle. This is a critical difference in dietary intervention studies between ours and others. We advise patients to continue a PBD as a lifestyle choice, whereas other interventions set certain periods for adherence to a recommended diet. 72 Dietary intervention should be comprehensive in patients with IBD who are genetically predisposed to IBD. There will be a limitation to mere exclusion of potentially untoward foodstuffs. 72–74
We believe that hospitalization plays a critical role in replacement of an omnivorous diet with a PBD. Hospitalization helps limit risk factors for IBD and ill health, including smoking, alcohol, sweets, and animal foods, while the patients benefit from preventive factors every day, including an increased intake of vegetables and fruits. In this study, 5 smokers stopped smoking after hospitalization. We provided a PBD from the beginning of and throughout hospitalization. Patients recovered during hospitalization and probably realized that the PBD contributed to their recovery. We believe that a PBD and learning about healthy habits during hospitalization contributed to enhancing self-management skills in maintaining remission. We already showed that educational hospitalization for mild UC achieved lower relapse rates compared with that of the current standard treatment. 14
There are several advantages of IPF therapy. Infliximab in IPF therapy was used only in the induction phase and not used as scheduled periodic maintenance in the quiescent phase. In addition, an immunosuppressant, such as azathioprine, was not coadministered with infliximab in the induction phase. IPF therapy is quite safe and provides swift induction without primary nonresponders. 21 There were no withdrawals due to adverse events in the induction phase. 21 Adherence to a PBD not only prevents IBD relapse but also reduces the likelihood of other chronic common diseases. 44,45,50 Altogether, it lowers medical costs. Patients are free from the worry associated with the adverse effects of medication. Our lacto-ovo-semi-vegetarian diet allowing fish once a week and meat every other week is not strict, but loose, and does not result in micronutrient deficiency. 50 There is no prohibited food in our PBD. 11 Above all, a long-term relapse-free outcome is the greatest benefit to patients with CD. A long-term relapse-free outcome obviously keeps the patient free from disability objectively and provides a high quality of life subjectively. Patient activities are the same as those before the disease. These benefits of IPF therapy far exceed the inconvenience of hospitalization in the induction phase.
Our study had some limitations. There was no control group, and the sample size was small. We hope that other larger, controlled studies will be conducted to validate our results.
The answer to the question of what kind of diet is suitable for IBD has long been sought. 72 By replacing a Westernized diet with PBD, we have shown far better outcomes in treating both CD and UC in either the active or the quiescent stage compared with the current standard treatments. 11,14,15,21,65 PBDs are effective for all degrees of severity, from mild to severe, in both diseases. Acceptance of the PBD was 100% in inpatients and around 75% in outpatients. 11 Granted, the evidence level of our single-group studies is low compared with conventional randomized controlled trials. It will take another decade to confirm the most suitable diet for IBD based on such trials because not only the short-term, but also the long-term effects of the suitable diet will need to be demonstrated. The content of PBD is consistent with the healthy reference diet. Considering the large number of patients suffering due to inappropriate dietary guidance, we believe recommendation of a PBD based on available evidence as the next best option is adequate, and it will provide great benefit to patients with IBD. 75
Supplementary Material
tpp_21.073-suppl-01.docx (90.7KB, docx)
Acknowledgments
The authors thank Mrs. Kimiko Yamada, registered dietitian, and other staff of the Dietary Division of Akita City Hospital for providing semi-vegetarian diet and dietary guidance.
Footnotes
Funding: None declared
Conflicts of Interest: None declared
Author Contributions: Mitsuro Chiba, MD, PhD, designed and conducted the study and wrote the manuscript. Tsuyotoshi Tsuji, MD, PhD, Kunio Nakane, MD, PhD, Satoko Tsuda, MD, Hideo Ohno, MD, Kae Sugawara, MD, PhD, Masafumi Komatsu, MD, PhD, and Haruhiko Tozawa, MD, contributed to the acquisition and interpretation of data and revision of the paper. All authors approved the final version of the manuscript for submission.
Supplementary Materials: Supplemental material is available at: https://www.thepermanentejournal.org/doi/10.7812/TPP/21.073#supplementary-materials
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 2.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17(6):1415–1422. 10.1002/ibd.21506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231(1):38–45. 10.1097/00000658-200001000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130(3):650–656. 10.1053/j.gastro.2005.12.019 [DOI] [PubMed] [Google Scholar]
- 5.Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol. 1995;30(7):699–706. 10.3109/00365529509096316 [DOI] [PubMed] [Google Scholar]
- 6.Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut. 2006;55(8):1124–1130. 10.1136/gut.2005.084061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solberg IC, Vatn MH, Høie O, et al. Clinical course in Crohn’s disease: Results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430–1438. 10.1016/j.cgh.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV Jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: A systematic review. Aliment Pharmacol Ther. 2002;16(1):51–60. 10.1046/j.1365-2036.2002.01140.x [DOI] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105(2):289–297. 10.1038/ajg.2009.579 [DOI] [PubMed] [Google Scholar]
- 10.Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):351–361. 10.1053/j.gastro.2016.09.046 [DOI] [PubMed] [Google Scholar]
- 11.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010;16(20):2484–2495. 10.3748/wjg.v16.i20.2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba M, Tsuda H, Abe T, Sugawara T, Morikawa Y. Missing environmental factor in inflammatory bowel disease: Diet-associated gut microflora. Inflamm Bowel Dis. 2011;17(8):E82-3. 10.1002/ibd.21745 [DOI] [PubMed] [Google Scholar]
- 13.Chiba M, Nakane K, Komatsu M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm J. 2019;23:18–107. 10.7812/TPP/18-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiba M, Nakane K, Tsuji T, et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: A single-group trial. Perm J. 2018;22:17–167. 10.7812/TPP/17-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba M, Nakane K, Tsuji T, et al. Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: A single group trial. Perm J. 2019;23:18–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiba M, Tsuda S, Komatsu M, Tozawa H, Takayama Y. Onset of ulcerative colitis during a low-carbohydrate weight-loss diet and treatment with a plant-based diet: A case report. Perm J. 2016;20(1):80–84. 10.7812/TPP/15-038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiba M, Sugawara T, Komatsu M, Tozawa H. Onset of ulcerative colitis in the second trimester after emesis gravidarum: Treatment with plant-based diet. Inflamm Bowel Dis. 2018;24(5):e8–e9. 10.1093/ibd/izy121 [DOI] [PubMed] [Google Scholar]
- 18.Chiba M, Tsuji T, Komatsu M, Watanabe H, Takahashi M. Ulcerative colitis in the postpartum period. Autops Case Rep. 2020;10(4):e2020187. 10.4322/acr.2020.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV Jr. Comparative efficacy of biologic therapy in biologic-naïve patients with Crohn disease: A systematic review and network meta-analysis. Mayo Clin Proc. 2014;89(12):1621–1635. 10.1016/j.mayocp.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 20.Siegel CA, Bernstein CN. Identifying patients with inflammatory bowel diseases at high vs low risk of complications. Clin Gastroenterol Hepatol. 2020;18(6):1261–1267. 10.1016/j.cgh.2019.11.034 [DOI] [PubMed] [Google Scholar]
- 21.Chiba M, Tsuji T, Nakane K, et al. Induction with infliximab and a plant-based diet as first-line (IPF) therapy for Crohn disease: A single-group trial. Perm J. 2017;21(4):17–009. 10.7812/TPP/17-009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiba M, Tsuji T, Nakane K, Ishii H, Komatsu M. How to avoid primary nonresponders to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2017;23(11):E55–E56. 10.1097/MIB.0000000000001281 [DOI] [PubMed] [Google Scholar]
- 23.Chiba M, Tsuji T, Komatsu M. How to optimize effects of infliximab in inflammatory bowel disease: Incorporation of a plant-based diet. Gastroenterology. 2020;158(5):1512. 10.1053/j.gastro.2019.12.050 [DOI] [PubMed] [Google Scholar]
- 24.Chiba M, Tanaka Y, Ono I. Early intestinal obstruction after infliximab therapy in Crohn’s disease. Autops Case Rep. 2019;9(1):e2018068. 10.4322/acr.2018.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomised trial. Lancet. 2008;371(9613):660–667. 10.1016/S0140-6736(08)60304-9 [DOI] [PubMed] [Google Scholar]
- 26.Hamdeh S, Aziz M, Altayar O, Olyaee M, Murad MH, Hanauer SB. Early vs late use of anti-TNFa therapy in adult patients with Crohn disease: A systematic review and meta-analysis. Inflamm Bowel Dis. 2020;26(12):1808–1818. 10.1093/ibd/izaa031 [DOI] [PubMed] [Google Scholar]
- 27.O’Dell JR. Treating rheumatoid arthritis early: A window of opportunity? Arthritis Rheum. 2002;46(2):283–285. 10.1002/art.10092 [DOI] [PubMed] [Google Scholar]
- 28.Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. 1980;9(4):469–483. 10.1016/0091-7435(80)90042-0 [DOI] [PubMed] [Google Scholar]
- 29.Chiba M, Nakane K, Takayama Y, et al. Development and application of a plant-based diet scoring system for Japanese patients with inflammatory bowel disease. Perm J. 2016;20(4):16–019. 10.7812/TPP/16-019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boirivant M, Leoni M, Tariciotti D, Fais S, Squarcia O, Pallone F. The clinical significance of serum C reactive protein levels in Crohn’s disease. J Clin Gastroenterol. 1988;10(4):401–405. 10.1097/00004836-198808000-00011 [DOI] [PubMed] [Google Scholar]
- 31.Click B, Vargas EJ, Anderson AM, et al. Silent Crohn’s disease: Asymptomatic patients with elevated C-reactive protein are at risk for subsequent hospitalization. Inflamm Bowel Dis. 2015;21(10):2254–2261. 10.1097/MIB.0000000000000516 [DOI] [PubMed] [Google Scholar]
- 32.Ferrante M, Colombel J-F, Sandborn WJ, et al. Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145(5):978–986. 10.1053/j.gastro.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 33.To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: The adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther. 2016;43(5):549–561. 10.1111/apt.13511 [DOI] [PubMed] [Google Scholar]
- 34.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006;55(6):749–753. 10.1136/gut.2005.082909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichtenstein GR, Hanauer SB, Sandborn WJ. The Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104(2):465–483. 10.1038/ajg.2008.168 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen GC, Nugent Z, Shaw S, Bernstein CN. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology. 2011;141(1):90–97. 10.1053/j.gastro.2011.03.050 [DOI] [PubMed] [Google Scholar]
- 37.Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145(5):996–1006. 10.1053/j.gastro.2013.07.041 [DOI] [PubMed] [Google Scholar]
- 38.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: A nationwide cohort study 1979–2011. Gut. 2014;63(10):1607–1616. 10.1136/gutjnl-2013-305607 [DOI] [PubMed] [Google Scholar]
- 39.Niewiadomski O, Studd C, Hair C, et al. Prospective population-based cohort of inflammatory bowel disease in the biologics era: Disease course and predictors of severity. J Gastroenterol Hepatol. 2015;30(9):1346–1353. 10.1111/jgh.12967 [DOI] [PubMed] [Google Scholar]
- 40.Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: A nationwide population-based study. Inflamm Bowel Dis. 2015;21(3):623–630. 10.1097/MIB.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 41.Okada M, Sakurai T, Yao T, et al. Clinical course and long-term prognosis of Crohn’s disease in Japan. J Gastroenterol. 1994;29(4):406–414. 10.1007/BF02361236 [DOI] [PubMed] [Google Scholar]
- 42.Wintjens D, Bergey F, Saccenti E, et al. Disease activity patterns of Crohn’s disease in the first ten years after diagnosis in the population-based IBD South Limburg Cohort. J Crohns Colitis. 2021;15(3):391–400. 10.1093/ecco-jcc/jjaa173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willett W, Rockström J, Loken B, et al. Food in the anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–492. 10.1016/S0140-6736(18)31788-4 [DOI] [PubMed] [Google Scholar]
- 44.Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans 2015-2020. 8th ed. US Department of Health and Human Services; 2015:35. [Google Scholar]
- 45.Wolf ID, Peterkin BB. Dietary guidelines: The USDA perspective. Food Technol. 1984;38(7):80–86. [Google Scholar]
- 46.Drewnowski A, Popkin BM. The nutrition transition: New trends in the global diet. Nutr Rev. 1997;55(2):31–43. 10.1111/j.1753-4887.1997.tb01593.x [DOI] [PubMed] [Google Scholar]
- 47.Crowley J, Ball L, Hiddink GJ. Nutrition in medical education: A systematic review. Lancet Planet Health. 2019;3(9):e379–e389. 10.1016/S2542-5196(19)30171-8 [DOI] [PubMed] [Google Scholar]
- 48.Amre DK, D’Souza S, Morgan K, et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol. 2007;102(9):2016–2025. 10.1111/j.1572-0241.2007.01411.x [DOI] [PubMed] [Google Scholar]
- 49.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–977. 10.1053/j.gastro.2013.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant JD. Time for change: Benefits of a plant-based diet. Can Fam Physician. 2017;63(10):744–746. [PMC free article] [PubMed] [Google Scholar]
- 51.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20(5):779–786. 10.1016/j.cmet.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee M, Chang EB. Inflammatory bowel diseases (IBD) and the microbiome—searching the crime scene for clues. Gastroenterology. 2021;160(2):524–537. 10.1053/j.gastro.2020.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parada Venegas D, De la Fuente MK, Landskron G, et al. Corrigendum: Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:1486. 10.3389/fimmu.2019.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohno H. The impact of metabolites derived from the gut microbiota on immune regulation and diseases. Int Immunol. 2020;32(10):629–636. 10.1093/intimm/dxaa041 [DOI] [PubMed] [Google Scholar]
- 58.Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353. 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao CK, Muir JG, Gibson PR. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016;43(2):181–196. 10.1111/apt.13456 [DOI] [PubMed] [Google Scholar]
- 60.Pittayanon R, Lau JT, Leontiadis GI, et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology. 2020;158(4):930–946. 10.1053/j.gastro.2019.11.294 [DOI] [PubMed] [Google Scholar]
- 61.Buhl S, Steenholdt C, Rasmussen M, et al. Outcomes after primary infliximab treatment failure in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(7):1210–1217. 10.1097/MIB.0000000000001117 [DOI] [PubMed] [Google Scholar]
- 62.Magnusson MK, Strid H, Sapnara M, et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis. 2016;10(8):943–952. 10.1093/ecco-jcc/jjw051 [DOI] [PubMed] [Google Scholar]
- 63.Yilmaz B, Juillerat P, Øyås O, et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med. 2019;25(2):323–336. 10.1038/s41591-018-0308-z [DOI] [PubMed] [Google Scholar]
- 64.Aden K, Rehman A, Waschina S, et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology. 2019;157(5):1279–1292. 10.1053/j.gastro.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 65.Chiba M, Tsuji T, Nakane K, et al. High remission rate with infliximab and plant-based diet as first-line (IPF) therapy for severe ulcerative colitis: Single-group trial. Perm J. 2020;24:1–10. 10.7812/TPP/19.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cammà C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohn’s disease: A meta-analysis adjusted for confounding variables. Gastroenterology. 1997;113(5):1465–1473. 10.1053/gast.1997.v113.pm9352848 [DOI] [PubMed] [Google Scholar]
- 67.Hyman MA, Ornish D, Roizen M. Lifestyle medicine: Treating the causes of disease. Altern Ther Health Med. 2009;15(6):12–14. [PubMed] [Google Scholar]
- 68.Bodai BI, Nakata TE, Wong WT, et al. Lifestyle medicine: A brief review of its dramatic impact on health and survival. Perm J. 2018;22:17–025. 10.7812/TPP/17-025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev. 2013;2(2):CD008722. 10.1002/14651858.CD008722.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. 10.1056/NEJMoa0708681 [DOI] [PubMed] [Google Scholar]
- 71.Atallah R, Filion KB, Wakil SM, et al. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: A systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2014;7(6):815–827. 10.1161/CIRCOUTCOMES.113.000723 [DOI] [PubMed] [Google Scholar]
- 72.Sasson AN, Ananthakrishnan AN, Raman M. Diet in treatment of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2021;19(3):425–435. 10.1016/j.cgh.2019.11.054 [DOI] [PubMed] [Google Scholar]
- 73.Schreiner P, Yilmaz B, Rossel J-B, et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United European Gastroenterol J. 2019;7(6):767–781. 10.1177/2050640619841249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Albenberg L, Brensinger CM, Wu Q, et al. A diet low in red and processed meat does not reduce rate of Crohn’s disease flares. Gastroenterology. 2019;157(1):128–136. 10.1053/j.gastro.2019.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiba M, Ishii H, Komatsu M. Recommendation of plant-based diets for inflammatory bowel disease. Transl Pediatr. 2019;8(1):23–27. 10.21037/tp.2018.12.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tpp_21.073-suppl-01.docx (90.7KB, docx)