Abstract
Bartonella henselae is the causative agent of human cat scratch disease as well as several serious sequelae of infections, including bacillary angiomatosis and bacillary peliosis. Conflicting reports describe the pathogenesis of B. henselae in the cat. In this study, we characterized a strain of B. henselae termed LSU16. This strain was isolated on rabbit blood agar from a naturally infected 10-month-old female cat during a recurrent episode of bacteremia. The bacterial species was confirmed by PCR-restriction fragment length polymorphism analysis. Nine cats were infected intradermally with 5 × 107 CFU of LSU16, and clinical signs, antibody responses, and bacteremia were monitored. All nine cats developed raised, erythematous areas at the site of inoculation within 72 h postinoculation; the swelling peaked at 14 days postinfection and was not palpable by 28 days postinfection. Fever developed in all nine cats between 6 and 16 days postinfection and lasted for 1 to 8 days. Between 6 and 16 days postinfection, all nine cats experienced lethargy which persisted 5 to 18 days. Seven of nine cats were bacteremic by day 7, and all nine cats had become bacteremic by 14 days postinfection. Bacteremia peaked at 14 to 28 days postinfection in all cats. In six of the nine infected cats, bacterial numbers reached nondetectable levels during the 7th week postinfection; however, a single animal maintained bacteremia to 18 weeks postinfection. All nine cats developed strong antibody responses to B. henselae, as determined by Western blot analysis and enzyme-linked immunosorbent assay. Subsequently, three naive cats were injected intradermally with blood from cats infected with LSU16 from a pure culture, and five naive cats were injected with feces from fleas which had been feeding on cats infected with a pure culture of LSU16. These cats developed signs similar to those described in the previous experiment and were euthanized at 5 weeks postinfection. We conclude that B. henselae LSU16 is a virulent strain of B. henselae in cats and propose that the virulence of B. henselae in cats is strain dependent.
Bartonella henselae, a gram-negative, oxidase-negative, nonflagellated, fastidious aerobic bacterium, is the causative agent of a wide range of clinical disease syndromes in human patients, including cat scratch disease (CSD). The typical CSD, which is seen in the majority of immunocompetent patients, is characterized by lymphadenopathy in the nodes draining the site of a cat scratch. Local lesions exhibiting redness, swelling, and/or a pustule at the site of the scratch may also develop but are not consistently seen. The involved lymph node usually regresses over a period of weeks to months. Patients may also experience fever, malaise, anorexia, and/or headache 7 to 10 days following exposure and painful lymphadenopathy in moderate or severe CSD. In approximately 10% of cases, the lymphadenopathy associated with CSD may proceed to suppuration. Serious sequelae, including ocular disease, encephalopathy, osteolytic lesions, and life-threatening bacillary angiomatous and bacillary peliosis, occur in a small percentage of immunocompetent individuals (2, 11, 19).
B. henselae is a zoonotic agent, and cats are the natural carrier (6, 22); however, the pathogenesis of B. henselae in the cat is not clearly understood. Cats naturally infected with B. henselae have recurrent periods of bacteremia, and during these periods of bacteremia they do not show clinical signs of disease (15). There are conflicting reports with regard to clinical signs in experimentally infected cats. Abbott et al. (1) and Regnery et al. (17) reported no clinical signs in experimentally infected cats. Guptill et al. (5) reported mild clinical signs which included mild fever as well as histopathologic lesions in some cats to 8 weeks postinfection. Kordick and Breitschwerdt (8) reported fever in six of eight experimentally infected cats and signs of abnormal central nervous system (CNS) disease in two cats. These findings are difficult to compare because of differences in the strain used to infect the cats, the route of infection, and the size of the inoculum.
Studies of the pathogenesis of B. henselae require a thorough characterization of the strain that is used. In this study, we characterized a strain of B. henselae, termed LSU16, which is pathogenic in experimentally infected cats and which produces a reproducible disease. We describe pathogenic events following intradermal (i.d.) inoculation of cats, including bacteremia and clinical signs, and discuss the usefulness of this strain for future studies involving characterization of B. henselae infection of cats.
MATERIALS AND METHODS
Cats.
Thirty-two 7- to 15-month-old cats, purchased either as conditioned pound source cats from the Louisiana State University Division of Laboratory Animal Medicine or as specific-pathogen-free (SPF) cats from Harlan Sprague-Dawley (Madison, Wis.), were used in these experiments. All of the cats were culture negative for B. henselae and antibody negative for B. henselae by Western blot analysis. The cats were housed individually and supplied with water and laboratory maintenance diet ad libidum. All cats were wormed and were vaccinated against the common infectious diseases of cats. The sources of individual cats are indicated in Tables 1 through 3.
TABLE 1.
Adverse clinical signs among cats inoculated with a pure culture of B. henselae LSU16
| Cat no. | Sourcea | Inoculum (CFU) | Ageb (mo) | No. of cats exhibiting:
|
|||
|---|---|---|---|---|---|---|---|
| Feverc
|
Depression
|
||||||
| Starting dayd | Duration (days) | Starting dayd | Duration (days) | ||||
| 37 | LSU | 5 × 107 | 14 | 10 | 4 | 10 | 8 |
| 39 | LSU | 5 × 107 | 15 | 11 | 5 | 13 | 9 |
| 40 | LSU | 5 × 107 | 14 | 10 | 3 | 10 | 8 |
| 50 | LSU | 5 × 107 | 15 | 16 | 1 | 13 | 7 |
| 54 | LSU | 5 × 107 | 15 | 6 | 8 | 15 | 5 |
| 58 | LSU | 5 × 107 | 14 | 10 | 3 | 10 | 8 |
| 182 | HSD | 5 × 107 | 7 | 13 | 5 | 11 | 8 |
| 184 | HSD | 5 × 107 | 7 | 16 | 2 | 11 | 11 |
| 223 | HSD | 5 × 107 | 7 | 14 | 5 | 11 | 12 |
| Mean ± SD | 5 × 107 | 12 | 12 ± 3 | 4 ± 2 | 12 ± 2 | 8 ± 2 | |
Cats were purchased from either the Division of Laboratory Animal Medicine at Louisiana State University (LSU) or Harlan Sprague-Dawley (HSD).
Age at the time of infection.
Rectal temperature of >103.0°F (39.4°C).
Day postinoculation that sign was first evident.
TABLE 3.
Adverse clinical signs in cats inoculated with feces from fleas feeding on cats infected with pure cultures of B. henselae LSU16
| Cat no. | Sourcea | Ageb (mo) | No. of cats exhibiting:
|
|||
|---|---|---|---|---|---|---|
| Feverc
|
Depression
|
|||||
| Starting dayd | Duration (days) | Starting dayd | Duration (days) | |||
| 353 | HSD | 5 | 15 | 4 | 15 | 5 |
| 359 | HSD | 5 | 16 | 3 | 16 | 7 |
| 357 | HSD | 5 | 12 | 6 | 14 | 8 |
| 350 | HSD | 5 | 18 | 1 | 15 | 5 |
| 225 | HSD | 7 | 12 | 6 | 7 | 14 |
| Mean ± SD | 5 ± 1 | 15 ± 3 | 4 ± 2 | 13 ± 4 | 8 ± 4 | |
Cats were purchased from Harlan Sprague-Dawley (HSD).
Age at the time of infection.
Rectal temperature of >103.0°F (39.4°C).
Day postinoculation that sign was first evident.
Isolation and propagation of B. henselae LSU16.
Cat no. 16, a female domestic short-haired cat that was antibody positive for Bartonella species, was purchased from the East Baton Rouge Parish Humane Society at approximately 16 weeks of age. After being conditioned, cat 16 was treated with Advantage (Bayer Corp., Shawnee Mission, Kans.) according to the manufacturer’s instructions to kill ectoparasites and with Ivermectin (Merck & Co., Inc., Rahway, N.J.) to kill ear mites and subsequently housed in a single-animal cage. Blood was collected weekly from cat 16, and serial dilutions of the blood were cultured for the presence of Bartonella sp. on rabbit blood agar plates. Cat 16 was negative by culture for 20 weeks. During the 21st week, 1,000 CFU of an organism consistent with B. henselae was cultured per ml of cat 16 blood. The isolate, termed LSU16, had a colony morphology consistent with that of B. henselae and was confirmed to be B. henselae by PCR-restriction fragment length polymorphism analysis as previously reported (8). The initial isolate of LSU16 was expanded twice on chocolate agar under an atmosphere of 5% CO2 at 37°C. Bacterial lawns were harvested after 5 to 8 days of culture, suspended in heart infusion broth with 25% glycerol, and stored as aliquots at −70°C. Before inoculation into cats, these aliquots of B. henselae were thawed and the culture was centrifuged to remove the freezing medium. The bacterial pellet was then suspended in saline to the appropriate concentration prior to inoculation, and the actual number of CFU per milliliter and the purity of the culture were confirmed by culturing serial dilutions on chocolate agar under an atmosphere of 5% CO2 at 37°C for 7 days. All animal inoculations were conducted with a second-passage preparation. Bacteremia in experimentally infected cats was determined by culturing serial dilutions of whole blood on chocolate agar under an atmosphere of 5% CO2 at 37°C for 7 days.
Infection of cats.
Nine cats were each given 5 × 107 CFU of LSU16 bacterial culture. Subsequently, three cats were given 1 ml of blood from one of three cats infected with a pure culture of LSU16 14 days previously. Five cats were injected with 45 mg of feces from fleas that had fed on LSU16-infected cats for 4 days as previously described (4). All cats were injected i.d. with 1.0 ml of the respective inocula, divided among six sites on the skin of the lateral trunk. The sites of inoculation were marked with a water-indelible marker and monitored daily.
Collection of samples from cats.
Prior to sample collection, cats were anesthetized with Telazol (Fort Dodge, Ames, Iowa) at the dosage recommended by the manufacturer. All cats were bled weekly by jugular puncture, using a 10-ml syringe and a 21-gauge needle, and the blood was distributed among 1.5-ml pediatric lysis-centrifugation isolator tubes (Wampole Laboratories, Cranbury, N.J.) for culture and among an EDTA tube and serum Vacutainer tubes (Becton-Dickinson, Franklin Lakes, N.J.) for complete blood counts and serological testing, respectively. Serum was collected by centrifugation of clotted blood at 800 × g and was frozen in aliquots at −20°C. Spinal fluid samples were aseptically collected by cerebellomedullary cisternal puncture from three anesthetized cats which had shown signs of possible CNS involvement. Cytologic preparations and creatine phosphokinase levels were examined, and the cerebrospinal fluid was cultured for the presence of B. henselae. Urine, spinal fluid, and tissues were collected from cats at necropsy for culture of B. henselae.
Clinical signs.
All cats were monitored daily for clinical signs of disease. To enumerate clinical disease, each of the following signs was assigned one point: swelling and/or redness at the site of inoculation, temperature of 39.5 to 40.5°C, temperature over 40.5°C, palpable lymphadenopathy (popliteal, prescapular, and/or submandibular lymph nodes), diarrhea, vomiting, anorexia, aggression, and lethargy. Cats received one point each week for each of the clinical signs which were observed within 1 week.
Analysis of antibody responses of cats to B. henselae LSU16.
The immunoglobulin G (IgG) and IgM responses to B. henselae LSU16 were monitored by enzyme-linked immunosorbent assay (ELISA) as previously described (4).
RESULTS
Bacteremia in cats infected with LSU16.
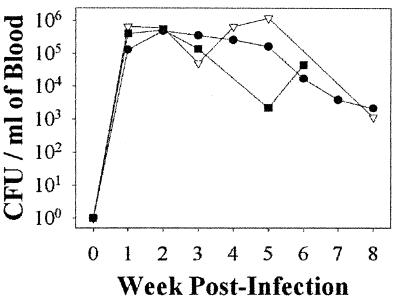
Nine animals were each given 5 × 107 CFU of LSU16, three animals were each given blood containing an average of 6.2 × 105 CFU/ml, and five animals were each given 45 mg of feces from fleas feeding on cats infected with LSU16. In addition, 15 cats not exposed to B. henselae were also monitored. All 17 cats infected with B. henselae LSU16 developed high levels of bacteremia, regardless of the inoculum used. All of the cats exposed to a pure culture of LSU16 were bacteremic by week 2, but seven of nine had developed bacteremia by 7 days (Fig. 1). Peak bacteremia occurred between week 2 (seven of nine cats) and week 4 (one of nine animals). The number of CFU of B. henselae in the blood inocula was lower than that in the pure-culture inocula (Table 2) and may have resulted in a slower onset of bacteremia in those cats given blood. The number of CFU in flea feces could not be accurately measured due to the presence of other organisms in the inoculum and was also presumed to be smaller than that of cats receiving pure-culture inocula. Only six of eight cats receiving blood or flea feces were bacteremic by week 2; however, all of the cats in both groups were bacteremic by week 3. Peak bacteremia occurred in most cats by week 2 (6 of 14) or week 3 (7 of 17). The number of bacteria began to wane after 4 weeks, and five of nine cats were negative by 8 weeks postinfection. Of the cats infected with pure-culture inoculum, only one (no. 58) was culture positive at 12 weeks, and that cat remained positive until 18 weeks postinfection and then remained negative until week 22, when the experiment was terminated. The eight cats infected with either blood or flea feces showed a similar pattern, with a slightly slower onset of bacteremia (Fig. 1); however, these cats were euthanized 8 weeks postinfection, while all eight cats were still bacteremic.
FIG. 1.
Mean levels of bacteremia in B. henselae-infected cats. Shown are mean CFU per milliliter values determined for blood of cats infected with a pure culture of LSU16 (●) (n = 9), blood from an LSU16-infected cat (▿) (n = 3), or feces from fleas feeding on a B. henselae LSU16-infected cat (■) (n = 5). Blood from cats infected with feces from fleas feeding on a B. henselae-infected cat was not cultured in weeks 7 and 8. No organisms were cultured from the blood of noninfected cats at any time during the course of this study.
TABLE 2.
Adverse clinical signs among cats inoculated with blood from B. henselae LSU16-infected cats
| Cat no. | Sourcea | Inoculum,b CFU | Agec (mo) | No. of cats exhibiting:
|
|||
|---|---|---|---|---|---|---|---|
| Feverd
|
Depression
|
||||||
| Starting daye | Duration (days) | Starting daye | Duration (days) | ||||
| 7 | LSU | Blood (223), 3.6 × 105 | 14 | 21 | 2 | 22 | 11 |
| 156 | HSD | Blood (182), 5.1 × 105 | 14 | 18 | 3 | 18 | 7 |
| 190 | HSD | Blood (184), 1.0 × 106 | 7 | 18 | 4 | 21 | 11 |
| Mean ± SD | 6.2 × 105 | 11.7 | 19 ± 2 | 3 ± 1 | 20 ± 2 | 10 ± 2 | |
Cats were purchased from either the Division of Laboratory Animal Medicine at Louisiana State University (LSU) or Harlan Sprague-Dawley (HSD).
The cat number in parentheses indicates the source of the blood and refers to a cat in Table 1.
Age at the time of infection.
Rectal temperature of >103.0°F (39.4°C).
Day postinoculation that sign was first evident.
Clinical disease in cats infected with LSU16.
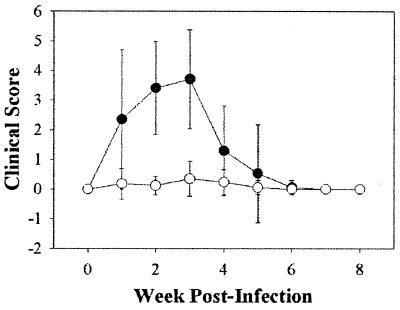
Table 4 lists the clinical signs that were seen in the cats, and the mean clinical scores for the animals are presented in Fig. 2. Every cat exposed to B. henselae developed fever and became somewhat sluggish and lethargic between 6 and 21 days postinoculation. In addition, 15 of the 17 cats developed strong, gross inflammatory lesions at the sites of inoculation, and 3 of the cats developed pustules. In most cats, these lesions started to develop by 72 hours postinoculation, became red and hard by 10 days postinoculation, and then gradually regressed over the next 14 to 18 days. All 17 cats were observed to have decreased appetites, but 8 of the 17 cats required force feeding and/or subcutaneous fluid replacement for up to 10 days. One infected animal was observed to have vomited once, and one infected cat had intermittent soft stools during the period of anorexia; however, since intermittent soft stools were seen in 2 of the 15 control cats (data not shown), this was probably not related to the infection. There were no significant differences in erythrocytes, lymphocytes, monocytes, or neutrophils, either in percentage or absolute number, between the infected and noninfected groups (data not shown).
TABLE 4.
Adverse clinical signs in cats inoculated with B. henselae LSU16
| Sign | No. of cats exhibiting sign/total no. (%) inoculated witha:
|
|||
|---|---|---|---|---|
| Uninfected (controls) | Pure culture of LSU16 | Infected blood | Infected flea feces | |
| Feverb | 0/15 (0) | 9/9 (100) | 3/3 (100) | 5/5 (100) |
| Lethargy | 0/15 (0) | 9/9 (100) | 3/3 (100) | 5/5 (100) |
| Swelling and/or redness at site of inoculation | 0/15 (0) | 9/9 (100) | 3/3 (100) | 4/5 (80) |
| Pustule at site of inoculation | 0/15 (0) | 3/9 (33) | 0/3 (0) | 0/3 (0) |
| Anorexia | 0/15 (0) | 9/9 (100) | 3/3 (100) | 5/5 (100) |
| Anorexia requiring force feeding and/or fluids | 0/15 (0) | 3/6 (50) | 0/3 (0) | 5/5 (100) |
| Vomiting | 0/15 (0) | 1/9 (11) | 0/3 (0) | 0/5 (0) |
| Muscle pain or stiffness | 0/15 (0) | 8/9 (89) | 2/3 (67) | 3/5 (60) |
| Abnormal or aggressive behavior | 0/15 (0) | 5/9 (56) | 0/3 (0) | 0/5 (0) |
| Lymphadenopathy | 7/15 (47) | 5/6c (83) | 3/3 (100) | 5/5 (100) |
Number of cats that showed the indicated sign at any time in the study.
Rectal temperature of >103.0°F (39.4°C).
Three cats receiving pure-culture inocula were not monitored for lymphadenopathy during the first 4 weeks postinfection and were excluded.
FIG. 2.
Mean clinical scores ± standard deviations for 17 cats exposed to strain LSU16 of B. henselae (●) and 15 noninfected cats (○). The clinical scores of the two groups for weeks 2 and 3 were significantly different (P < 0.05).
Palpable lymph node enlargements were observed in five of six pure-culture-infected cats and in all of the cats that received the blood or flea feces inoculum. The enlargement of the lymph nodes was first observed at week 2 postinfection and continued for 2 to 4 weeks postinfection. These cats did not show any significant sensitivity in the area of nodal enlargement. We observed sporatic enlargement of lymph nodes in 7 of the 15 noninfected cats.
Several cats (13 of the 17) showed a variety of other signs, including muscle tenderness or pain, particularly along the spine. Mild signs, consistent with CNS involvement, were seen in 3 of the 17 cats. These included unusual sensitivities to noise, light, and touch which resulted in an exaggerated startle response or a vacant staring and reduced responsiveness to external stimuli. These changes usually were observed 7 to 10 days postinfection and continued to up to 3 weeks postinfection. In addition, two cats showed abrupt behavior changes, becoming aggressive and difficult to handle, in contrast to their docile preinfection behavior. These two cats reverted to their normal, nonaggressive behavior within 3 weeks.
Cerebrospinal fluid from three cats was tested during the period of bacteremia and fever because the clinical signs suggested possible CNS involvement. B. henselae was not cultured from the single spinal fluid that did not have blood contamination. The organism was cultured from two cats, but both samples were contaminated with blood, and in both cases the number of CFU per milliliter of spinal fluid was less than 0.5% of the CFU per milliliter value of the matched blood sample (data not shown). Further, creatine phosphokinase in the cerebrospinal fluid samples was within reference ranges (data not shown).
B. henselae was not cultured from urine or spinal fluid but was cultured from all tissues (lymph nodes, heart, lung, spleen, liver, kidney, pancreas, thymus, testes or ovary, bone marrow, and brain) collected from the euthanized cats. Although all eight cats had one or more lymph nodes which appeared to be enlarged, the number of organisms isolated from the tissue samples was not significantly different from that in the blood, and blood contamination of the tissues could not be ruled out (data not shown).
Antibody responses to infection with LSU16.
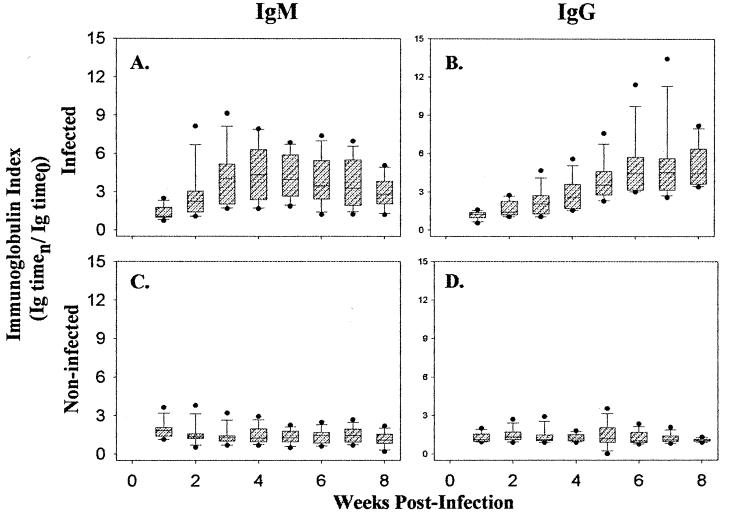
Antibody responses to infection with LSU16 were monitored by enzyme-linked immunosorbent assay. As shown in Fig. 3, all of the cats exposed to B. henselae LSU16 had high levels of IgG by 4 weeks postinfection, and these levels were maintained for the duration of the study. We also examined the IgM response to LSU16 and observed measurable levels of this immunoglobulin by week 3 postexposure. The IgM response began to wane by week 6; however, compared to the preinfection levels, IgM levels remained elevated in the exposed cats for the duration of the study.
FIG. 3.
Box-and-whisker plot representation of the antibody responses of the 17 B. henselae LSU16-infected cats (A and B) and the 15 noninfected cats (C and D) at each time point. IgM (A and C) and IgG (B and D) indices for each cat were calculated by dividing the ELISA optical density (450 nm) determined at each week by the preinfection optical density. The index range which includes the 25th to the 75th percentiles is indicated by the hatched boxes. The 10th and 90th percentiles are indicated by the lower and upper bars, respectively. The mean index for each week is indicated by the center bar. The black dots represent the outliers.
DISCUSSION
The LSU16 strain of B. henselae caused a reproducible, clinically characteristic disease in cats. We observed fever, anorexia, and lethargy in all 17 cats; redness and swelling at the site of exposure in 16 of the 17 cats infected with a pure-culture or blood inoculum; and myalgia, behavioral and/or neurological changes, and lymphadenopathy in many others. These signs are compatible with those reported for human patients with moderate to severe CSD (19). In contrast to human CSD, we did not observe tenderness in those cats that had lymphadenopathy. All cats in this study were inoculated in the left or right thorax, but the accessable lymph nodes (submandibular, prescapular, and popliteal) were not the nodes draining the site of inoculation. One has to consider the possibility that if the inoculations had been done in a more peripheral site, the lymphadenopathy might have been more similar to that reported in human patients.
The biology of B. henselae and the relationship of this organism to its feline host are poorly understood. It was reported that naturally infected cats have periods of recurrent bacteremia and do not show overt signs of clinical disease during these periods of bacteremia (9, 14). In fact, cat no. 16, from which the strain LSU16 was isolated, did not show clinical signs during the period of recurrent bacteremia. Studies using experimentally infected cats have provided conflicting evidence on the relationship of B. henselae infection to disease in the cat. Abbott et al. (1) infected 21 cats, 2 to 18 months of age, as well as 4 pregnant queens by different routes and with different doses ranging from 8 × 102 to 3.2 × 107 CFU and did not observe signs of clinical disease in any of the animals. Similarly, Regnery et al. (17) infected a total of 31 cats with 1 × 103 to 2 × 107 CFU of B. henselae (Houston-1 isolate), subcutaneously and i.d., and did not observe clinical disease in any of the animals. Guptill et al. (5) infected 12 cats intravenously with doses ranging from 106 to 1010 CFU and observed fever and mild anorexia in 4 of the 12 animals. Kordick and Breitschwerdt (8) infected 18 cats with blood or urine and observed that 4 had low-level fevers (39 to 40.3°C) and 8 had signs of lymphadenopathy by 16 days postinfection which regressed within 12 days. In addition, two of their infected cats developed signs of CNS involvement, characterized by abnormal behavior and vacant staring. In all of the experimental infections of cats reported to date, a measurable bacteremia occurred as early as 7 days postinoculation and was transient, reaching undetectable levels in most cats by 12 weeks. (1, 5, 8, 17). In addition, large numbers of organisms were seen in most cats, sometimes exceeding 106/ml of blood. The bacteremia observed in LSU16-inoculated cats was consistent with that seen in these other studies, both in timing and in magnitude. Antibody responses in cats infected with LSU16 were also similar to those reported by others. We observed an initial increase in IgM levels, which subsequently waned over the course of the study but did not return to baseline, suggesting continued antigen stimulation. In addition, the levels of IgG increased during week 2, continued to rise through week 8, and were henceforth maintained throughout the duration of the study. This finding is in contrast to that for the noninfected cats, which showed no detectable changes in levels of either IgM or IgG antibodies to B. henselae LSU16.
The adverse clinical signs that we observed in our cats following infection with B. henselae LSU16 are consistent with the timing and clinical signs observed by other researchers, i.e., fever, anorexia, lymphadenopathy, and abnormal behavior; however, the signs observed with LSU16 appear to be less variable and more severe than those previously reported. The reasons for the observed differences can be hypothetically assessed. First, differences in the host could account for the apparent increase in pathogenicity observed in our study. In the studies reported to date, including the present study, SPF cats were used; however, different vendors were used for each study. Due to the care required to maintain SPF cats, differences in the genetic makeup of the cats might develop over time and could account for the variability of pathogenesis of B. henselae. However, like Guptill et al. (5), we used cats from Harlan Sprague-Dawley, but in contrast to their study, we saw evidence of consistent, reproducible disease in our cats. In addition, we saw a nearly identical disease in outbred cats. Age may be another factor. At 7 to 14 months, the cats used in our study were significantly older than those used by Guptill et al. (5). However, in another study we are currently conducting, 12-week-old kittens were inoculated with strain LSU16 and we still observed fever, lethargy, and redness and swelling at the site of inoculation (unpublished data).
Second, differences in the route of inoculation or the size of the inoculum may affect the onset of clinical disease. The inocula in the previously reported studies included blood and urine (5) as well as pure cultures (1, 5, 8, 17), and the routes of exposure used have included intravenous (1, 5, 7), i.d. (1, 17), and intramuscular (8). Abbott et al. (1) compared routes of inoculation and reported the most efficient infection via the i.d. route. Based on that report, we chose an i.d. route, which might account for our 100% infection rate. Significantly, we used as inocula pure cultures, blood from infected cats, and feces from fleas feeding on infected cats and were able to demonstrate infection in all cases. Furthermore, we infected cats with a pure culture of 5 × 107 CFU of LSU16, a significantly larger inoculum than was used by Abbott et al. for i.d. inoculation. It is possible that many of the clinical signs we observed were due to a response to endotoxin from the large inoculum; however, we feel that this is unlikely because we used 500-fold less inoculum than Guptill et al. (5), who did not observe significant disease. The blood inoculum in this study contained 50- to 140-fold fewer CFU of B. henselae than that used in the pure-culture inocula. While the inoculation of blood resulted in slightly slower kinetics, the observed disease was indistinguishable from that seen in cats receiving pure-culture inocula of LSU16. Further, we did not observe increased inflammation at the site of inoculation in cats receiving flea feces compared to that seen in animals given pure-culture or blood inocula, even though we were aware of the presence of other organisms in the flea feces. Additionally, we injected B. henselae-negative flea feces into naive cats (4) and did not observe inflammation at the site of inoculation. Together, these observations imply that the response to foreign antigens other than B. henselae did not contribute significantly to the inflammation observed in this study.
A third and highly likely explanation is that there are differences in the virulence of strains of B. henselae. Strain LSU16 was isolated from a naturally infected cat during a recurrent bacteremic episode. This cat was not clinically ill at the time that we isolated this strain. We kept the passage of the organism on artificial medium to a minimum; the organism used in this study was from only a second-passage culture. Relman (18) suggested that there are differences in virulence among strains of B. henselae and that it is possible that particular clones or strains are associated with specific clinical syndromes. We are presently examining LSU16 to determine if we can identify further differences between it and other strains of B. henselae.
If the disease we have observed in association with LSU16 is characteristic of many strains of B. henselae, this observation could have important implications in both veterinary and human medicine. In veterinary medicine, fever and lethargy of unknown etiology are commonly seen in cats. It is possible that the initial infection of a kitten or cat with B. henselae will result in fever and malaise of several days duration. Once the animal then recovers from the primary infection, bacteremia could then recur without adverse clinical signs. It is recommended that pet owners and veterinarians screen cats with fevers of unknown etiology for the presence of B. henselae. We believe that transmission of B. henselae to humans most likely occurs during periods of bacteremia in the cat. We have recently demonstrated that feces from fleas feeding on bacteremic cats are an infectious source for B. henselae (4), and consequently flea control may reduce the potential for transmission of B. henselae among cats and from cats to man. Clearance of B. henselae from chronically infected cats by the use of antibiotics is difficult (17). Antibiotic intervention may be more effective during the initial infection, before the establishment of chronic infection, and this approach may provide a means for decreasing the incidence of human CSD and its sequelae.
The behavioral changes seen in the LSU16-infected cats probably are of significance. It has been known for several years that signs of CNS disease are a serious sequela of CSD, particularly in children (10, 13, 21). Recent reports have suggested that B. henselae may also play an important role in some AIDS-related encephalopathies (20). Two of the cats in this study were observed to become aggressive and excitable. The reasons for these changes are not known; however, aggressive and excitable behavior has been observed in human patients with CSD (20). We were not able to isolate the organism from the spinal fluid, although that does not preclude the possibility that the organism was present in the brain. Even if the organism does not cross the blood-brain barrier, damage to brain tissue could occur via imbalances induced by the organism. For example, induction of high systemic levels of cytokines such as tumor necrosis factor alpha, produced in response to infection, could lead to temporary or permanent damage to brain tissue. A strain like LSU16, which produces these behavioral changes in a relatively large percentage of cats (5 of 17 in the present study), might be useful in experimental investigations into the role of B. henselae in CNS disease.
Little is known about the interaction of B. henselae and its host or about the virulence factors responsible for the observed disease. B. henselae was reported to be intraerythrocytic (7, 12). It may also be a facultative intracellular organism that avoids being killed intracellularly by macrophages via mechanisms similar to those employed by Brucella abortus, an organism to which it exhibits a high degree of evolutionary homology (3). It has also been suggested that B. henselae is somehow sequestered in the human host and that the resulting antigenic stimulation and immune response account for the ensuing disease (16); thus, a strain that is more difficult to clear from the body could have increased disease potential. The LSU16 strain is more pathogenic in cats than the previously reported B. henselae strains, and comparison of LSU16 with other strains could generate useful information on the virulence factors of this organism and its interaction with its human and feline hosts.
ACKNOWLEDGMENTS
This work was supported by NIH grant 1 R15 AI39720-01.
We thank Joseph Tabota and Mark Neer for assisting in the evaluation of the cats for CNS signs; Earl Andress, Leslie Birke, Laura Blanke, Tracy Brown, David Good, Victor Goss, Malgorzata Mikolajczyk, Katy Parr, Kenneth Ransom, Roxanne Rutledge, and Jeff Taylor for technical assistance; Melanie Rembert and Laurie Henderson for assistance with the cats; and Michael Groves and Daniel Scholl for helpful discussions.
REFERENCES
- 1.Abbott R C, Chomel B B, Kasten R W, Floyd-Hawkins K A, Kikuchi Y, Koehler J E, Pedersen N C. Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis. 1997;20:41–51. doi: 10.1016/s0147-9571(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 2.Adal K A, Cockerell C J, Petri W A., Jr Cat scratch disease, bacillary angiomatosis, and other infections due to Rochalimaea. N Engl J Med. 1994;330:1509–1515. doi: 10.1056/NEJM199405263302108. [DOI] [PubMed] [Google Scholar]
- 3.Brenner D J, O’Connor S P, Winkler H H, Steigerwalt A G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 4.Foil L, Andress E, Freeland R L, Roy A F, Rutledge R, Triche P C, O’Reilly K L. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera:Pulicidae) feces. J Med Entomol. 1998;35:625–628. doi: 10.1093/jmedent/35.5.625. [DOI] [PubMed] [Google Scholar]
- 5.Guptill L, Slater L, Wu C C, Lin T L, Glickman L T, Welch D F, HogenEsch H. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 6.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA. 1994;271:553–554. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 7.Kordick D L, Breitschwerdt E B. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655–1656. doi: 10.1128/jcm.33.6.1655-1656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kordick D L, Breitschwerdt E B. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res. 1997;58:492–497. [PubMed] [Google Scholar]
- 9.Kordick D L, Wilson K H, Sexton D J, Hadfield T L, Berkhoff H A, Breitschwerdt E B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33:3245–3251. doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusumanto Y H, Veenhoven R H, Bokma J A, Schellekens J F. Two patients with atypical manifestations of cat-scratch disease. Ned Tijdschr Geneeskd. 1997;141:385–387. [PubMed] [Google Scholar]
- 11.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 12.Mehock J R, Greene C E, Gherardini F C, Hahn T-W, Krause D C. Bartonella henselae invasion of feline erythrocytes in vitro. Infect Immun. 1998;66:3462–3466. doi: 10.1128/iai.66.7.3462-3466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noah D L, Bresee J S, Gorensek M J, Rooney J A, Cresanta J L, Regnery R L, Wong J, del Toro J, Olson J G, Childs J E. Cluster of five children with acute encephalopathy associated with cat-scratch disease in South Florida. Pediatr Infect Dis J. 1995;14:866–869. doi: 10.1097/00006454-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Regnery R, Martin M, Olson J. Naturally occurring “Rochalimaea henselae” infection in domestic cat. Lancet. 1992;340:557–558. doi: 10.1016/0140-6736(92)91760-6. [DOI] [PubMed] [Google Scholar]
- 15.Regnery R L, Olson J G, Perkins B A, Bibb W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet. 1992;339:1443–1445. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 16.Regnery R L, Tappero J. Unraveling mysteries associated with cat-scratch disease, bacillary angiomatosis, and related syndromes. Emerg Infect Dis. 1995;1:16–21. doi: 10.3201/eid0101.950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regnery R L, Rooney J A, Johnson A M, Nesby S L, Manzewitsch P, Beaver K, Olson J G. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714–1719. [PubMed] [Google Scholar]
- 18.Relman D A. Are all Bartonella henselae strains created equal? Clin Infect Dis. 1998;26:1300–1301. doi: 10.1086/516347. [DOI] [PubMed] [Google Scholar]
- 19.Schwartzman W A, Patnaik M, Barka N E, Peter J B. Rochalimaea antibodies in HIV-associated neurologic disease. Neurology. 1994;44:1312–1316. doi: 10.1212/wnl.44.7.1312. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzman W A. Infections due to Rochalimaea: the expanding clinical spectrum. Clin Infect Dis. 1992;15:893–900. doi: 10.1093/clind/15.6.893. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler S W, Wolf S M, Steinberg E A. Cat-scratch encephalopathy. Neurology. 1997;49:876–878. doi: 10.1212/wnl.49.3.876. [DOI] [PubMed] [Google Scholar]
- 22.Zangwill K M, Hamilton D H, Perkins B A, Regnery R L, Plikaytis B D, Hadler J L, Cartter M L, Wenger J D. Cat-scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. Vet Rec. 1993;133:27–28. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]