Cancer Metastasis is a multistep process in which cancer cells disseminate from primary site enter circulation. After surviving the harsh environments of the vasculature, they arrive at a distal site and colonize it. Development of metastatic lesions diminishes therapeutic options and often results in patient death. Therefore, understanding the mechanisms that govern the efficacy of this long process are important in identifying novel targets or improving use of available drugs to alleviate disease progression.
In 1889, Stephen Paget continued to build on the hypothesis of “seed and soil” after postmortem examination of women with breast cancer. He determined that sites of metastasis are not random. Instead, he proposed that cancer cells “the seeds” intrinsically express proteins that preferentially grown in specific organs “soil”. Overtime, studies have focused on cell surface proteins mediating cell-cell and/or cell-Extracellular Matrix interaction to facilitate cancer cell adhesion and proliferation at a new site. In 1928, James Ewing added to this theory that the options for cancer cell destinations are limited by vascular flow. For example, prostate cancer metastasizes to bone in over 80% of cases due to expression of bone tropic proteins, however, their trip is facilitated their route to the lumbar vertebrae by the Baston’s plexus of draining lymph nodes.
Recent studies demonstrate that cancer cells release secreted factors that promote primary tumor growth, but also travel through circulation to alter the landscape of distal sites to facilitate the homing, adhesion, and proliferation of circulating tumor cells. For a while, the focus has been on soluble factors, such as growth factors, chemokines, and cytokines, this category has expanded to include another secreted factor: extracellular vesicles. What are extracellular vesicles?
Extracellular vesicles are vesicles released by all cells of different sizes carrying a variety of proteins, lipids, nucleic acids (RNA and DNA), and metabolites (1). Extracellular vesicles can be of various sizes to include apoptotic bodies of 1 μm diameter, microvesicles of 30–300 nm diameter, and smaller exosomes of 10–200 nm diameter (1). Each sized vesicle is released through different mechanisms (1, 2). Exosomes are released through the exocytosis of multivesicular bodies via the Rab27a/b pathway, while microvesicles and apoptotic bodies result from blebbing of the plasma membrane (1, 2). Composition of the extracellular vesicle surface and the status of the recipient cells determine the route of uptake and the fate of the extracellular vesicle cargo (3). Together, this suggests that cells release vesicles as a means of intercellular communication. As cancer cells aberrantly express excess or mutant proteins and other biomolecules, extracellular vesicles released from cancer cells, thus, potentially sharing oncogenic material. The cancer cell extracellular vesicles are released throughout circulation, but what is their effect on proximal and distal tissue?
In 2012, David Lyden’s group demonstrated that a subset of cancer-derived vesicles called exosomes were pro-tumorigenic and pro-metastatic effectors (4). They showed that exosomes from cancer cells are more protein rich than exosomes from normal cells, and that melanoma patients of advanced stages of disease contain exosomes in circulation that are more protein rich than patients of early stages (4). To demonstrate the role of exosomes in cancer progression, they set up the following experiment. GFP mice were treated regularly treated with 10ug of B16F10 exosomes or PBS over 4 weeks to “educate” the bone marrow (4). This educated or naïve BM was then transplanted to non-GFP mice that had undergone lethal irradiation (4). The mice were given 4 weeks to recover before inoculated with B16F10mCherry subcutaneous tumors (4). The differential fluorescent colors allow them to track the migratory patterns of educated bone marrow derived cells and cancer cells (4).
Their first observation was that tumors in mice with educated bone marrow grew at a faster rate than tumors in mice with naïve bone marrow (4). They then examined the effect of exosome education on metastatic potential and found that mice with educated bone marrow had greater infiltration of tumor cells and bone marrow derived cells that ultimately increased metastatic burden (4). My study then focused on the question: If these cancer-derived vesicles are in circulation, why are other tissues not affected?
We hypothesized that cells possess an innate protective mechanism against extracellular vesicle incorporation/ “education”. We posited that loss of this defense facilitates tumor growth, formation of the pre-metastatic niche, and increased cancer-related death. We suspected that the defense mechanism would involve a cell surface protein that would respond to external stimuli. Therefore, we started with plasma membrane profiling using Stable Isotope Labeling with Amino acids in Cell culture (aka SILAC). Labeling cell surface proteins with biotin and using streptavidin beads allowed us to examine the differences in cell surface landscape between cells receiving PBS or cells receiving tumor-derived extracellular vesicles. When examining proteins that were significantly downregulated by tumor derived extracellular vesicles, we identified IFNAR1 (5).
We then confirmed using flow cytometry that these vesicles indeed downregulated IFNAR1 on the cell surface of target cells (5). Cells treated with extracellular vesicles from melanoma patients also resulted in decreased IFNAR1 on the cell surface while extracellular vesicles from healthy donors did not (5). Loss of protein can be affected by protein degradation or changes in transcription. However, QPCR analysis of treated cells showed no statistical difference in IFNAR1 mRNA (5). We then set out to determine role IFNAR1 and type I interferon played in tumor-derived extracellular vesicle (TEV) driven cancer progression.
IFNAR1 is a subunit of the type I interferon receptor. It binds type I IFN ligands (such as IFN alpha and IFN beta) to elicit anti-viral, anti-tumorigenic, and anti-metastatic responses (6). The FDA approved the use of IFN1 in melanoma patients at risk of developing severe metastatic disease (6). And while this treatment was curative a subset of patients, many patients showed no response (6). We now posit that the lack of response to interferon therapy is due to loss of IFNAR1 and type I interferon signaling during cancer progression. IFN1 signaling is regulated to prevent an uncontrollable inflammatory response (7). One method is ligand-mediated downregulation of IFNAR1 (in which after ligand binding) IFNAR1 is phosphorylated and then polyubiquitinated before lysosomal degradation (7). Unfortunately, during tumor progression, cancer cells create an environment that activates intracellular kinases to induce phosphorylation and ubiquitination of IFNAR1 and its subsequent degradation (5, 8).
To show that stable IFNAR1 expression and type I signaling is a defense against cancer progression mediated by tumor derived extracellular vesicles, I used a mouse generated by Serge Fuchs at the University of Pennsylvania in which the Serine 526 of IFNAR1 is mutated to alanine, rendering the molecule exempt from phosphorylation and subsequent ubiquitination (5, 8). The Serine 526 corresponds to the Serine 535 found in human IFNaR1 (5, 7, 8). The Fuchs laboratory refers to this mouse as SA.
Splenocytes were isolated from WT and SA mice and treated in vitro with extracellular vesicles from B16F10 cultures. Treatment with the vesicles were able to reduce WT IFNAR1 but not SA (5). Tumor derived extracellular vesicles from B16F10 cells were labeled with a lipophilic dye to track in vitro incorporation by WT and SA splenocytes (5). We found that splenocytes from SA did not incorporate the vesicles as readily as WT splenocytes (5). Similarly labeled vesicles were injected intravenously into WT and SA. 24 hours after injection, splenocytes and bone marrow were collect. Vesicle uptake was again limited in SA tissues in vivo compared to WT (5). Because these tumor derived vesicles cannot downregulate the SA molecule and are not readily incorporated into cells expressing SA, we posited that SA tissue is exempt from tumor vesicle education (5). If we recall the experiment from Lyden group, they found that vesicle education drove tumor growth and metastasis (4). We followed a similar protocol to include our SA mice. We confirmed that WT mice receiving educated WT bone marrow had faster tumor growth (5). However, WT mice with educated SA bone marrow did not elicit an increase in tumor growth compared to naïve SA bone marrow (5). We confirmed that mice with WT educated BM also increased lung metastasis (5). However, WT mice with SA bone marrow, though also educated with tumor derived extracellular vesicles did not increase metastatic burden (5). We can, therefore, conclude that TEV incorporation by bone marrow cells over a month altered IFNAR1 expression and function sufficiently to promote metastatic progression once engrafted in the bone marrow recipient mice with tumors (5).
In 2011, Peinado and Lyden proposed in a review article that these tumor-derived vesicles also facilitate the formation of a pre-metastatic niche (9). A place rich in pro-tumorigenic myeloid cells and extracellular matrix molecules that promote cancer cell adhesion, survival, and colonization (9). However, this was not tested in their 2012 study. We wondered however: Do tumor-derived vesicles aid in the formation of the pre-metastatic niche? And is loss of IFNAR1 part of the signature of this niche?
To examine these possibilities, mice underwentintravenous injections of either PBS or 8ug of B16F10 vesicles thrice a week for 3 weeks (5). After 3 weeks treatment, lungs were collected for immunofluorescence. We found that the vesicles induced loss of IFNAR1 in WT lungs but not SA (5). Moreover, the WT lungs treated with vesicles exhibited numerous clusters of myeloid cells and more fibronectin deposition while SA lungs did not (5).
We, therefore conclude, that during tumor growth, cancer cell release extracellular vesicles that induce loss of IFNAR1 in the lung, increase myeloid infiltration and fibronectin deposition (5). Thus creating a pre-met niche to facilitate metastasis. This means that IFNAR1 expression is needed to limit TEV incorporation to suppress formation of pre-met niche and subsequent metastatic burden.
We then set out to determine the mechanism by which IFNAR1 is downregulated following TEV exposure. First, we must remember that during tumor progression, massive cancer cell proliferation creates a hypoxic, nutrient-limited environment, which activate intracellular kinases able to phosphorylate IFNAR1 for subsequent polyubiquitination and lysosomal degradation (5, 7, 8). And we can see how that will affect IFNAR1 downregulation in the tumor, so we ask: Can tumor derived extracellular vesicles induce ligand-independent phosphorylation and downregulation of IFNAR1? YES! After 30 minutes treatment with tumor derived extracellular vesicles, we see increased IFNAR1 phosphorylation corresponding to increased IFNAR1-ubiquitination and decrease in total IFNAR1 (5). We then set out to determine if this loss of IFNAR1 protein correspond also resulted in loss of type I interferon signaling and expression of interferon stimulated genes (ISGs). Splenocytes from WT and SA animals were treated with PBS, extracellular vesicles from primary fibroblasts (FEV), or tumor-derived extracellular vesicles for four hours before RNA isolation for QPCR (5). We found that tumor-derived extracellular vesicles dampened the expression of interferon stimulated genes in WT splenocytes but not SA (5). Moreover, the fibroblast vesicles had no effect on the expression levels of interferon stimulated genes (5). Similarly, vesicles from melanoma patients dampen expression of interferon stimulated genes while vesicles from healthy donors do not (5). So far, we know that stable expression of IFNAR1 (via the expression of the SA mutation) is needed to limit vesicle incorporation. Thus, we wondered if expression of IFNAR1-SA protein is sufficient or if the receptor need to be activated by ligand.
Splenocytes from WT and SA mice were pre-treated with interferon beta before treatment with labeled vesicles (5). We found that pre-treatment with interferon limited vesicle incorporation in WT splenocytes and was almost non-existent in SA splenocytes (5). To demonstrate that the limited vesicle incorporation in SA was due to stable signaling and not due to expression of a mutant protein, we used an anti-IFNAR1 blocking neutralizing antibody (5). Pre-treatment with the neutralizing antibody made SA susceptible to vesicle incorporation suggesting that a signaling through IFNAR1 is important (5).
As the antitumorigenic and antimetastatic function of type 1 interferon are mediated by the expression of ISGS, we set out to determine what ISG is suppressed by vesicle incorporation that contributes to TEV-mediated cancer progression. In 2013, a study demonstrated that the enzyme cholesterol-25-hydroxylase was a type I interferon stimulated gene (10). Cholesterol-25-hydroxylase converts cholesterol into the oxysterol 25-hydroxycholesterol (10). Treatment with 25-hydroxycholesterol was able to limit viral uptake even in the absence of the gene (10). Therefore, we wondered if expression of this interferon stimulated gene is also affected by tumor-derived extracellular vesicles. Cells treated with vesicles from melanoma patients exhibited lower levels of Ch25h mRNA compared to cells treated with PBS or vesicles from healthy donors (5). When we analyzed Ch25h mRNA expression in peripheral blood leukocytes of healthy donors and melanoma patients, we found patients had lower levels of Ch25h expression and that patients with verifiable lymph node metastasis had even lower expression levels (5).
We wanted to understand how loss of Ch25h expression affected vesicle incorporation, so we isolated splenocytes from WT and Ch25hNull mice and pre-treated them with interferon beta or 25-hydroxycholesterol. Again, we found that interferon limited vesicle incorporation in WT cells but had no effect on Ch25h−/− cells (5). However, pre-treatment with 25-hydroxycholesterol limited vesicle incorporation in WT and in Ch25h−/− cells (5).
When DiD-labeled vesicles were injected into mice lacking Ch25h, we found more vesicle incorporation (5). Even if cells expressed SA, when there is lack of Ch25h there is more vesicle incorporation in vivo (5). Mice lacking Ch25h are also susceptible to formation of pre-metastatic niche after prolonged vesicle treatment, via numerous myeloid clusters and fibronectin deposition (5). Moreover, mice lacking Ch25h are susceptible to lung metastasis and poor survival, even if they express the SA knock-in molecule (5). When analyzing peripheral blood leukocytes from melanoma patients in different stages of disease, we found that patients with low Ch25h corresponded to presence of distal metastasis and poor survival (5).
Prolonged exposure to the oxysterol 25-hydroxycholesterol induced apoptosis in cells in vitro and attempts to incorporate thoroughly 25-hydroxycholesterol into solution for intraperitoneal injection proved difficult. We, therefore, set out to determine if there was a drug that literature showed affected membrane fluidity and may mimic 25-hydroxycholesterol. We tested several drugs that were FDA approved or under FDA review (5) to determine which suppressed TEV uptake and suppress TEV-mediated cancer progression. Therefore, we set up a TEV uptake assay and found reserpine was able to limit vesicle incorporation in both splenocytes and bone marrow cells (5). Reserpine in an alkaloid found in the roots of Rauwolfia serpentina and Rauvolfia vomitoria. It functions as an adrenergic blocking agent used to treat mild to moderate hypertension by disrupting norepinephrine vesicular storage. We found that reserpine pre-treatment was able to limit in vivo incorporation of tumor-derived vesicles in splenocytes and bone marrow cells (5). Moreover, reserpine treatment during frequent tumor-derived vesicle treatment over three weeks also suppressed vesicle-induced loss of IFNAR1 (5). Reserpine treatment was also able to suppress formation of myeloid clusters and fibronectin deposition (5).
Considering the efficacy of reserpine in vitro and in vivo to suppress TEV-mediated downregulation of IFNAR1 and formation of the pre-metastatic niche, we set out to determine if reserpine also could be used as an adjuvant therapy agent. After various adjustments of dose, exposure and vehicle, I modified a treatment regimen in which mice are treated with vehicle or reserpine once tumors are 50mm^2 until they reach a size for surgical resection (5). After surgery, mice receive weekly treatment until all vehicle treated animals are moribund. We found that reserpine treatment was able to delay growth of primary tumor (5). And though the tumors are similar size upon resection, the mice receiving reserpine adjuvant therapy did not develop lung metastasis and exhibited improved survival (5).
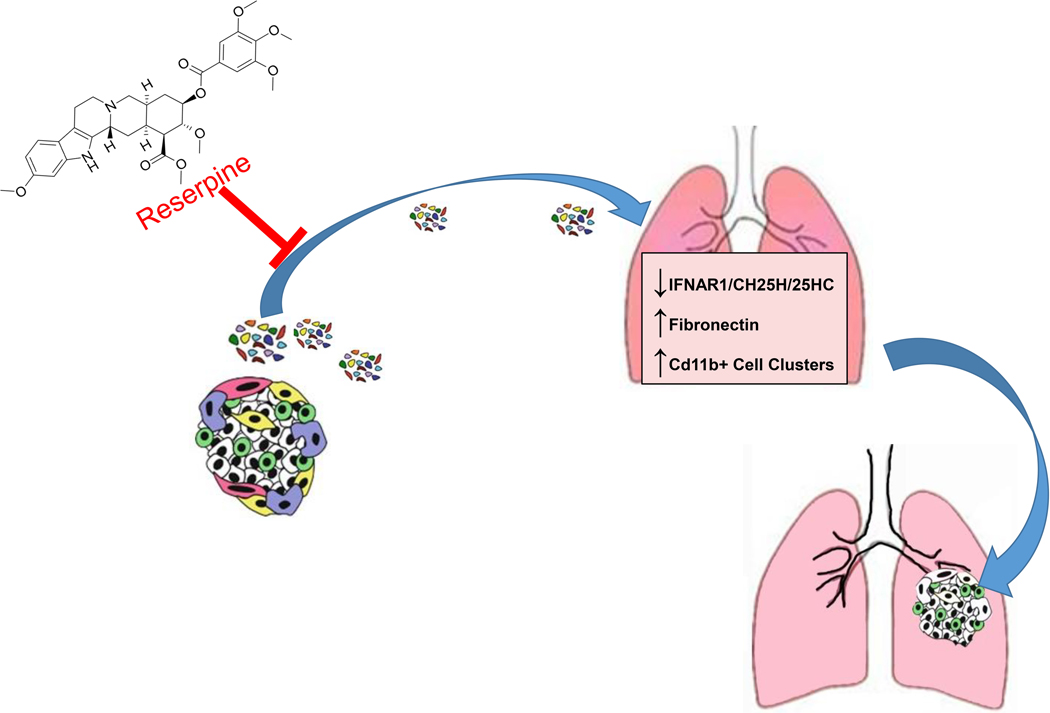
In summary, cancer cells in primary tumors release extracellular vesicles. If interferon ligand activates signaling through IFNAR1 to induce cholesterol 25-hydroxylase expression and increases synthesis of 25-hydroxycholesterol to suppress vesicle education and vesicle-mediated metastasis. However, tumor-derived vesicles can overtime downregulate IFNAR1 resulting in the loss of cholesterol 25-hydroxylase and 25-hydroxycholesterol and the subsequent vesicle-induced metastatic progression (Fig. 1). Reserpine treatment can limit vesicle-mediated education and disease progression (Fig. 1).
Figure 1. Summary Figure.
Cartoon depicts the role of extracellular vesicles from cancer cells on altering distal tissue to promote colonization of cancer cells, and how suppressing extracellular vesicle incorporation using reserpine can be suppress cancer progression.
CH25H= cholesterol 25-hydroxylase;
25HC= 25-hydroxycholesterol
Taking all this into account, we set out to expand our understanding of tumor-derived extracellular vesicles in cancer progression. A tumor is comprised of cancer cells and cancer associated stromal cells (fibroblasts, endothelial cells, and immune cells). However, even the cancer cells within the tumor have different protein, genetic and epigenetic profiles. The variety in cancer cells within a single tumor and between primary and metastatic tumors is attributed to either a progenitor cancer cells (cancer stem cell-like cell) whose genetic instability accumulates with each cell division resulting in increased genetic variability in each resulting cells, as well as changes in the microenvironment along with this genetic instability. However, it is possible that aggressive cancer cells release extracellular vesicles that carry oncogenic biomolecules (protein, RNA, DNA, lipids, and metabolites) that when incorporated by normal cells induce oncogenic transformation resulting in cancer cells or cancer-like cells that contribute to cancer progression. Primary mouse fibroblasts were isolated and treated with either PBS or 30 μg of extracellular vesicles isolated from B16F10 mouse melanoma cells for 5 days before the cells were counted and seeded in equal number (in triplicate) into soft agar. After the cells were incubated for 5 days, the cells treated with PBS remained as single cells and some appeared as a group of no more than 5 cells. However, fibroblasts treated with extracellular vesicles from the melanoma cells formed large spheres in the soft agar. The experiment was repeated reducing the extracellular vesicle treatment from 30 μg to 10 μg for 7 days before plating into soft agar and in the second experiment we treated primary fibroblasts from WT, SA, Ch25h−/− and Ch25−/−;SA mice. Cells lacking Ch25h formed larger colonies that WT and the cells lacking Ch25h and SA. The B16F10 melanoma cell line is riddle with various mutations and expresses a variety of oncogenic material, so we also isolated extracellular vesicles from a cell line derived from KPC mouse pancreatic adenocarcinoma tumor. These vesicles were also able to confer all primary fibroblasts (except SA) with anchorage independent growth in soft agar. This suggests that extracellular vesicles carry oncogenic material with the capacity to transform normal cells to cancer cells, thus contributing to the diversity of cancer cells within a tumor and promoting cancer progression.
Acknowledgments
Funding Sources
The work presented at the conference was primarily supported by an NIH/NCI PO1 CA165997 grant (to C. Koumenis., J.A. Diehl., and S.Y. Fuchs), R01 grants CA092900, CA216936, and CA188575 (to S.Y. Fuchs and H. Rui), the PA Department of Health FY18 2017 Health Research Formula Fund (to S.Y. Fuchs.), as well as by additional support from F32 CA206431 (to A.Ortiz). Other funding sources are written in the original manuscript (Ortiz et al. (5)).
References
- 1.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. Epub 2018/01/18. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 2.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30; sup pp 1–13. Epub 2009/12/08. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 3.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. Epub 2014/08/22. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91. Epub 2012/05/29. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz A, Gui J, Zahedi F, Yu P, Cho C, Bhattacharya S, Carbone CJ, Yu Q, Katlinski KV, Katlinskaya YV, Handa S, Haas V, Volk SW, Brice AK, Wals K, Matheson NJ, Antrobus R, Ludwig S, Whiteside TL, Sander C, Tarhini AA, Kirkwood JM, Lehner PJ, Guo W, Rui H, Minn AJ, Koumenis C, Diehl JA, Fuchs SY. An Interferon-Driven Oxysterol-Based Defense against Tumor-Derived Extracellular Vesicles. Cancer Cell. 2019;35(1):33–45 e6. Epub 2019/01/16. doi: 10.1016/j.ccell.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz A, Fuchs SY. Anti-metastatic functions of type 1 interferons: Foundation for the adjuvant therapy of cancer. Cytokine. 2017;89:4–11. Epub 2016/01/30. doi: 10.1016/j.cyto.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Katlinski KV, Reichert M, Takano S, Brice A, Zhao B, Yu Q, Zheng H, Carbone CJ, Katlinskaya YV, Leu NA, McCorkell KA, Srinivasan S, Girondo M, Rui H, May MJ, Avadhani NG, Rustgi AK, Fuchs SY. Triggering ubiquitination of IFNAR1 protects tissues from inflammatory injury. EMBO Mol Med. 2014;6(3):384–97. Epub 2014/02/01. doi: 10.1002/emmm.201303236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katlinski KV, Gui J, Katlinskaya YV, Ortiz A, Chakraborty R, Bhattacharya S, Carbone CJ, Beiting DP, Girondo MA, Peck AR, Pure E, Chatterji P, Rustgi AK, Diehl JA, Koumenis C, Rui H, Fuchs SY. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell. 2017;31(2):194–207. Epub 2017/02/16. doi: 10.1016/j.ccell.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21(2):139–46. Epub 2011/01/22. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Liu SY, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, Pernet O, Guo H, Nusbaum R, Zack JA, Freiberg AN, Su L, Lee B, Cheng G. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38(1):92–105. Epub 2013/01/01. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]