Abstract
Background
Palmar-plantar atopic dermatitis is a common and debilitating condition with significant diagnostic and management challenges.
Case Presentation
The paper presents the successful treatment of dupilumab in five female patients with recalcitrant palmar-plantar atopic dermatitis.
Methods
The Hand Eczema Severity Index (HECSI) and the Foot Eczema Severity Index (FECSI), eosinophils and IgE levels were evaluated before and after the treatment.
Results
Five recalcitrant palmar-plantar atopic dermatitis patients had substantial responses to dupilumab and the persistent pruritus was soon alleviated during the therapy. By week 16, all patients’ palmar-plantar eczema has been almost clear, with significant decrease of HECSI and FECSI. The count of eosinophils and IgE levels returned to normal in all patients.
Conclusion
Dupilumab significantly improves disease severity and reduces eosinophils and IgE in refractory palmar-plantar AD patients.
Keywords: atopic dermatitis, dupilumab, palmar-plantar
Introduction
Atopic dermatitis (AD) is a systemic inflammatory disorder that occurs as a result of epidermal barrier disruption and dysregulation of type 2 immune response. Palmar-plantar dermatitis is a common and debilitating condition of AD with significant diagnostic and management challenges. Hand eczema alternatively called palmar eczema has various etiological subtypes, of which atopic hand eczema is the most common subtype and accounts for over 50%. According to the UK Working Party criteria, it is characterized by the presence (previously or currently) of hand eczema in patients with AD.1 For treatment, steroids, alitretinoin, hydration, emollients, and calcineurin inhibitors are the usual options,2 but up to 65% of cases have not been well alleviated after treatment.3
Dupilumab, a monoclonal antibody inhibiting interleukin (IL)-4 and IL-13 signaling, has been approved for the treatment of AD. IL-4 and IL-13 are central drivers of the type 2 pathway and play critical roles in the pathogenesis of AD. However, limited information is available on dupilumab for treating AD with confined skin to the palmar-plantar. Since the palm, including the fingers, makes up only 1% of the body surface area. Patients with limited lesions in their hands and feet had a small EASI score, so they were hardly included in the study to evaluate the therapeutic efficacy of dupilumab. Several case series and ongoing phase II clinical trials focus on hand provide additional data. Oosterhaven and Matthew first reported 1 and 3 cases of dupilumab successfully treating hand eczema in atopic patients, respectively.4,5 In addition, an observational study using dupilumab in AD patients with comorbidity hand eczema found significant improvement in hand eczema based on the Hand Eczema Severity Index (HECSI) and quality of life measures.6 The results of these cases and observational studies have prompted an ongoing phase 2 clinical trial involving 94 patients with moderate to severe hand eczema.7 Besides, Stingeni et al conducted a retrospective analysis of 139 AD patients treated with dupilumab and analyzed the effect of the drug depending on AD phenotypes. The authors found that dupilumab is especially effective for diffuse eczema type and has comparable efficacy on other types including hand eczema type.8 However, dupilumab treatment of AD patients with palmar-plantar or plantar alone lesions has not yet been described.
In this study, we evaluated the clinical effectiveness and safety of five recalcitrant hand and foot atopic dermatitis after the treatment of dupilumab. The biomarkers such as IgE and eosinophils involved in the pathogenesis of AD were also assessed.
Methods
All cases in this study satisfied at least three major features and three minor features of the Hanafin and Rajka criteria for diagnosis of atopic dermatitis including pruritus, chronic or chronically-relapsing dermatitis, personal or family history of atopy such as asthma, allergic rhinitis, atopic dermatitis, xerosis, elevated serum immunoglobulin E, itch when sweating, course influence by environmental/emotional factors, food intolerance. All patients received dupilumab at a 600 mg loading dose, with 300 mg every two weeks for at least 16 weeks. They had tried more than four systemic or topical treatments including immunosuppressive/immunomodulatory drugs. The following data was collected before and after treatment: gender, age at diagnosis of palmar-plantar AD, information on personal and family history of atopy, the count of eosinophils and IgE, Eczema Severity Index of hands and feet, Pruritus Rating Scales (NRS) and adverse event occurring during treatment. HECSI was used to record the severity prior and response to the dupilumab, and Foot Eczema Severity Index (FECSI) is referenced as HECSI.9 Two dermatologists measured the HECSI and FECSI at baseline, and every time before the injection until week 16. Meanwhile, timing of discontinuations of Dupilumab in the patients and outcomes were recorded in Table 1.
Table 1.
The Characteristics of Patients and the Details of Dupilumab Therapy
| No. | Age (y)/Sex | Duration (y) | Prior Therapies | Other Allergic Diseases | Eosinophils (Norm:0.02–0.52×109/L) | IgE (0–100 IU/mL) | Family History of Atopy | Positive Allergen Test | Treatment Duration (Dupilumab) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32/F | 4 | Prednisone, tacrolimus, alitretinoin | AR AC |
0.3×109 | 644 | AR (Father) |
ND | 600 mg loading dose 300 mg/2w (2–16w) 300 mg/4w (17w-1y) |
Relief |
| 2 | 65/F | 7 | Corticosteroids, Loratadine, cetirizine |
COPD AR |
1.63×109 | 501 | AR Asthma (Father) |
Cashew cuts Mixed grass Mulberry Tree pollen assemblage |
600 mg loading dose 300 mg/2w (2–22w) 300 mg/4w (23w-1y) ongoing treatment |
Relief |
| 3 | 68/F | 8 | Hydroxychloroquine sulfate, Ebastine, cetirizine |
CU | 1.8×109 | 8.6 | AR, AB (Mother) |
ND | 600 mg loading dose 300 mg/2w (2–16w) |
Recrudescence after withdrawal |
| 4 | 72/F | 3 | Prednisone, tacrolimus, alitretinoin |
AR | 1.08×109 | 214 | Asthma (Father) |
ND | 600 mg loading dose 300 mg/2w (2–20w) |
Relief |
| 5 | 55/F | 9 | Corticosteroids, Antihistamines |
AR | 0.2×109 | 185 | AR (Mother) |
Mulberry | 600 mg loading dose 300 mg/2w (2–24w) ongoing treatment |
Relief |
Abbreviations: F, Female; Y, Year; W, Week; COPD, Chronic obstructive pulmonary disease, AR, Allergic Rhinallergosis; CU, Chronic urticaria, AC, Allergic conjunctivitis, AB, Allergic bronchitis, ND, Not done.
Results
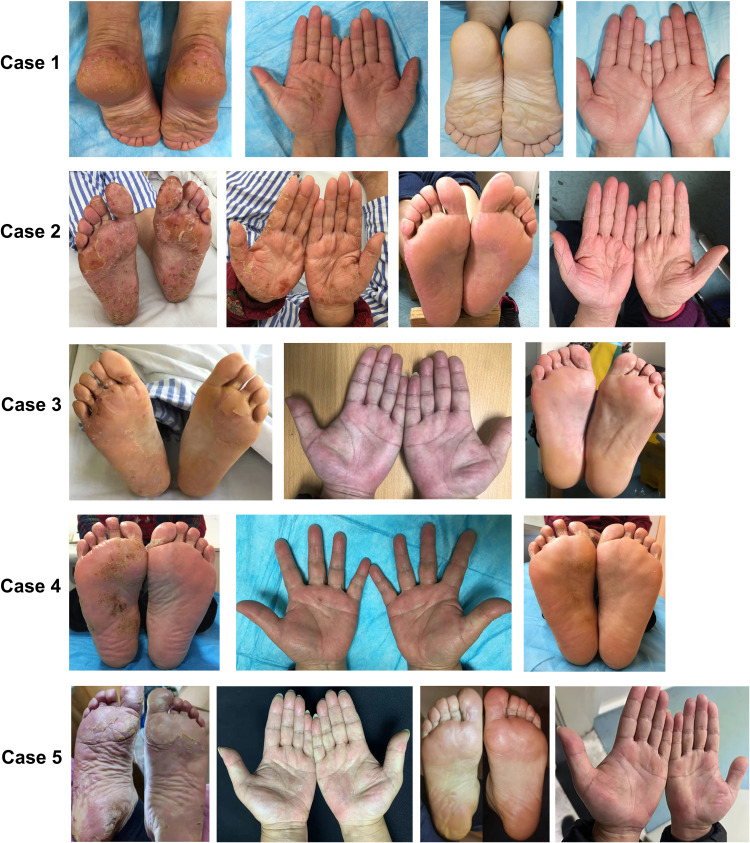
The characteristic of patients was summarized in the Table 1. Five patients were consecutively treated with dupilumab for AD with concomitant palmar-plantar eczema. Case 3 and Case 4 only had a plantar-alone lesion. The other three patients had palmar-plantar involvement. Case 2 and Case 5 had positive allergen test results. The five female patients consulted by dermatology department had a mean age of 58.4 years old with average disease duration of 6.2 years. All five patients complained of itching hyperkeratosis and scaly skin on both feet and (or) hands without the involvement of other body sites. They all had the atopic disease of rhinallergosis or allergic conjunctivitis since younghood. Case 3 had a history of chronic urticaria, while Case 1 also had chronic obstructive pulmonary disease. The patients were treated with prednisone, tacrolimus or alitretinoin without significant remission. All patients received dupilumab at a 600 mg loading dose with 300 mg biweekly maintenance doses at the same injection schedule for at least 16 weeks. Case 2 and Case 5 were prescribed with maintenance doses of dupilumab until now. Hyperkeratosis and erythema gradually recovered during the therapy (Figure 1).
Figure 1.
The pictures of patients’ hands and feet before and after the treatment of dupilumab. From case 1 to case 5.
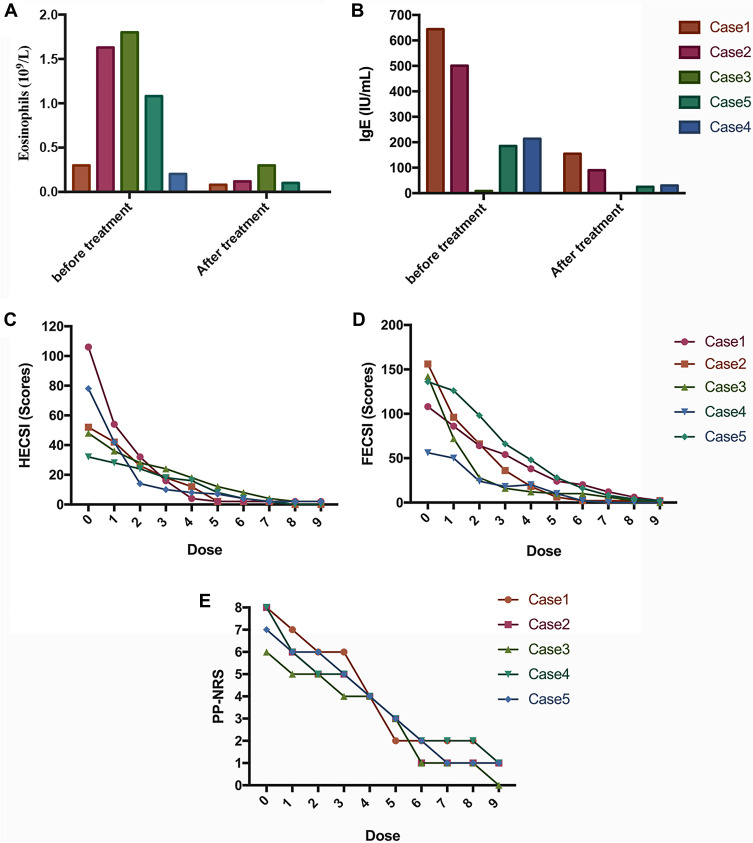
All patients presented with elevations of eosinophils and (or) IgE, with the estimated amount of 1.03×109/L and 228 IU/mL. After 16 weeks, all patients’ eosinophil and IgE levels returned to normal level. Meanwhile, the HECSI and FECSI also gradually decreased during the treatment, and finally five patients’ eczema was “almost clear”. The pruritus of all the patients was also markedly alleviated, which obviously improved the life quality of patients (Figure 2). What’s more, no adverse events were observed during the therapy. However, Case 3 was relapsed after discontinuations of Dupilumab in 2 months. Case 1 and Case 4 showed good response to Dupilumab without recurrence.
Figure 2.
Dynamic changes of various parameters during the dupilumab therapy. The sequence of five charts was as follows: (A) Eosinophils, (B) IgE, (C) HECSI, (D) PESCI and (E) PP-NRS.
Discussion
Herein, we report five recalcitrant palmar-plantar eczemas, that have been successfully treated with dupilumab. Five patients tried at least four treatments before dupilumab, including allergen or irritant avoidance, urea, corticosteroids, chlorpheniramine injection, hydroxychloroquine sulfate, oral antihistamines, compound glycyrrhizin tablets, and calcineurin inhibitors. These drugs respectively target the calcineurin pathway (cyclosporine and tacrolimus), the retinoid receptor (alitretinoin), and the folic acid pathway (methotrexate).10 Dupilumab presented good therapeutic response in all five patients, which targets the shared alpha subunit IL-4 receptor and blocks signaling from IL-4 and IL-13.
IL-4 and IL-13 were identified as type 2 immune response cytokines that could activate B cell class switching to IgE and play a role in eosinophil migration into allergic inflammatory tissues.11 After 16-week treatment, we observed normalization of eosinophil and IgE levels in all five patients. This efficacy has also been observed in other studies.12 The previously published results of one observational study showed that dupilumab improved HECSI-75 was achieved by 28 (60%).6 In the present article, we also find that the HECSI of five cases was nearly wholly cleared after the dupilumab treatment. Meanwhile, all of our five cases had plantar lesions, which is often neglected in the study of atopic dermatitis. The feet of five patients also showed promising results, as well as the hands.
However, there is no evidence of how long we should use dupilumab for AD. One case presented here was shortly relapsed after discontinuation of dupilumab. Two cases were still received maintenance doses of 300 mg per four weeks. Only two cases showed good response without recurrence after discontinuation of dupilumab. Therefore, more cases need to be included in multicentric trails to elaborate this thesis.
Dupilumab has become widely available for the treatment of atopic dermatitis.13–15 The positive results in these five patients suggest that dupilumab can be considered an off-label treatment in cases of severe, highly treatment-refractory hand and foot eczema that meets the diagnostic criteria for atopic dermatitis.
Funding Statement
The work was supported by Health Technology Programme of Hangzhou (A20210285).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
Written informed consent for publication was provided by the patients. Institutional approval was not required for the case report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Williams HC, Jburney PG, Pembroke AC, Hay RJ. The U.K. working party’s diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131(3):406–416. doi: 10.1111/J.1365-2133.1994.TB08532.X [DOI] [PubMed] [Google Scholar]
- 2.Barrett A, Hahn-Pedersen J, Kragh N, Evans E, Gnanasakthy A. Patient-reported outcome measures in atopic dermatitis and chronic hand eczema in adults. Patient. 2019;12(5):445–459. doi: 10.1007/S40271-019-00373-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee GR, Maarouf M, Hendricks AK, Lee DE, Shi VY. Current and emerging therapies for hand eczema. Dermatol Ther. 2019;32(3). doi: 10.1111/DTH.12840 [DOI] [PubMed] [Google Scholar]
- 4.Oosterhaven JAF, Romeijn GLE, Schuttelaar MLA. Dupilumab treatment of very severe refractory atopic hand eczema. JAMA Dermatol. 2018;154(8):969–970. doi: 10.1001/JAMADERMATOL.2018.2027 [DOI] [PubMed] [Google Scholar]
- 5.Zirwas MJ. Dupilumab for hand eczema. J Am Acad Dermatol. 2018;79(1):167–169. doi: 10.1016/J.JAAD.2018.02.073 [DOI] [PubMed] [Google Scholar]
- 6.Oosterhaven JAF, Voorberg AN, Romeijn GLE, de Bruin-Weller MS, Schuttelaar MLA. Effect of dupilumab on hand eczema in patients with atopic dermatitis: an observational study. J Dermatol. 2019;46(8):680. doi: 10.1111/1346-8138.14982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efficacy and safety of dupilumab chronic hands eczema refractory to highly potent topical corticosteroids - full text view - clinicaltrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03861455. Accessed July 6, 2022.
- 8.Stingeni L, Bianchi L, Antonelli E, et al. Moderate-to-severe atopic dermatitis in adolescents treated with dupilumab: a multicentre Italian real-world experience. J Eur Acad Dermatol Venereol. 2022;36(8):1292–1299. doi: 10.1111/jdv.18141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Held E, Skoet R, Johansen JD, Agner T. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. A study of inter- and intraobserver reliability. Br J Dermatol. 2005;152(2):302–307. doi: 10.1111/J.1365-2133.2004.06305.X [DOI] [PubMed] [Google Scholar]
- 10.Dubin C, Del Duca E, Guttman-Yassky E. Drugs for the treatment of chronic hand eczema: successes and key challenges. Ther Clin Risk Manag. 2020;16:1319. doi: 10.2147/TCRM.S292504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akdis CA, Arkwright PD, Brüggen MC, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–1605. doi: 10.1111/ALL.14318 [DOI] [PubMed] [Google Scholar]
- 12.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMOA1304048/SUPPL_FILE/NEJMOA1304048_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 13.Kamata M, Tada Y, Literature A. Review of real-world effectiveness and safety of dupilumab for atopic dermatitis. JID Innov Ski Sci Mol Popul Heal. 2021;1(3):100042. doi: 10.1016/J.XJIDI.2021.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puar N, Chovatiya R, Paller AS. New treatments in atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126:21–31. doi: 10.1016/j.anai.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 15.Da J, Ali K, Lu K, et al. Off-label use of dupilumab for the treatment of moderate to severe atopic dermatitis in children aged below 6 years of age: a case series. Clin Exp Dermatol. 2022;47:423–425. doi: 10.1111/ced.14925 [DOI] [PubMed] [Google Scholar]