Abstract
Objective:
To examine predictors of uptake (never start), adherence (drop out), and completion of pulmonary rehabilitation (PR), as well as PR treatment response based on minimal clinically important difference (MCID) on the 6-minute walk test (6MWT) distance and Chronic Respiratory Questionnaire–Self-Report (CRQ-SR).
Design:
Retrospective, cohort study.
Setting:
Veterans Health Administration.
Participants:
U.S. veterans with chronic obstructive pulmonary disease (COPD) (N=253) referred to PR between 2010 and 2018.
Interventions:
Outpatient PR program.
Main Outcome Measures:
Participants completed baseline (time 1) measures of depression (Beck Depression Inventory-II), health-related quality of life (CRQ-SR), self-efficacy (Exercise Self-Regulatory Efficacy Scale [Ex-SRES]), and COPD knowledge. Exercise capacity was assessed with the 6MWT. Participants who completed all 18 sessions of PR repeated assessments (time 2). Logistic regression models examined predictors of uptake, adherence, and completion of PR as well as treatment response based on MCID.
Results:
Participants were referred to PR with 24.90% never starting, 28.90% dropping out, and 46.20% completing. No differences emerged between never starters and dropouts. Having a history of any cancer increased the likelihood of completing PR (vs never starting; odds ratio [OR], 3.18; P=.003). Greater CRQ-SR dyspnea score, indicating less dyspnea, was associated with increased likelihood of completing PR (OR, 1.12; P=.006). Past smoking compared with current smoking was associated with increased likelihood of completion (OR, 3.89; P≤.002). Those without a history of alcohol use disorder had increased likelihood of completing PR (OR, 2.23; P=.048). Greater baseline 6MWT distance was associated with lower likelihood of achieving MCID in 6MWT (OR, 0.99; P<.001). Greater Ex-SRES was associated with decreased likelihood of achieving 6MWT MCID (OR, 0.98; P=.023).
Conclusions:
Findings suggest that early psychoeducation on dyspnea management and smoking and alcohol cessation may increase completion of PR.
Keywords: Pulmonary disease, chronic obstructive, Rehabilitation
Chronic obstructive pulmonary disease (COPD), a progressive disease with no cure, is associated with significant public health burden.1 The prevalence of COPD is higher in U.S. veterans than civilians, which is attributed in large part to higher rates of smoking within the Veteran population.2,3 Within the Veterans Health Administration (VHA), COPD is the fourth most common discharge diagnosis and accounts for one-third of all medical admissions in the VHA system.4
Pulmonary rehabilitation (PR) is standard of care and improves numerous COPD-related outcomes.5–8 Despite the established evidence base of PR, it remains significantly underutilized worldwide.9 Uptake of PR ranges from 8.3%–50%, and among those who do start, 9.7%–32% do not complete it.10–12 Physician referral rate is low, ranging from 3%–16% across countries.9 Over a 5-year period between 2007 and 2011, only 1.5% of 32,856 U.S. veterans discharged from VHA hospitals after an acute COPD exacerbation participated in at least 1 session of PR.13
In civilian samples, prior research suggests that reduced PR uptake is associated with disruption to routines, inadequate travel and/or transportation, long-term oxygen therapy, living alone, lack of perceived benefit, and inconvenient timing and is influenced by the patient’s physician (eg, limited education provided on the benefit of exercise and/or PR).10,12 Similar barriers to adherence and completion of PR include illness and comorbidities, inadequate travel and transport, smoking, depression, lack of support, and lack of perceived benefit.11,14 Poorer exercise tolerance and prior hospitalization in the past 12 months have also been found to predict lower PR completion.10 Depression has been found to be most consistently associated with PR engagement in civilian samples.11 Although the mechanisms linking depression and PR engagement are not fully understood, symptoms such as low motivation, social withdrawal, hopelessness, and low purpose in life could all affect an individual’s willingness to engage in PR. Anxiety has not been linked to PR engagement, although it has been shown to be associated with physical activity levels and PR outcomes in COPD.15,16 Known barriers to reduced PR uptake and completion, such as mental health conditions and current smoking, are more prevalent in veterans than civilians,17–19 warranting investigation of veterans’ engagement in PR. However, there is limited research examining predictors of treatment engagement and response among U.S. veterans.
Given substantial barriers to PR, guidelines have called for more systematic study of PR uptake, adherence, and completion rates that includes a comprehensive examination of potential patient-level predictors.20 The current study extends the body of research by examining predictors of PR uptake (ie, never starters), adherence (ie, dropouts), and completion in a US Veteran sample. The study also investigates baseline predictors of treatment response based on established minimal clinically important difference (MCID) on the 6-minute walk test (6MWT) distance and Chronic Respiratory Questionnaire–Self-Report (CRQ-SR) scores. Finally, we explore patient reported reasons for either not initiating PR or dropping out of PR.
Methods
Participants
The data set is composed of information collected from 253 U.S. Veterans with a diagnosis of COPD who were referred and completed their initial evaluation to the VA Boston Healthcare System outpatient PR program between 2010 and 2018. The criteria for diagnosis of COPD was defined as forced expiratory volume in first second of expiration/forced vital capacity (FEV1/FVC) <0.70.21 Contraindications were unstable cardiovascular disease and inability to safely use exercise equipment. The VA Boston Healthcare System Institutional Review Board (no. 3182) approved procedures to analyze the data obtained as part of routine clinical practice and to perform medical chart reviews.
Outpatient pulmonary rehabilitation program
The VA Boston Healthcare System PR program is nationally accredited by the American Association of Cardiovascular and Pulmonary Rehabilitation, and as such, our exercise intervention meets national standards. The PR program includes an initial evaluation followed by twice weekly, 2-hour classes composed of supervised exercise and education, for a total of 18 sessions based on guidelines for PR published by the American Thoracic Society.5 Aerobic exercise was progressive and performed on tread-mills, stationary bicycles, and arm ergometers. Exercise prescriptions were personalized based on exercise and functional tests. The education classes focused on increasing knowledge of COPD, symptom management, medications and supplemental oxygen use, behavior change as it pertains to physical activity, and disease self-management. PR was delivered by a multidisciplinary team.
Measures
Group and treatment response definitions
Never starters were defined as participants who were referred to PR and had an initial evaluation but did not return for their first PR exercise session. Dropouts were defined as participants who started PR but dropped out before session 18. Completers were defined as participants who completed 18 sessions of PR. We defined treatment response based on MCID established in the literature for outcome measures of 6MWT distance and CRQ-SR. For 6MWT distance, we used ≥30 m as indicator of MCID.22,23 MCID of the CRQ-SR is 0.50 for each subscale.24 Time 1 refers to baseline measures; time 2 refers to postcompletion measures for those who completed PR. For patients who did not start or continue PR, clinic scheduling staff queried by telephone and recorded patient-reported reasons. They asked, “Would you mind sharing why you do not want to attend PR at this time?” Patients’ response was recorded in a spreadsheet as part of routine clinical data collection.
At the initial PR evaluation, age and sex were recorded. Medical chart extraction coded for race and ethnicity, marital status, body mass index, medical and mental health comorbidities at the time of the pulmonary rehabilitation consult appointment, and lung function. The 6MWT was conducted at the initial evaluation and final session (approximately 9 weeks later). The Modified Medical Research Council (mMRC) is a 0–4 point category scale of perceived dyspnea, with higher scores associated with worse dyspnea.25 The Bristol COPD Knowledge Questionnaire is a multiple choice questionnaire that assesses COPD knowledge across 13 topic areas, each of which are assessed by 5 true or false statements.26 Higher scores indicate greater COPD knowledge. The CRQ-SR is a 20-item self-report measure of disease-specific health-related quality of life.27 Four subscales are produced: dyspnea (5 items), fatigue (4 items), mastery (4 items), and emotional function (7 items), and a total score is calculated from the sum of all items.27 Participants respond on a 7-point scale ranging from 1 (maximal impairment) to 7 (no impairment). Higher scores are indicative of better health-related quality of life. The Beck Depression Inventory-II is a 21-item self-report of depression symptom severity based on the Diagnostic and Statistical Manual of Mental Disorders (fourth edition) criteria.28 Higher scores indicate greater depression symptom severity.29 The Exercise Self-Regulatory Efficacy Scale (Ex-SRES) is a 16-item measure developed for patients with COPD to assess their ability to persist in exercise despite barriers.30 Total scores are summed and divided by 10, with higher scores indicative of greater self-efficacy. Ex-SRES has been validated in patients with COPD.30
Medical chart review
Medical chart extraction identified relevant clinical variables chosen a priori based on relevance to PR participation and outcomes.11,15 The following were extracted from the VA Boston electronic medical record: current smoking status, past smoking status, medical diagnoses (cardiovascular and metabolic diseases, obesity, cancer, pain, obstructive sleep apnea, dementia), mental health diagnoses (mood, anxiety, insomnia, posttraumatic stress disorder [PTSD], adjustment, psychotic spectrum, alcohol use disorder [AUD], substance use), and current mental health treatment. Clinical variables were coded as either a 0 (no) or 1 (yes). Pain condition was coded as 1 (yes) if there was chart diagnosis of arthritis, sciatica, back pain, spinal stenosis, and/or gout. Mental health treatment, including psychiatry and/or psychotherapy, received during the time of PR was recorded. Spirometry data including FEV1% predicted, FVC% predicted, and FEV1/FVC were extracted from the PR initial evaluation note.
Statistical analyses
Data analyses were conducted with SPSS version 26.a Of those participants who completed all 18 sessions, missing data ranged from 0.9%–18.8%, depending on the clinical variable. When patients (n=39) completed more than 1 course of PR, data from the first course of PR were used in these analyses. Multiple imputation was conducted to handle missing data because it produces less biased estimates even with small samples sizes.31 One-way between-participants analysis of variance (ANOVA) with Tukey post hoc test comparisons determined which baseline continuous variables differed between groups (never starters vs completers; dropouts vs completers; never starters vs dropouts). Chi-square tests were used for categorical variables. For treatment response variables, we first conducted separate 1-way between-participants ANOVAs for each dependent variable (MCID for 6MWT and CRQ-SR subscales) by baseline measures. Variables were entered as predictors in subsequent logistic regression models if they were significantly associated with group status in the univariate analyses. Logistic regression models were constructed predicting group (dropouts vs completers, etc). To examine predictors of treatment response in participants who completed PR (n=117), we constructed categorical variables for 6MWT and the CRQ-SR subscales. Patient-reported reasons for never starting or dropping out of PR were combined based on category of response, as illustrated in fig 1. Significance for P values was set at <.05.
Fig 1.
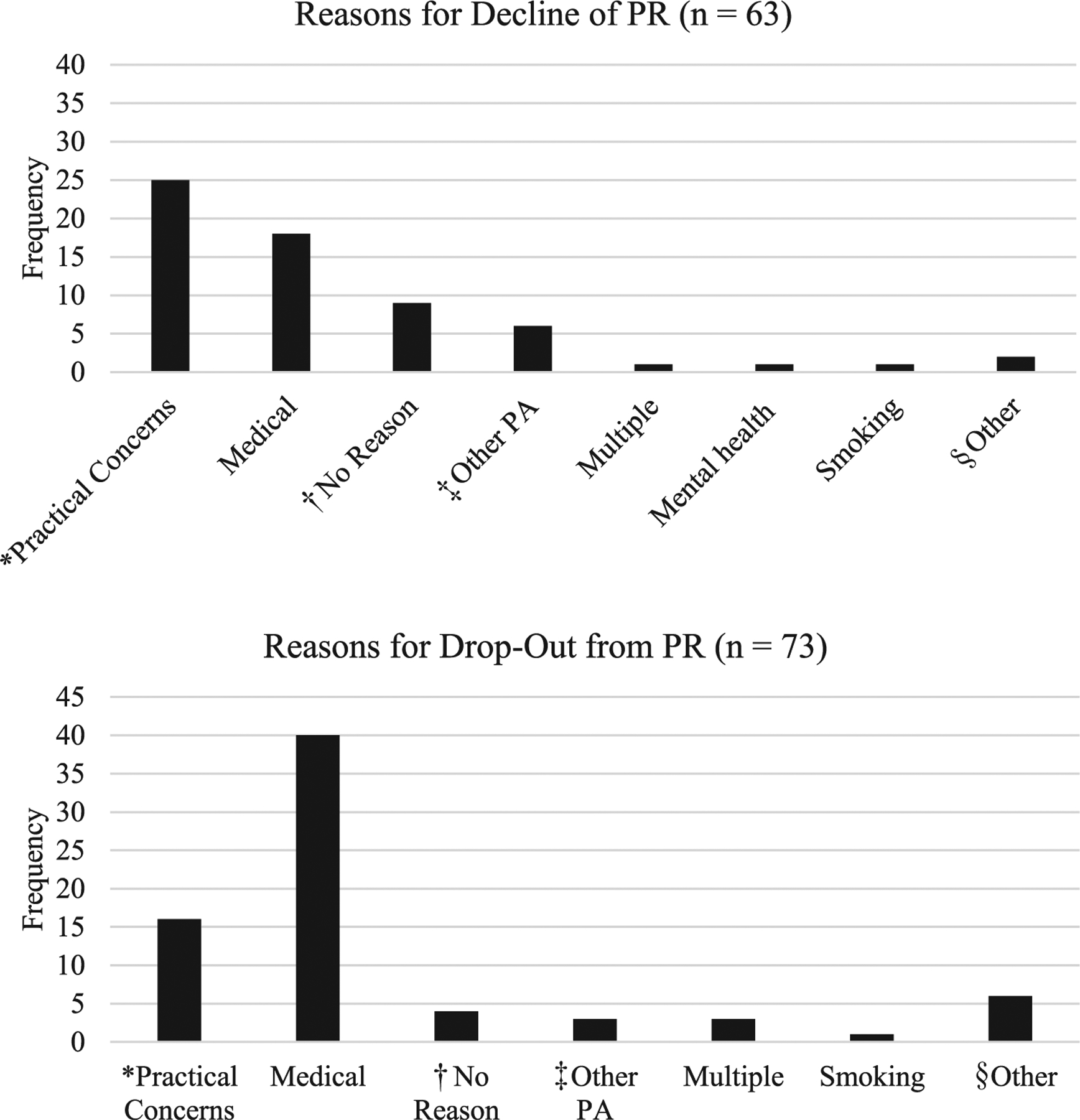
Reasons for patients never starting and dropping out from PR. Abbreviation: PA, physical activity. *Practical concerns included barriers such as travel, employment, competing medical appointments, and finances. †No reason indicated reasons that were not clearly provided by participants. ‡Other PA indicated when participants chose another physical activity program over PR. §Other included responses that did not fall into other categories such as desire to delay engagement, noninterest in PR, or scheduling difficulties.
Results
Participant characteristics
Figure 2 depicts veterans referred to PR between 2010 and 2018. Of the 253 male patients referred to PR and who underwent the initial evaluation, 63 (24.9%) never started, 73 (28.9%) dropped out, and 117 (46.2%) completed PR. Table 1 describes sample demographics. Average lung function fell in the moderate range across groups (52.48%±21.93% predicted).32 Self-reported reasons for never starting and dropping out of PR are summarized in fig 2. Of 117 participants (46.2%) who completed the PR program, 42 (35.9%) met or exceeded the MCID for the 6MWT distance. On the CRQ-SR, 85 participants (72.6%) met or exceeded the MCID for dyspnea, 86 (73.5%) for fatigue, 75 (64.1%) mastery, and 82 (70.1%) for emotional function.
Fig 2.

Flowchart of patients who were referred to and attended their initial consult appointment for PR between 2010 and 2018 and are included in the analyses.
Table 1.
Characteristics of male Veterans referred for PR between 2010 and 2018
| Variable | Mean ± SD or n (%) |
|---|---|
| Age (y) | 70.0 ± 8.0 |
| Race | |
| White | 229 (90.5) |
| Black | 15 (5.9) |
| Asian | 1 (0.4) |
| American Indian or Alaska Native | 2 (0.8) |
| Did not specify | 3 (1.2) |
| Ethnicity | |
| Hispanic or Latino | 1 (0.4) |
| Marital status | |
| Single/never married | 28 (11.1) |
| Married/partnered | 110 (43.5) |
| Divorced/separated | 74 (29.2) |
| Widowed | 38 (15.0) |
| Unknown | 1 (0.4) |
| FEV1% predicted | 52.48 ± 21.9 |
| FVC% predicted | 80.38 ± 21.9 |
| FEV1/FVC | 65.32 ± 21.4 |
| BMI | 29.69 ± 7.9 |
| No. of comorbidities | 2.15 ± 1.1 |
| Any mental health condition | 166 (65.6) |
| Current mental health treatment | 91 (36.0) |
NOTE. N varies 242–253 because of missing data.
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Baseline comparison of groups
Baseline comparison of groups are illustrated in table 2. Compared with completers, never starters had lower 6MWT distance and greater mMRC score at baseline (P<.05). No differences emerged between never starters and dropouts. Compared with completers, dropouts reported lower CRQ-SR total scores, lower dyspnea and mastery subscale scores, and greater mMRC (P<.05).
Table 2.
Differences in variables between never starters, dropouts, and completers at initial consult
| Variable | Never Starters | Dropouts | Completers | P Value |
|---|---|---|---|---|
| n (%) | 63 (24.90) | 73 (28.90) | 117 (46.20) | |
| Age (y) | 71.17 ± 8.32 | 69.00 ± 7.47 | 70.18 ± 8.14 | .282 |
| FEV1% predicted | 51.90 ± 20.46 | 50.57 ± 25.05 | 53.98 ± 20.68 | .570 |
| FVC% predicted | 77.90 ± 22.06 | 79.67 ± 23.00 | 82.16 ± 21.00 | .448 |
| FEV1/FVC | 66.62 ± 20.66 | 63.68 ± 25.40 | 65.65 ± 19.13 | .716 |
| BMI | 30.40 ± 7.79 | 28.07 ± 6.00 | 30.27 ± 8.89 | .144 |
| 6MWT distance (m) | 297.36 ± 105.16* | 325.34 ± 107.08 | 341.23 ± 96.78 | .024 |
| BDI-II | 12.91 ± 7.91 | 12.57 ± 8.84 | 11.82 ± 9.84 | .308 |
| CRQ-SR | 78.42 ± 14.49 | 76.78 ± 15.66† | 83.83 ± 17.62 | .009 |
| Dyspnea | 13.82 ± 4.60 | 12.64 ± 3.82† | 14.80 ± 4.86 | .006 |
| Emotional Function | 32.17 ± 6.71 | 32.41 ± 8.27 | 34.08 ± 8.38 | .204 |
| Fatigue | 14.59 ± 3.64 | 14.82 ± 4.34 | 15.76 ± 4.40 | .140 |
| Mastery | 17.83 ± 4.81 | 16.91 ± 5.07† | 19.19 ± 5.00 | .008 |
| Ex-ERES | 85.24 ± 28.43 | 84.71 ± 36.88 | 93.25 ± 31.78 | .132 |
| mMRC | 1.92 ± 1.04* | 2.01 ± 0.99† | 1.55 ± 0.95 | .004 |
| BCKQ | 30.14 ± 10.29 | 30.94 ± 8.68 | 28.18 ± 8.40 | .100 |
NOTE. Data are presented as mean ± SD unless otherwise indicated.
Abbreviations: BCKQ, Bristol Knowledge COPD Knowledge Questionnaire; BDI-II, Beck Depression Inventory-II; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Ex-ERES, Exercise Self-Efficacy Scale.
Significant difference between never starters vs completers.
Significant difference between dropouts vs completers.
Chi-square tests examined the association between group and baseline clinical and psychosocial variables. There was a significant association between pain condition and group (never starters vs completers; P=.043), such that those with a pain condition were more likely to complete PR. There was a significant association between cancer and group (never starters vs completers; P=.004), such that those with a history of cancer were more likely to complete PR. There was a significant association between groups (dropouts vs completers) and PTSD (P=.020) and AUD (P=.004), such that those without a history of these conditions were more likely to complete PR. Past smoking was associated with increased likelihood of completion of PR compared with never smoking or currently smoking (P=.003).
Predictors of group status
Variables significant in the univariate analyses were entered as independent variables in the following multivariate models. In the first model, the effect of cancer, pain, 6MWT distance, and mMRC dyspnea score on the likelihood of never starting vs completing PR was assessed with logistic regression model. The overall model was significant (χ2[4]=21.68, P<.001). The model explained 15.6% of the variance in group status (Nagelkerke R2). Veterans with a history of cancer were 3.18 times more likely to complete PR than those without a cancer diagnosis (odds ratio [OR], 3.18; 95% CI, 1.37–7.34; P=.007). There was a trend for mMRC score predicting group membership. Specifically, increasing mMRC score, indicating worse dyspnea, was associated with decreased likelihood of completing PR (OR, 0.717; 95% CI, 0.505–1.02; P=.063). No other variables were significant in the regression model.
In the second model, the effect of PTSD, AUD, smoking status (never, past, current), mMRC score, and CRQ-SR dyspnea, emotional function, and mastery subscales on the likelihood of dropping out vs completing was examined. The overall model was significant (χ2[7]=38.20, P<.001, pseudo R2=0.25). Greater baseline CRQ-SR dyspnea score, indicating better dyspnea, was associated with an increased likelihood of completing PR (OR, 1.11; 95% CI, 1.03–1.21; P=.006). Past smoking was associated with increased likelihood of completion compared with current smoking (OR, 3.89; 95% CI, 1.66–9.12; P=.002). Those without a history of AUD were more likely to complete PR than those with a history of AUD (OR, 2.23; 95% CI, 1.01–4.93; P=.048).
Predictors of treatment response among completers
Univariate results showed that baseline 6MWT distance, CRQ-SR fatigue and emotional function subscales, and Ex-SRES significantly differed between those whose 6MWT was ≥30 m compared with those with 6MWT<30 m after PR (P<.05) (table 3). Significant baseline variables were entered into the logistic regression model predicting achieving 6MWT MCID. The overall model was significant (χ2[4]=38.79, P<.001, pseudo R2=0.39). Greater 6MWT distance at baseline was associated with lower likelihood of achieving 6MWT MCID (OR, 0.99; 95% CI, 0.99–0.99; P<.001). Greater baseline Ex-SRES was associated with a decreased likelihood of achieving 6MWT MCID (OR, 0.98; 95% CI, 0.97–0.99; P=.013. No other variables were significant predictors of achieving 6MWT MCID.
Table 3.
Study variables at initial consult by 6MWT treatment response
| Variable | Did Not Achieve MCID 6MWT (<30m) | Achieved MCID 6MWT (≥30m) | P Value |
|---|---|---|---|
| n (%) | 75 (64.30) | 42 (35.70) | |
| Age (y) | 70.89 ± 7.82 | 68.90 ± 9.33 | .221 |
| FEV1% predicted | 55.70 ± 20.86 | 50.87 ± 20.23 | .232 |
| FVC% predicted | 83.03 ± 20.94 | 80.59 ± 22.14 | .559 |
| FEV1/FVC | 67.04 ± 18.83 | 63.17 ± 19.65 | .302 |
| BMI | 30.52 ± 10.11 | 29.81 ± 6.15 | .682 |
| 6MWT distance (m) | 373.53 ± 83.12 | 283.53 ± 93.33 | <.001 |
| BDI-II | 10.85 ± 8.88 | 13.57 ± 11.25 | .151 |
| CRQ-SR | 86.63 ± 17.03 | 78.84 ± 17.74 | .021 |
| Dyspnea | 14.90 ± 5.17 | 14.63 ± 4.31 | .779 |
| Emotional Function | 35.55 ± 7.75 | 31.46 ± 8.89 | .011 |
| Fatigue | 16.66 ± 4.24 | 14.16 ± 4.26 | .003 |
| Mastery | 19.52 ± 4.87 | 18.60 ± 5.23 | .339 |
| Ex-ERES | 101.39 ± 30.15 | 78.71 ± 29.65 | <.001 |
| mMRC | 1.48 ± 0.97 | 1.67 ± 0.92 | .300 |
| BCKQ | 28.49 ± 8.16 | 27.62 ± 8.88 | .595 |
NOTE. Data are presented as mean ± SD unless otherwise indicated.
Abbreviations: BCKQ, Bristol Knowledge COPD Knowledge Questionnaire; BDI-II, Beck Depression Inventory-II; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Ex-ERES, Exercise Self-Efficacy Scale.
Univariates results showed that participants who met MCID for CRQ-SR dyspnea subscale were younger in age and had lower (worse) baseline CRQ-SR dyspnea scores (P<.05). Achieving MCID in emotional function was associated with baseline fatigue, emotional function, and mastery (P<.05). MCID in fatigue was associated with lower baseline 6MWT and worse scores on CRQ-SR fatigue subscale (P<.05). MCID in mastery was only associated with baseline mastery (P<.05).
Logistic regression models were conducted predicting the likelihood of achieving MCID across the CRQ-SR subscales. In the first model, based on the univariate results, baseline dyspnea and age were entered as predictors of MCID in dyspnea. Lower baseline dyspnea, indicating worse dyspnea, was associated with lower likelihood of meeting MCID in dyspnea (OR, 0.81; CI, 0.73–0.90; P<.001; model χ2[2]=24.02; P<.001; pseudo R2=0.27). Age was not associated with achieving MCID in dyspnea (P>.05).
In the second model, baseline CRQ-SR emotional function, fatigue, and mastery subscales were entered as predictors of achieving MCID in emotional function. Greater baseline emotional function, indicating better emotional functioning, was associated with lower likelihood of achieving MICD in emotional function (OR, 0.84; CI, 0.75–0.94; P=.003; model χ2[3]=18.26; P<.001; pseudo R2=0.20), but CRQ-SR fatigue and mastery subscales were no longer significant in the multivariate model.
In the third model, baseline 6MWT and CRQ-SR fatigue subscale were entered as predictors of achieving MCID in CRQ-SR fatigue. Only baseline fatigue was associated with likelihood of achieving an MCID in fatigue, with greater baseline fatigue, indicating less perceived fatigue, associated with lower likelihood of achieving an MCID of fatigue (OR, 0.83; CI, 0.74–0.93; P=.002; model χ2[2]=16.97; P<.001; pseudo R2=0.20). We did not examine predictors of MCID in mastery because 1-way ANOVA showed only baseline mastery was associated with treatment response. Please see supplemental results (available online only at http://www.archives-pmr.org/) for all logistic regression models described above.
Discussion
The current study advances our understanding of predictors of uptake, adherence, completion, and treatment response to conventional PR in the US Veteran population with COPD. We found that logistical and practical barriers represent the most common self-reported reason for low uptake of PR and nonadherence. The consistency of these barriers across studies highlights the importance of offering alternatives to hospital-based PR, such as tele-health and technology-mediated home-based programs.33,34
A history of cancer was found to increase the likelihood of completing a course of PR. Survivors of cancer may be more attuned to symptom management from their experience in cancer treatment,35,36 thereby recognizing and appreciating how PR may improve COPD symptoms. Moreover, survivors of cancer may be more adept at navigating the healthcare system. Such factors are important for PR referrals and uptake because individuals who accept PR referrals report feeling supported by healthcare professionals.10,11,37
Lower baseline dyspnea predicted completion of PR. Many individuals with COPD experience fear of dyspnea and anxiety about the effect of exercise on breathlessness contributing to low PR uptake.38,39 Holding more positive views of exercise and believing that exercise will improve COPD symptoms, including dyspnea, facilitates PR uptake.37 Personalizing educational PR content according to an individual’s baseline PR assessment may increase completion rate.37 For example, individuals with greater CRQ-SR dyspnea may be provided early education on breathing techniques and exercises and education on managing dyspnea in COPD.40
Consistent with the civilian literature, past smoking compared with current smoking was associated with greater adherence to PR.12,41 Pairing PR and smoking cessation programs has demonstrated highest rates of smoking cessation among individuals with COPD.42 Similarly, we found that a history of AUD was associated with lower likelihood of PR completion. AUD is understudied in patients with COPD and has been found in 1 previous study to be associated with lower action and knowledge scores on a measure of disease self-management in COPD.43 Findings suggest that AUD may be an underappreciated barrier to PR adherence.43 In contrast to prior research, the current study did not replicate findings linking baseline depression to poorer PR uptake and completion.11 This may be because of differences in depression measurement across studies and retrospective coding for depression diagnosis. There was no association between lung function and PR engagement consistent with a mixed body of literature linking lung function and PR engagement.11,12,14,44
Veterans who achieved MCID for the 6MWT had lower 6MWT at baseline, replicating results in civilian samples.45,46 We also found that greater baseline self-efficacy was associated with lower likelihood of achieving 6MWT. Prior research has found no association between baseline self-efficacy and PR outcomes, although positive change in self-efficacy across PR is associated with more favorable PR outcomes.47,48 The self-efficacy literature in older adults suggests that a recalibration of self-efficacy occurs across exercise interventions whereby barrier-related self-efficacy declines throughout the course of the intervention as participants face the real challenges of consistent exercise.49 Although this finding requires replication, it preliminarily indicates that attention to baseline self-efficacy is important and requires repeated measurement over the course of PR. Finally, the study revealed no significant predictors of achieving MCID on CRQ-SR subscales beyond baseline levels, replicating 1 previous study.50
Study limitations
The study was conducted at a single center, composed of an all-male sample with limited racial and ethnic diversity, which limits generalizability. Future research with a larger, more diverse sample is needed to explore whether sex, race, and ethnicity affects rates of referral, uptake, and drop out, which would allow for targeted education and referral strategies. Future research is needed that includes more comprehensive psychosocial variables (eg, measures of dyspnea-related anxiety, social support, socioeconomic indicators). Results of our study do not establish causality. Thus, it is impossible to know exactly how variables, such as cancer history and smoking history, translate to better or worse patient engagement. Patient-reported reasons were collected with 1 open-ended question by clinic scheduling staff that was designed to be a screen of barriers to attendance. The current data lacked the richness of a qualitative study design. While prior qualitative studies have been conducted in civilian populations,51–53 they have yet to be examined in veterans. Finally, given the relatively small sample size, it is possible differences between never starters, dropouts, and completers were not found because of low power. Despite the study’s limitations, the results reflect clinical care on the front lines and offers practical recommendations for PR programs.
Conclusions
The current results identify veterans with COPD who would benefit from increased support and motivation and point to targeting dyspnea, smoking, and alcohol use early in the course of PR to increase completion. There is limited literature on interventions to increase PR participation and a clear need for controlled trials testing the efficacy of preparatory interventions.37,54 Future research is needed to improve utilization of conventional PR both within and outside of the VHA healthcare system.
Supplier
a. SPSS Version 26; IBM.
Supplementary Material
Acknowledgments
We thank the VA Boston Healthcare Pulmonary Rehabilitation Staff (Christine Stella, NP, Laura Fiore, PA, Robert Burke, KT, and Rachel Kelley, RRT) and the VA Boston Psychology Trainees (Rachel Weiskittle, PhD, and Chelsea Wiener, PhD).
List of abbreviations:
- ANOVA
analysis of variance
- AUD
alcohol use disorder
- COPD
chronic obstructive pulmonary disease
- CRQ-SR
Chronic Respiratory Questionnaire–Self-Report
- Ex-SRES
Exercise Self-Regulatory Efficacy Scale
- FEV1
forced expiratory volume in first second of expiration
- FVC
forced vital capacity
- MCID
minimal clinically important difference
- mMRC
Modified Medical Research Council
- OR
odds ratio
- PR
pulmonary rehabilitation
- PTSD
posttraumatic stress disorder
- 6MWT
6-minute walk test
- US
United States
- VHA
Veterans Health Administration
Footnotes
Disclosures: none
References
- 1.May SM, Li JT. Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy Asthma Proc 2015;36:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis 2010;5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc 2013;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med 2011;176:552–60. [DOI] [PubMed] [Google Scholar]
- 5.Rochester CL, Vogiatzis I, Holland AE, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med 2015;192:1373–86. [DOI] [PubMed] [Google Scholar]
- 6.Ryrsø CK, Godtfredsen NS, Kofod LM, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulmon Med 2018;18:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spitzer KA, Stefan MS, Priya A, et al. A geographic analysis of racial disparities in use of pulmonary rehabilitation after hospitalization for COPD exacerbation. Chest 2020;157:1130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindenauer PK, Stefan MS, Pekow PS, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among Medicare beneficiaries. JAMA 2020;323:1813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston K, Grimmer-Somers K. Pulmonary rehabilitation: overwhelming evidence but lost in translation? Physiother Can 2010;62:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating A, Lee AL, Holland AE. Lack of perceived benefit and inadequate transport influence uptake and completion of pulmonary rehabilitation in people with chronic obstructive pulmonary disease: a qualitative study. J Physiother 2011;57:183–90. [DOI] [PubMed] [Google Scholar]
- 11.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis 2011;8:89–99. [DOI] [PubMed] [Google Scholar]
- 12.Hayton C, Clark A, Olive S, et al. Barriers to pulmonary rehabilitation: characteristics that predict patient attendance and adherence. Respir Med 2013;107:401–7. [DOI] [PubMed] [Google Scholar]
- 13.Vercammen-Grandjean C, Schopfer DW, Zhang N, Whooley MA. Participation in pulmonary rehabilitation by veterans health administration and medicare beneficiaries after hospitalization for chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2018;38:406–10. [DOI] [PubMed] [Google Scholar]
- 14.Selzler A-M, Simmonds L, Rodgers WM, Wong EY, Stickland MK. Pulmonary rehabilitation in chronic obstructive pulmonary disease: predictors of program completion and success. COPD 2012;9:538–45. [DOI] [PubMed] [Google Scholar]
- 15.Pirraglia PA, Casserly B, Velasco R, Borgia ML, Nici L. Association of change in depression and anxiety symptoms with functional outcomes in pulmonary rehabilitation patients. J Psychosom Res 2011;71:45–9. [DOI] [PubMed] [Google Scholar]
- 16.Schüuz N, Walters JA, Cameron-Tucker H, Scott J, Wood-Baker R, Walters EH. Patient anxiety and depression moderate the effects of increased self-management knowledge on physical activity: a secondary analysis of a randomised controlled trial on health-mentoring in COPD. COPD 2015;12:502–9. [DOI] [PubMed] [Google Scholar]
- 17.Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health 2015;105:2564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisen SA, Griffith KH, Xian H, et al. Lifetime and 12-month prevalence of psychiatric disorders in 8,169 male Vietnam War era veterans. Mil Med 2004;169:896–902. [DOI] [PubMed] [Google Scholar]
- 19.Brown DW. Smoking prevalence among US veterans. J Gen Intern Med 2010;25:147–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults: accredited by NICE. Thorax 2013;68(2):ii1–30. Suppl. [DOI] [PubMed] [Google Scholar]
- 21.Simel DL, Rennie D. The rational clinical examination: evidence-based clinical diagnosis. New York: McGraw Hill Professional; 2008. [Google Scholar]
- 22.Polkey MI, Spruit MA, Edwards LD, et al. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med 2013;187:382–6. [DOI] [PubMed] [Google Scholar]
- 23.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997;155:1278–82. [DOI] [PubMed] [Google Scholar]
- 24.Schüunemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). COPD 2005;2:81–9. [DOI] [PubMed] [Google Scholar]
- 25.Perez T, Burgel PR, Paillasseur J-L, et al. Modified Medical Research Council scale vs Baseline Dyspnea Index to evaluate dyspnea in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015;10:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White R, Walker P, Roberts S, Kalisky S, White P. Bristol COPD Knowledge Questionnaire (BCKQ): testing what we teach patients about COPD. Chron Respir Dis 2006;3:123–31. [DOI] [PubMed] [Google Scholar]
- 27.Wijkstra P, TenVergert E, Van Altena R, et al. Reliability and validity of the Chronic Respiratory Questionnaire (CRQ). Thorax 1994;49:465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 29.Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychol Assess 1998;10:83. [Google Scholar]
- 30.Davis AH, Figueredo AJ, Fahy BF, Rawiworrakul T. Reliability and validity of the Exercise Self-Regulatory Efficacy Scale for individuals with chronic obstructive pulmonary disease. Heart Lung 2007;36:205–16. [DOI] [PubMed] [Google Scholar]
- 31.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods 2002;7:147. [PubMed] [Google Scholar]
- 32.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–55. [DOI] [PubMed] [Google Scholar]
- 33.Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD. Chest 2015;148:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selzler A, Wald J, Sedeno M, et al. Telehealth pulmonary rehabilitation: a review of the literature and an example of a nationwide initiative to improve the accessibility of pulmonary rehabilitation. Chron Respir Dis 2018;15:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leach CR, Troeschel AN, Wiatrek D, et al. Preparedness and cancer-related symptom management among cancer survivors in the first year post-treatment. Ann Behav Med 2017;51:587–98. [DOI] [PubMed] [Google Scholar]
- 36.Vaske I, Kenn K, Keil DC, Rief W, Stenzel NM. Illness perceptions and coping with disease in chronic obstructive pulmonary disease: effects on health-related quality of life. J Health Psychol 2017;22:1570–81. [DOI] [PubMed] [Google Scholar]
- 37.Early F, Wellwood I, Kuhn I, Deaton C, Fuld J. Interventions to increase referral and uptake to pulmonary rehabilitation in people with COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 2018;13:3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssens T, De Peuter S, Stans L, et al. Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest 2011;140:618–25. [DOI] [PubMed] [Google Scholar]
- 39.Kosteli M-C, Heneghan NR, Roskell C, et al. Barriers and enablers of physical activity engagement for patients with COPD in primary care. Int J Chron Obstruct Pulmon Dis 2017;12:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts NJ, Kidd L, Kirkwood K, Cross J, Partridge MR. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respir Med 2018;145:161–81. [DOI] [PubMed] [Google Scholar]
- 41.Oates GR, Hamby BW, Stepanikova I, et al. Social determinants of adherence to pulmonary rehabilitation for chronic obstructive pulmonary disease. COPD 2017;14:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill K, Vogiatzis I, Burtin C. The importance of components of pulmonary rehabilitation, other than exercise training, in COPD. Eur Respir Rev 2013;22:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowson CA, Town GI, Frampton C, Mulder RT. Psychopathology and illness beliefs influence COPD self-management. J Psychosom Res 2004;56:333–40. [DOI] [PubMed] [Google Scholar]
- 44.Fischer MJ, Scharloo M, Abbink JJ, et al. Drop-out and attendance in pulmonary rehabilitation: the role of clinical and psychosocial variables. Respir Med 2009;103:1564–71. [DOI] [PubMed] [Google Scholar]
- 45.Vagaggini B, Costa F, Antonelli S, et al. Clinical predictors of the efficacy of a pulmonary rehabilitation programme in patients with COPD. Respir Med 2009;103:1224–30. [DOI] [PubMed] [Google Scholar]
- 46.Boutou AK, Tanner RJ, Lord VM, et al. An evaluation of factors associated with completion and benefit from pulmonary rehabilitation in COPD. BMJ Open Respir Res 2014;1:e000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold R, Ranchor AV, Koëter GH, et al. Changes in personal control as a predictor of quality of life after pulmonary rehabilitation. Patient Educ Couns 2006;61:99–108. [DOI] [PubMed] [Google Scholar]
- 48.Garrod R, Marshall J, Jones F. Self efficacy measurement and goal attainment after pulmonary rehabilitation. Int J Chron Obstruct Pulmon Dis 2008;3:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McAuley E, Jerome GJ, Marquez DX, Elavsky S, Blissmer B. Exercise self-efficacy in older adults: social, affective, and behavioral influences. Ann Behav Med 2003;25:1–7. [DOI] [PubMed] [Google Scholar]
- 50.Hogg L, Garrod R, Thornton H, McDonnell L, Bellas H, White P. Effectiveness, attendance, and completion of an integrated, system-wide pulmonary rehabilitation service for COPD: prospective observational study. COPD 2012;9:546–54. [DOI] [PubMed] [Google Scholar]
- 51.Guo SE, Bruce A. Improving understanding of and adherence to pulmonary rehabilitation in patients with COPD: a qualitative inquiry of patient and health professional perspectives. PLoS One 2014;9:e110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore L, Hogg L, White P. Acceptability and feasibility of pulmonary rehabilitation for COPD: a community qualitative study. Prim Care Respir J 2012;21:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison SL, Robertson N, Apps L, Steiner MC, Morgan MD, Singh SJ. We are not worthy”–understanding why patients decline pulmonary rehabilitation following an acute exacerbation of COPD. Disabil Rehabil 2015;37:750–6. [DOI] [PubMed] [Google Scholar]
- 54.Jones AW, Taylor A, Gowler H, O’Kelly N, Ghosh S, Bridle C. Systematic review of interventions to improve patient uptake and completion of pulmonary rehabilitation in COPD. ERJ Open Res 2017;3:00089–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


