Abstract
Background
Despite the use of platinum-based chemotherapy, lung cancer continues to be the leading cause of cancer-related death in the world. To overcome the rate of lung cancer–related death, scientists discovered advanced therapies, including mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.
Observations
We conducted a meta-analysis to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced non–small cell lung cancer (NSCLC). Included in this study are 9 phase 3 randomized controlled trials designed to study the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. The study showed that mutant EGFR-TK inhibitors have an incidence of adverse effects that is less reported when compared with platinum-based chemotherapy.
Conclusions
We recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. Adverse effects caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
METHODS
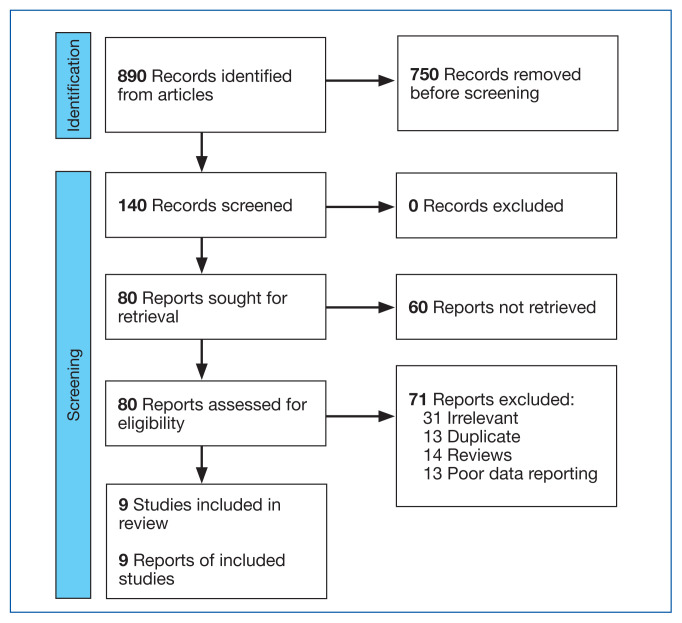
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFRTK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
FIGURE 1.
PRISMA Flow Diagram
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
RESULTS
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
TABLE 1.
Meta-analysis Study Characteristics
| Study, y | Trial name | Phase (design) | EGFR inhibitor dosage | Treatment groupa |
|---|---|---|---|---|
| Zhong et al, 201819 | ADJUVANT/CTONG1104 | 3 (RCT) | Gefitinib 250 mg/d for 24 mo | EGFR inhibitors |
| Wu et al, 201820 | LUX LUNG 6 | 3 (RCT) | Afatinib 40 mg/d with increment of 10 mg/d if no adverse effects | EGFR inhibitors |
| Shi et al, 201721 | CONVINCE | 3 (RCT) | Icotinib 125 mg 3 times daily | EGFR inhibitors |
| Soria et al, 201522 | IMPRESS | 3 (RCT) | Gefitinib 250 mg/d for 24 mo | EGFR inhibitors |
| Goss et al, 201323 | NCIC CTG BR19 | 3 (RCT) | Gefitinib 250 mg/d for 24 mo | EGFR inhibitors |
| Mu Sun et al, 201224 | KCSG-LU08-01 | 3 (RCT) | Gefitinib 250 mg/d for 24 mo | EGFR inhibitors |
| Mitsudomi et al, 201125 | WJTOG3405 | 3 (RCT) | Gefitinib 250 mg/d | EGFR inhibitors |
| Lee et al, 201026 | ISTANA | 3 (RCT) | Gefitinib 250 mg/d | EGFR inhibitors |
| Kim et al, 200827 | INTEREST | 3 (RCT) | Gefitinib 250 mg/d | EGFR inhibitors |
Abbreviations: EGFR, epidermal growth factor receptor; RCT, randomized controlled trial.
All control groups treated with platinum-based chemotherapy.
TABLE 2.
Adverse Effects
| Study | Group | Rash, No. (%) | Diarrhea, No. (%) | Elevated ALT, No. (%) | Elevated AST, No. (%) | Stomatitis, No. (%) | Nausea, No. (%) | Leucopenia, No. (%) | Fatigue, No. (%) | Neutropenia, No. (%) | Anorexia, No. (%) | Anemia, No. (%) | Cough, No. (%) | Vomiting, No. (%) | Fever, No. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADJUVANT/CTONG110419 | Treatment | 43 (40.6) | 28 (26.4) | 29 (27.4) | 12 (11.3) | 8 (7.5) | 3 (2.8) | 4 (3.7) | 4 (3.7) | 3 (2.9) | 2 (1.8) | 2 (1.8) | 11 (10.4) | 5 (4.7) | 1 (0.9) |
| Control | 0 (0) | 4 (4.6) | 3 (3.5) | 1 (1.2) | 5 (5.7) | 38 (43.7) | 41 (47) | 4 (4.6) | 46 (52.9) | 20 (23) | 44 (50.6) | 15 (17.2) | 36 (41.4) | 9 (10.3) | |
| LUX LUNG 620 | Treatment | 144 (82.3) | 153 (87.4) | 39 (22.3) | 32 (18.3) | 88 (50.3) | 11 (6.3) | 5 (2.8) | 14 (8) | 3 (1.7) | 11 (6.3) | 9 (5.2) | — | 14 (8) | — |
| Control | 9 (9.5) | 10 (10.5) | 16 (17) | 10 (10.5) | 5 (5.3) | 72 (75.8) | 53 (55.8) | 37 (39) | 53 (55.8) | 39 (41) | 26 (27.4) | — | 75 (78.9) | — | |
| CONVINCE21 | Treatment | 22 (15) | 11 (7.4) | 10 (6.7) | 12 (81) | — | 4 (2.7) | 11 (7.4) | 5 (3.4) | 5 (3.4) | 3 (2) | 4 (2.7) | 2 (1.4) | 2 (1.4) | 0 (0) |
| Control | 2 (1.5) | 6 (4.3) | 19 (13.9) | 15 (11) | — | 63 (46) | 60 (43.8) | 20 (14.6) | 58 (42.3) | 32 (23.4) | 17 (12.4) | 3 (2.2) | 40 (29.2) | 4 (2.9) | |
| IMPRESS22 | Treatment | 14 (11) | 44 (33.3) | 17 (12.9) | 30 (22.7%) | 14 (10.6) | 85 (64.4) | 27 (20.5) | 28 (21.2) | 29 (22) | 65 (49.2) | 0 (0) | 18 (13.6) | 55 (41.6) | 22 (16.7) |
| Control | 11 (8) | 19 (14.9) | 23 (17) | 29 (22%) | 5 (3.7) | 81 (61.4) | 22 (16.7) | 23 (17.4) | 28 (21.2) | 45 (34.1) | 1 (0.7) | 15 (11.4) | 44 (33.3) | 14 (10.6) | |
| NCIC CTG BR1923 | Treatment | 21 (8.5) | 18 (7.2) | — | — | — | 6 (2.4) | — | 15 (6) | — | — | — | — | 3 (1.2) | — |
| Control | 1 (0.5) | 5 (2) | — | — | — | 1 (0.4) | — | 6 (2.5) | — | — | — | — | 0 (0) | — | |
| KCSG-LU08-0124 | Treatment | 31 (46) | 18 (26) | — | — | — | 11 (16.2) | — | 15 (22.1) | 0 (0) | 22 (32.4) | — | 25 (36.7) | — | — |
| Control | 3 (4.5) | 3 (4.4) | — | — | — | 11 (16.4) | — | 14 (21) | 1 (1.5) | 20 (29.8) | — | 24 (35.8) | — | — | |
| WJTOG340525 | Treatment | 74 (85) | 47 (54) | 61 (70) | 61 (70%) | 19 (21.8) | 15 (17.2) | 13 (14.9) | 34 (39.1) | 7 (8.1) | — | 33 (37.9) | — | — | — |
| Control | 7 (8) | 35 (40) | 35 (40) | 17 (19.3%) | 13 (14.7) | 83 (94) | 82 (93.2) | 73 (82.9) | 81 (92) | — | 79 (89.7) | — | — | — | |
| ISTANAl26 | Treatment | 61 (75) | 21 (26) | — | — | 3 (3.7) | 13 (16) | — | 20 (24.7) | — | 29 (35.8) | — | 25 (30.8) | 4 (4.9) | 4 (4.9) |
| Control | 6 (8) | 12 (15.7) | — | — | 9 (1.8) | 14 (18.4) | — | 28 (36.9) | — | 36 (47.4) | — | 25 (32.9) | 8 (10.5) | 8 (10.5) | |
| INTEREST27 | Treatment | 360 (49) | 255 (35) | — | — | 67 (9.2) | 148 (20.3) | — | 182 (25) | 35 (4.8) | 159 (21.8) | 34 (4.7) | 108 (14.8) | 109 (15) | — |
| Control group | 73 (10.3) | 177 (24.7) | — | — | 93 (13) | 187 (26.2) | — | 334 (46.7) | 514 (72) | 151 (21.2) | 84 (11.7) | 102 (14.3) | 123 (17.2) | — |
Abbreviations ALT, alanine transaminase; AST, aspartate aminotransferase.
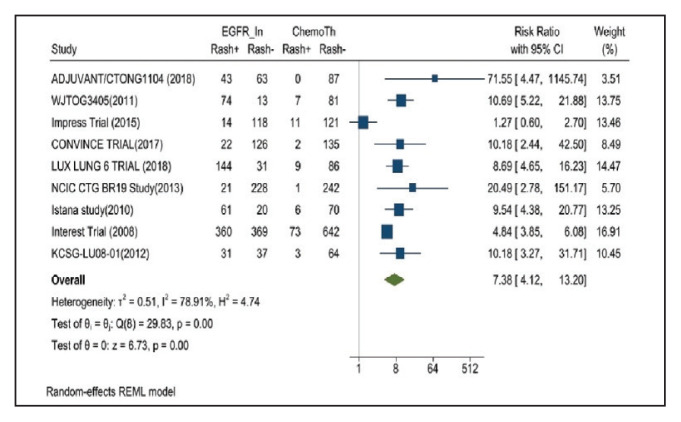
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
FIGURE 2.
Rash Adverse Events
Risk ratio > 1 indicates higher rash event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
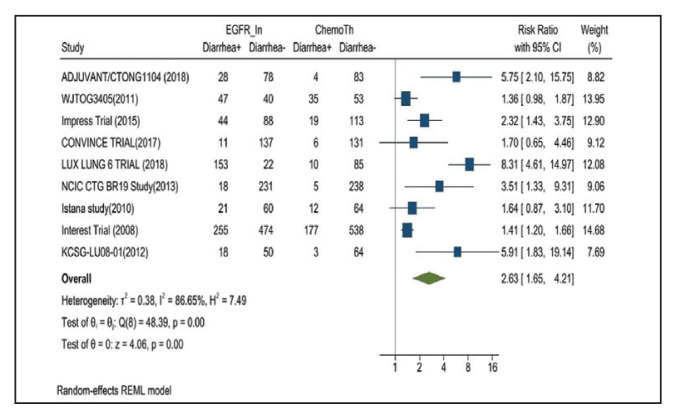
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
FIGURE 3.
Diarrhea Adverse Events
Risk ratio > 1 indicates higher diarrhea event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
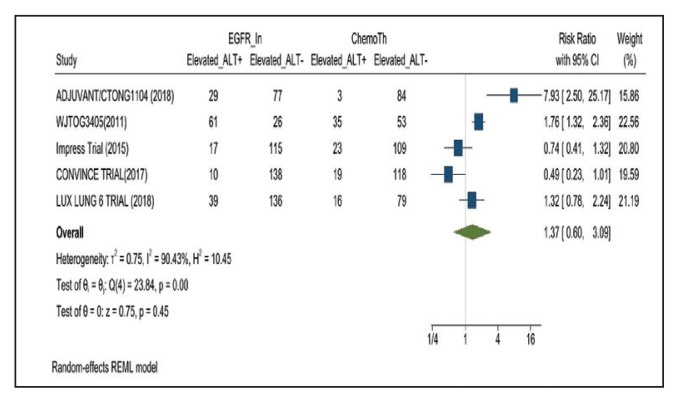
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
FIGURE 4.
Elevated Alanine Transaminase Adverse Events
Risk ratio > 1 indicates higher elevated alanine transaminase event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
FIGURE 5.
Elevated Aspartate Aminotransferase Adverse Events
Risk ratio > 1 indicates higher elevated aspartate aminotransferase event rates in the estimated epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
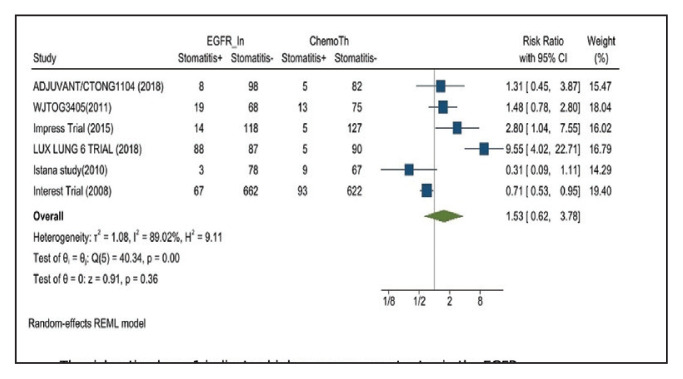
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFRTK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
FIGURE 6.
Stomatitis Adverse Events
Risk ratio > 1 indicates higher stomatitis event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
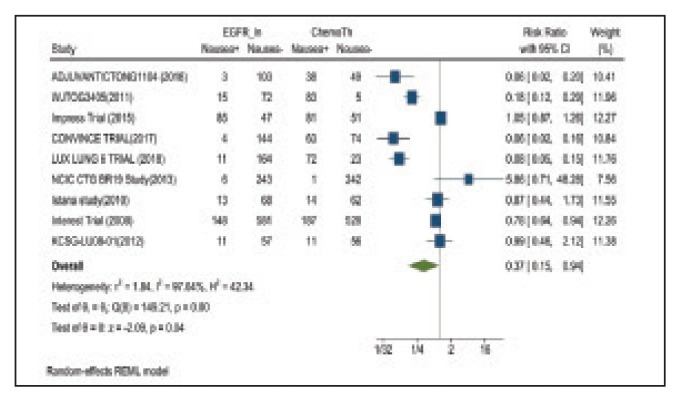
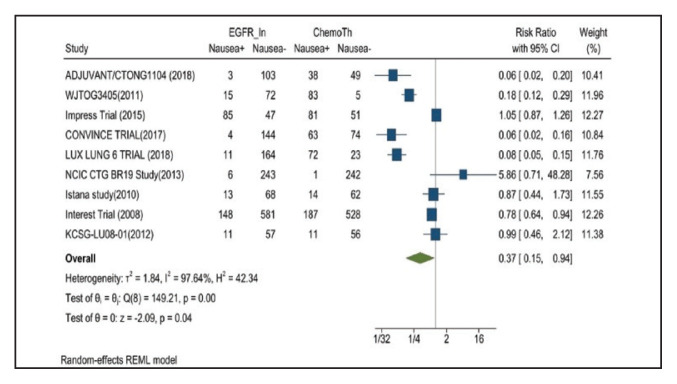
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
FIGURE 7.
Nausea Adverse Events
Risk ratio > 1 indicates higher nausea event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
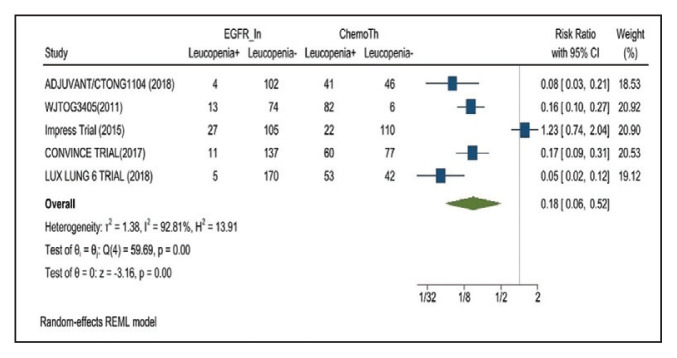
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
FIGURE 8.
Leucopenia Adverse Events
Risk ratio > 1 indicates higher leucopenia event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
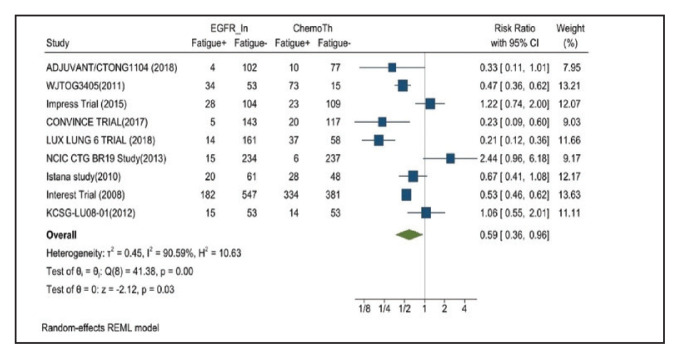
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
FIGURE 9.
Fatigue Adverse Events
Risk ratio >1 indicates higher fatigue event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
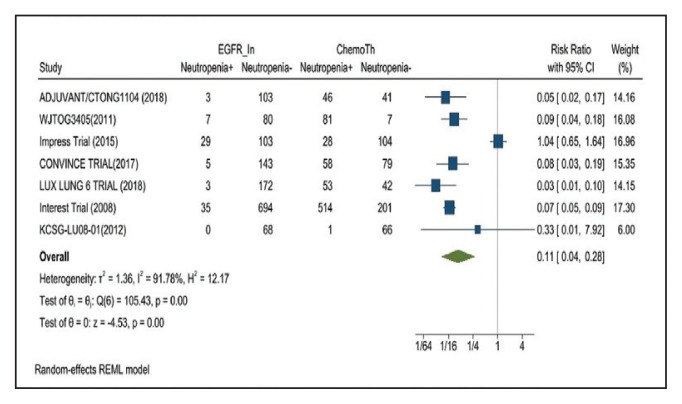
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
FIGURE 10.
Neutropenia Adverse Events
Risk ratio > 1 indicates higher neutropenia event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
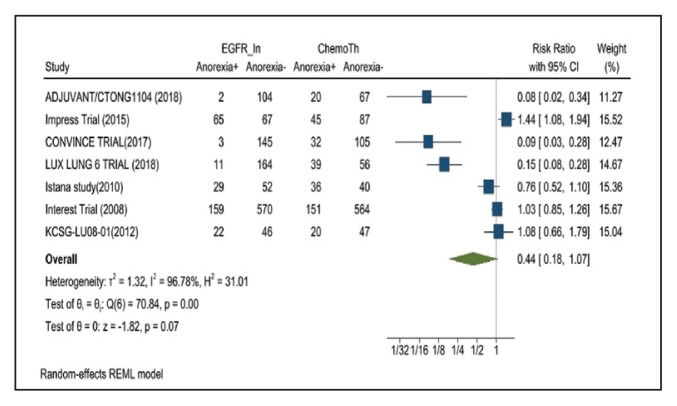
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
FIGURE 11.
Anorexia Adverse Events
Risk ratio > 1 indicates higher anorexia event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
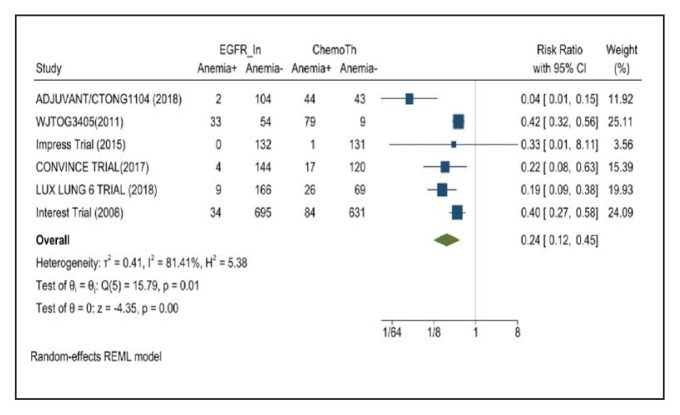
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
FIGURE 12.
Anemia Adverse Events
Risk ratio > 1 indicates higher anemia event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
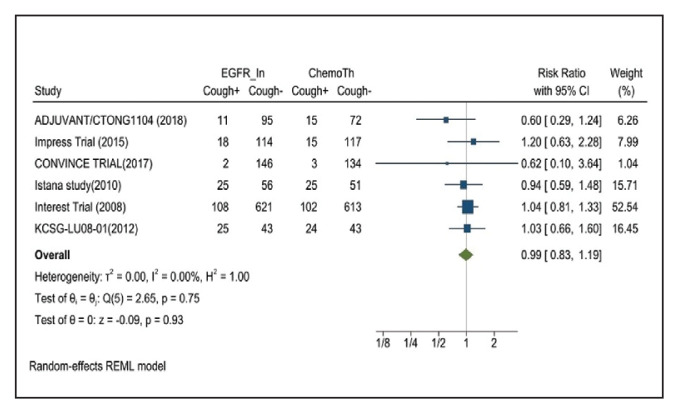
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
FIGURE 13.
Cough Adverse Events
Risk ratio > 1 indicates higher cough event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
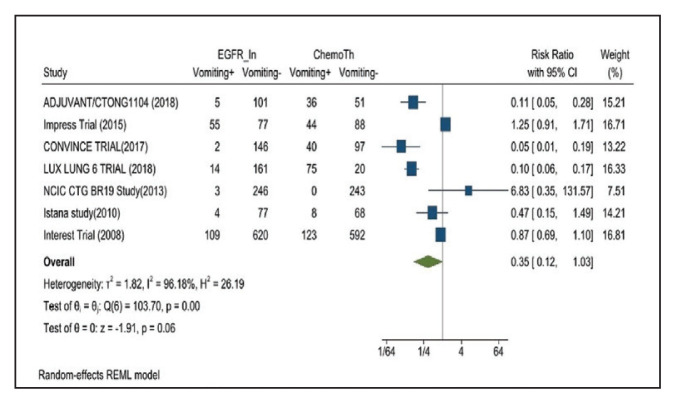
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
FIGURE 14.
Vomiting Adverse Events
Risk ratio > 1 indicates higher vomiting event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
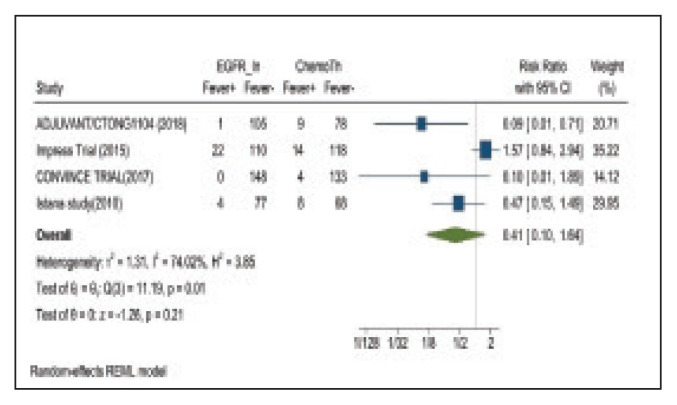
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
FIGURE 15.
Fever Adverse Events
Risk ratio > 1 indicates higher fever event rates in the epidermal growth factor receptor inhibitor group compared with the chemotherapy group.
DISCUSSION
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes mutant EGFR-TK inhibitors, including erlotinib, gefitinib, dacomitinib, lapatinib, and osimertinib.4
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
CONCLUSIONS
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Footnotes
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Ethics and consent
This is a meta-analysis including already published clinical trials.
Author disclosures
The authors report no actual or potential conflicts of interest or outside sources of funding with regard to this article.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 3.Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337–3352. doi: 10.2174/092986712801215973. [DOI] [PubMed] [Google Scholar]
- 4.Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653–660. doi: 10.1586/14737140.2016.1170596. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 9.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin- paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 10.Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049–1056. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 12.Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235–2245. doi: 10.1200/JCO.19.00075. [DOI] [PubMed] [Google Scholar]
- 13.Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124–136. doi: 10.1200/JCO.19.01154. [DOI] [PubMed] [Google Scholar]
- 14.Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. doi: 10.1371/journal.pone.0061561. Published 2013 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229–237. doi: 10.1007/s40264-017-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 17.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969–2979. doi: 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713–722. doi: 10.1200/JCO.20.01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 21.Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450. doi: 10.1093/annonc/mdx359. [DOI] [PubMed] [Google Scholar]
- 22.Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 23.Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320–3326. doi: 10.1200/JCO.2013.51.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234–6242. doi: 10.1200/JCO.2013.51.1816. [DOI] [PubMed] [Google Scholar]
- 25.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307–1314. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 27.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;372(9652):1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]