Abstract
There is an epidemiological association between influenza virus infection and meningococcal disease. Proposed mechanisms are the destruction of the normal epithelial barrier function of the upper respiratory tract by influenza virus or the expression of human or viral surface-exposed proteins that enhance bacterial adherence and/or invasion. To test these hypotheses, human nasopharyngeal mucosa specimens from a total of 19 individual donors were successfully infected with influenza B virus and then inoculated with serogroup B Neisseria meningitidis. Subsequent bacterial association with the epithelial surface was measured in three separate series of experiments by using transmission electron microscopy (n = 6), scanning electron microscopy (n = 6), and counting of viable bacteria within homogenates of explants (n = 7). Penetration of the mucosa was estimated by measuring the count of viable bacteria recovered from explants after exposure to sodium taurocholate. Bacterial association with the surface of explants was time dependent over 24 h of superinfection. Influenza virus did not positively or negatively influence bacterial attachment to or penetration of explant mucosa compared to those of uninfected controls, even when the period of preincubation with virus was extended to 7 days. When proteins were purified from mucosal epithelium and immobilized on nitrocellulose membranes, N. meningitidis attached predominantly to bands corresponding to proteins of 210 and 130 kDa. In the presence of influenza virus infection, these proteins were gradually lost over the course of 72 h. In conclusion, influenza B virus did not increase association of serogroup B N. meningitidis with human nasopharyngeal mucosa.
Neisseria meningitidis may cause invasive disease following a period of colonization of the upper respiratory tract. There is an epidemiological association of meningococcus-associated disease with other infections that result in disease of the upper respiratory tract mucosa, including influenza A virus (3), influenza B virus (8), and Mycoplasma (11) infections. This could be due to a number of factors, including increased binding to or penetration of virus-infected airway mucosa and subtle dysregulation of the innate response to N. meningitidis. Soon after the onset of symptoms of influenza, airway tissue derived from affected patients demonstrates edema, vacuolation of columnar epithelial cells, loss of cilia, and desquamation (12). Organ cultures of human adenoid tissue experimentally infected with influenza virus A demonstrate epithelial vacuolization and sloughing commencing 24 h after inoculation (7), which tend to be more severe in tissue obtained from nonimmune donors.
When human nasopharyngeal explants are inoculated with N. meningitidis, the organism binds predominantly to nonciliated cells and elicits parasite-directed epithelial responses, including formation of microvillus extensions and endocytosis (14, 16). Adherence to and penetration of human nasopharyngeal mucosa in vitro by N. meningitidis are accompanied by phase variation of multiple surface features of this organism, including capsule production, sialylation of lipopolysaccharide, and outer membrane protein expression (5).
In view of the association between influenza virus infection and meningococcus-associated disease, we used an organ culture model of human nasopharyngeal mucosa to determine whether experimental infection of human mucosa with influenza B virus modifies subsequent binding to and penetration of N. meningitidis.
MATERIALS AND METHODS
Microorganisms.
A single serogroup B case isolate (K454), typed as B15P1.7,16L3,7,9, sulfonamide resistant, and electrophoretic type ET-5, was used in this study (4). This strain is piliated and carries opc and pilC (data not shown). For each experiment, a suspension of approximately 105 CFU of washed mid-log-phase N. meningitidis per ml in 3 ml of minimal essential medium (MEM) (Gibco, Uxbridge, United Kingdom) was made. Stocks of influenza virus B/Singapore/222/79 were prepared by the allantoic inoculation of 10-day embryonated eggs and stored at −80°C in 100-μl aliquots, each of which contained approximately 640 hemagglutinating units (fowl red cells were used) of virus. This was the lowest concentration that consistently resulted in detection of influenza virus B by enzyme-linked immunosorbent assay of supernatants of organ cultures after 48 and 72 h of incubation.
Organ culture.
Human respiratory mucosa samples were derived from inferior turbinates resected from patients with nonallergic nasal obstruction. An established organ culture technique was used (14), in which 3- to 4-mm squares of mucosa were initially treated with antibiotics and then supported in a petri dish with the epithelial surface projecting above a bed of nonnutrient agar. MEM (without antibiotics) was added to immerse the tissue. Explants that demonstrated ciliary activity were subsequently incubated in a humidified atmosphere of 5% CO2 in air at 37°C. Tissue culture medium was then aspirated, and the 100-μl aliquot of virus or phosphate-buffered saline (PBS) as a control was placed onto the surface of the explant and left for 1 h. MEM was then replaced, and the whole was incubated for variable lengths of time up to 7 days, according to the requirements of each experiment conducted. To superinfect with meningococci, at a given point, tissue culture medium was aspirated and replaced with 3 ml of MEM containing either N. meningitidis or MEM control and the incubation was continued.
Verification of influenza virus infection.
Supernatants of influenza virus-infected and control organ cultures were sampled at intervals from 18 to 72 h, and the viral antigen load was determined by enzyme immunoassay. This was performed with a ladder of reagents, from the solid phase up, consisting of the following: (i) rabbit antiserum (raised against influenza virus B/Singapore/222/79), (ii) organ culture supernatant, (iii) mouse antiserum (raised against influenza virus B/Singapore/222/79), (iv) goat anti-mouse immunoglobulin G (IgG) conjugated with horseradish peroxidase. Following incubation for 20 min, the reaction was stopped with sulfuric acid and the chromogen product was measured by reading absorbance at 492 nm.
Explants infected for 24, 48, and 72 h and 7 days were fixed in 4% paraformaldehyde, and viral antigens were demonstrated within the epithelium by a standard immunoperoxidase (peroxidase-antiperoxidase method) technique, with partial trypsin digestion. To monitor influenza viral infection of organ cultures used in experiments, homogenates of explants were inoculated into MDCK cells to detect cytopathic effect. If a control explant was infected with influenza virus, the experiment was abandoned.
Measurement of bacterial association and invasion by homogenization of explants.
At specified points during incubation, explants were removed from their petri dishes, washed, weighed, and then homogenized in a modified French press (Constant Systems, Warwick, United Kingdom), at 10 lb/in2. This pressure caused no loss of viability of an inoculum containing 107 CFU of N. meningitidis (data not shown). Viable bacteria within the homogenized explant were enumerated by a dilutional technique. To estimate invasion, explants were immersed in 0.25% sodium taurocholate (bile salts) for 30 s, washed in PBS, and homogenized. This concentration kills 107 CFU of N. meningitidis within 30 s. Explants that are treated with sodium taurocholate and then washed with PBS prior to homogenization do not exhibit any bactericidal activity against the same number of meningococci, suggesting that sodium taurocholate does not penetrate the tissue at concentrations that are detectable by simple bioassay (data not shown). Therefore, the viable bacteria recovered were assumed to have been protected from sodium taurocholate by penetration of the mucosa; the invasive fraction was calculated by dividing this number by the number of viable bacteria recovered from tissue not pretreated with sodium taurocholate.
Electron microscopy.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were conducted by a blinded observer. For TEM, a randomly selected ultrathin section (typically consisting of 100 to 500 epithelial cells) from the central part of each specimen was examined. The proportions of the surface of the explant demonstrating ciliated and nonciliated cells, stripping to the basal cell layer or basement membrane, or dead cells were noted. When N. meningitidis cells were seen in the section, the frequency of association of bacteria was calculated by dividing the total number of bacteria in the section by the number of epithelial cells per section. For SEM, the center of each specimen was set at 0° tilt, magnification was increased to ×2,500, and the features of the surface were examined in a box pattern (13) over a calculated surface area of 0.06 mm2. The percentages of the surface of the mucosa that were ciliated, nonciliated, covered with normal mucosa, or extruded or stripped (damaged) were noted. Bacteria were counted, and the numbers associated with these surface features were noted.
Detection of expression of novel epithelial receptors following influenza virus infection.
Tissue explants that were either uninfected or infected with influenza virus B for 24, 48, or 72 h were gently washed and placed in solubilization buffer (10 mM Tris-HCl [pH 7.0], 1 mM EDTA, 1% sodium dodecyl sulfate, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride), and warmed at 37°C for 15 min. Solid material was removed by centrifugation at 1,500 × g for 5 min, and aliquots of the cleared lysate were loaded on sodium dodecyl sulfate–10% polyacrylamide gels. The proteins were separated by electrophoresis and then transferred to nitrocellulose membranes by electroblotting. The nitrocellulose membranes were blocked by using 1% bovine serum albumin in 50 mM Tris-HCl (pH 8.0) and then incubated with approximately 106 CFU of N. meningitidis K454 (grown to late log phase in Dulbecco’s MEM [DMEM] containing 2% fetal calf serum) per ml in fresh DMEM containing 2% fetal calf serum for 6 h. After this time, membranes were washed in fresh medium to remove unattached bacteria and finally washed in PBS. Bacteria were fixed to the nitrocellulose membranes by incubation in PBS containing 2% formaldehyde for 10 min, and the residual formaldehyde was removed by repeated washing in 50 mM Tris-HCl (pH 8.0). Attachment of N. meningitidis was detected by incubation with monoclonal antibody (MAb) TbpB1, a murine MAb which recognizes the TbpB proteins from the majority of N. meningitidis isolates tested. The MAb TbpB1 is of IgG class 2a and recognizes native TbpB protein. Membranes were incubated with the primary antibody, MAb TbpB1, for 2 h at room temperature, washed three times in 50 mM Tris-HCl (pH 8.0), and then incubated with a rabbit anti-mouse IgG 2a conjugated with alkaline phosphatase in 50 mM Tris-HCl (pH 8.0) for 1 h. Membranes were then washed in 50 mM Tris-HCl (pH 8.0), and the positions of bacteria adherent to protein bands on the membrane were detected by incubation with the chromogenic substrate 5-bromo-4-chloro-3-indolylphosphate toluidinium–nitroblue tetrazolium.
Statistical analysis.
Data were collected for experiments using explants from six different human donors for each of the studies employing SEM and TEM and explants from seven donors for the studies in which explants were homogenized for dilutional counting of bacteria. One-way analysis of variance (general linear model) was conducted by using the Minitab 9 statistical package, with each donor treated as a blocking factor, and virus infection, duration of virus infection, and length of bacterial incubation as covariates. Approximate normality of residuals was assessed graphically using Normal Scores plots, and data were also logarithmically transformed before analysis, to approximate normality.
RESULTS
Verification of infection of explants by influenza virus.
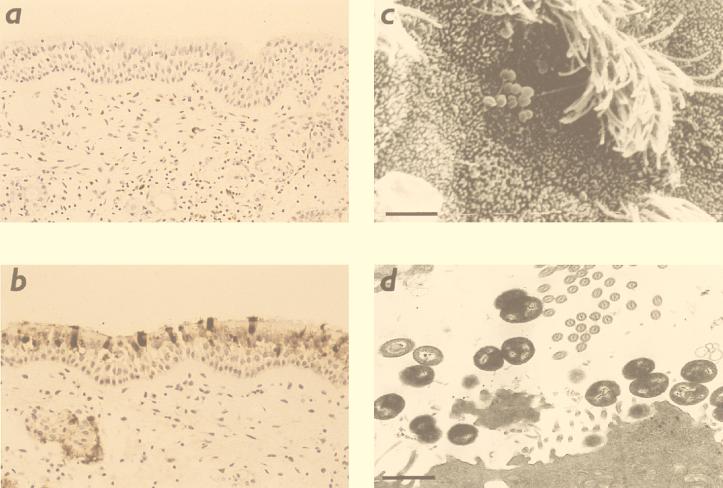
By enzyme immunoassay, influenza virus antigen was detected in supernatants of organ cultures over 72 h of infection with influenza virus, and it was detected at higher concentrations in complete organ cultures infected with influenza virus than in identical preparations lacking tissue (infected sham control) (Table 1). The concentration in supernatants of infected explants measured by enzyme immunoassay reached a maximum by 48 h. Following this period of incubation, virus could be cultured from organ culture tissue and supernatant. By immunohistochemistry, a strong signal was demonstrated within 5 to 20% of individual sustentacular cells at 48 h of infection (Fig. 1a and b). There was no detectable virus in either basal cells or Bowman’s glandular cells. Virus was not detected in uninfected control preparations.
TABLE 1.
Results of enzyme immunoassays of supernatants of organ cultures infected with influenza virus
| Organ culture | Absorbance at 492 nm (mean ± SEM) (n = 3) after infection of organ culture with influenza virus for:
|
|||
|---|---|---|---|---|
| 16 h | 24 h | 48 h | 72 h | |
| Uninfected control | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 |
| Infected culture | 0.70 ± 0.01 | 0.76 ± 0.01 | 0.84 ± 0.03 | 0.71 ± 0.01 |
| Infected sham control | 0.54 ± 0.01 | 0.52 ± 0.01 | 0.49 ± 0.04 | 0.50 ± 0.04 |
FIG. 1.
Microscopy of human nasopharyngeal explants. Immunohistochemistry of control explant (a) and explant infected for 72 h with influenza B virus (b). The latter shows strong signal from individual sustentacular cells, in contrast to absent staining in the control section. (c) Scanning electron micrograph of human nasopharyngeal explant incubated with PBS as a control for 48 h and superinfected with N. meningitidis for 24 h; bacteria are seen in association with nonciliated epithelial cells (original magnification, ×2,500). (d) Transmission electron micrograph of an explant treated similarly to that shown in panel c. Bacteria are seen adhering to the epithelial surface (bar = 10 μm).
Morphology of influenza virus-infected explants.
When examined by light microscopy, explants infected with influenza virus for 24, 48, and 72 h retained apparently normal pseudostratified ciliated epithelium, but after 7 days there was widespread stripping of epithelium which was not present in controls. Therefore, for TEM and SEM, only explants incubated with influenza virus for 48 h and subsequently superinfected with N. meningitidis or treated with PBS control for a further 6 and 24 h were examined. When examined by TEM and SEM, these explants infected with virus for 54 and 72 h demonstrated patchy extrusion of epithelial cells and loss of cilia from ciliated cells compared to explants that were not infected with virus.
Effect of influenza virus on interaction of N. meningitidis with nasopharyngeal explants.
Bacterial association with explants was greater after 24 h than after 6 h (Table 2). By TEM and SEM, bacteria were seen to be predominantly associated with nonciliated cells in both virus-infected and uninfected explants (Fig. 1c and d). There was marked variation in frequency of bacterial association among individual donors, but no overall positive or negative effect of influenza virus was observed over the three series of experiments employing viable counts of homogenized explants, TEM, and SEM. The relative effects of virus (after controlling for incubation times and subject) on viable bacteria recovered from homogenates and invasive fraction were 0.66 (95% confidence interval [CI], 0.28 and 1.54) and 1.53 (95% CI, 0.75 to 3.15), respectively; the effect on bacteria observed on explants by SEM was 0.41 (95% CI, 0.18 to 1.36). There were no significant differences demonstrated by analysis of variance. The data from the TEM experiment were not formally analyzed because of the small numbers of explants displaying bacterial association. The mean invasive fraction was higher in influenza virus-infected explants than in uninfected explants, but the effect was inconsistent across individual donors and nonsignificant overall.
TABLE 2.
Effect of 48 h influenza B virus infection of human nasopharyngeal explants on subsequent bacterial association by N. meningitidis 6 and 24 h after inoculation
| Treatment of explant, period of superinfection with N. meningitidis | Viable N. meningitidis (CFU [103] ± SEM) recovered per mg of homogenized explant (n = 7)
|
SEM (n = 6)
|
TEM (n = 6)
|
||||
|---|---|---|---|---|---|---|---|
| After washing alone | After washing plus bile treatment | Invasive fraction | No. of explantsa | Mean no. of bacteria in sample area (0.06 mm2) | No. of explantsb | Ratio of bacteria to epithelial cells in section | |
| Influenza B virus for 48 h | |||||||
| 6 h | 3.43 ± 0.99 | 0.28 ± 0.13 | 0.17 ± 0.06 | 4 | 86 ± 35 | 1 | 0.012 |
| 24 h | 48.74 ± 10.93 | 7.67 ± 4.44 | 0.11 ± 0.06 | 6 | 150 ± 47 | 3 | 0.042 ± 0.015 |
| Uninfected control at 48 h | |||||||
| 6 h | 3.43 ± 1.05 | 0.13 ± 0.05 | 0.05 ± 0.02 | 6 | 381 ± 170 | 2 | 0.021 ± 0.008 |
| 24 h | 157.46 ± 81.32 | 3.91 ± 1.94 | 0.03 ± 0.01 | 6 | 555 ± 172 | 3 | 0.516 ± 0.457 |
No. of explants with N. meningitidis on epithelial surface.
No. of explants displaying N. meningitidis on or within epithelium.
When the period of incubation of explants with virus prior to superinfection with N. meningitidis was extended to 7 days, subsequent bacterial recovery from explant homogenates was significantly greater, but there was no effect of virus on total bacterial recovery from explants or on the invasive fraction (n = 7; data not shown).
Binding of N. meningitidis to solubilized epithelium from influenza virus-infected explants.
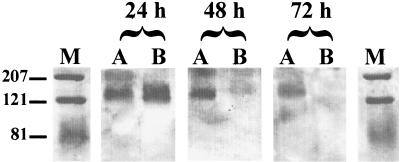
The immunoblots revealed that bacteria attached to the nitrocellulose membranes predominantly at positions corresponding to proteins with sizes of approximately 210 and 130 kDa (Fig. 2). Autoradiographs from similar experiments using radiolabelled bacteria grown in leucine-deficient medium containing 10 kBq of 14C-labelled leucine/ml confirmed these binding positions but also gave high background on autoradiographs, possibly due to excessive bacterial lysis during the labelling procedure.
FIG. 2.
Immunoblot of N. meningitidis bound to solubilized epithelium. M, molecular weight markers; A, uninfected control epithelium; B, epithelium infected with virus for 24, 48, or 72 h, as indicated. Molecular weights are indicated on the left.
Some variation in binding pattern could be observed between the virus-infected and noninfected solubilized explants over the course of the incubation. With solubilized tissue from explants incubated for 24 h, bacteria attached to two distinct bands corresponding to proteins of 210 and 130 kDa. By 72 h of incubation, attachment to the 210-kDa band from all explants by bacteria was considerably reduced, indicating that changes in the explant tissue had occurred during in vitro culture. The 130-kDa band of control explants remained a constant and significant target for N. meningitidis throughout the course of the experiment, but in virus-infected explants, a reduction in binding to the nitrocellulose membranes at this position could be observed by 48 h of virus infection, indicating that this protein was probably being lost from virus-infected tissue.
DISCUSSION
In this study an organ culture technique was employed because we wanted to establish whether prior influenza virus infection of an intact mucosal surface enhances meningococcal association with it. We verified that explants were infected with virus and conducted three series of experiments, each using a separate method to detect meningococcal association with the mucosa. No effect of virus was demonstrated.
The majority of the data were collected from explants preinfected with influenza virus for 48 h, because after this period virus could be demonstrated within the mucosa, while the epithelium retained sufficient integrity for orientation and identification of surface structures by electron microscopy. Explants infected with virus for more than 72 h displayed gross exfoliation of epithelial cells, but even when the incubation with influenza virus was extended to 7 days, we demonstrated no effect on recovery of subsequently inoculated meningococci from homogenates of explants.
Meningococcal association was assessed after 6 and 24 h of superinfection, because it can be detected in experimentally infected mucosae from the majority of donors at these time points by this technique (14). One turbinate from an average donor will usually yield no more than 12 viable explants after dissection, which limits the sample size of the experiments. Therefore, to provide sufficient replicates for statistical power, we had to choose only one strain each of N. meningitidis and influenza virus. The N. meningitidis strain K454 is a clinical isolate that has been shown by using this method to successfully invade mucosal tissue (14), and it originates from a clone that was prevalent at a time when epidemic influenza activity was associated with an exceptional increase in the number of cases of meningococcus-associated disease (3).
We examined attachment of N. meningitidis to solubilized extracts of virus-infected explants because we wanted to test the hypothesis that influenza virus infection of nasopharyngeal epithelial cells results in export of novel receptors for meningococcal binding to the epithelial surface. However, we found that at the point at which meningococcal superinfection was conducted (48 h after virus infection) proteins available for meningococcal attachment were apparently more scarce. This could result either from reduction in receptor expression by virus-infected cells or from shedding of terminally differentiated cells and replacement by regenerating epithelium that lacks the receptors present in control epithelia. That epithelial shedding occurs is supported by our microscopy data.
We used influenza virus B to simplify detection of natural influenza infection of donor tissue and contamination of controls. However, virulence of influenza virus B in humans is very similar to that of influenza virus A (12), and the epidemiological association between meningococcus-associated disease and influenza applies to both subtypes.
The damage to virus-infected mucosa in organ culture at 54 and 72 h was relatively mild. Edwards et al. (7) demonstrated marked epithelial cytotoxicity in human adenoidal tissue infected with influenza virus A for 24 h, with extensive epithelial stripping by 48 h. This disparity may be because nasal turbinate tissue is more robust than other respiratory mucosal tissues. Also, individual strains of influenza virus B vary widely in their cytotoxicities in animal tracheal organ cultures (15).
The overall result is consistent with some epidemiological observations. A number of studies have failed to demonstrate any relationship between acquisition of nasopharyngeal carriage of meningococcus and intercurrent virus infection (1, 2, 6). During epidemics of influenza virus A infection in the United Kingdom in 1976 and 1989, the observed rise in meningococcal disease occurred at least 2 weeks after the peak of influenza infection (3), but if increased susceptibility is mediated by increased binding to and invasion of epithelial cells, then the peaks in influenza and meningococcus-related disease should be almost synchronous, in view of the stable background prevalence of community carriage.
What are the alternative mechanisms for the epidemiological observation? Perhaps meningoccoci have tropism for the relatively undifferentiated epithelium during initial recovery from influenza infection, but this is not supported by our data. Interference with humoral immunity is likely to be irrelevant, because most disease occurs in nonimmune individuals. One possibility that our quantitative study has not addressed is that influenza qualitatively changes the interaction of meningococci in a manner that is permissive to invasion of the host by a small number of bacteria. Clearly virus causes morphometric changes in epithelial cells, but what about other cell species? Influenza virus has profound effects on phagocytic components of innate defenses. Once N. meningitidis penetrates the nasopharyngeal epithelium, defense against bloodstream invasion depends upon recognition and killing by macrophages and, if necessary, recruited polymorphonuclear leukocytes. Influenza virus induces apoptosis in macrophages and also modifies cytokine and chemokine secretion. Following influenza virus infection, macrophages secrete predominantly mononuclear-cell-attracting chemokines, whereas neutrophil chemoattractants, e.g., interleukin 8, are entirely suppressed (10). Influenza-infected neutrophils are defective in chemotaxis and phagocytic function (9). In the light of this study, such mechanisms may be pathophysiologically relevant.
ACKNOWLEDGMENTS
This work was supported by the Special Trustees of the Former United Sheffield Hospitals. R.C. Read is supported by the National Meningitis Trust.
We thank Mark Pickett and Ian Palmer for technical assistance and Pauline Whitaker for her help with preparation of the manuscript.
REFERENCES
- 1.Artenstein M S, Rust J H, Hunter D H, Lamson T H, Buescher E L. Acute respiratory disease of meningococcal infection in army recruits. JAMA. 1967;201:1004–1008. [PubMed] [Google Scholar]
- 2.Blakebrough I S, Greenwood B M, Whittle H C, Bradley A K, Gilles H M. The epidemiology of infections due to Neisseria meningitidis and Neisseria lactamica in a northern Nigerian community. J Infect Dis. 1982;146:626–637. doi: 10.1093/infdis/146.5.626. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright K A V, Jones D M, Smith A J, Stuart J M, Kaczmarski E B, Palmer S R. Influenza A and meningococcal disease. Lancet. 1991;338:554–557. doi: 10.1016/0140-6736(91)91112-8. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright K A V, Stuart J M, Jones D M, Noah N T. The Stonehouse Survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987;99:591–601. doi: 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vries F P, Van der End A, Van Putten J P M, Dankert J. Invasion of primary nasopharyngeal cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect Immun. 1996;64:2998–3006. doi: 10.1128/iai.64.8.2998-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Wals P, Gilquin C, de Maeyer S, Bouikaert A, Nowel A, Lechat M F, Lafontaine A. Longitudinal study of asymptomatic meningococcal carriage in two Belgian populations of schoolchildren. J Infect. 1983;6:147–156. doi: 10.1016/s0163-4453(83)92756-1. [DOI] [PubMed] [Google Scholar]
- 7.Edwards K M, Snyder P N, Stephens D S, Wright P F. Human adenoid organ culture: a model to study the interaction of influenza A with human nasopharyngeal mucosa. J Infect Dis. 1986;153:41–47. doi: 10.1093/infdis/153.1.41. [DOI] [PubMed] [Google Scholar]
- 8.Harrison L H, Armstrong C W, Jenkins S R, Harmon M W, Ajello G W, Miller G B, Broome C V. A cluster of meningococcal disease on a school bus following epidemic influenza. Arch Intern Med. 1991;151:1005–1009. [PubMed] [Google Scholar]
- 9.Hartshorn K L, Liou L S, White M R, Kazhdan M M, Tauber J L, Tauber A I. Neutrophil deactivation by influenza A virus. Role of haemagglutinin in binding to specific sialic acid-bearing cellular proteins. J Immunol. 1995;154:3952–3960. [PubMed] [Google Scholar]
- 10.Hofmann P, Sprenger H, Kaufmann A, Bender A, Hasse C, Nain M, Gemsa D. Susceptibility of mononuclear phagocytes to influenza A virus infection and possible role in the antiviral response. J Leukoc Biol. 1997;65:408–414. doi: 10.1002/jlb.61.4.408. [DOI] [PubMed] [Google Scholar]
- 11.Moore P S, Hierholzer J, De Witt W, Gouan K, Djore D, Lippeveld T, Plikaytis B, Broom C V. Respiratory viruses and Mycoplasma as co-factors for epidemic group A meningococcal meningitis. JAMA. 1990;264:1271–1275. [PubMed] [Google Scholar]
- 12.Murphy B R, Webster R G. Orthomyxoviruses. In: Fields B N, Knipe D M, Chanock R M, et al., editors. Fields virology. 2nd ed. Vol. 1. New York, N.Y: Raven Press; 1990. pp. 1091–1152. [Google Scholar]
- 13.Rayner C F J, Jackson A D, Rutman A, Dewar A, Mitchell T J, Andrew P W, Cole P J, Wilson R. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995;63:442–447. doi: 10.1128/iai.63.2.442-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read R C, Fox A, Miller K, Gray T, Jones N, Borrow R, Jones D M, Finch R G. Experimental infection of human nasal mucosal explants with Neisseria meningitidis. J Med Microbiol. 1995;42:353–361. doi: 10.1099/00222615-42-5-353. [DOI] [PubMed] [Google Scholar]
- 15.Reeve P, Pibermann M, Gerendas B. Studies with some influenza B viruses in cell cultures, hamsters and hamster tracheal organ cultures. Med Microbiol Immunol. 1981;169:179–186. doi: 10.1007/BF02123591. [DOI] [PubMed] [Google Scholar]
- 16.Stephens D S, Hoffman L H, McGee Z A. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J Infect Dis. 1983;148:369–377. doi: 10.1093/infdis/148.3.369. [DOI] [PubMed] [Google Scholar]