Abstract
Introduction
Osler-Weber-Rendu syndrome, or hereditary hemorrhagic telangiectasia, is a rare genetic disease that causes recurrent epistaxis and anemia. Numerous bleeding vascular malformations can be found throughout the body.
Case presentation
A 75-year-old woman presented to her hematologist with recurrent epistaxis, iron deficiency anemia, menorrhagia, and hypothyroidism. Her mother had similar nosebleeds, and physical examination revealed small vascular malformations on the conjunctiva, oropharynx, tongue, lip, and palate. Heavy epistaxis occurred several times per week. Multiple nasal and gastrointestinal endoscopic procedures were performed. She received over 100 iron infusions and multiple blood transfusions. Overall treatment involved integrated care with multiple medical specialties.
Conclusion
Hereditary hemorrhagic telangiectasia and other complex diseases are best treated with a multidisciplinary approach within an integrated health care setting.
Keywords: chronic conditions, clinical, diagnosis, hematology, otolaryngology, teamwork
Introduction
Imagine having a nosebleed every day. Imagine not knowing if the next nosebleed might result in your hospitalization or death. For some patients with hereditary hemorrhagic telangiectasia (HHT), nosebleeds can be a serious, life-changing problem. When asked about her health concerns, “I’m worried I will bleed to death ...” was the pressing response from a patient with recurrent epistaxis for 45 years. Through the clinical and personal account of a patient with HHT, this article aims to help clinicians develop an integrated clinical approach to this multifaceted disease. This case report was prepared following the CARE Guidelines.1
HHT, also known as Osler-Weber-Rendu syndrome, is an autosomal dominant disorder occurring in 1:5000–8000 individuals.2 HHT is most commonly diagnosed using the Curaçao criteria: recurrent and spontaneous epistaxis, telangiectasias, arteriovenous malformations (AVMs), and/or a family history of HHT.2 The diagnosis is definitive if at least 3 of the 4 criteria are present.
The presentation of HHT can be challenging to recognize. A variety of nonspecific manifestations may occur, such as: migraine, seizure, back pain, hypoxemia, hypoesthesia of extremities, heart failure, dyspnea, fatigue, and stroke. The disease may result in difficulty completing activities of daily living. Epistaxis is the most common reason for presentation to otolaryngologists. More than half of patients with HHT report recurrent nosebleeds negatively impact their work, social activities, and overall quality of life.3 Pain and discomfort due to epistaxis, nasal packing, and other treatments are noted as principal factors in decreasing disease-related quality of life and overall energy level.3 HHT may have an immense impact on the patient’s well-being. Thus, establishing the correct clinical diagnosis is critical for treatment and to improve health outcomes.
Developing an effective management strategy can be a monumental task. The multisystem involvement of HHT demands a multidisciplinary approach.4 Frigerio et al reported that structured, multidisciplinary programs with a variety of specialists help improve patient care by avoiding extraneous examinations and ensuring proper follow-up. Clinicians within such integrated care settings provide an earlier diagnosis, faster treatment, and a decreased chance of serious complications.5 This framework allows a team of health care specialists to design and establish an all-inclusive treatment plan and carefully coordinate each aspect of patient care. The following case illustrates the complexities involved in the diagnosis and management of HHT within a modern, large US integrated health care setting.
Case Narrative
Presenting concerns
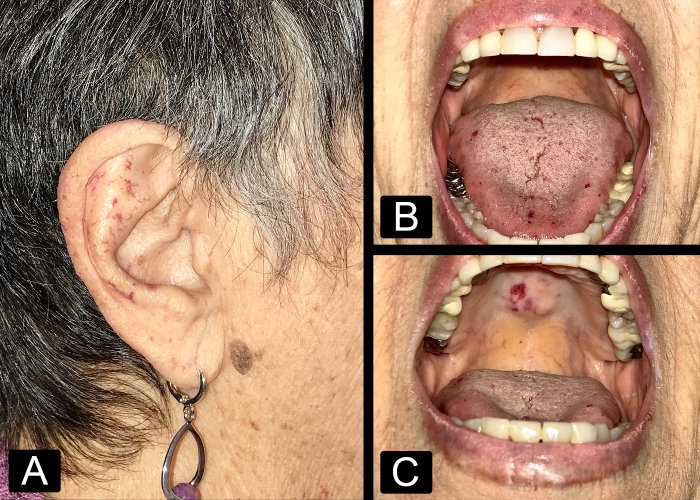
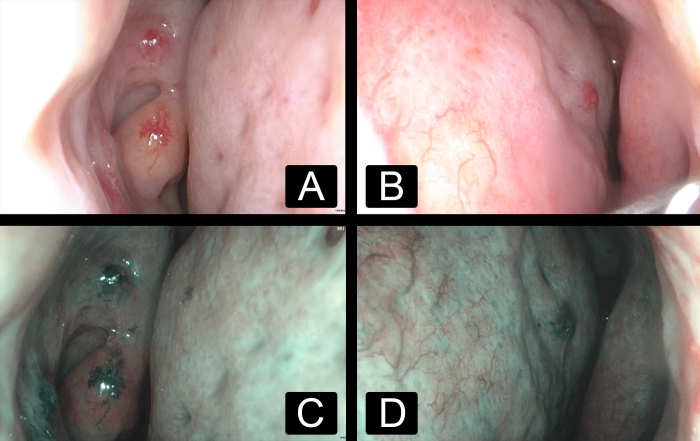
A 75-year-old woman with a past medical history of recurrent epistaxis, iron deficiency anemia, menorrhagia, and hypothyroidism first presented to her hematologist in 2008. She reported nosebleeds every few days that lasted 20 to 60 minutes, often requiring “nose clamps” to stop the bleeds due to “thin membranes” in her nose. Her mother had similar nosebleeds. The patient also experienced heavy menses. Physical examination revealed small vascular malformations on the face, conjunctiva, oral cavity, oropharynx, and bleeding from the nasal mucosa membrane (Figure 1). Her skin demonstrated innumerable cherry hemangiomas and telangiectasias throughout most of her body. HHT was diagnosed based on the clinical criteria. Genetic testing was offered and consultation with otolaryngology and gastroenterology were arranged immediately. Nasal endoscopy revealed telangiectasias in the nasal cavity and nasopharynx (Figure 2). In 2015, the patient experienced bloody stools, severe stomach pain, generalized fatigue, and nausea. Gastrointestinal endoscopy revealed multiple AVMs in the stomach. The patient opted to decline imaging to evaluate for pulmonary and brain AVMs.
Figure 1:
Clinical photographs demonstrating cutaneous telangiectasias involving (A) the facial skin and auricle, (B) the oral tongue, and (C) the hard palate.
Figure 2:
Nasal endoscopy images demonstrating nasal cavity mucosal telangiectasias in the (A) right and (B) left nasal cavities under white light and the (C) right and (D) left nasal cavities under narrow band imaging, consisting of 415 nm blue light and 540 nm green light, allowing improved visibility of blood vessels.
Therapeutic Intervention and Treatment
Initial preventative measures included a humidifier, nasal saline spray, saline gel, hydration, and intravenous iron supplementation. During epistaxis episodes, topical mupirocin ointment, Afrin spray, and nasal clamps were often used. Nasal packing was avoided to minimize trauma-induced epistaxis. Regular consultation with hematology was maintained to manage anemia and blood transfusions.
Otolaryngologists performed epistaxis control using medications and specialized endoscopic procedures. Additionally, intranasal silicone splints were placed on the nasal septum long-term to prevent dryness and decrease epistaxis severity.
Gastroenterologists performed endoscopic procedures to treat AVMs in the gastric body and antrum using submucosal epinephrine injections and argon plasma coagulation. Between 2013 and 2020, the patient underwent 5 nasal endoscopic procedures, 4 gastrointestinal endoscopic procedures, over 100 iron infusions, and 5 blood transfusions.
Follow-Up and Outcomes
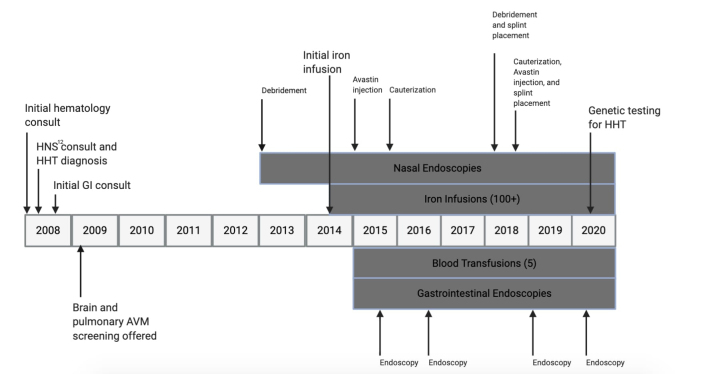
The patient tolerated iron infusions. There was no diarrhea from excess iron intake, and the patient was pleased with the feeling of increased energy. Her nasal procedures successfully decreased the frequency and severity of epistaxis based on a patient-assessed Epistaxis Severity Score. The Epistaxis Severity Score provides 6 questions that measure nosebleed frequency and length, and plots the score on a scale between 0–10. A score of 0 indicates no disease, while 10 indicates severe disease. Before a nasal endoscopy procedure in 2018 was performed, a score of 9.5 (severe disease) was attained. A follow-up score of 3.8 (mild disease) 2 months later suggested marked improvement, with decreases in nosebleed frequency and postcauterization bleeding. Gastrointestinal procedures improved the severity of bleeding AVMs, but the patient still experienced melena, nausea, and bloating as new AVMs developed. Hemoglobin values also improved from 5.2 g/dL in 2015, indicating severe anemia, to 11.6 g/dL in 2019, a value within typical limits. A representative timeline demonstrates the patient’s clinical course over a 12-year period (Figure 3).
Figure 3:
Timeline demonstrating the clinical course of the patient as it relates to her hereditary hemorrhagic telangiectasia care. AVM = arteriovenous malformations; HHT = hereditary hemorrhagic telangiectasia.
A multidisciplinary team of specialists and primary care physicians collaborated to ensure regular follow-up and monitor treatment progress. Early integration of care and close communication between the health care team facilitated the complex management of the patient’s surgical, pharmacologic, and holistic medical treatment.
Discussion
HHT requires specific clinical indicators for an accurate diagnosis. The patient’s history of recurrent and spontaneous epistaxis, telangiectasias, and family history of similar epistaxis resulted in the clinical diagnosis of HHT. The complex nature of this disease lends itself to underdiagnosis. Analysis of the Kaiser Permanente Northern California adult patient population from 2010 to 2012 revealed that 1:24,000 patients had undiagnosed HHT, using ICD-9-CM code criteria.6 Although the prevalence is substantially less than the true national average of 1:5000–8000, clinical manifestations must be recognized to limit underdiagnosis, ensure patient safety, and maximize efficient treatment of HHT. It is of utmost importance for the physician to maintain sharp clinical acumen to make a timely diagnosis. Once diagnosed, the physician will require the assistance of multiple specialty clinicians to coordinate and oversee disease management.
A multidisciplinary approach has been proven to be the most effective way to treat and manage HHT patients. Medical centers, such as the Vaudois University Hospital Center in Lausanne, Switzerland, coordinate the efforts of several specialists to avoid gaps in performing exams and procedures, achieving better outcomes.5 Other Geneva University hospitals utilize a similar structure of systematizing management of HHT patients. This multidisciplinary approach includes otolaryngologists, pulmonologists, gastroenterologists, dermatologists, hematologists, radiologists, and geneticists to increase efficacy in the treatment of HHT and other complex diseases. A report of 19 cases from the Brain Vascular Malformation Consortium HHT project also suggests that a multidisciplinary approach is essential in determining whether neurosurgical treatment is the best course of action for HHT patients with brain AVMs.7
Kaiser Permanente addresses a patient’s needs across the continuum of care with an integrated, multidisciplinary approach. This integrated system coordinates efficient and comprehensive health care, encompassing physicians in all specialties who share an organization-wide electronic health record system. For HHT patients, this means notes, lab findings, endoscopy results, and biopsy results can be easily shared among multidisciplinary team members who can then better manage the disease and communicate with the patient. In this case, the ability to quickly share the patient’s medical record between the primary medical physician, hematologist, otolaryngologist, and gastroenterologist facilitated a rapid and efficient diagnosis. Overall, Kaiser Permanente’s integrated process comprises a focused team approach, demonstrating practicality, convenience, and value in prioritizing prompt patient care, putting the patient at the center of decision making.
Genetics is an essential part of the integrated approach. Genetic counselors assist with genetic testing and examination of genetic risks. They may help detect pathogenic variants during prenatal care and affected offspring of undiagnosed parents.8 Typically, HHT genetic screening encompasses analysis of 5 genes: ACVRL-1 (also known as ALK-1), ENG, GDF2, RASA1, and SMAD4. ENG on chromosome 9 and ALK-1 on chromosome 12 are a few of many genes expressed on the vascular endothelium responsible for preserving vascular structure.9 Up to 80% of people with clinical signs of HHT are found to have ENG and ALK-1 mutations, but 10–15% of mutations are still unknown.10 In this case, genetic testing resulted in no known mutations, only a variant of uncertain significance was found. Although genetic testing is not required for a definitive diagnosis of HHT, integration of genetics as part of multidisciplinary care is beneficial in early detection of family-specific pathogenic mutations.11
The management of HHT requires a multipronged approach. The primary medical team and the patient should be acquainted with epistaxis preventative measures. Patients are often their own primary caretakers, managing the social and personal implications of HHT outside of controlled medical settings. An example in current times is nasal swab testing for COVID-19; such procedures may ultimately be fatal for HHT patients with sensitive nasal mucosa and AVMs. Therefore, patients must assume a crucial role in disease surveillance, symptom management, and effective communication as a member of the medical team.
Provided below is an account of the patient’s personal experience with HHT, which demonstrates how this disease impacts every aspect of her life.
Conclusion
Greater clinical awareness for all practitioners may be the first step in identifying patients with HHT, and a multidisciplinary approach to treatment in the context of an integrated health care system prioritizes safe and quality care for patients.
| Patient Perspective |
|---|
|
1. How does HHT affect your daily life?
“I don’t know when my nose will start bleeding. These things can start at any time, and I have to be ready. That’s why I carry a nose clamp, many tissues, and an Afrin spray wherever I go.” “When I was first diagnosed, I was forced to retire. I had to build up confidence and learn how to effectively take care of my symptoms. That helps you make choices and live your life normally.” 2. What are your concerns for your future health? “I’m worried I will bleed to death, because it is unclear how long a bleed will last. For the future, I am worried about living by myself in case I go into hypovolemic shock. Now, I measure the bleeding by how long it lasts, and decide if I should go to the hospital if it takes too long to stop. COVID-19 makes the situation more complicated because simply getting a nasal swab will make me bleed even more.” 3. What are the psychological impacts of having HHT? “Until you learn how to stop a nosebleed and stomach pain confidently, you wonder, is this the one going to take me to the hospital? In social situations when I am dressed nicely, I want to avoid stained clothes out in public or with friends.” 4. Why did you choose to share your diagnosis of HHT? “I want everyone in the world to know about HHT; if I can save 1 person 1 night of stress, or show them what to do if they start to bleed, then it’s worth it. Other people with HHT do not want to tell others because they don’t know what it is; it’s nothing to be ashamed of, it’s how your body works.” 5. What do you wish health practitioners knew about diagnosing future patients with HHT? “If someone suspects HHT from heavy nose bleeding, they need to call an [ear, nose, and throat doctor] or hematologist for them to look in the nose, and see ‘ground meat’ for AVMs. The physician should notice that the bleeding is uncontrollable and the use of foam packs or even clamps should give some insight into the problem. Primary care physicians should also be more aware of patients that are having exceptionally long, heavy, and consistent nosebleeds and that there is more to a nosebleed than just a nosebleed. Listen more to what the patient experiences, especially if they have other comorbidities.” |
Footnotes
Funding: None declared
Conflicts of Interest: None declared
Author Contributions: Michael Zhitnitsky, BS and Jason Gilde, MD both participated in the critical review, drafting, and submission of the final manuscript.
Consent: Informed consent was received from case patient.
References
- 1.Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: Explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. 10.1016/j.jclinepi.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 2.Pahl KS, Choudhury A, Wusik K, et al. Applicability of the curaçao criteria for the diagnosis of hereditary hemorrhagic telangiectasia in the pediatric population. J Pediatr. 2018;197:207–213. 10.1016/j.jpeds.2018.01.079 [DOI] [PubMed] [Google Scholar]
- 3.Geirdal AØ, Dheyauldeen S, Bachmann-Harildstad G, Heimdal K. Quality of life in patients with hereditary hemorrhagic telangiectasia in Norway: A population based study. Am J Med Genet A. 2012;158A(6):1269–1278. 10.1002/ajmg.a.35309 [DOI] [PubMed] [Google Scholar]
- 4.Lupa MD, Wise SK. Comprehensive management of hereditary hemorrhagic telangiectasia. Curr Opin Otolaryngol Head Neck Surg. 2017;25(1):64–68. 10.1097/MOO.0000000000000319 [DOI] [PubMed] [Google Scholar]
- 5.Frigerio C, Aebischer N, Baud D, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): Clinical manifestations and multidisciplinary management. Rev Med Suisse. 2016;12(517):896–901. [PubMed] [Google Scholar]
- 6.Saparia T, Faughnan ME, Schneider JL, et al. Assessing the hereditary hemorrhagic telangiectasia algorithms in a community-based patient population. Perm J. 2019;23:18–145. 10.7812/TPP/18-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meybodi AT, Kim H, Nelson J, et al. Surgical treatment vs nonsurgical treatment for brain arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia: A retrospective multicenter consortium study. Neurosurgery. 2018;82(1):35–47. 10.1093/neuros/nyx168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald J, Pyeritz RE, et al. Hereditary Hemorrhagic Telangiectasia. In: Adam MP, ed. GeneReviews. University of Washington, Seattle; 1993. [PubMed] [Google Scholar]
- 9.Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: Current views on genetics and mechanisms of disease. J Med Genet. 2006;43(2):97–110. 10.1136/jmg.2005.030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girit S, Senol E, Karatas Ö, Yıldırım Aİ. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. Respir Med Case Rep. 2020;30:101137. 10.1016/j.rmcr.2020.101137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg N, Khunger M, Gupta A, Kumar N. Optimal management of hereditary hemorrhagic telangiectasia. J Blood Med. 2014;5:191–206. 10.2147/JBM.S45295 [DOI] [PMC free article] [PubMed] [Google Scholar]