Abstract
Oedaleus asiaticus (Bey-Bienko) is an economically devastating locust species found in grassland and pastoral areas of the Inner Mongolia region of northern China. In this study, resistance to three frequently used insecticides (beta-cypermethrin, matrine, and azadirachtin) was investigated in six field populations of O. asiaticus using the leaf-dip bioassay method. The inhibitory effects of synergists and the activities of detoxification enzyme activities in the different populations were determined to explore potential biochemical resistance mechanisms. The results showed that the field populations SB (resistance ratio [RR] = 7.85), ZB (RR = 5.64), and DB (RR = 6.75) had developed low levels of resistance to beta-cypermethrin compared with a susceptible control strain. Both the SB (RR = 5.92) and XC (RR = 6.38) populations had also developed low levels of resistance against matrine, with the other populations remaining susceptible to both beta-cypermethrin and matrine. All field populations were susceptible to azadirachtin. Synergism analysis showed that triphenyl phosphate (TPP) and diethyl-maleate (DEM) increased the toxicity of beta-cypermethrin significantly in the SB population, while the synergistic effects of TPP, piperonyl butoxide (PBO), and DEM on the toxicity of matrine were higher in SB (SR 3.86, 4.18, and 3.07, respectively) than in SS (SR 2.24, 2.86, and 2.29, respectively), but no synergistic effects of TPP, PBO, and DEM on azadirachtin were found. Biochemical assays showed that the activities of carboxylesterases (CarEs) and glutathione-S-transferases (GSTs) were significantly raised in all field populations of O. asiaticus, with a significant positive correlation observed between beta-cypermethrin resistance and CarE activity. The activities of cytochrome P450 monooxygenases (P450) and multi-function oxidases (MFO) were elevated in all six field populations, and P450 activity displayed strong positive correlations with the three insecticides. Our findings suggest that resistance to beta-cypermethrin in O. asiaticus may be mainly attributed to elevated CarE and GST activities, while P450 plays an important role in metabolizing matrine and azadirachtin. Our study provides insights that will help improve insecticide resistance management strategies.
Keywords: resistance monitoring, Oedaleus asiaticus, synergism, detoxification enzyme, insecticide
The band-winged locust, Oedaleus asiaticus (Orthoptera, Acrididae), is an economically important pest that is widely distributed throughout the northern Asian grasslands (Chen and Kang 2000), and is particularly abundant on the steppes of the Inner Mongolian, Gansu, Hebei, Shanxi, Shandong region of northern China, and mainly in Mongolia, Russia, and other regions abroad. (Li and Ma 1990, Zhou et al. 2012). This locust species prefers to live on overgrazed steppes and mainly feeds on gramineaceous plants such as Stipa krylovii, Stipa grandis, Neurolepidium chinense, and Cleistogenes squarrosa (Huang et al. 2016, 2017). In Inner Mongolia, O. asiaticus is characterized by one generation annually and occasionally exhibits migratory behavior (Cease et al. 2010). This pest causes great damage to the vegetation on grasslands and they have been suggested to serve as indicators of habitat deterioration in typical steppe zone of Inner Mongolia (Kang and Chen 1995, Liu and Guo 2004). With the gradual exacerbation of climate change and heavy livestock pressure, O. asiaticus has become increasingly destructive, threatening agriculture, and animal husbandry in northern China, especially in Inner Mongolia (Liu et al. 2013, Zhang et al. 2014).
Although diverse strategies have been developed to control outbreaks of locusts and grasshoppers, the use of broad-spectrum insecticides remains the most effective control tactic (Cease et al. 2012, Zhang et al. 2019). Over the last 20 years, conventional insecticides, including pyrethroids and neonicotinoids, have been widely recommended against locusts in steppe regions (Mao et al. 2021). However, the frequent use of insecticides has led to the development of resistance and consequent loss of efficacy (Li et al. 2014, Cao et al. 2017). Therefore, determining the susceptibility of field populations of O. asiaticus to common insecticides and elucidating its resistance mechanisms are important for the implementation of suitable management strategies against this key pest.
In recent years, O. asiaticus has developed resistance to numerous insecticides, including beta-cypermethrin and deltamethrin (Dong et al. 2016, Cao et al. 2017). Beta-cypermethrin is frequently used to control O. asiaticus on the Inner Mongolian steppe, where populations have a low level of resistance (Dong et al. 2016). Two botanical insecticides, azadirachtin and matrine, also play an important role in locust control, and their long-term usage has thus far raised little concern about the development of resistance (Zhang et al. 2018). Previous studies have observed that pyrethroid resistance is associated with elevated levels of detoxification enzymes such as cytochrome P450 monooxygenases (P450s) (Sonoda 2010, Silva et al. 2015, Zibaee et al. 2018), multi-function oxidases (MFOs) (Chen et al. 2019), carboxylesterases (CarEs) (Xu et al. 2021), and glutathione-S-transferases (GSTs) (Vontas et al. 2001; Yang et al. 2004) in a wide range of insect species. The use of botanical pesticides has led to adaptation in detoxification enzymes, possibly resulting in resistance to a variety of chemicals (Tan et al. 2019). Giraudo et al. (2015) found that overexpression of P450 genes was induced by azadirachtin and showed the important role of the CYP3 enzymes in the metabolism of botanical pesticides. Shu et al. (2021) speculated that the up-regulation of P450 and GST enzymes may be involved in the degradation of azadirachtin in Spodoptera frugiperda larvae.
In the present study, we monitored the resistance levels of O. asiaticus against beta-cypermethrin, azadirachtin, and matrine. Populations collected from six regions of the Inner Mongolian steppe were exposed to these three insecticides. In addition, to investigate the metabolic mechanisms that are involved in resistance, three synergist compounds, piperonyl butoxide (PBO), diethyl-maleate (DEM), and triphenyl phosphate (TPP), were used to determine the effects of the detoxifying enzyme activities on the sensitivity of these field populations of O. asiaticus towards these insecticides. Detoxification enzyme activities were measured in the different field populations to determine underlying biochemical resistance mechanisms. Our results provide a framework for an integrated management strategy using commonly used insecticides against the locust on the Inner Mongolian steppes.
Materials and Methods
Insects
A susceptible strain (SS) of O. asiaticus was collected in June 2018 from the Daqing Mountain grassland area (111° 38ʹ16.2″ E, 41° 21ʹ12.6″ N) in Hohhot, Inner Mongolia, China. Daqing Mountain area is a national nature reserve, where pesticides have not been used and O. asiaticus resistance to insecticides has never been reported. The susceptible strain has been reared in the laboratory without exposure to insecticides. Geographical populations of O. asiaticus were collected from six sampling areas in the Inner Mongolian steppe region in June 2020. These areas included typical steppe regions in Xilinhot City (XC) and Zarut Banner (ZB), desert steppes in Siziwang Banner (SB), Alxa Left Banner (AB), and Damao Banner (DB), and meadow steppes in Xinbaerhu Banner (XB) (Fig. 1). All locust strains were reared on wheat seedlings in isolated cages with mesh protection and maintained in the laboratory without any exposure to insecticides at 26 ± 1°C, 60 ± 5% relative humidity (RH), and a photoperiod of 14:10 (L:D) h. The wheat variety used was Changfeng 2112, and the seeds were purchased from Shaanxi Xichangfeng Seed Industry Co., Ltd. (China). The different populations were kept separately in the laboratory and the third-instar nymphs were immediately processed for bioassays or frozen in liquid nitrogen and stored at −80°C for biochemical determinations.
Fig. 1.
Collection locations of the six O. asiaticus populations tested.
Reagents
The active insecticide ingredients beta-cypermethrin (96%), azadirachtin (30%), and matrine (98%) were provided by Beijing Qingyuanbao Biological Technology Co., Ltd. (Beijing, China). Piperonyl butoxide (PBO; 98%) and diethyl maleate (DEM; 97%) were purchased from Sigma-Aldrich Co. (St Louis, MO). Triphenyl phosphate (TPP), α-naphthol, α-naphthyl acetate (α-NA), β-naphthyl acetate (β-NA), and fast blue B salt (O-dianisidine, tetrazotized) were acquired from Shanghai No.1 Chemical Reagent Company (China); bovine serum albumin (BSA) was from Bio-Rad (Hercules, CA); sodium dodecyl sulfate (SDS) was bought from Sigma Chemical Co.; Coomassie brilliant blue G-250, sucrose, and Triton X-100 were bought from Amresco Co. (Solon, OH); acrylamide (Acr) and disodium ethylenediaminetetraacetate (EDTA-Na2) were obtained from Solarbio (Beijing, China). In addition, 1,2-dichloro-4-nitrobenzene (DCNB), acetylthiocholine iodide (ATChI), and catechol,1-chloro-2,4-dinitrobenzene (CDNB) were bought from Sigma Chemical Co.). Reduced glutathione, p-nitrophenol, p-nitroanisole, and coenzyme NADPH were obtained from Shanghai Dingguo Biotech Development Co. (Shanghai, China). N,N,Nʹ,Nʹ-tetramethyl ethylenediamine (TEMED) and other reagents were purchased from commercial suppliers in China.
Bioassay and Synergism Determination
The susceptibility of O. asiaticus to insecticides was determined using the leaf-dip bioassay method as described by Shelton (1993) with minor modifications. Stock solutions of each of the three insecticides were prepared in acetone and seven dilutions, causing 10–90% mortality, were prepared. Fresh wheat seedling bundles were cut into small 3-cm-diameter pieces. Each bundle was dipped in a solution of the diluted insecticide or acetone as a control for 10 s, and allowed to air-dry at room temperature. The bundles were placed individually inside a plastic feeding box (20 cm × 15 cm × 6 cm). Third-instar nymphs of O. asiaticus were selected and used for the bioassay. Each treatment was replicated three times with each replication containing 20 test locusts. The control nymphs were treated with a 1% acetone solution. All third-instar nymphs were kept at 26 ± 1°C, 60 ± 5% RH, and a photoperiod of 14:10 (L:D) h, and the mortality was recorded after 48 h exposure. The individuals were considered dead if they failed to move or twitch slightly when touched with a fine brush.
To study which detoxifying enzymes might play critical roles in the mechanism of insecticide resistance, bioassays were performed in the absence or presence of the synergists TPP, PBO, and DEM. The mixing ratio was 3:1 (synergist/insecticide) according to He et al. (2009). The field population with the highest resistance level to the three insecticides was used for synergism determination.
Detoxification Enzyme Activity Determination
Enzyme Extract Preparation
Thorax samples of third-instar nymphs from each geographical population were dissected on ice and homogenized in phosphate-buffered saline (PBS) (0.1 mol L−1, pH 7.5) containing 0.3% (v:v) Triton X-100. The homogenates were centrifuged at 10,000 g for 10 min at 4°C. The supernatants were transferred to new centrifuge tubes and centrifuged again at 15,000 × g for 20 min at 4°C. The supernatants were then used for the immediate measurement of protein concentrations and enzyme activities.
Carboxylesterase (CarE) Activity
Carboxylesterase (CarE) activity was assayed using the method described by Zhu and He (2000). Enzyme-containing solutions were added to microplate wells with the substrates α-NA (3 × 10−4 mol L−1) and physostigmine (1 × 10−4 mol L−1) and incubated at 37°C for 10 min. The chromogenic agent included 5% SDS and 1% fast blue B salt (v/v = 5:2). OD values at 600 nm were measured in a microplate reader (Synergy H4, Burton Co.).
Glutathione S-transferase Activity
The activity of glutathione S-transferase (GST) was determined according to the method of Oppenoorth et al. (1979) with minor modifications. The enzyme-containing solutions were added to microplate wells with the substrates CDNB (0.6 mmol L−1) and GSH (6 mol L−1), and then incubated at 37°C for 20 min. Absorbances at 340 nm were read at 30-second intervals for 5 min.
Multifunction Oxidase Activity
Multifunction oxidase (MFO) activity was measured using a modification of the method of Shang and Soderlund (1984). The enzyme solution was added to microplate wells with the substrates nitroanisole (0.05 mol L−1 in acetone) and NADPH (9.6 mmol L−1) and incubated for 30 min at 37°C. The reaction was terminated with 1 mol L−1 hydrochloric acid, and extracted with chloroform and NaOH (0.5 mol L−1). The OD values were measured at 405 nm using a microplate reader.
Cytochrome P450 Monooxygenase (P450) Activity
The activity of P450 was evaluated by measuring p-nitroanisole (PNOD) activities according to the method of Chang and Hodgson (1975) with minor modifications. The detection system contained 100 µL of enzyme solution, 25 mM p-nitroanisole, and 100 mM potassium phosphate buffer (pH 7.2). After the addition of 100 mM D-glucose-6-phosphate sodium salt and 100 U/mL glucose-6-phosphate dehydrogenase, the reaction was initiated by the addition of 5 mM beta-nicotinamide adenine dinucleotide phosphate. After incubation for 10 min at 30°C, the reaction was stopped by adding acetone containing 2 mM glycine and 2 U/mL sodium hydroxide. Absorbances at 405 nm were measured in the microplate reader and the amount of product formed was calculated from the p-nitrophenol standard curve. CarE, GST, P450, and MFO activities were measured in all field populations and were compared to the susceptible strain (SS).
For all the experiments of detoxification enzyme activity determination, each geographical population was as one treatment group, there are a total of 7 geographical populations or 7 treatment groups including susceptible strain (SS) (SS, AB, DB, SB, XC, XB, and ZB). The experiments were performed three determinations with three biological replicates per geographical sample (treatment group).
Protein Quantification
Protein concentration was determined by the method of Bradford (1976), using bovine serum albumin as the standard.
Data Analysis
Mortality data were corrected using Abbott’s formula (1987) and analyzed by probit analysis using POLO-Plus (Robertson et al. 2007). Differences were considered significant if the 95% confidence limits of the two LC50 values did not overlap. Differences in enzyme activities were analyzed using one-way analysis of variance (ANOVA) with Tukey’s test for means separation and multiple linear regression was used to analyze the correlation between resistance ratio to insecticide and the enzymatic activity of the population using SPSS 18.0 software. Enzymatic activities were expressed as mean ± SE (mol μg−1min−1 protein for CarEs, GSTs, P450s, and MFOs). Softmax Pro 6.1 was used to record and analyze the protein data.
Resistance levels were classified based on the resistance ratio (RR) using LC50 values compared with susceptible populations. Resistance levels were classified as follows: susceptible (RR = 1–5 fold), low resistance (RR = 5–10 fold), medium resistance (RR = 10–40 fold), or high resistance (RR = 40–160 fold) (Kim et al. 1999).
Results
Monitoring of Resistance of O. asiaticus to Insecticides
The LC50 values of the SS population with beta-cypermethrin, matrine, and azadirachtin were 0.21 µg L–1, 0.43 µg L–1, and 0.54 µg L–1, respectively (Table 1). This showed that the SS population of O. asiaticus was more susceptible to beta-cypermethrin, matrine, and azadirachtin than several of the field collected populations.
Table 1.
Resistance ratios towards beta-cypermethrin, matrine, and azadirachtin of field populations of O. asiaticus from Inner Mongolia compared to a susceptible strain (SS)
| Insecticides | Population | χ2a | Slope ± SE | LC50 (µg L−1) | 95% CL | RRb (R/S) |
|---|---|---|---|---|---|---|
| Beta-cypermethrin | SS | 2.36* | 2.30 ± 0.51 | 0.21 | 0.17–0.30 | 1.00 |
| AB | 1.67* | 1.83 ± 0.39 | 0.77 | 0.46–1.13 | 3.68 | |
| DB | 4.55* | 2.67 ± 0.41 | 1.41 | 0.82–1.94 | 6.72 | |
| SB | 2.14* | 3.13 ± 0.58 | 1.65 | 0.73–2.15 | 7.85 | |
| XC | 0.97* | 1.16 ± 0.36 | 0.78 | 0.46–1.14 | 3.74 | |
| XB | 2.18* | 2.34 ± 0.42 | 0.99 | 0.49–1.36 | 4.76 | |
| ZB | 1.45* | 1.68 ± 0.33 | 1.13 | 0.67–1.45 | 5.64 | |
| Matrine | SS | 0.83* | 1.63 ± 0.38 | 0.43 | 0.42–0.65 | 1.00 |
| AB | 1.97* | 1.97 ± 0.41 | 1.71 | 1.32–3.75 | 3.98 | |
| DB | 2.34* | 1.82 ± 0.36 | 2.05 | 1.75–4.25 | 4.76 | |
| SB | 1.20* | 2.63 ± 0.47 | 2.55 | 2.30–4.16 | 5.92 | |
| XC | 3.19* | 2.06 ± 0.41 | 2.74 | 2.43–4.70 | 6.38 | |
| XB | 4.15* | 1.89 ± 0.39 | 0.92 | 0.83–2.27 | 2.13 | |
| ZB | 1.74* | 2.24 ± 0.41 | 1.49 | 1.31–3.75 | 3.46 | |
| Azadirachtin | SS | 2.13* | 3.34 ± 0.64 | 0.54 | 0.31–0.59 | 1.00 |
| AB | 3.62* | 3.22 ± 0.61 | 1.25 | 0.62–1.74 | 2.32 | |
| DB | 2.27* | 3.01 ± 0.54 | 1.31 | 0.67–1.59 | 2.41 | |
| SB | 5.01* | 2.15 ± 0.46 | 1.39 | 0.73–1.66 | 2.57 | |
| XC | 0.92* | 1.79 ± 0.29 | 1.05 | 0.29–1.82 | 1.95 | |
| XB | 1.67* | 1.68 ± 0.28 | 0.95 | 0.35–1.57 | 1.76 | |
| ZB | 2.54* | 2.10 ± 0.31 | 0.85 | 0.32–1.62 | 1.56 |
*Meant pass the χ2 test.
bPearson chi-square, goodness-of-fit test.
cRR: resistance ratio = LC50 of the field strain/LC50 of the susceptible strain.
The LC50 values of field collected populations ranged from 0.773 to 1.648 µg L − 1 for beta-cypermethrin, and the resistance ratios (RRs) ranged from 3.68 to 7.85. Table 1 indicates that the SB, ZB, and DB populations had developed low resistance to beta-cypermethrin. The SB and XC populations had developed low resistance towards matrine, with 5.92- and 6.38-fold higher LC50 values, respectively, while the other populations remained susceptible (Table 1). The resistance levels in the field populations of O. asiaticus exhibited low LC50 values for azadirachtin, with RRs ranging from 1.56 to 2.57, suggesting that the six populations remained susceptible to azadirachtin (Table 1).
Synergism Effects
The SB population, with a trend for being more resistant to beta-cypermethrin, matrine, and azadirachtinhad than the other field populations, was chosen to analyze the potential biochemical mechanism of resistance in O. asiaticus. Both TPP and DEM increased the beta-cypermethrin toxicity for the SB population, with synergistic ratios (SRs) of 5.29 and 4.92, respectively, significantly higher than those in the SS (SRs of 2.05 and 2.67, respectively). No synergistic effects of PBO were observed in both the SS and the SB populations (SR < 2.0). The synergistic effects of TPP, PBO, and DEM on the toxicity of matrine were higher in the SB (SRs 3.86, 4.18, and 3.07, respectively) than in the SS (SRs 2.24, 2.86, and 2.29, respectively). However, no synergistic effects of TPP, PBO, and DEM on azadirachtin were found in the SS or SB populations (Table 2).
Table 2.
Synergism with beta-cypermethrin, matrine, and azadirachtin between SS and SB strains
| Insecticides | population | χ2a | Slope ± SE | LC50 (µg L−1) | 95% CL | SRb |
|---|---|---|---|---|---|---|
| Beta-cypermethrin | SS | 2.36* | 2.30 ± 0.51 | 0.21 | 0.17–0.30 | – |
| SB | 2.14* | 3.13 ± 0.58 | 1.65 | 0.73–2.15 | – | |
| Beta-cypermethrin + TPP | SS | 1.95* | 1.67 ± 0.36 | 0.10 | 0.09–0.18 | 2.05 |
| SB | 3.01* | 2.81 ± 0.42 | 0.31 | 0.25–0.66 | 5.29 | |
| Beta-cypermethrin + PBO | SS | 0.94* | 1.17 ± 0.27 | 0.20 | 0.17–0.28 | 1.02 |
| SB | 1.64* | 1.85 ± 0.42 | 0.83 | 0.59–1.28 | 1.98 | |
| Beta-cypermethrin + DEM | SS | 1.26* | 1.54 ± 0.32 | 0.08 | 0.07–0.15 | 2.67 |
| SB | 0.92* | 2.15 ± 0.41 | 0.33 | 0.26–0.59 | 4.92 | |
| Matrine | SS | 0.83* | 1.63 ± 0.38 | 0.43 | 0.42–0.65 | – |
| SB | 1.20* | 2.63 ± 0.47 | 2.55 | 2.30–4.16 | – | |
| Matrine + TPP | SS | 2.67* | 0.63 ± 0.21 | 0.19 | 0.16–0.27 | 2.24 |
| SB | 3.55* | 1.14 ± 0.32 | 1.14 | 0.94–1.41 | 3.86 | |
| Matrine + PBO | SS | 3.47* | 1.89 ± 0.54 | 0.15 | 0.13–0.19 | 2.86 |
| SB | 6.24* | 4.67 ± 0.36 | 0.61 | 0.40–0.71 | 4.18 | |
| Matrine + DEM | SS | 2.47* | 1.54 ± 0.39 | 0.18 | 0.17–0.20 | 2.29 |
| SB | 1.69* | 0.72 ± 0.24 | 0.83 | 0.58–0.93 | 3.07 | |
| Azadirachtin | SS | 2.13* | 3.34 ± 0.64 | 0.54 | 0.31–0.59 | – |
| SB | 2.89* | 0.88 ± 0.24 | 1.38 | 0.73–1.66 | – | |
| Azadirachtin + TPP | SS | 3.44* | 1.96 ± 0.41 | 0.25 | 0.19–0.35 | 2.16 |
| SB | 3.53* | 2.11 ± 0.38 | 0.42 | 0.28–0.56 | 3.28 | |
| Azadirachtin + PBO | SS | 1.24* | 2.99 ± 0.57 | 0.26 | 0.16–0.41 | 2.08 |
| SB | 3.14* | 1.36 ± 0.32 | 0.33 | 0.21–0.49 | 4.19 | |
| Azadirachtin + DEM | SS | 3.68* | 2.94 ± 0.39 | 0.24 | 0.12–0.38 | 2.25 |
| SB | 2.86* | 1.71 ± 0.51 | 0.52 | 0.37–0.63 | 2.65 |
*Meant pass the χ2 test.
aPearson chi-square, goodness-of-fit test.
bSR: synergistic ratio = LC50 of insecticides/LC50 of (synergist + insecticides).
Detoxification Enzyme Activities
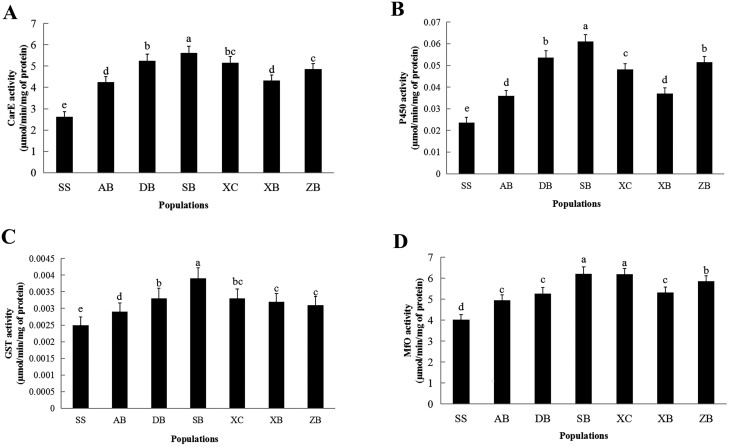
Activities for CarE ranged from 4.26 to 5.62 in the field populations compared with 2.63 μmol/min/mg protein in the SS (Fig. 2A) (F = 191.62; df = 6, 56; P < 0.0001) These results indicated that the CarE activity was significantly higher in the six field populations than in the susceptible population (P < 0.05); the CarE activity ratios of the SB, DB, XC, ZB, XB, and AB populations were 2.14, 2.00, 1.96, 1.85, 1.65, and 1.62-fold, respectively. Correlation analysis showed a significant positive correlation between the RRs to beta-cypermethrin and CarE activity in different field populations (Table 3).
Fig. 2.
Activities of detoxifying enzymes in O. asiaticus (locust) populations from Inner Mongolia. The activities of Carboxylesterase (CarEs) (A), Cytochrome P450 monooxygenase (B), Glutathione S-transferase (C), and Mixed-function oxidase (D) were determined for six field populations and a susceptible strain (SS) of O. asiaticus. The results are means ± SE, bars marked with different letters are significantly different at P < 0.05 by ANOVA with Bonferroni multiple comparison test [F(6,56) = 191.62, P < 0.0001 (CarE); F(6,56) = 225.94, P < 0.0001 (P450); F(6,56) = 127.65, P = 0.0001 (GSTs); F(6,56) = 5.97, P = 0.0027].
Table 3.
Correlation coefficients (r) between insecticide toxicities and enzyme activities in O. asiaticus
| Insecticides | CarEs | GST | MFO | P450 |
|---|---|---|---|---|
| Beta-cypermethrin | 0.932* (0.016) | 0.757 (0.212) | 0.416 (0.562) | 0.842 (0.324) |
| Matrine | 0.556 (0.445) | 0.463 (0.452) | 0.327 (0.614) | 0.905 (0.486) |
| Azadirachtin | 0.572 (0.383) | 0.379 (0.687) | 0.285 (0.796) | 0.917 (0.227) |
The figures in parentheses indicate the probability rejecting null hypothesis that r = 0.
*Represents significant correlation at P > 0.01.
The activities of cytochrome P450 monooxygenases (P450s) were elevated in all six field populations, ranging from 0.04 to 0.06 μmol/min/mg of protein compared with 0.02 μmol/min/mg of protein in the SS population (F = 225.94; df = 6, 56; P < 0.0001), with the SB population showing the highest activity ratio of 2.54-fold (Fig. 2B). Correlation coefficients between the RRs for the three insecticides and P450 activities were not significant (Table 3).
GST activity ranged from 2.9 to 3.9 nmol/min/mg protein in the six field populations compared with 2.5 nmol/min/mg protein in the SS (Fig. 2C) (F = 127.65; df = 6, 56; P = 0.0001). The activity ratios were 1.16–1.56-fold compared to the SS population (P < 0.05). The correlation coefficients between GST and the RRs for beta-cypermethrin, matrine, and azadirachtin were not significant (Table 3).
MFO activities were elevated in all six field populations, ranging from 4.96 to 6.21 μmol/min/mg protein compared with 4.02 μmol/min/mg protein in the SS population (F = 5.97; df = 6, 56; P = 0.0027) (Fig. 2D). The highest activity ratio was 1.54-fold in the SB population. The correlation coefficients between MFO activity and RRs for beta-cypermethrin, matrine, and azadirachtin were not significant (Table 3).
Discussion
Development of resistance in locusts to various groups of insecticides is a major concern for sustainable management of this pest. Our results showed that field populations of locusts from different regions of the Inner Mongalian steppes had varying levels of resistance to three insecticides. Compared with the SS population, resistance levels of six field populations to beta-cypermethrin were still at a low level. The SB and XC populations had low-level resistance to matrine with resistance ratios (RRs) of 5.92 and 6.38, respectively, which is likely associated with the frequent use of matrine for locust control in recent years (Bilal et al. 2018). In the past 10 years, beta-cypermethrin has been used for significant locust outbreaks, while matrine is used more frequently for routine control (Ma et al. 2018). All field populations were susceptible to azadirachtin.
Increased levels of detoxification enzyme activities can play an important role in the early stages of resistance development (Sonoda 2010, Lira et al. 2020). The synergistic inhibition of detoxification enzymes may increase the sensitivity of locusts to the tested insecticides. Based on our results, TPP and DEM had a synergistic effect on beta-cypermethrin resistance with activity ratios of 5.29 and 4.92, respectively, for the SB population, indicating that both CarE and GST play important roles in the resistance of O. asiaticus to beta-cypermethrin. Results also showed that PBO and TPP had a greater synergistic effects with matrine and azadirachtin, suggesting that CarE and MFO are closely associated with the resistance of O. asiaticus to these botanical insecticides. Many secondary metabolites that are produced in plants, including alkaloids and terpenes, have strong insecticidal activity that has been exploited for developing botanical insecticides. In response, insects have evolved multiple detoxification strategies for metabolizing plant toxins (Tan et al. 2019).
In the present study, elevated CarE and GST activities appeared to be associated with the resistance of O. asiaticus to beta-cypermethrin. CarE activity was significantly elevated in all field populations and a significant positive correlation was shown between beta-cypermethrin resistance and CarEs activity in O. asiaticus. Similarly, an association between elevated CarE levels and resistance to beta-cypermethrin has been reported in the house fly, Musca domestica (Zhang et al. 2007, 2010). Elevated CarE activities have been implicated as a biochemical resistance mechanism in many insect species due to their ability to hydrolyze the ester bond of insecticides (Hemingway 2000, Wu et al. 2011). Increased GST activity was found in the six field populations, but statistical analysis failed to establish any significant correlation between GST activity and beta-cypermethrin resistance. Previous studies have demonstrated that GST plays an important role in pyrethroid resistance in many insect species (Vontas et al. 2001, Enayati and Haghi 2007, Achaleke et al. 2009, Cao et al. 2017). Therefore, the enhanced GST activity in these populations may be due to resistance developed against other insecticides that had been previously applied. P450 activity was significantly increased in all six field populations and the activity ratios were between 1.57 and 2.54-fold higher than in the SS. Correlation coefficients between resistance to botanical insecticides (matrine and azadirachtin) and P450 activity were 0.91 and 0.92 for matrine and azadirachtin, respectively, suggesting that these insecticides might trigger P450-associated detoxification mechanisms. Tan (2019) also reported the important role of P450s induced by azadirachtin in xenobiotic metabolism.
In conclusion, the populations of O. asiaticus from the AB, DB, SB, XC, XB, and ZB regions of Inner Mongolia have developed different degrees of resistance to the three selected insecticides. The high selection pressure with beta-cypermethrin and matrine insecticides in the field likely contributed to the resistance emergence of O. asiaticus populations, which are resistant to more than one insecticide. Potential biochemical resistance mechanisms in O. asiaticus against these insecticides were explored using synergists. Our study showed that the activities of detoxification enzymes of O. asiaticus were associated with resistance levels. The results suggested that the variations in O. asiaticus susceptibility to beta-cypermethrin might be attributed to increased activities of CarE and GST, while CarE and MFO might play important roles in metabolizing the plant toxins matrine and azadirachtin. Based on the resistance monitoring data, rotation of insecticides with different modes of action may be needed to delay or prevent the development of resistance.
Acknowledgments
This study was supported by Natural Science foundation of Inner Mongolia (2021MS0321); Central Public-interest Scientific Institution Basal Research Fund (1610332020008); National Science & Technology Fundamental Resources Investigation Program of China (2019FY100400).
Contributor Information
Shujing Gao, Institute of Grassland Research, Chinese Academy of Agricultural Science, Hohhot 010010, Inner Mongolia, China.
Yao Tan, Research Center for Grassland Entomology, Inner Mongolia Agricultural University, Hohhot 010020, China.
Haibin Han, Institute of Grassland Research, Chinese Academy of Agricultural Science, Hohhot 010010, Inner Mongolia, China.
Na Guo, Institute of Grassland Research, Chinese Academy of Agricultural Science, Hohhot 010010, Inner Mongolia, China.
Haiyan Gao, Institute of Grassland Research, Chinese Academy of Agricultural Science, Hohhot 010010, Inner Mongolia, China.
Linbo Xu, Institute of Grassland Research, Chinese Academy of Agricultural Science, Hohhot 010010, Inner Mongolia, China.
Kejian Lin, Institute of Grassland Research, Chinese Academy of Agricultural Science, Hohhot 010010, Inner Mongolia, China.
Author Contributions
Conceptualization, S.J.G., K.J.L. and Y.T.; methodology, S.J.G. and Y.T.; software, H.B.H., N.G.; validation, S.J.G. and Y.T.; formal analysis, H.Y.G and L.B.X.; investigation, H.B.H., N.G., H.Y.G and L.B.X; resources, H.B.H.; writing—original draft preparation, S.J.G. and Y.T.; writing—review and editing, S.J.G., K.J.L. and Y.T.; supervision, K.J.L.. All authors have read and agreed to the published version of the manuscript.
References Cited
- Abbott, W. S. 1987. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 3: 302–303. [PubMed] [Google Scholar]
- Achaleke, J., Martin T., Ghogomu R. T., Vaissayre M., and Brevault T... 2009. Esterase-mediated resistance to pyrethroids in field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) from Central Africa. Pest Manag. Sci. 65: 1147–1154. [DOI] [PubMed] [Google Scholar]
- Bilal, M., Freed S., Ashraf M. Z., and Rehan A... 2018. Resistance and detoxification enzyme activities to bifenthrin in Oxycarenus hyalinipennis (Hemiptera: Lygaeidae). Crop Prot. 111: 17–22. [Google Scholar]
- Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Cao, G., Jia M., Zhao X., Wang L., Tu X., Wang G., Nong X., and Zhang Z... 2017. Effects of chlorantraniliprole on detoxification enzymes activities in Locusta migratoria L. J. Asia-Pac. Entomol. 20: 741–746. [Google Scholar]
- Cease, A. J., Hao S., Kang L., Elser J. J., and Harrison J. F... 2010. Are color or high rearing density related to migratory polyphenism in the band-winged grasshopper, Oedaleus asiaticus? J. Insect Physiol. 56: 926–936. [DOI] [PubMed] [Google Scholar]
- Cease, A. J., Elser J. J., Ford C. F., Hao S., Kang L., and Harrison J. F... 2012. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science. 335: 467–469. [DOI] [PubMed] [Google Scholar]
- Chang, L. L., and Hodgson E... 1975. Biochemistry of detoxification in insects: microsomal mixed-function oxidase activity in the housefly, Musca domestica. Insect Biochem. 5: 93–103. [Google Scholar]
- Chen, H. H., and Kang L... 2000. Olfactory responses of two species of grasshoppers to plant odours. Entomol. Exp. Appl. 95: 129–134. [Google Scholar]
- Chen, H. M., Gao J. P., Shi H., Li X. Y., Peng H., and Ma Y. J... 2019. Role of metebolic detoxification enzyme activity and knockdown resistance gene mutations in resistance of Aedes albopictus to pyrethroid insecticides. Acad. J. Sec. Milit. Med. Univ. 40: 512–519. [Google Scholar]
- Dong, W., Zhang X., Zhang X., Wu H., Zhang M., Ma E., and Zhang J... 2016. Susceptibility and potential biochemical mechanism of Oedaleus asiaticus to beta-cypermethrin and deltamethrin in the Inner Mongolia, China. Pest. Biochem. Physiol. 132: 47–52. [DOI] [PubMed] [Google Scholar]
- Enayati, A. A., and Haghi F. M... 2007. Biochemistry of pyrethroid resistance in German cockroach (Dictyoptera, Blatellidae) from hospitals of Sari, Iran. Iran. Biomed. J. 11: 251–258. [PubMed] [Google Scholar]
- Giraudo, M., Hilliou F., Fricaux T., Audant P., Feyereisen R., and Le Goff G... 2015. Cytochrome P450s from the fall armyworm (Spodoptera frugiperda): responses to plant allelochemicals and pesticides. Insect. Mol. Biol. 24: 115–128. [DOI] [PubMed] [Google Scholar]
- He, L., Xue C. H., Wang J. J., Li M., Lu W. C., and Zhao Z. M... 2009. Resistance selection and biochemical mechanism of resistance to two Acaricides in Tetranychus cinnabarinus (Boiduval). Pest. Biochem. Physiol 95: 56–56. [DOI] [PubMed] [Google Scholar]
- Hemingway, J. 2000. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem. Mol. Biol. 30: 1009–1015. [DOI] [PubMed] [Google Scholar]
- Huang, X., McNeill M., and Zhang Z... 2016. Quantitative analysis of plant consumption and preference by Oedaleus asiaticus (Acrididae: Oedipodinae) in changed plant communities consisting of three grass species. Environ. Entomol. 45: 163–170. [DOI] [PubMed] [Google Scholar]
- Huang, X. B., McNeill M. R., Ma J. C., Qin X. H., Tu X. B., Cao G. C., Wang G. J. X., Nong Q., and Zhang Z. H... 2017. Biological and ecological evidences suggest Stipa krylovii (Pooideae), contributes to optimal growth performance and population distribution of the grasshopper Oedaleus asiaticus. Bull. Entomol. Res. 107: 401–409. [DOI] [PubMed] [Google Scholar]
- Kang, L., and Chen Y. L... 1995. Dynamics of grasshopper communities under different grazing intensities in Inner Mongolian steppes. Entomologia Sinica 2: 265–281. [Google Scholar]
- Kim, Y. J., Lee H. S., Lee S. W., Kim G. H., and Ahn Y. J... 1999. Toxicity of tebufenpyrad to Tetranychus urticae (Acari: Tetranychidae) and Amblyseius womersleyi (Acari: Phytoseiidae) under laboratory and field conditions. J. Econ. Entomol. 92: 187–192. [Google Scholar]
- Li, H. C., and Ma Y... 1990. Fauna and regional distribution of Acridoidea Inner Mongolia. Entomotaxon. 12: 171–193. in Chinese. [Google Scholar]
- Li, H., Zhang H. Z., Pang B., and Chang J... 2014. Toxicities of four pyrethroids and their inhibition to ATPase in Oedaleus asiaticus. Plant Prot. 40: 90–94. [Google Scholar]
- Lira, E. C., Bolzan A., Nascimento A. R. B., Amaral F. S. A., Kanno R. H., Kaiser I. S., and Omoto C... 2020. Resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to spinetoram: inheritance and cross-resistance to spinosad. Pest Manag. Sci. 76: 2674–2680. [DOI] [PubMed] [Google Scholar]
- Liu, L., and Guo A... 2004. Analysis of meteorological and ecological conditions of grasshopper infestation in Inner Mongolia in 2004. Meteorol. Mon. 30: 55–57. [Google Scholar]
- Liu, G., Hao S., Shao X., Zhang Y., and Wang S... 2013. Diet composition and trophic niche of Oedaleus asiaticus (Orthoptera: Acrididae) in natural grasslands under different grazing pressure in Inner Mongolia, northern China. Acta Entomol. Sinica. 56: 537–547. [Google Scholar]
- Ma, C. Y., Du G. L., Zhang Z. R., Yao G. M., Luo J. P., and Na R. H... 2018. Research of grassland locust regionalization and its green prevention-control matching technology in Inner Mongolia. Acta Agrestia Sinica. 26: 804–810. [Google Scholar]
- Mao, L. G., Tu X. B., Liu X. G., Zhang L., Zhang Y., Zhu L. Z., Zhang Y. Q., and Jiang I. H... 2021. Advances and prospects of pesticides registered for controlling locusts and grasshoppers in China. Mod. Agrochem. 20: 1–7. [Google Scholar]
- Oppenoorth, F. J., Van der Pas L. J., and Houx N. W... 1979. Glutathione S-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of housefly and their influence on resistance. Pestic. Biochem. Physiol. 11: 176–178. [Google Scholar]
- Robertson, J. L., Russel R. M., Preisler H. K., and Savin N. E... 2007. Bioassays with arthropods. CRC Press, Boca Raton, FL. [Google Scholar]
- Shang, C. C., and Soderlund D. M... 1984. Monooxygenase activity of tobacco budworm (Heliothis virescens F.) larvae: tissue distribution and optimal assay conditions for the gut activity. Comp. Biochem. Physiol. B. 79: 407–411. [Google Scholar]
- Shelton, A. M., Robertson J. L., Tang J. D., Perez C., Eigenbrode S. D., Priesler H. K., Wilsey W. T., and Cooley J... 1993. Resistance of diamondback moth (Lepidoptera: Plutellidae) to Bacillus thuringiensis subspecies in the field. J. Econ. Entomol. 86: 697–705. [Google Scholar]
- Shu, B., Yu H., Li Y., Zhong H., Li X., Cao L., and Lin J... 2021. Identification of azadirachtin responsive genes in Spodoptera frugiperda larvae based on RNA-seq. J. Econ. Entomol. 172: 104745. [DOI] [PubMed] [Google Scholar]
- Silva, W. M., Berger M., Bass C., Balbino V. Q., Amaral M. H., Campos M. R., and Siqueira H. A... 2015. Status of pyrethroid resistance and mechanisms in Brazilian populations of Tuta absoluta. J. Econ. Entomol. 122: 8–14. [DOI] [PubMed] [Google Scholar]
- Sonoda, S. 2010. Molecular analysis of pyrethroid resistance conferred by target insensitivity and increased metabolic detoxification in Plutella xylostella. Pest Manag. Sci. 66: 572–575. [DOI] [PubMed] [Google Scholar]
- Tan, W. H., Acevedo T., Harris E. V., Alcaide T. Y., Walters J. R., Hunter M. D., Gerardo N. M., and de Roode J. C... 2019. Transcriptomics of monarch butterflies (Danaus plexippus) reveals that toxic host plants alter expression of detoxification genes and down-regulate a small number of immune genes. Mol. Ecol. 28: 4845–4863. [DOI] [PubMed] [Google Scholar]
- Vontas, J., Small G., and Hemingway J... 2001. Glutathione s-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 357: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S., Yang Y., Yuan G., Campbell P. M., Teese M. G., Russell R. J., Oakeshott J. G., and Wu Y... 2011. Overexpressed esterases in a fenvalerate resistant strain of the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 41: 14–21. [DOI] [PubMed] [Google Scholar]
- Xu, J. J., Chang Y. M., Lu M., Tie Y., Dong Y. L., Chen G. Y., Ma Z. Q., Liu X. L., and Li Y. Q... 2021. Two single mutations in carboxylesterase 001C improve fenvalerate hydrolase activity in Helicoverpa armigera. Pest. Biochem. Physiol. 179: 104969. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Wu Y., Chen S., Devine G., Denholm I., Jewess P., and Moores G... 2004. The involvement of microsomal oxidases in pyrethroid resistance in helicoverpa armigera from Asia. Insect Biochem. Mol. Biol. 34: 763–773. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Gao X., and Liang P... 2007. Beta-cypermethrin resistance associated with high carboxylesterase activities in a strain of house fly, Musca domestica (Diptera: Muscidae). Pest. Biochem. Physiol. 9: 65–72. [Google Scholar]
- Zhang, L., Shi J., Shi X., Liang P., Gao J., and Gao X... 2010. Quantitative and qualitative changes of the carboxylesterase associated with beta-cypermethrin resistance in the housefly, Musca domestica (Diptera: Muscidae). Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 156: 6–11. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Elser J. J., Cease A. J., Zhang X., Yu Q., Han X., and Zhang G... 2014. Grasshoppers regulate N:P stoichiometric homeostasis by changing phosphorus contents in their frass. PLoS One. 9: e103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Sun T., Sun Z., Li H., Qi X., Zhang G., and Yi X... 2018. Azadirachtin acting as a hazardous compound to induce multiple detrimental effects in Drosophila melanogaster. J. Hazard. Mater. 359: 338–347. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Lecoq M., Latchininsky A., and Hunter D... 2019. Locust and grasshopper management. Annu. Rev. Entomol. 64: 15–34. [DOI] [PubMed] [Google Scholar]
- Zhou, X. R., Chen Y., Guo Y. H., and Pang B. P... 2012. Population dynamics of Oedaleus asiaticus on desert grasslands in Inner Mongolia. J. Appl. Entomol. 49: 1598–1603. [Google Scholar]
- Zhu, K. Y., and He F. Q... 2000. Elevated esterases exhibiting arylesterase-like characteristics in an organophosphate-resistant clone of the greenbug, Schizaphis graminum (Homoptera: Aphididae). Pest. Biochem. Physiol. 67: 155–167. [Google Scholar]
- Zibaee, I., Mahmood K., Esmaeily M., Bandani A. R., and Kristensen M... 2018. Organophosphate and pyrethroid resistances in the tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) from Iran. J. Appl. Entomol. 142: 181–191. [Google Scholar]