Abstract
The role of CD8+ T cells was evaluated in a mouse model of disseminated Mycobacterium avium infection. C57BL/6J and C57BL/6Jβ2−/− (β2−/−) mice were infected intravenously, and the number of viable bacteria in each liver and spleen was determined. No significant difference between the number of bacteria in the two strains of mice was observed at 2, 4, 6, and 8 weeks after infection. Histopathological examination of granulomas from C57BL/6J and β2−/− mice did not show any difference either in the number of organisms per granuloma or in the size of the granulomas. Investigation of the cytokine profile in the spleen demonstrated that the β2−/− strain of mice produced a significantly lower amount of gamma interferon at 8 weeks after infection and significantly increased concentrations of tumor necrosis factor alpha compared with that from the wild-type mouse. Interleukin-12 and transforming growth factor β1 levels did not differ between the two strains of mice at 2, 4, 6, and 8 weeks. Although previous work had found that host response against Mycobacterium tuberculosis involves major histocompatibility complex class I-restricted T cells, our results indicate that chronic deficiency of CD8+ T cells does not lead to a different expression of the disease and that if CD8+ T cells are involved in the host response, their function can be assumed by other immune cells.
Organisms of the Mycobacterium avium complex are intracellular pathogens associated with disseminated disease in patients in advanced stages of AIDS (15, 17). Immunity to M. avium initially requires the stimulation of NK cells (5, 7) and later the activation of specific T lymphocytes, which respond to the infection by secreting cytokines that increase the ability of monocytes and macrophages to inhibit M. avium growth. In addition, a number of researchers point to a plausible role of CD8+ cytotoxic cells against infected monocytes or macrophages in the lysing of infected cells (1, 20).
There is substantial experimental evidence that CD4+ T cells are important for an effective host defense against M. avium (1, 20). The role of CD8+ T lymphocytes, however, is controversial. Cytotoxic CD8+ T cells have been shown to play an important role in the host defense against Mycobacterium tuberculosis as demonstrated both by studies of CD8 T-lymphocyte depletion by specific antibodies and by studies with β2 microglobulin knockout (KO) (also referred to in this work as β2−/−) mice (13, 16). In contrast, no information is available about the role of CD8+ T cells on M. avium growth in mouse models of infection. More recently, Saunders and Cheers (23) showed that in a mouse intranasal model of M. avium infection, depletion of CD8+ T lymphocytes from infected mice had no effect on bacterial growth and CD4+ T-cell activation, indicating that the immune response against M. avium may differ from the response against M. tuberculosis.
In this study, we evaluated the role of CD8+ T cells in the host defense against M. avium by using the β2−/− mice, devoid of major histocompatibility complex (MHC) class I-restricted T cells, including cytotoxic T lymphocytes, and CD1+ restricted cytotoxic T cells (no murine equivalent to human CD1+ has previously been described).
MATERIALS AND METHODS
M. avium.
M. avium 101 (serovar 1) was isolated from the blood of a patient with AIDS. Strain 101 is a virulent strain in mice and is associated with reproducible levels of tissue infection in C57BL/6J mice. Mycobacteria were cultured on Middlebrook 7H11 agar (Difco Laboratories, Detroit, Mich.) for 10 days at 37°C. Transparent colonies were harvested and resuspended in Hanks’ balanced salt solution and washed twice. The final suspension was then adjusted to 108 bacteria/ml according to a McFarland turbidimetric standard. A sample obtained from the final suspension was plated to confirm the number of bacteria in the inoculum.
Mice.
Female, 6- to 8-week-old, C57BL/6J and C57BL/6J β2−/− (β2−/−) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were infected with 5 × 107 viable bacteria injected into the tail vein. We decided to use this inoculum based on previous experience with a variety of inocula and preliminary experiments using 105, 107, and 108 bacteria as inocula (data not shown). All mice were maintained in a pathogen-free environment and were found to be free of the four more common mouse pathogens (data not shown). Experiments were repeated twice, and 16 or 17 mice were used per group for each time point.
Harvesting.
Spleens and livers were removed aseptically at 2, 4, 6, and 8 weeks and, after weighing, were homogenized in 5 ml of Middlebrook 7H9 broth as previously described (8). Serial 10-fold dilutions were plated onto 7H11 agar supplemented with oleic acid, albumin, dextrose, and catalase. Colonies on the plates were counted after 10 to 14 days of incubation at 37°C and 5% CO2.
Cytokine assays.
Spleens were obtained from infected and uninfected mice at 2, 4, 6, and 8 weeks and were prepared as previously described (2). Because splenic macrophages are heavily infected, we measured the extracellular release of cytokines by splenocytes (lymphocytes plus macrophages). Cytokines in the supernatant were obtained after 24 h and filtered through a 0.45-μm-pore-size filter, and gamma interferon (IFN-γ), interleukin-10 (IL-10), IL-12, tumor necrosis factor alpha (TNF-α) (Biosource, Camarillo, Calif.), and transforming growth factor β1 (TGF-β1) were measured by enzyme-linked immunosorbent assay (R and D Systems, Minneapolis, Minn.) as recommended by the manufacturers.
Histopathology.
Sections (5 μm thick) from paraffin blocks containing livers and spleens were cut and stained with hematoxylin and eosin or by the Ziehl-Neelsen method for acid-fast bacilli (AFB). The mean number of AFB was evaluated by counting the organisms with granulomas in 20 random fields per section (magnification, ×400).
Statistical analysis.
The results were represented as means ± standard errors. The comparison between experimental groups and control was done by using analysis of variance at the same time point. A P value of <0.05 was considered significant.
RESULTS
M. avium infection in β2−/− and control mice.
A total of 16 or 17 C57BL/6J and 16 or 17 β2−/− mice were infected with 5 × 106 M. avium organisms intravenously per experimental group for each time point. Mice were monitored for 2 to 8 weeks. No mortality was observed in either group at 2 weeks; however, 1 of 17 β2−/− mice died and 3 of 17 control C57BL/6J mice died after 4 weeks (P > 0.05 for all comparisons). At 6 weeks, 4 of 16 C57BL/6J mice died and 1 of 16 of β2−/− mice died, while 4 of 16 died at 8 weeks in the control group and 4 of 16 β2−/− mice died.
As shown in Table 1, the numbers of viable bacteria in both liver and spleen at 2, 4, 6, and 8 weeks were similar between C57BL/6J and C57BL/6J β2−/− mice. Limited data obtained with 105, 107, and 108 bacteria showed similar results to the ones obtained with 106 organisms (data not shown).
TABLE 1.
Number of viable bacteria in mouse organs following infection
| Time point and exptl groupa | CFU of bacteria/g of:
|
|
|---|---|---|
| Liver | Spleen | |
| 2 wk | ||
| C57BL/6J | (1.2 ± 0.3) × 108 | (1.1 ± 0.3) × 108 |
| β2−/− | (1.4 ± 0.2) × 108 (P = 0.602)b | (1.0 ± 0.1) × 108 (P = 0.833) |
| 4 wk | ||
| C57BL/6J | (2.8 ± 0.4) × 108 | (3.8 ± 0.5) × 108 |
| β2−/− | (1.5 ± 0.3) × 108 (P = 0.060) | (3.0 ± 0.4) × 108 (P = 0.195) |
| 6 wk | ||
| C57BL/6J | (1.6 ± 0.3) × 109 | (3.6 ± 0.4) × 109 |
| β2−/− | (1.8 ± 0.3) × 109 (P = 0.514) | (5.0 ± 0.8) × 109 (P = 0.157) |
| 8 wk | ||
| C57BL/6J | (2.3 ± 0.3) × 109 | (1.4 ± 0.1) × 1010 |
| β2−/− | (1.3 ± 0.1) × 109 (P = 0.156) | (1.1 ± 0.1) × 1010 (P = 0.100) |
Either 16 or 17 mice of each strain were used per time point.
P values are for comparisons between mouse strains.
Histopathology studies.
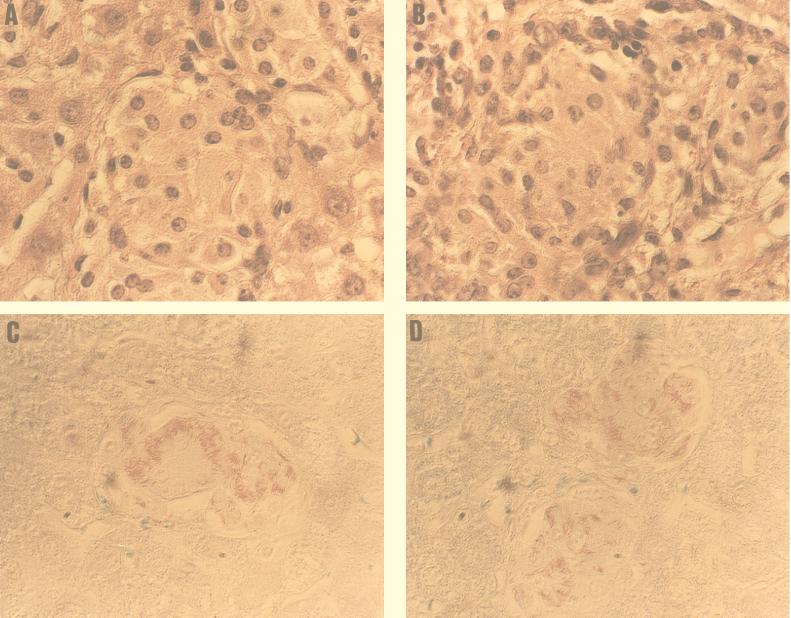
Histopathologic sections of spleen and liver from both C57BL/6J control and β2−/− mice had well-demonstrated granulomas composed of epithelioid macrophages. The numbers of AFB in granulomas from C57BL/6J and β2−/− mice were similar at both 2, 4, and 6 weeks (data not shown) and 8 weeks (Fig. 1 and Table 2). By 8 weeks the number of bacteria in spleen was significantly greater than that by 2 and 4 weeks, and some of the granulomas were confluent.
FIG. 1.
Histopathology (hematoxylin and eosin stain) of spleens C57BL/6J (A) and β2−/− (B) mice at 8 weeks after infection. Ziehl-Neelsen staining of necrotizing granulomas at 8 weeks after infection in C57BL/6J (C) and β2−/− (D) mice. Magnification, ×170.
TABLE 2.
Number of bacteria per granuloma in mouse spleen after infection
| Mouse | Wk post-infection | No. of granulomas containing indicated no. of bacteria/20 granulomasa
|
||||
|---|---|---|---|---|---|---|
| 1–10 | 10–50 | 50–100 | 100–200 | >200 | ||
| Wild type | 2 | 3 ± 1 | 8 ± 3 | 6 ± 1 | 3 ± 1 | 0 |
| 8 | 0 | 6 ± 3 | 7 ± 2 | 4 ± 1 | 3 ± 1 | |
| CD8+ KO | 2 | 2 ± 1 | 9 ± 4 | 8 ± 2 | 2 ± 1 | 0 |
| 8 | 0 | 5 ± 2 | 6 ± 2 | 5 ± 1 | 4 ± 1 | |
Values are means ± standard deviations determined on slides of samples taken from two mice.
Cytokine production.
IFN-γ, IL-12, and TNF-α have been shown to be important in the control of M. avium infection (6, 12, 24), whereas IL-10 and TGF-β1 have been associated with the progression of disease both in vitro and in vivo (3, 4).
As shown in Table 3, the cytokine profile did not differ significantly between splenic cells from C57BL/6J control and β2−/− mice at 2 and 4 weeks after infection; however, significant differences in the levels of IFN-γ and TNF-α were observed at week 8 of infection.
TABLE 3.
Cytokine profiles in mice
| Time point and exptl group | Concn (pg/ml) of cytokinea
|
||||
|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-12 | IL-10 | TGF-β1 | |
| 2 wk | |||||
| C57BL/6J | 35 ± 10 | 56.4 ± 4.2 | 166 ± 2.2 | 150 ± 0.4 | 8.5 ± 0.8 |
| β2−/− | 46 ± 7 (P = 0.300)b | 65.9 ± 4.4 (P = 0.828) | 209 ± 27 (P = 0.248) | 266 ± 90 (P = 0.326) | 10.0 ± 0.4 (P = 0.239) |
| 4 wk | |||||
| C57BL/6J | 45 ± 0.2 | 63.1 ± 5.5 | 84 ± 42 | 131 ± 21 | 10.9 ± 2.2 |
| β2−/− | 39 ± 1.5 (P = 0.426) | 82.4 ± 4.8 (P = 0.115) | 82.2 ± 39 (P = 0.156) | 179 ± 13 (P = 0.188) | 11.4 ± 0.9 (P = 0.853) |
| 6 wk | |||||
| C57BL/6J | 27 ± 2 | 40 ± 9 | 109 ± 19 | 428 ± 61 | 169 ± 18 |
| β2−/− | 25 ± 3 (P = 0.270) | 44 ± 3 (P = 0.351) | 106 ± 23 (P = 0.410) | 258 ± 37 (P < 0.05) | 159 ± 26 (P = 0.0240) |
| 8 wk | |||||
| C57BL/6J | 51 ± 7 | 30 ± 9 | 75 ± 16 | 121 ± 20 | 181 ± 27 |
| β2−/− | 35 ± 5 (P < 0.05) | 66 ± 8 (P < 0.05) | 84 ± 8 (P = 0.235) | 118 ± 9 (P = 0.634) | 159 ± 31 (P = 182) |
Cytokines were measured in the supernatants of splenic cells 24 h after being cultured in vitro.
P values are for comparisons between mouse strains.
DISCUSSION
The susceptibility of AIDS patients with low CD4+ T-cell counts to M. avium infection illustrates the importance of this T-cell subpopulation in the mechanisms of acquired resistance against M. avium infection. Our present results with β2 microglobulin KO mice confirm the finding of previous studies using specific antibodies to deplete CD8+ T-cell population that CD8+ T lymphocytes are not an absolute requirement for the control of M. avium infection (1, 23).
In contrast to their role in M. avium infection, CD8+ T cells appear to play a role in immunity of M. tuberculosis-infected mice (13, 16). These observations suggest that the immune mechanisms involved in the host defense against these pathogens are different. For example, there is plenty of evidence that NK cells play an important role in the innate immune response against M. avium (5, 6) but have no established role in M. tuberculosis infection (11). Recent work has shown that lack of both perforin and granzyme, which represent known mechanisms of CD8+ T-cell-mediated cytotoxicity, does not influence the outcome of M. tuberculosis infection in mice (10, 18), raising the possibility that cytokine production is more likely the way CD8+ cells participate in the defense against M. tuberculosis. More recently it was also demonstrated that production of IFN-γ is a major function of CD8+ T cells in tuberculosis (26). Results with the M. avium model, however, demonstrate that CD8+ T-cell-mediated cytotoxicity is not an obligatory mechanism of host defense against the organism. In addition, production of cytokines by CD8+ T cells does not appear to participate in the immune response. It is interesting that β2−/− mice produce significantly less IFN-γ than the wild-type mice at 8 weeks, without any impact in the level of infection. The drop in IFN-γ concentration does not seem to be dependent upon the IL-12 level. Perhaps the increased TNF-α levels at 8 weeks are compensatory for the drop in IFN-γ levels. It is intriguing that the role of CD8+ T cells in the host defense is completely different in infections with M. avium and M. tuberculosis, although it has also been demonstrated that lack of MHC class I expression did not compromise the ability to control Mycobacterium bovis BCG infection (13). While M. avium infections of SCID mice are slow to progress and do not end in augmented mortality (1), infection of SCID mice with M. tuberculosis is fatal within approximately 30 days (11). It is possible that the well-known hypertrophic NK cell compartment in SCID mice protects against M. avium. In our experiments, greater mortality was seen in control mice (wild type) than in CD8 KO mice. It is possible that CD8 T cells, although they do not participate in the defense against M. avium infection, do secrete or stimulate the secretion of inflammatory cytokines which ultimately participate in mortality.
The finding that splenic cells from β2−/− mice and C57BL/6J mice produced equal amounts of IFN-γ, TNF-α, IL-12, IL-10, and TGF-β1 at 2 and 4 weeks after infection with M. avium was unexpected. By 8 weeks, however, a significant difference between IFN-γ produced by control mice and KO mice was observed. Work by our and other laboratories has suggested the importance of IFN-γ and TNF-α as key players in the host defense against M. avium (12, 24). Saunders and Cheers, using an intranasal infection model for M. avium lung infection, have determined that CD8+ T cells from mice infected with M. avium do not produce IFN-γ (23). This observation was in contrast to the reports showing that both CD4+ and CD8+ T cells produce IFN-γ following activation by M. tuberculosis infection (26).
The results of our study were supported by the histopathologic sectioning of the spleens, which demonstrated that granulomas from β2−/− mice and C57BL/6J mice did not differ in size or number of organisms contained. The lack of evidence for CD8+ T-cell contribution in the host defense against M. avium is consistent with the intravacuolar residence of M. avium in macrophages (25) and is similar to the role of CD8+ T cells in the protection against Salmonella enterica infection (14). Although experimental evidence clearly demonstrates that CD8+ T cells respond to Salmonella antigens in vivo, the relevance of antigen-specific CD8+ T cells has not been proved. In contrast, CD8+ T cells have been shown to be important for the defense against Listeria monocytogenes (21), an intracellular pathogen that lyses its vacuole membrane and lives within the cytoplasm.
A report by McDonough and Kress (19) showed that M. tuberculosis can escape from vacuoles in macrophages. Although this observation has not been confirmed by other laboratories (9, 22), it may be that under certain circumstances (for example, bacterial growth conditions before the uptake by macrophages) it may occur, which would better explain the differences observed between CD8+ T-cell roles in M. avium and M. tuberculosis infections. Although a recent study (27) suggests that β2−/− mice possess a limited repertoire of self-MHC class I-restricted CD8+ T cells, which can be explained by selection on the remaining low levels of MHC class I, the different results obtained with M. avium and M. tuberculosis indicate that at least CD8+ T-cell participation in host defense against M. avium is not indispensable. Clear evidence exists, for example, for the importance of CD8+ T cells in L. monocytogenes infection, as well as in M. tuberculosis infection. For Salmonella and M. avium, CD8+ T cells appear not to participate in the immunity against the pathogens, although antigen-specific CD8+ T-cell clones can be identified.
In summary, we have demonstrated in β2−/− mice that CD8+ T cells are not essential for the host defense against M. avium. Further studies will be necessary to confirm these findings in an oral infection model more similar to the infection in humans.
ACKNOWLEDGMENTS
We thank Karen Allen for preparing the manuscript.
This work was supported by contract NOI-AI-25140 of the National Institutes of Health.
REFERENCES
- 1.Appelberg R, Castro A G, Pedrosa J, Silva R A, Orme I M, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azouaou N, Petrofsky M, Young L S, Bermudez L E. Mycobacterium avium infection in mice is associated with time-related expression of TH1 and TH2 CD4+ T-lymphocyte response. Immunology. 1997;91:414–420. doi: 10.1046/j.1365-2567.1997.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J Immunol. 1993;150:1838–1845. [PubMed] [Google Scholar]
- 4.Bermudez L E, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–3096. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E, Kolonoski P, Young L S. Natural killer cell activity and macrophage dependent inhibition of growth or killing of Mycobacterium avium complex in a mouse model. J Leukoc Biol. 1990;47:135–142. doi: 10.1002/jlb.47.2.135. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez L E, Wu M, Young L S. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium complex. Infect Immun. 1995;63:4099–4105. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermudez L E, Young L S. Natural killer cell dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991;146:265–269. [PubMed] [Google Scholar]
- 8.Champsi J, Young L S, Bermudez L E. Production of TNF-α, IL-6 and TGFβ and expression of receptors for TNFα and IL-6 during murine Mycobacterium avium infection. Immunology. 1995;84:549–554. [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper A M, D’Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty T M, Sher A. Defects in cell-mediated immunity after chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 13.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess J, Kaufmann S H E. Salmonella enterica infection. Res Immunol. 1997;36:581–586. doi: 10.1016/s0923-2494(97)85225-x. [DOI] [PubMed] [Google Scholar]
- 15.Horsburgh C R., Jr Mycobacterium avium complex in the acquired immunodeficiency syndrome (AIDS) N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 16.Howard A D, Trans O J, Weisbrode S E, Zwilling B S. Plenotypic changes in T cell populations during the reactivation of tuberculosis in mice. Clin Exp Immunol. 1998;111:309–315. doi: 10.1046/j.1365-2249.1998.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laochumroonvapong P, Wang J, Liu C C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonough K, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orme I M, Furney S K, Roberts A D. Dissemination of enteric Mycobacterium avium infections in mice rendered immunodeficient by thymectomy and CD4 depletion or by prior infection with murine AIDS retrovirus. Infect Immun. 1992;60:4747–4753. doi: 10.1128/iai.60.11.4747-4753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts A D, Ordway D J, Orme I M. Listeria monocytogenes infection in β2 microglobulin-deficient mice. Infect Immun. 1993;61:1113–1116. doi: 10.1128/iai.61.3.1113-1116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell D G. Mycobacterium and Leishmania: stowaways in the endosomal network. Trends Cell Biol. 1995;5:125–128. doi: 10.1016/s0962-8924(00)88963-1. [DOI] [PubMed] [Google Scholar]
- 23.Saunders B M, Cheers C. Inflammatory response following intranasal infection of Mycobacterium avium complex: role of T-cell subsets and interferon gamma. Infect Immun. 1995;63:2282–2287. doi: 10.1128/iai.63.6.2282-2287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders B M, Zhan Y, Cheers C. Interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–4015. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 26.Tascon R E, Stavropoulos E, Lukacs K V, Colston M J. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vugmeyster Y, Glas R, Perarnau B, Lemonnier F A, Eisen H, Ploegh H. Major histocompatibility complex (MHC) class I KdDb−/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci USA. 1998;95:12492–12497. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]