Abstract
Malaria vector control interventions in Sumba, Indonesia, have not been able to eliminate malaria. Human drivers of exposure to Anopheles bites were investigated as part of a larger clinical trial evaluating the impact of a spatial repellent product on malaria incidence. Human behavioral observations (HBOs) evaluating temporal and spatial presence, sleeping behaviors, and insecticide treated net (ITN) use, were collected parallel to entomological collections—indoor and outdoor human landing catches (HLCs), and house hold surveys. Data demonstrates that mosquito access to humans, enabled by structurally open houses, is evident by the similar entomological landing rates both inside and outside households. The presence of animals inside houses was associated with increased mosquito entry–however, the number of humans present inside houses was not related to increased mosquito landing. Analyzing mosquito landing rates with human behavior data enables the spatial and temporal estimation of exposure to Anopheles bites, accounting for intervention (ITN) presence and usage. Human behavior adjusted exposure to Anopheles bites was found to be highest in the early in the evening, but continued at lower levels throughout the night. Over the night, most exposure (53%) occurred when people were indoors and not under the protection of nets (asleep or awake) followed by exposure outside (44%). Characterized gaps in protection are outdoor exposure as well as exposure indoors–when awake, and when asleep and not using ITNs. Interestingly, in the primary trial, even though there was not a significant impact of the spatial repellent on vector biting rates by themselves (16%), when factoring in human behavior, there was approximately 28% less exposure in the intervention arm than in the placebo arm. The treated arm had less human behavior adjusted bites in all spaces evaluated though there was proportionally higher exposure indoors. This analysis points to the importance of using HBOs both towards understanding gaps in protection as well as how interventions are evaluated. To mitigate ongoing transmission, understanding context specific spatial and temporal exposure based on the interactions of vectors, humans and interventions would be vital for a directed evidence-based control or elimination strategy.
Introduction
The Indonesian national strategic plan to control malaria is conducted through the malaria elimination program. Intervention activities include early diagnosis with prompt and accurate treatment, surveillance, and vector control [1,2]. The primary vector control interventions include the distribution of insecticide-treated bed nets (ITNs), indoor residual spraying (IRS), and larval source management (LSM) that includes larvicide, biological control, and environmental management. However, the decentralized system with various local (district) priorities in health financing and implementation may pose a barrier to the elimination agenda especially in the eastern part of Indonesia—since malaria may not be either considered or be a priority [2–4]. Environmental and human drivers both expose or protect people from malaria. Environmental drivers of malaria that determine vector population include the ecology, weather, and temperature, while human drivers include local cultural practices and behavior [5,6], implemented interventions and their usage, house construction, and mobility. For example, even though malaria was eliminated in Sabang Municipality, Aceh, Indonesia, a comprehensive analysis including human, vector and other components of the transmission system demonstrated a significant potential for reintroduction of malaria. A mobile and susceptible population resulted in high vulnerability, the presence of endemic populations of malaria vectors including Anopheles sundaicus, An. minimus, An. aconitus and An. dirus [7] resulted in high receptivity, while local factors that specifically contributed to malaria in this area include the importation of malaria infections, as well as Plasmodium knowlesi transmission stemming from long-tailed macaques and endemic Anopheles in the Leucosphyrus group [8].
Although more than half of districts have been declared malaria free, Indonesia is still one of nine malaria-endemic countries in the South-East Asia region, and accounts for 21% of the region’s reported cases and 16% of malaria deaths [1,9]. In order to achieve the goal of malaria elimination in Indonesia by 2030, a strategy that focuses on local drivers of transmission may be required. A successful implementation of this approach was demonstrated in the Purworejo area of Central Java, Indonesia, where multiple local factors that enable transmission were identified, including drivers of vector populations, locally relevant interventions, the implementation of epidemiological control measures, and the political system [10].
Even though the need for characterizing where and when human and vectors overlap was recognized in the 1960s [11], the incorporation of human behavior has not been included in many studies [12–17]. Key measures include understanding when and where the vector and human overlap occurs as well as understanding the human activities that put people at risk [13]. Activities that may increase risk include routine household and community activities, large scale socio-cultural events, as well as regular livelihood or economy-based activities [18,19].
Most residents in rural Southwest Sumba and West Sumba districts are subsistence agriculturalists such as farming and raising livestock, where night-time activity such as cooking and/or protecting stable animals (pigs and horses), hunting, and fishing is a necessity [20]. Moreover, traditional Sumba houses with open construction allow for vector entry and indoor exposure. The use of ITNs as well as factors that may increase use are not well documented. Documentation of human behaviors in conjunction with vector behaviors would allow insights into local gaps in protection that could lead to continued transmission and limit the impact of intervention efforts such as suboptimal ITN usage [13]. Towards addressing this knowledge gap, data was collected on indoor and outdoor vector biting behavior and corresponding human behaviors including time spent indoors versus outdoors, awake versus asleep, and under the protection of an ITN throughout the night.
Methods
Study site
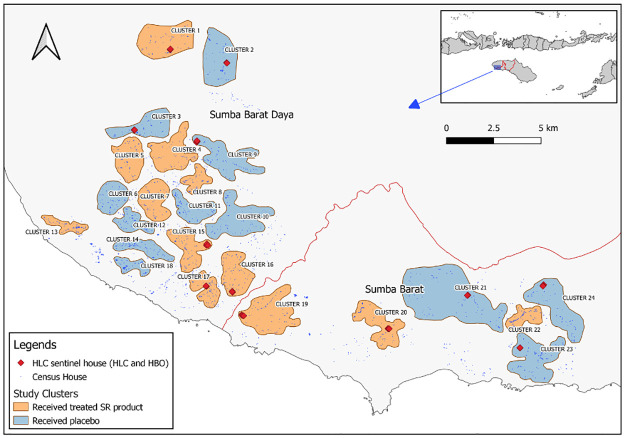
This study was conducted in parallel with an epidemiological trial to determine the effect of spatial repellents on malaria transmission in Southwest and West Sumba, East Nusa Tenggara, Indonesia [21]. Sumba island, a part of East Nusa Tenggara province, has an area of around 11 thousand square kilometers, an estimated population of more than 755,000 (2015), and is divided into 4 districts. The geography is generally of low elevation and consists of limestone hills. The dry season extends from May to November, and the wet season from December to April. The districts of Southwest Sumba and West Sumba are included in the 22 districts that still had the highest annual parasite incidence (API) in Indonesia in 2019. The dominant malaria parasites in Sumba are P. falciparum and P. vivax, with the occasional case of P. malariae. Studies in Southwest Sumba, West Sumba and Central Sumba districts in 2007 demonstrated malaria seasonal prevalence of 6.83% in the wet season and 4.95% in the dry season [22]. In the wet season P. falciparum accounted for 70% of infections while in dry season P. falciparum and P. vivax were present in equal proportion. Malaria vectors detected from previous studies include An. aconitus, An. annularis, An. barbirostris, An. flavirostris, An. maculatus, An. sundaicus, An. tesellatus. An. subpictus, and An. vagus [21–25]. There were more than 430,000 residents in the two study districts occupying 92 villages and 13 small-sized towns [26,27]. Thirteen village groups, with populations ranging from 1,067 to 3,904 (avg. 2,132), served as the 12 study clusters included in this study (Fig 1).
Fig 1. Primary study area.
Primary study area with treated clusters in orange and untreated clusters in blue. Diamonds indicate HLC/HBO sentinel houses. The inset indicates the location of the study site in Sumba Island, Nusa Tenggara Timur Province. Map source: Natural Earth (https://www.naturalearthdata.com/).
Study population
This study included human participants that are residents of West and Southwest Sumba Districts, East Nusa Tenggara Province, Indonesia (Fig 1). Most participants are of Sumba Ethic groups that adopt both traditional beliefs and Christianity. The majority of the study participants are subsistence farmers, who also perform animal husbandry and fishing. Livestock includes pigs, buffalos that are traditionally and culturally used as currency for social and cultural activities [21,28].
Human Landing Catch (HLC) collections
HLCs were conducted in 12 clusters continuously every two weeks from June 2015 to April 2018 with six clusters each from placebo (untreated) and intervention (treated) clusters [21]. For the adjusted biting rate analysis, only HLC data that overlapped with human behavioral observations (HBOs) were used (February 2018 to April 2018), and included five HLC periods from the parent study intervention period (HLC #I48 to #I52). Four sentinel houses within each of the 12 clusters were selected for paired (indoor and outdoor/house verandah) HLC collections. Mosquito collections were conducted from 1800 h until 0600 h the next day. At the end of each HLC, a household questionnaire was conducted that documented the presence of fire and/or smoke, and animals both inside and outside the structure during the HLC (S1 File. HLC Questionnaire). Human landing rates (HLR) were used as a proxy for human biting rates (HBRs) and were calculated as bites per person per night (bpn) or bites per person per night (bpn) for the location of collection (inside and outside).
Human Behavioral Observations (HBOs)
HBOs were conducted hourly from 1800 h to 0600 h alongside entomology collections in HLC households. HBO data was collected during five HLC periods (February 2018 to April 2018) in 12 clusters—resulting in data from 48 houses over five nights each (S2 File. HBO Questionnaire). HBOs focused on temporal (hourly, over the night), location (domestic or peri-domestic) and bed net usage. HBO data was limited to the perimeter of the sentinel structure (usually less than 20 m around the structure). Data was collected by the HLC volunteer on paper questionnaires at the end of each HLC hour, with spot-checks conducted by a supervisor, and double data entry checks after data collection towards ensuring quality. The HLC volunteers were part of the community and their presence was excluded from the HBO data and analysis.
Household and bed net (ITN) surveys
Household and ITN surveys were conducted in every household during household enrollment in the parent study [21]. The household questionnaire collected data on structure materials, practices against mosquito bites, the presence of livestock and ITN presence (S3 File. Household and ITN questionnaire).
Analysis
Analysis of quantitative data was carried out using Microsoft Office Excel basic functions and open-source software, RStudio version 1.3.1056 based on R version 4.0.2 [29,30]. Clustering of house type analysis was done using the klaR package clustering of categorical variables [31]. The effect of fire/smoke (indoor or outdoor) and large animals (indoor or outdoor) on mosquito landing rates, were separated into time periods (1800h – 0000h, and 0000h to 0600h), and were evaluated using Kruskal-Wallis test. With mosquito landing rates being normally distributed, the number of people in each house were evaluated using a regression analysis towards determining if they had an effect on mosquito numbers. Human behavior-adjusted biting rates and the protections afforded by ITNs were generated using HLRs, HBOs and ITN survey data as in Monroe et. al, 2020 [12,13].
Ethical review
Ethical review and approval for this study was granted by the Ethics Committee (EC) of the Faculty of Medicine, Universitas Hasanuddin, Indonesia (Protocol #UH14070385), the University of Notre Dame, USA (Protocol #14-01-1448), and endorsed by the Eijkman Institute Research Ethics Committee, Jakarta, Indonesia.
Results
Demographic characteristics
All households (n = 2910) were characterized before inclusion in the parent study [21] (Table 1). Most household structures enrolled were made from traditional materials including bamboo and wood with thatch roofs. A clustering analysis of wall, roof and floor materials (household dataset n = 2842) demonstrated an optimal characterization into four house type groups—Type 1: The traditional house type (n = 1985, 69.85%) with bamboo structure and a bamboo floors; Type 2: (n = 339, 11.93%) bamboo structure with dirt floors and thatch roofs (some were replaced with metal); Type 3 (n = 297, 10.45%) were households with concrete floors and metal roof, with walls made of are plaster or bamboo; and Type 4 (n = 221, 7.78%) consisted of wood houses with thatch or metal roofs. The traditional Sumba house has floors made of bamboo (Type 1), with a raised floor (mean floor height is 145.3 cm ± 38.8 cm) under which livestock is reared. Type 1 houses do not have doors or windows, and have open exits—one each at the front and rear of the house. These structures have hollow bamboo walls. The other types of houses have doors and windows, without screening material (only four houses had windows with screening material). Overall, 2864 households (98.5%) had open eaves. Household cooking is usually conducted at an indoor central location using firewood (84.3% of households), resulting in the presence of smoke indoors. Food preparation for livestock was performed outdoors in 34.9% of households. Approximately 81.3% of the households did not have a source of electricity. All structures are open and permeable to mosquito entry with clear demarcations of inside versus outside spaces irrespective of house type.
Table 1. Household survey results (total household n = 2910).
| Variable | Category | Total (percentage) |
|---|---|---|
| Wall type | Bamboo | 2399 (82.5%) |
| Plaster | 229 (7.9%) | |
| Wood | 211 (7.3%) | |
| Split wood | 31 (1.1%) | |
| Others (concrete, brick, etc) | 39 (1.2%) | |
| Roof type | Thatch | 2273 (78.1%) |
| Metal | 632 (21.7%) | |
| Tiles and sticks | 4 (0.1%) | |
| Floor type | Bamboo | 2155 (74.1%) |
| Concrete | 365 (12.5%) | |
| Dirt | 318 (10.9%) | |
| Tiles | 41 (1.4%) | |
| Wood | 21 (0.7%) | |
| Other | 9 (0.3%) | |
| Eaves opened | Yes | 2864 (98.5%) |
| No | 45 (1.5%) | |
| Door number | Mean (SD) | 2.0 (± 0.4) |
| Window number | Mean (SD) | 0.6 (± 1.5) |
| Screened windows | Yes | 4 (0.1%) |
| No | 2706 (99.4%) | |
| Floor height for Sumba traditional bamboo house (in cm) (subset n = 1985) | Mean (SD) | 145.3 (± 38.8) |
| Usually fire/burning indoor | Yes | 2295 (84.3%) |
| No | 427 (15.7%) | |
| Usually fire/burning outdoor | Yes | 950 (34.9%) |
| No | 1772 (65.1%) | |
| Electrical source | Yes | 507 (18.6%) |
| No | 2214 (81.3%) | |
| IRS | Never | 2500 (98.1%) |
| Don’t know | 45 (1.8%) | |
| < 3 months | 1 (0.0%) | |
| 3–6 months | 0 (0.0%) | |
| > 6 months | 3 (0.1%) | |
| Protection from mosquito bite | Coil (included emanator) | 2 (0.1%) |
| Other | 3 (0.1%) | |
| Not used | 2717 (99.8%) | |
| Livestock indoor/under the house | Has animal | 2040 (74.9%) |
| Large animals | 1952 (71.7%) | |
| Fowl | 1437 (52.8%) | |
| Others | 14 (0.5%) | |
| No animal | 678 (24.9%) | |
| Livestock outdoor | Has animals | 848 (31.1%) |
| Large animals | 764 (28.1%) | |
| Fowl | 530 (19.5%) | |
| Others | 4 (0.1%) | |
| No animal | 1880 (69.0%) |
Drivers of human landing rates (HLR)
A total of 1488 female Anopheles mosquitoes were caught during these HLCs, of which 833 came from untreated clusters and 655 from treated clusters. There was significant variation between clusters with cluster 2 sampling the most (n = 655) and cluster 15 the least (n = 15) (Table 2). Equal proportions of Anopheles were seen landing indoors and outdoors with no significant difference in total mosquitos caught between indoor and outdoor collections in either arm (Welch Two Sample t-test result p-value = 0.8993). Anopheles were host-seeking on humans throughout the night, both indoors and outdoors, in all clusters, although there was cluster-specific variation seen (Fig 2).
Table 2. Total collected Anopheles mosquitoes during HLCs per cluster (treated cluster: Shaded; untreated cluster: Unshaded).
| Cluster | HLC #I48 | HLC #I49 | HLC #I50 | HLC #I51 | HLC #I52 | Total |
|---|---|---|---|---|---|---|
| 01 | 1 | 4 | 4 | 9 | 3 | 21 |
| 02 | 78 | 66 | 242 | 171 | 98 | 655 |
| 03 | 2 | 5 | 8 | 17 | 9 | 41 |
| 09 | 5 | 3 | 13 | 2 | 1 | 24 |
| 15 | 0 | 1 | 0 | 0 | 14 | 15 |
| 16 | 75 | 23 | 94 | 74 | 70 | 336 |
| 17 | 20 | 11 | 7 | 4 | 6 | 48 |
| 19 | 7 | 28 | 34 | 26 | 16 | 111 |
| 20 | 26 | 35 | 13 | 42 | 8 | 124 |
| 21 | 0 | 1 | No HLC a | 8 | 10 | 19 |
| 23 | 13 | 13 | 5 | 30 | 14 | 75 |
| 24 | 5 | 0 | 6 | 8 | 0 | 19 |
| Total Treated | 129 | 102 | 152 | 155 | 117 | 655 |
| Total Untreated | 103 | 88 | 274 | 236 | 132 | 833 |
| ALL | 232 | 190 | 426 | 391 | 249 | 1488 |
a No HLC activities.
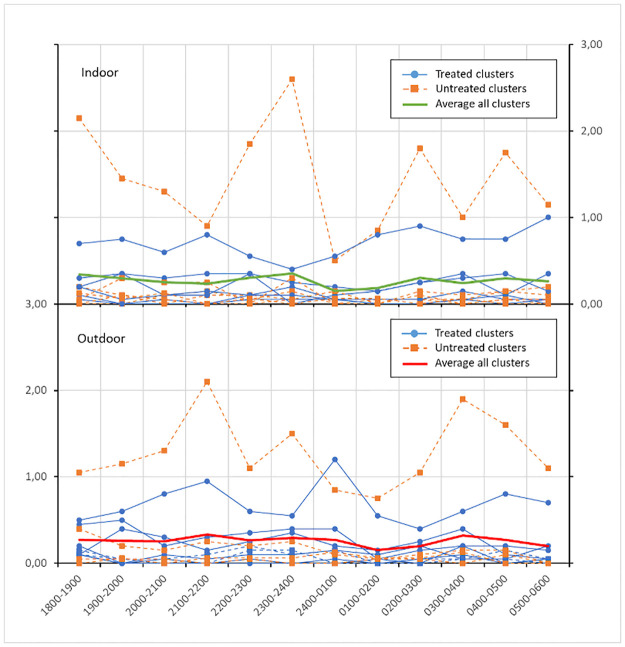
Fig 2. Indoor (A) and outdoor (B) HLRs by cluster over the course of the night.
Though there were mosquitoes present throughout the night, there was high heterogeneity in mosquito landing rates between clusters. The solid green and red line indicate average HLRs indoors and outdoors.
The number of Anopheles per cluster per night varied significantly (min = 0, max = 242, mean 24.8 ± 42.50) (Table 2). When evaluating mosquito numbers per cluster per total observation (a five-night collection), there was a minimum of 15 and a maximum of 655 mosquitos sampled, with a mean mosquito number of 124 ± 189.90. Each cluster had different landing rates and consequently differential exposure to Anopheles bites. The Shapiro-Wilk’s W test on HLRs over the night or just indoor HLRs demonstrates that the data is not normally distributed (p-value of HLR indoor per night is < 2.2e-16). This also indicated that there was no correlation between the number of mosquitoes collected per house and the total number of humans indoors. There was also no correlation between mosquitoes caught and the presence of fire/smoke indoors, fire/smoke outdoors, and animals outdoors (p-values are 0.4971, 0.5519 and 0.3742 respectively). However, there was a positive relationship between the presence of animals indoors and the numbers of mosquitoes caught indoors (p-value = 0.04882) (data not shown). The two data points describing “no animal indoors all night” and “presence of animals indoors all night” demonstrated that houses with animals tended to be visited by more mosquitoes.
Malaria vectors in South West Sumba and West Sumba Districts [21,23–25] did not demonstrate species-specific indoor or outdoor human landing preferences (Table 3). Indoor and outdoor biting rates of An. aconitus, An. annularis, An. barbirostris, An. flavirostris, An. maculatus, An. tessellatus and An. vagus (also including An. sundaicus and An. subpictus that consisted of only one mosquito each), were sampled at equivalent rates both indoors and outdoors (Table 3).
Table 3. Total number of collected female Anopheles mosquito species.
| Species | Indoor | Outdoor | Total |
|---|---|---|---|
| An. aconitus | 237 | 208 | 445 |
| An. annularis | 42 | 46 | 88 |
| An. barbirostris | 40 | 41 | 81 |
| An. flavirostris | 94 | 101 | 195 |
| An. kochi | 54 | 60 | 114 |
| An. leucosphyrus | 2 | 3 | 5 |
| An. maculatus | 15 | 22 | 37 |
| An. sundaicus | 1 | 0 | 1 |
| An. subpictus | 1 | 0 | 1 |
| An. tesellatus | 63 | 74 | 137 |
| An. vagus | 209 | 170 | 379 |
| Total | 759 | 729 | 1488 |
Human behavior
The communities, in general, move indoors after nightfall (dark) at 1800h and start to go to sleep at about 2000 h (Fig 3a and 3d). Both indoor and outdoor activity during the night is limited since only a proportion (18.6%) of households in the study site have a source of electricity (Table 1). Primary evening and night-time activities before sleep included cooking and eating, where 93.9% of HLC sentinel houses reported the presence of fire/smoke (cooking) indoors after 1800 h (Table 4). Though people spent time outdoors in the peri-domestic area in the evening until midnight, some were documented outdoors throughout the night. ITNs were not utilized outdoors even though about 11% of the population preferred to sleep outdoors on the house verandah. Besides ITNs, only 0.1% of the population used other mosquito bite prevention tools (e.g. mosquito coils) (Table 1).
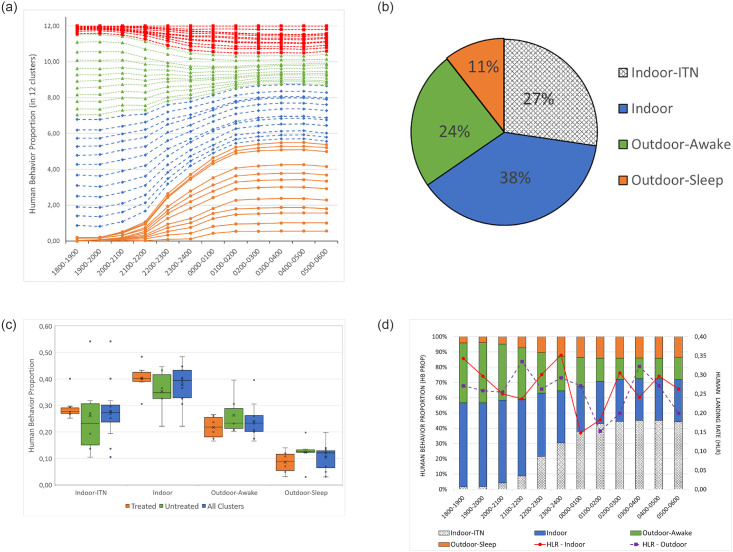
Fig 3.
a. Human behavior proportions from all clusters. ●: Indoor-ITN, ♦: Indoor, ▲: Outdoor-awake and ■: Outdoor-sleep. b. Average human behaviors. Average human behaviors spent in different categories over the course of a night. c. Average human behavior per treated and untreated clusters. d. Stacked bar graph of the proportions of human behavior overlaid with entomological indoor and outdoor landing rates.
Table 4. HLC household characterization.
| Any fire burning/smoke inside the structure during 1800–2400 h | ||
| Yes | 93.9% | |
| No | 6.1% | |
| Any fire burning/smoke inside the structure during 2400–0600 h | ||
| Yes | 39.1% | |
| No | 60.9% | |
| Any fire burning/smoke outside the structure during 1800–2400 h | ||
| Yes | 9.6% | |
| No | 90.4% | |
| Any fire burning/smoke outside the structure during 2400–0600 h | ||
| Yes | 7.8% | |
| No | 92.2% | |
| Any large animals inside (under) the structure during 1800–2400 h | ||
| Yes | 79.6% | |
| No | 20.4% | |
| Any large animals inside (under) the structure during 2400–0600 h | ||
| Yes | 79.6% | |
| No | 20.4% | |
| Any large animals outside the structure during 1800–2400 h | ||
| Yes | 39.6% | |
| No | 60.4% | |
| Any large animals outside the structure during 2400–0600 h | ||
| Yes | 40.0% | |
| No | 60.0% | |
| Number of people slept indoor during HLC | ||
| Mean | 5.6 | |
| SD | 1.9 | |
| Number of people slept under ITN during HLC | ||
| Mean | 2.8 | |
| SD | 1.5 | |
| Any efforts to avoid mosquito bites during HLC | ||
| ITN | 93.0% | |
| No protection | 7.0% | |
Indoor ITN usage varied from house to house and cluster to cluster (See indoor-ITN in Fig 3a and 3d). Overall, when looking at person time spent in each behavioral category, 24% of time was spent outdoors awake, 11% time was spent outdoors asleep (not under ITN protection), 38% of time was spent indoors not under ITN protection, 27% of time was spent under ITNs (Fig 3b). There remained a proportion of people in all behavioral categories over the course of HBO observations. Fig 3c depicts the box and whisker graphs of the proportion of human behaviors by treated clusters, untreated clusters and all clusters together. The household survey demonstrated large variations in household ITN coverage and use, for example, although the mean number of ITNs per house in cluster #21 was only 2.19, the highest proportion of ITN use was 54%. Paradoxically, the cluster with the highest mean ITN presence per house was 4.28 ITNs in cluster #24, but only 14% actually used the ITNs. The average number of ITNs per household was 1.73 (± 1.27). Fig 3d illustrates the proportion of human behaviors overlaid with the overall vector human landing rate. Although 57% of the population were indoors (including the proportion indoors and using an ITN) by 1800 h, and reached 72% at 0600 h, both indoor and outdoor landing rates remained relatively consistent throughout the night. Excluding the 27% of people indoors using an ITN, the remaining 73% of people remain unprotected from mosquito bites.
Human behavior adjusted exposure to Anopheles
Towards understanding the relationship between human behavior and vector landing rates, adjusted exposure rates were derived [12]. Overall, as the biting rate drops to a minimum at 2400–0200 h (Fig 2), behavior adjusted bites also decrease (Fig 4a and 4b). The proportion of the population exposed to mosquito bites was highest at early in the evening (1800 h), is lowest in the middle of the night (0100–0200 h), and then increases until early morning—0500 h.
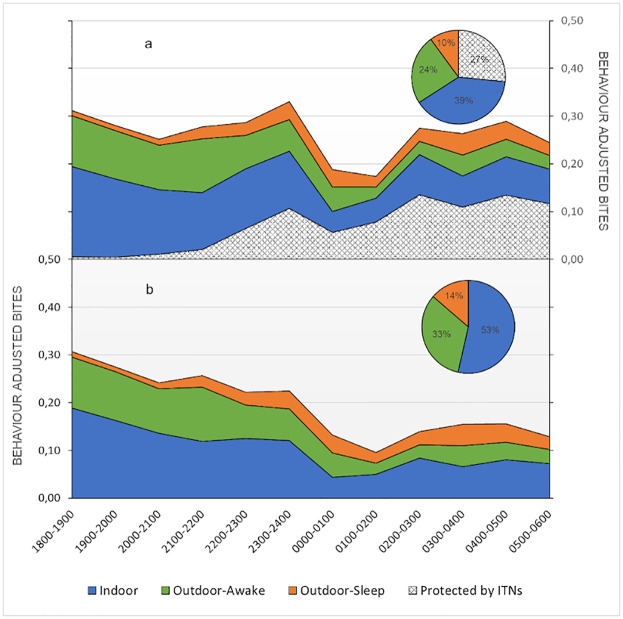
Fig 4. Human behavior adjusted.
4a. Human behavior adjusted temporal exposure and ITN protection over the night with proportional spatial exposure and ITN protection in the inset. ITNs reduce exposure by approximately 27%. 4b. Quantified temporal and spatial (inset) gaps in protection i.e. human behavior adjusted exposure alone, demonstrate that the majority of exposure (53%) occurs when people are indoor and not protected by ITNs.
Indicators demonstrative of exposure to Anopheles bites based on human behavior [13] have been summarized in Table 5. There were 0.66 bpn occurring indoors for an unprotected individual (including periods when an individual cannot be under an ITN). The proportion of vector bites occurring while asleep for an unprotected individual is 0.27 bpn. Overall, there were 0.24 bpn prevented by using an ITN. Even ITN-protected individuals are exposed to mosquito bites when awake and this proportion of remaining exposure occurring indoors for a user of an ITN is 0.55 bpn while the proportion of exposure occurring outdoors for a protected individual is 0.45 bpn. Meanwhile, the proportion of exposure prevented by current levels of ITN use in the population is relatively low (12%), resulting in spaces and times when people are not under ITN protection or when ITNs cannot be used.
Table 5. Human exposure patterns to Anopheles bites.
| Indicator | Anopheles bites |
|---|---|
| Directly measured biting | |
| • Proportion biting indoors | 0.51 |
| Behavior-adjusted exposure–unprotected individual | |
| • Proportion of vector bites occurring indoors for an unprotected individual | 0.66 |
| • Proportion of vector bites occurring while asleep indoors for an unprotected individual | 0.27 |
| Exposure prevented by ITN use | |
| • Proportion of all vector bites prevented by using an ITN | 0.24 |
| Remaining exposure for an ITN-user | |
| • Proportion of remaining exposure occurring indoors for a protected user of an ITN | 0.55 |
| • Proportion of remaining exposure occurring outdoors for a protected user of an ITN | 0.45 |
| Population mean exposure based on observed level of ITN use | |
| • Proportion of exposure prevented by current levels of ITN use in the population | 0.12 |
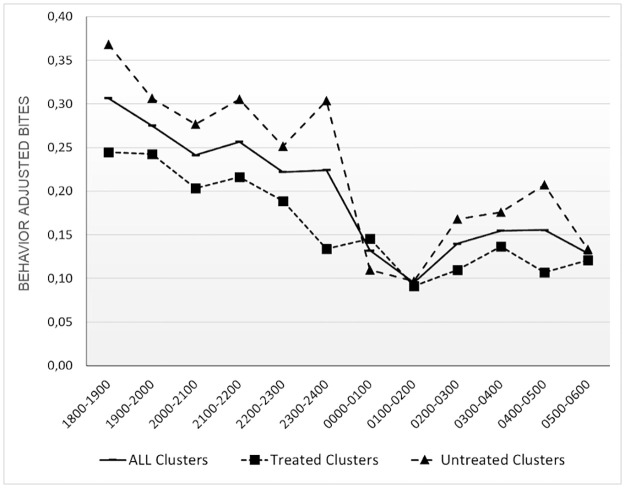
There was a difference in behavior adjusted bites seen in treated and untreated clusters, the sum of behavior adjusted bites per person for untreated clusters was higher than treated clusters (Fig 5).
Fig 5. Overall behavior adjusted exposure.
Overall behavior adjusted exposure over the course of a night for treated, untreated and all clusters together. Treated clusters demonstrate less exposure overall with key differences seen during times when people are indoors and not under ITN protection, that consistent with expectations from a spatial repellent product.
When looking at spatial exposure indoors, outdoors when awake, and outdoors when asleep in the untreated arm, there were 1.36, 0.93 and 0.41 behavior adjusted bpn respectively. All these HBO-based rates were lower in the treated arm (1.10, 0.61 and 0.23 indoors, outdoors when awake, and outdoors when asleep respectively). Proportionally, spatial exposure was higher indoors in the treated arm (57% in the treated arm versus 50% in the untreated arm), but lower in both outdoor behavioral spaces (Outdoors when awake: 31% treated versus 35% untreated; outdoors when asleep: 12% treated versus 15% untreated). Adjusted exposure in the treated clusters was 1.94 bpn while that in the untreated clusters were 2.7 bpn–representing approximately 28% less exposure in the treated arm over the course of a night.
Discussion
The impact of a spatial repellent on malaria incidence was evaluated in a cluster-randomized, double-blinded, placebo-controlled trial, in Sumba, Indonesia [21]. Temporal and routine indoor and outdoor HLC sampling, as part of this parent study, enabled the documentation of Anopheles mosquito landing rates while parallel HBO collections (corresponding to five HLC periods) recorded the spatial and temporal presence of humans along with ITN usage. Household surveys documented house construction, the presence of animals, intervention usage, and other potential drivers of malaria.
The open nature of all four types of characterized house types in Sumba–especially the presence of open eaves in 98.5% of houses, enable entry and exit of vectors. Mosquito access to humans inside houses is evident by the similar entomological landing rates both inside and outside houses. Open eaves are documented entry points for malaria vectors [32,33] and may significantly contribute to indoor biting in this area. House design has been documented to impact disease [34–39], and even in areas with high ITN coverage and usage, primary exposure to malaria may occur indoors [40,41]. The openness of the houses with consistent airflow may have also impacted the protective efficacy of the spatial repellent intervention. The similar indoor and outdoor landing rates documented in multiple vector species suggest that indoor interventions like ITNs may impact species that feed both indoors and outdoors [42,43]. Biting throughout the night, including when people are outdoors and inside before going to bed, suggests the need for supplementary protection measures in addition to ITNs.
The presence of animals inside houses, a cultural aspect of Sumba [28,44] was also associated with increased mosquito entry. Interestingly, the number of humans present was not found to be related to the number of mosquitoes indoors. Most Anopheles species found here feed on both humans and animals but are generally considered zoophagic [23] potentially explaining this outcome. The presence of indoor fires (cooking) or animals outside did not impact mosquito entry.
Human behavior by itself was a driver of exposure to mosquito bites. Over the course of a night, people spent time both indoors and outdoors. There was almost no mosquito personal protection in use other than ITNs. Overall, the population evaluated spent 27% of the night under ITNs, while approximately 35% of the night was spent outdoors and 38% indoors unprotected. Though this site did not have any ITN access issues (there was a mass ITN distribution in 2018) [21], ITN presence and usage was both house and cluster specific demonstrating that, unconnected to intervention presence, household behaviors will impact exposure to mosquito bites. This points to the value of passive interventions, like the spatial repellent product evaluated in the primary trial [21], where human compliance, a large factor in determining intervention efficacy, can be mitigated. Other behaviors that increased exposure included outdoor sleeping as well as indoor activities, such as cooking, where ITNs cannot be used. Appropriate social and behavior change communication (SBCC) directed at increasing ITN use both indoors and outdoors may reduce a proportion of this exposure. Relating setting-specific human behaviors with complementary entomological and epidemiological interventions that function within the spaces and times of exposure may better impact disease transmission [45].
Analyzing mosquito landing rates with human behavior data enables the spatial and temporal estimation of where and when exposure to Anopheles bites occurs based on intervention (ITN) presence and usage [12,15]. Human behavior and ITN use were found to impact exposure considerably relative to vector-biting-only estimations of exposure. Even though vector biting based on HLCs, peaked at the beginning of the night, midnight and then early in the morning, human behavior adjusted exposure was found to predominate early in the evening, and present throughout the night. Reduced human behavior adjusted exposure was based on people being indoors and under ITNs, and away from outdoor exposure. Over the night, most exposure (53%) occurred when people were indoors and not under the protection of ITNs (asleep or awake) followed by exposure outside (44%). This outcome immediately points to the possibility of reducing indoor exposure by increasing ITN use when asleep, while using complementary interventions like spatial repellents to combat remaining exposure occurring indoors. Alongside this observation and the use of HBO’s, the spatial repellent (SR) arm had decreased human behavior adjusted exposure in all spaces (inside and outside), though it may have proportionally increased exposure indoors, possibly attributed to people wanting to be in a space with the least biting and possibly using ITNs less. Evidence on the impact of spatial repellents on the use of other interventions (ITNs) and human behavior with ensuing remedial measures need to be evaluated.
Towards understanding the impact of human behavior on spatial repellent protection, intervention (treated) and placebo (untreated) clusters were analyzed separately with regards to adjusted exposure. Interestingly, although there was not a significant impact on vector biting rates by themselves [21], there was approximately 28% less human behavior adjusted bites in the intervention arm that had the spatial repellent over the course of the night during the period that HBOs were documented. Key differences are seen during times when people are awake indoors and not under ITN protection that consistent with expectations from a spatial repellent product. This observation is particularly important since, when looking at HLC clusters only, the primary study [21] documented a statistically inconclusive (16.4%) reduction of bites in the intervention clusters, with a statistically significant epidemiological impact (60% protective efficacy). This higher-than-expected epidemiological outcome relative to a smaller entomological impact may be explained by the impact of human behaviors on exposure as documented above i.e., actual exposure to bites was not reflected by HLC data alone. In addition, the 60% protective efficacy seen in the primary study [21] was in children under five who generally have a higher ITN use as compared to the overall population documented in this analysis. Here a greater than 28% impact on exposure in this under five cohort would be expected that would better relate to the 60% protective efficacy seen.
Characterized gaps in protection are outdoor exposure as well as exposure indoors such as when awake, and when asleep and not using ITNs. Analysis incorporating human behaviors indicate that overall, ~55% of exposure occurs indoors, suggesting that the protection from increasing ITN use can be augmented by paradigms such as spatial repellents that function when ITNs cannot be used. In this study spatial repellents fill specific gaps in protection, primarily indoors when people are not under the protection of ITNs. Overall, only about 24% of exposure is mitigated by ITN use in these communities. Even when using ITNs, 55% of remaining or residual exposure occurs indoors and 45% occurs outdoors pointing to the need for additional protective measures. Continuing gaps in exposure even with optimal ITN and spatial repellent use includes bites that continue to happen outdoors as well as indoors due to the open housing structure, limitations with the spatial repellent product, ITN durability, and so on. Solutions here may include more improved housing, evidence-based SBCC, improved intervention products, or increased complementary epidemiological interventions (e.g., mass drug administration or increased testing and treatment towards reducing the parasite reservoir).
Study limitations include the HBOs not extending through the entire timeline of the primary study which would have enabled the better evaluation of human behavioral components of protection and exposure–the limited timeline however still does demonstrate HBO-based differences in the two arms of the study. The evaluation of behaviors of children under five (the cohort followed in the primary study [21]) would have allowed for determining a relationship between protective efficacy and entomological (adjusted) exposure as well. In addition, the high heterogeneity in entomological landing rates (two separate clusters, clusters 2 and 16, were outliers in the number of mosquitoes collected) were possible confounders.
This analysis points to the importance of using HBOs both towards understanding gaps in protection as well as how interventions are being evaluated and may serve as a proxy for entomological outcomes when and if characterized in relationship to measured entomological and epidemiological endpoints. To mitigate ongoing transmission, understanding context specific spatial and temporal exposure based on the interactions of vectors, humans and interventions would be vital for a directed evidence-based control or elimination strategy.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We gratefully thank for the supports of all local field staffs and local volunteers in Southwest and West Sumba Districts, East Nusa Tenggara Province, for their long-serving dedication to participate in this study. We are also grateful for the support of the Eijkman Institute for Molecular Biology (EIMB) Jakarta, Ministry of Health Republic of Indonesia, District health departments of Southwest and West Sumba, and East Nusa Tenggara Province. We thank local data entry clerks, Petronela Ndode and her team, and Indrawan Agus Permadi for their assistance to quality of data, and also to the entomology team for the long-nights collecting mosquitoes during HLC.
Data Availability
All relevant data are within the article and its Supporting information files.
Funding Statement
This study was funded by a substantial award from the Bill and Melinda Gates Foundation (BMGF) to the University of Notre Dame (Grant# OPP1081737) and the Government of Indonesia, Ministry of Research and Technology/National Research and Innovation Agency through Eijkman Institute for Molecular Biology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1."Zero malaria starts with me”: history of malaria elimination in Indonesia helps to shape a malaria-free future [Internet]. [cited 2020 Sep 17]. https://www.who.int/indonesia/news/feature-stories/detail/zero-malaria-starts-with-me-history-of-malaria-elimination-in-indonesia-helps-to-shape-a-malaria-free-future.
- 2.Mahendradhata Y, Trisnantoro L, Listyadewi S, Soewondo P, MArthias T, Harimurti P, et al. The Republic of Indonesia Health System Review. Vol. 7. 2017. 1 p. [Google Scholar]
- 3.Gani A, Budiharsana MP. The Consolidated Report on Indonesia Health Sector Review 2018. 2018;56.
- 4.Elyazar IRF, Hay SI, Baird JK. Malaria distribution, prevalence, drug resistance and control in Indonesia. In: Advances in Parasitology. 2011. [DOI] [PMC free article] [PubMed]
- 5.Moshi IR, Manderson L, Ngowo HS, Mlacha YP, Okumu FO, Mnyone LL. Outdoor malaria transmission risks and social life: A qualitative study in South-Eastern Tanzania. Malar J [Internet]. 2018;17(1):1–11. Available from: doi: 10.1186/s12936-018-2550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn CE, Le Mare A, Makungu C. Malaria risk behaviours, socio-cultural practices and rural livelihoods in southern Tanzania: Implications for bednet usage. Soc Sci Med [Internet]. 2011;72(3):408–17. Available from: doi: 10.1016/j.socscimed.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 7.Herdiana H, Fuad A, Asih PB, Zubaedah S, Arisanti RR, Syafruddin D, et al. Progress towards malaria elimination in Sabang Municipality, Aceh, Indonesia. Malar J. 2013;12(May 2014). doi: 10.1186/1475-2875-12-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herdiana H, Irnawati I, Coutrier FN, Munthe A, Mardiati M, Yuniarti T, et al. Two clusters of Plasmodium knowlesi cases in a malaria elimination area, Sabang Municipality, Aceh, Indonesia. Malar J [Internet]. 2018;17(1):1–10. Available from: doi: 10.1186/s12936-018-2334-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitohang V, Sariwati E, Fajariyani SB, Hwang D, Kurnia B, Hapsari RK, et al. Malaria elimination in Indonesia: halfway there. Lancet Glob Heal. 2018;6(6):e604–6. [DOI] [PubMed] [Google Scholar]
- 10.Murhandarwati EEH, Fuad A, Sulistyawati, Wijayanti MA, Bia MB, Widartono BS, et al. Change of strategy is required for malaria elimination: A case study in Purworejo District, Central Java Province, Indonesia. Malar J. 2015;14(1):1–14. doi: 10.1186/s12936-015-0828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett-Jones C. A method for estimating the man-biting rate / by C. Garrett-Jones [Internet]. World Health Organization; 1964. https://apps.who.int/iris/handle/10665/65193.
- 12.Martin JA, Hendershot AL, Saá Portilla IA, English DJ, Woodruff M, Vera-Arias CA, et al. Anopheline and human drivers of malaria risk in northern coastal, Ecuador: A pilot study. Malar J [Internet]. 2020;19(1):1–11. Available from: doi: 10.1186/s12936-020-03426-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monroe A, Moore S, Okumu F, Kiware S, Lobo NF, Koenker H, et al. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar J 2020 191 [Internet]. 2020. Dec 16 [cited 2020 Jun 29];19(1):1–14. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-020-03271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monroe A, Moore S, Okumu F, Kiware S, Lobo NF, Koenker H, et al. Erratum: Methods and indicators for measuring patterns of human exposure to malaria vectors (Malar J (2020) 19: 207 10.1186/s12936-020-03271-z). Malar J [Internet]. 2020;19(1):12936. Available from: doi: 10.1186/s12936-020-03308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monroe A, Msaky D, Kiware S, Tarimo BB, Moore S, Haji K, et al. Patterns of human exposure to malaria vectors in Zanzibar and implications for malaria elimination efforts. Malar J [Internet]. 2020. Dec 22 [cited 2020 Jun 29];19(1):212. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-020-03266-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monroe A, Moore S, Koenker H, Lynch M, Ricotta E. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in sub-Saharan Africa: A review of the published literature. Malar J [Internet]. 2019;18(1):1–12. Available from: doi: 10.1186/s12936-019-2638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monroe A, Moore S, Olapeju B, Merritt AP, Okumu F. Unlocking the human factor to increase effectiveness and sustainability of malaria vector control. Malar J [Internet]. 2021;1–6. doi: 10.1186/s12936-021-03943-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SC O, VS M, GW P, CCJ C. Malaria: Obstacles and Opportunities [Internet]. Washington, D.C.: National Academies Press; 1991 [cited 2020 Aug 28]. http://www.nap.edu/catalog/1812. [PubMed]
- 19.Heggenhougen HK, Hackethal V, Vivek P. The behavioural and social aspects of malaria and its control. Cdrwww.Who.Int. 2003.
- 20.JRI Research. Socio-Economic-Gender Baseline Survey. 2013;(February). https://sumbaiconicisland.org/wp-content/uploads/2018/11/jri_socio_economic_gender_survey_-_sumba_iconic_island_round12_-_hivos_2013.pdf.
- 21.Syafruddin D, Asih PBS, Rozi IE, Permana DH, Hidayati APN, Syahrani L, et al. Efficacy of a spatial repellent for control of malaria in Indonesia: A cluster-randomized controlled trial. Am J Trop Med Hyg. 2020;103(1):344–58. doi: 10.4269/ajtmh.19-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syafruddin D, Krisin, Asih P, Sekartuti, Dewi RM, Coutrier F, et al. Seasonal prevalence of malaria in West Sumba district, Indonesia. Malar J. 2009;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbara KA, Sukowati S, Rusmiarto S, Susapto D, Bangs MJ, Kinzer MH. Survey of anopheles mosquitoes (Diptera: Culicidae) in West Sumba District, Indonesia. Southeast Asian J Trop Med Public Health. 2011;42(1):71–82. [PubMed] [Google Scholar]
- 24.Syafruddin D, Bangs MJ, Sidik D, Elyazar I, Asih PBS, Chan K, et al. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. Am J Trop Med Hyg [Internet]. 2014. Dec 1 [cited 2020 Jul 29];91(6):1079–87. Available from: https://pubmed.ncbi.nlm.nih.gov/25311699/. doi: 10.4269/ajtmh.13-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nixon CP, Nixon CE, Arsyad DS, Chand K, Yudhaputri FA, Sumarto W, et al. Distance to anopheles sundaicus larval habitats dominant among risk factors for parasitemia in meso-endemic Southwest Sumba, Indonesia. Pathog Glob Health. 2014;108(8):369–80. doi: 10.1179/2047773214Y.0000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumba Barat Daya Regency in Figures 2015 [Internet]. BPS—Statistics of Sumba Barat Daya Regency; 2015. https://sumbabaratdayakab.bps.go.id/.
- 27.Sumba Barat in Figures 2015 [Internet]. BPS—Statistics of Sumba Barat Regency; 2015. https://sumbabaratkab.bps.go.id/.
- 28.Adams RL. Household Ethnoarchaeology and Social Action in a Megalith-Building Society in West Sumba, Indonesia. Asian Perspect. 2019;58(2):331–65. [Google Scholar]
- 29.R: The R Project for Statistical Computing [Internet]. [cited 2020 Jul 24]. https://www.r-project.org/.
- 30.RStudio | Open source & professional software for data science teams—RStudio [Internet]. [cited 2020 Jul 24]. https://rstudio.com/.
- 31.Weihs C, Ligges U, Luebke K, Raabe N. klaR Analyzing German Business Cycles. In: Data Analysis and Decision Support [Internet]. Springer-Verlag; 2005 [cited 2020 Aug 3]. p. 335–43. https://link.springer.com/chapter/10.1007/3-540-28397-8_36.
- 32.Lindsay SW, Snow RW. The trouble with eaves; house entry by vectors of malaria. Trans R Soc Trop Med Hyg [Internet]. 1988. Jul 1;82(4):645–6. Available from: doi: 10.1016/0035-9203(88)90546-9 [DOI] [PubMed] [Google Scholar]
- 33.SNOW WF. Studies of house-entering habits of mosquitoes in The Gambia, West Africa: experiments with prefabricated huts with varied wall apertures. Med Vet Entomol [Internet]. 1987. Jan 1;1(1):9–21. Available from: doi: 10.1111/j.1365-2915.1987.tb00318.x [DOI] [PubMed] [Google Scholar]
- 34.Schofield CJ, White GB. House design and domestic vectors of disease. Trans R Soc Trop Med Hyg [Internet]. 1984. Jan 1;78(3):285–92. Available from: doi: 10.1016/0035-9203(84)90097-x [DOI] [PubMed] [Google Scholar]
- 35.Pinder M, Conteh L, Jeffries D, Jones C, Knudsen J, Kandeh B, et al. The RooPfs study to assess whether improved housing provides additional protection against clinical malaria over current best practice in The Gambia: Study protocol for a randomized controlled study and ancillary studies. Trials [Internet]. 2016;17(1):1–11. Available from: 10.1186/s13063-016-1400-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Keeping the vector out: Housing improvements for vector control and sustainable development [Internet]. World Health Organization. 2017. http://apps.who.int/iris/bitstream/handle/10665/259404/9789241513166-eng.pdf;jsessionid=2A5A4422CA689EED32C324CC3EC94033?sequence=1.
- 37.Jatta E, Jawara M, Bradley J, Jeffries D, Kandeh B, Knudsen JB, et al. How house design affects malaria mosquito density, temperature, and relative humidity: an experimental study in rural Gambia. Lancet Planet Heal [Internet]. 2018;2(11):e498–508. Available from: 10.1016/S2542-5196(18)30234-1 [DOI] [PubMed] [Google Scholar]
- 38.Lindsay SW, Jawara M, Paine K, Pinder M, Walraven GEL, Emerson PM. Changes in house design reduce exposure to malaria mosquitoes. Trop Med Int Heal. 2003;8(6):512–7. doi: 10.1046/j.1365-3156.2003.01059.x [DOI] [PubMed] [Google Scholar]
- 39.Kaindoa EW, Finda M, Kiplagat J, Mkandawile G, Nyoni A, Coetzee M, et al. Housing gaps, mosquitoes and public viewpoints: A mixed methods assessment of relationships between house characteristics, malaria vector biting risk and community perspectives in rural Tanzania. Malar J [Internet]. 2018;17(1):1–16. Available from: doi: 10.1186/s12936-018-2450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, Githeko AK, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasites and Vectors. 2014;7(1):1–13. doi: 10.1186/1756-3305-7-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lwetoijera DW, Kiware SS, Mageni ZD, Dongus S, Harris C, Devine GJ, et al. A need for better housing to further reduce indoor malaria transmission in areas with high bed net coverage. Parasites and Vectors. 2013;6(1):1–9. doi: 10.1186/1756-3305-6-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell TL, Beebe NW, Bugoro H, Apairamo A, Chow WK, Cooper RD, et al. Frequent blood feeding enables insecticide-treated nets to reduce transmission by mosquitoes that bite predominately outdoors. Malar J. 2016;15(1):1–9. doi: 10.1186/s12936-016-1195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Killeen GF, Seyoum A, Sikaala C, Zomboko AS, Gimnig JE, Govella NJ, et al. Eliminating malaria vectors. Parasites and Vectors. 2013;6(1):1–10. doi: 10.1186/1756-3305-6-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mross JW. Cultural and Architectural Transitions of Southwestern Sumba Island, Indonesia. Acs4 2000 Int Conf. 2000;260–5.
- 45.Rodríguez-Rodríguez D, Katusele M, Auwun A, Marem M, Robinson LJ, Laman M, et al. Human Behavior, Livelihood, and Malaria Transmission in Two Sites of Papua New Guinea. J Infect Dis. 2021;223(2):S171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the article and its Supporting information files.