Keywords: cytokine, epithelial transport, hypertension, mineralocorticoid receptor, sodium
Abstract
Hypertension is characterized by increased sodium (Na+) reabsorption along the aldosterone-sensitive distal nephron (ASDN) as well as chronic systemic inflammation. Interleukin-6 (IL-6) is thought to be a mediator of this inflammatory process. Interestingly, increased Na+ reabsorption within the ASDN does not always correlate with increases in aldosterone (Aldo), the primary hormone that modulates Na+ reabsorption via the mineralocorticoid receptor (MR). Thus, understanding how increased ASDN Na+ reabsorption may occur independent of Aldo stimulation is critical. Here, we show that IL-6 can activate the MR by activating Rac1 and stimulating the generation of reactive oxygen species (ROS) with a consequent increase in thiazide-sensitive Na+ uptake. Using an in vitro model of the distal convoluted tubule (DCT2), mDCT15 cells, we observed nuclear translocation of eGFP-tagged MR after IL-6 treatment. To confirm the activation of downstream transcription factors, mDCT15 cells were transfected with mineralocorticoid response element (MRE)-luciferase reporter constructs; then treated with vehicle, Aldo, or IL-6. Aldosterone or IL-6 treatment increased luciferase activity that was reversed with MR antagonist cotreatment, but IL-6 treatment was reversed by Rac1 inhibition or ROS reduction. In both mDCT15 and mpkCCD cells, IL-6 increased amiloride-sensitive transepithelial Na+ current. ROS and IL-6 increased 22Na+ uptake via the thiazide-sensitive sodium chloride cotransporter (NCC). These results are the first to demonstrate that IL-6 can activate the MR resulting in MRE activation and that IL-6 increases NCC-mediated Na+ reabsorption, providing evidence for an alternative mechanism for stimulating ASDN Na+ uptake during conditions where Aldo-mediated MR stimulation may not occur.
INTRODUCTION
Hypertension (HTN) affects more than 1 billion people worldwide, and a significant portion of the US population is considered “salt-sensitive” (1). Furthermore, underlying and chronic inflammation clearly plays a role in the pathogenesis of HTN, along with the activation of the renin-angiotensin-aldosterone system (2–4). Indeed, multiple studies have linked HTN with increased numbers of activated immune cells in the kidney (5–8). Once activated, the immune cells secrete cytokines, which mediate intercellular communication between cells and initiate immune responses. One in particular, interleukin 6 (IL-6), has been positively correlated with resistant HTN, independent of other comorbidities and factors such as age, sex, and obesity (4, 9–12). IL-6 knockout (KO) mice do not have a hypertensive response to angiotensin II (Ang II), and administration of IL-6-neutralizing antibody reduced mean arterial pressure (MAP) in Dahl salt-sensitive hypertensive rats (5, 13). Together, these studies suggest a strong association between IL-6 and HTN.
The distal nephron expresses two main sodium-transporting proteins, the sodium chloride cotransporter (NCC) and the epithelial sodium channel (ENaC) (14, 15). This portion of the nephron, from the second part of the distal convoluted tubule (DCT2) through the cortical collecting duct (CCD), responds to aldosterone due to 11-β hydroxysteroid dehydrogenase 2, which converts active cortisol to inactive cortisone, reducing specificity for glucocorticoids and is referred to as the aldosterone-sensitive distal nephron (ASDN) (16, 17). Patients with hypertension frequently do not have increased levels of serum aldosterone, the agent that conventional wisdom suggests is the primary ligand for the mineralocorticoid receptor (MR) in the distal nephron (17–20). However, these patients present as if they have a mineralocorticoid excess, including positively responding to MR antagonists (9, 21). This suggests some alternative activator of the MR (21). Clinically, the use of inhibitors for the sodium chloride cotransporter (NCC) and the MR reveals the important role of the MR in increased distal sodium (Na+) transport (22). Understanding the mechanisms of increased distal tubular Na+ reabsorption during HTN is important in developing future treatment options (23). Until now, there has been no evidence that the activation of MR is involved in cytokine-mediated increases in Na+ transport. Here, we show that increased levels of IL-6 can activate the MR leading to increases in Na+ transporter activity.
MATERIALS AND METHODS
All drugs were purchased from Sigma Aldrich (St. Louis, MO), unless otherwise specified.
Cell Culture
The murine distal convoluted tubule 2 cells (mDCT15) used for our in vitro studies have been previously described (24). As part of the prior development and use, a stable puromycin-resistance gene was stably inserted in the cells. Therefore, mDCT15 cells should not be used in applications requiring puromycin selection. Mouse cortical collecting duct (mpkCCD) cells were used for in vitro studies investigating the CCD. Both of these cell lines have been used extensively as a model for the DCT2 and CCD and are not a primary cell line (16, 24–31). The cells were plated on cell culture dishes. mDCT15 cells were grown in growth medium containing a 50:50 mix of DMEM/F12, 5% heat-inactivated fetal bovine serum (FBS), and 1% penicillin/streptomycin/neomycin (P/S/N), at 37°C. mpkCCD cells were grown in the same base medium supplemented with 50 nM dexamethasone, 1 nM triiodothyronine, 20 mM HEPES, 2 mM l-glutamine, 0.1% P/S, and 2% heat-inactivated FBS at 5% CO2/37°C.
Two innate immune cell lines were used, DC2.4 as a dendritic cell line and RAW as a macrophage cell line (ATCC, Alexandria, MN). Cell culture conditions were used as per manufacturer’s suggestion as follows. Cells were plated on cell culture dishes. DC2.4 cells were grown in RPMI 1640 (Gibco/ThermoFisher) growth medium containing 5% FBS, nonessential amino acids, 2-mercaptoethanol, HEPES, and 0.1% P/S. RAW cells were grown in DMEM with l-glutamine, supplemented with 5% FBS and 0.1% P/S.
Vector Design
The eGFP-tagged MR and MRE-luciferase constructs were a generous gift of Dr. David Pearce (University of California, San Francisco). Cloned and purified vectors were verified by sequencing (Genewiz, South Plainfield, NJ). Dominant-negative Rac1 (DN Rac1) was used, which contains a threonine to asparagine substitution at residue 17 (Rac1 T17N), abolishing the affinity for GTP and reducing GDP. Site-directed mutagenesis was done with the pENTRY-Rac1 using the primers:
mRacT17N Forward: GGA GCT GTT GGT AAA AAC TGC CTG CTC ATC AG
mRac1T17N Reverse: CTG ATG AGC AGG CAG TTT TTA CCA ACA GCT CC
mRac1Q61L Forward: CTA TGG GAC ACA GCT GGA CTA GAA GAT TAT GAC AGA TTG
mRac1Q61L Reverse: CAA TCT GTC ATA ATC TTC TAG TCC AGC TGT GTC CCA TAG
For constitutively active Rac1 (CA Rac1), murine Rac1 (GenBank NM_001347530.1) was cloned from mouse kidney by RT-PCR and introduced into the plasmid pENTRY using the primers:
mRac1-pENTRStart, CACCATG CAG GCC ATC AAG TGT GTG
mRac1-Stop, TTA CAA CAG CAG GCA TTT TCT C
After confirming the sequence, both were introduced into the Gateway plasmid pCLX-pTF-R1DEST-R2-EBR (Addgene plasmid 45952 polyswitch lentivectors) (32). The Rac1 vector is in the pCLX-pTF DEST backbone [pCLX-R4-DEST-R2 was a gift from Patrick Salmon (Addgene plasmid No. 45956; http://n2t.net/addgene:45956; RRID:Addgene_45956)].
MR-eGFP Translocation
mDCT15 cells were plated on Mat-Tek dishes (Ashland, MA) and grown to ∼75% confluency. The cells were then transfected using the X-fect transfection reagent (Clontech, Mountain View, CA) as per manufacturer’s instructions. The cells were transfected with a vector containing the mineralocorticoid receptor (MR) tagged with eGFP (MR-eGFP; 5 μg total DNA). The cells were allowed to grow for 48 h, media was refreshed with Optimem (ThermoFisher), and treated with either vehicle (ethanol), aldosterone (100 nM), or IL-6 (100 ng/mL) (PreproTech, Rocky Hill, NJ) for 30 min. The cells were washed and fixed (4% PFA) before mounting with DAPI-containing mounting media (VectaShield, Burlingame, CA). The cells were visualized on Olympus Fluoview 1000.
Luciferase Reporter Assay
mDCT15 cells were grown to ∼65% confluency, and then transfected with vectors using the X-fect transfection reagent (Clontech, Mountain View, CA) as per manufacturer’s instructions. The cells were transfected with a vector containing the mineralocorticoid response element (MRE) with a luciferase reporter (MRE-Luc, resp.; 5-μg total DNA), pcDNA3 (2.5-μg total DNA) + MRE-Luc (2.5-μg total DNA), or a dominant-negative (DN) Rac1 vector (2.5-μg total DNA) + MRE-Luc (2.5 μg total DNA). The cells were washed after 4 h with complete media and then used for experiments. The cells were treated with vehicle and/or inhibitors in fresh media twice in 48 h. Transfected (MRE-luciferase) mDCT15 cells were treated with vehicle (DMSO/ethanol/water), Aldo (100 nM), IL-6 (40, 100, 300 ng/mL), IL-10 (40, 100, 300 ng/mL; PreproTech), spironolactone [Spiro, MR antagonist, (10 μM) Tocris, Minneapolis, MN], EHT-1864 [Rac inhibitor, (20 μM) Tocris], or tempol [superoxide dismutase mimetic, (250 μM) Cayman Chemical, Ann Arbor, MI] for the indicated times and concentrations for 48 h.
Cells were lysed using Passive Lysis Buffer (Promega, WI), and luciferase assay was performed using 1:1 dilution of cell lysate as per manufacturer’s (Bright-Glo Luciferase Reporter Assay, Promega) instructions and luminescence was read on luminometer. Data are expressed as a fold change over MRE-transfected cells (no treatment).
RT-PCR
mDCT15 cells were treated with angiotensin II [Ang II (100 nM) 2–24 h] or aldosterone [Aldo (100 nM) 2–24 h], and total RNA was then isolated (Trizol, ThermoScientific, Waltham, MA) as per manufacturer’s instructions; after which the RNA was reverse transcribed (TaqMan Superscript II, ThermoScientific) and equal quantities of cDNA were used for RT-PCR (BioRad, Hercules, CA) using the following primers for IL-6 receptor alpha (IL-6Rα) (Sigma Aldrich, St. Louis, MO) and GAPDH. Technical replicates were performed (x3) and averaged for each biological replicate (n) described.
IL-6Rα
Forward: GAG GAT ACC ACT CCC AAC AGA CC
Reverse: AAG TGC ATC ATC GTT GTT CAT ACA
GAPDH
Forward: TGG CCT TCC GTG TTC CTA CC
Reverse: TGT AGG CCA TGA GGT CCA CCA C
22Na+-Uptake Assay
mDCT15 cells were seeded in 12-well plates and incubated in a serum-free growth media (Opti-Mem) for 24 h before being assayed. The cells were then treated with vehicle (DMSO), aldosterone (100 nM), IL-6 (40, 100, 300 ng/mL), IL-10 (40, 100, 300 ng/mL), spironolactone (10 nM), eplerenone (5 μM), losartan (Ang II receptor blocker; 100 μM), EHT-1864 (20 μM), and tempol (250 μM) for the indicated times and concentrations. Thirty minutes before uptake, an NCC inhibitor, metolazone (0.1 mM) or vehicle (DMSO) was added to the media to ensure NCC inhibition during the uptake period. The medium was then changed to a 22Na+-containing medium [140 mM NaCl, 1 mM CaCl, 1 mM MgCl, 5 mM HEPES/Tris pH 7.4, 1 mM amiloride (ENaC inhibitor), 0.1 mM bumetanide (NKCC inhibitor), 0.1 mM benzamil (ENaC/Na-Ca exchanger inhibitor), 1 mM ouabain (Na-K-ATPase inhibitor), and 1 μCi/mL of 22Na+] with or without thiazide [(0.1 mM) metolazone] and incubated for 20 min. Tracer uptake was then stopped via washes with ice-cold wash buffer. The cells were subsequently lysed with 0.1% sodium dodecyl sulfate (SDS). Radioactivity was measured via liquid scintillation and protein concentrations of the lysates were determined (BCA Protein Assay, Pierce). Uptakes were normalized to nanomoles per milligram. Thiazide-sensitive uptake was given by the difference in the uptakes with and without thiazide.
Standard Immunoblotting
Total protein was isolated in RIPA lysis buffer with a phosphatase inhibitor cocktail (Thermo Fisher). Total protein (50 µg) was run on an SDS-PAGE gel and transferred electrophoretically to PVDF membranes following activation in methanol (30 s). After blocking with 3%–5% BSA or 5% milk, the membranes were probed with corresponding primary antibodies: IL-6Rα (No. 39837 Cell Signaling, Danvers, MA; 1:1,000) and gp130 (No. 3732 Cell Signaling; 1:1,000) overnight at 4°C. The blots were washed in TBST (Tris-buffered saline, Tween 0.5%) and secondary antibodies were HRP-linked (1:5,000; Amersham, Marlborough, MA). Supersignal West Pico was used for chemiluminescence (ThermoScientific). Chemiluminescence was detected with G:Box (Gelbox) and analyzed by Genetools software (Syngene, Frederick, MD).
Reactive Oxygen Species Detection
mDCT15 cells were either used directly for DHE experiments or transfected prior. The mDCT15 cells were grown to ∼65% confluency and then transfected with vectors using the X-fect transfection reagent (Clontech, Mountain View, CA) as per manufacturer’s instructions. The cells were transfected with vectors (described previously) containing the pcDNA3, DN Rac1, Rac1 Wt, or CA Rac1 (all 5-μg total DNA). The cells were washed after 4 h with complete media and then used for experiments. The cells were then serum-starved (24 h; Optimem) and treated with vehicle or IL-6 (100 ng/mL), washed, and then incubated with dihydroethidium [(5 μM), 35 min; Molecular Probes, Waltham, MA] for 30 mins, and again washed with PBS and then visualized using dipping objective Olympus Fluoview (Upright FV1000, Emory Integrated Cellular Imaging Core). Experiments were performed to ensure that transfection alone and/or transfection in conjunction with treatment (Aldo) did not affect ROS production/DHE staining before combining. All images were analyzed using FIJI (33). Random images were obtained (4–6) per well, and fluorescence intensity was quantified.
Transepithelial Current
Transepithelial current in mDCT15 and mpkCCD cells was measured using an epithelial voltmeter (EVOM). Cells were grown on transwell inserts (Corning, Corning, NY) for 3–5 days after confluence. For some experiments, the cells were transfected on transwell inserts. The cells were treated with aldosterone (100 nM), IL-6 (100 ng/mL), or IL-6 + gp130 inhibitor [SC144, (2 μM) Cayman Chemical]. EVOM measurements for voltage and resistance were obtained, and current was calculated using Ohm’s Law.
Statistical Analysis
Data were analyzed using Prism GraphPad 9 (San Diego, CA), using appropriate parametric (ANOVA, Student’s t test) or nonparametric analyses (Kruskal–Wallis, Wilcoxon signed-rank sum). All data are shown at means ± SE; “n” denotes separate experiments (biological replicates).
RESULTS
Interleukin 6 Induces Nuclear Translocation
We used our previously established in vitro model of the DCT2, mDCT15 cells (23, 24, 34) to confirm that the IL-6 receptor (IL-6Rα) is expressed in mDCT15 cells. We performed immunoblots using total protein from mDCT15 and mpkCCD cells. As a positive control, we also used cell lysates from two standard innate immune cell lines, DC2.4 (dendritic cell) and RAW (macrophages) known to express IL-6Rα (Fig. 1) (35). We also performed RT-PCR on total RNA from whole cell lysates from mDCT15 cells. In Fig. 1A, we confirm that both distal nephron epithelial cell lines (mDCT15/mpkCCD) express IL-6Rα. When comparing with the dendritic cell and macrophage cell lines, a strong banding profile is observed between 70 and 80 kDa, with greater expression in the immune cell lines. We hypothesize that variations in glycosylation may account for the slight variations in band size. IL-6Rα is not functional unless coupled to the IL-6Rβ or gp130 (36). We also performed immunoblot assays and confirmed gp130 expression in both mDCT15 and mpkCCD cells. Interestingly, we notice that mDCT15 cells had increased expression.
Figure 1.
Interleukin 6 receptor alpha expression. Total protein (A) was isolated from RAW264.7, DC2.4, mDCT15, and mpkCCD cells and interleukin 6 (IL-6) receptor alpha (IL-6Rα) (top) and IL-6Rβ or gp130 (bottom) expression was determined. mRNA (B) from mDCT15 cells was isolated and quantitative PCR was performed to determine whether IL-6Rα expression is physiologically altered. mDCT15 cells were serum-starved (Optimem, 24 h) and treated with aldosterone (100 nM) or angiotensin II (100 nM) for 24 h. IL-6Rα expression was significantly increased in Aldo-treated mDCT15 cells (over baseline). Cycle threshold (CT) values were obtained and normalized to housekeeping (GAPDH) gene expression. Data were analyzed using ΔΔCT/fold change, and are expressed as means ± SE, n = 5, performed in triplicates (averaged). *P < 0.05; one-sample t test.
In addition, to show that receptor expression can be physiologically modulated, we used angiotensin II (Ang II) and aldosterone as physiological stimulants. Cells were treated with Ang II and aldosterone (24 h) (Fig. 1B) before total RNA was isolated. We observed a significant increase in IL-6Rα mRNA following Aldo treatment (fold change ΔΔCT, 1.704 ± 0.17; P < 0.05 t test), but no change after Ang II treatment suggesting that mDCT15 cells express baseline IL-6Rα, which can be increased following Aldo stimulation.
Nuclear MR translocation is the first step in MR activation (37). mDCT15 cells were transfected with the enhanced green fluorescence protein (eGFP)-tagged MR construct (5 μg of total DNA) (37) and treated with either vehicle, aldosterone (100 nM), or IL-6 (100 ng/mL). Transfected, but otherwise untreated, cells show diffuse cytosolic MR expression (Fig. 2A, vehicle), but following Aldo treatment (Fig. 2B), we observed rapid (30 min) nuclear translocation of eGFP-tagged MR (n = 4–6/group). mDCT15 cells treated with IL-6 also showed a similar nuclear translocation (Fig. 2C). These observations occurred as early as 15 min following either Aldo or IL-6 treatment. These data show that IL-6 can induce exogenously transfected MR nuclear translocation, similar to Aldo, and that late distal nephron epithelia express IL-6Rα.
Figure 2.
Interleukin 6 induces nuclear translocation of the mineralocorticoid receptor (MR). mDCT15 cells were transfected with eGFP-tagged MR and treated with either vehicle (ethanol), aldosterone (100 nM), or IL-6 (100 ng/mL) for 30 min. The cells were fixed and counterstained with DAPI before imaging (Fluoview 1000). Two representative images (n = 4–6) are shown for vehicle (A), Aldo (B), and IL-6 (C). Representative images from n = 4–6.
Interleukin 6 Activates Mineralocorticoid Response Elements
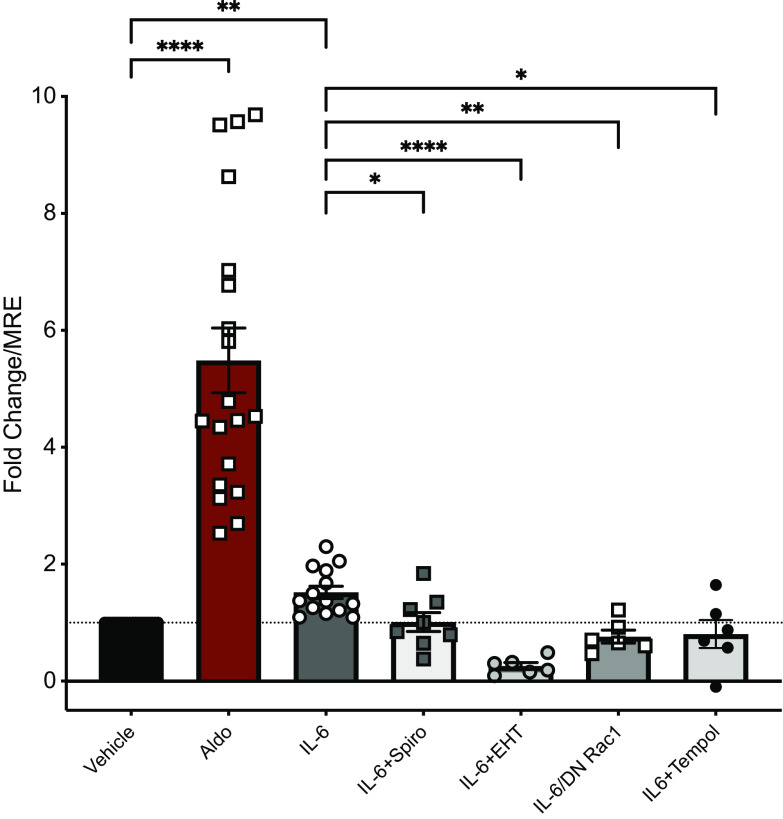
Following MR translocation, the MR binds to mineralocorticoid response elements (MRE) on DNA leading to the canonical genomic effects associated with MR stimulation. To demonstrate that activation of nuclear MR targets occurs following MR translocation, mDCT15 cells were transfected with a construct containing an MRE-luciferase reporter where MRE activation increases luciferase expression allowing for semi-quantification of MRE activity (17, 38, 39). Following transfection, cells were washed and treated with vehicle, Aldo, or IL-6 alone or together with pharmacological inhibitors (48 h) (Fig. 3). Total cell lysates were then used to obtain luciferase values, indicating MRE activity. As expected, aldosterone treatment significantly increased MRE activity over unstimulated MRE-transfected cells (a 5.49 ± 0.55-fold increase, ****P < 0.0001 vs. vehicle, n = 6–20). Notably, IL-6 incubation (100 ng/mL) also induced a significant increase in MRE activity (1.52 ± 0.11-fold increase, **P < 0.05 vs. vehicle), compared with unstimulated MRE-transfected cells. MRE-luciferase experiments were also performed in the presence of the MR antagonist, spironolactone, to ensure that IL-6-mediated increases in luciferase is, in fact, occurring through MR activation. We observed a significant inhibition of IL-6-mediated increases in MRE-luciferase activity (1.01 ± 0.16-fold increase, *P < 0.05 vs. IL-6). These data show that IL-6 can induce nuclear MR translocation, leading to subsequent MRE activation.
Figure 3.
Interleukin 6 induces activation of the mineralocorticoid response element (MRE). mDCT15 cells were transfected with a mineralocorticoid response element (MRE)-luciferase reporter vector, with a subset also transfected with a dominant-negative Rac1 (DN Rac1). Cells were treated with vehicle, aldosterone (100 nM), IL-6 (100 ng/mL), IL-6 + spironolactone (10 nM), IL-6 + EHT-1864 (20 μM), or IL-6 + Tempol (250 μM). Cell lysates were used for luciferase assay. Data are expressed as a fold change over MRE-luciferase transfected cells only (MRE-only); means ± SE, n = 6–19. *P < 0.05, **P < 0.01, ****P < 0.0001; Kruskal–Wallis ANOVA on ranks.
Previously, studies by Fujita and colleagues have suggested that the small GTPase, Rac1, can transactivate the MR via production of reactive oxygen species (ROS), in an Aldo-independent mechanism (40, 41). We hypothesized that our IL-6-mediated MRE activation may be through a ROS and/or Rac1 activation pathway. To investigate this, we used a Rac inhibitor (EHT-1864) or cells transfected with a dominant-negative construct for Rac1 (DN Rac1); both pharmacological inhibition and Rac1 knockdown prevented IL-6-mediated activation of the MR (0.26 ± 0.06 and 0.76 ± 0.11-fold change, respectively, ****P < 0.0001, **P < 0.01 vs. IL-6). To investigate the role of ROS in IL-6-mediated MR activation, MRE-luciferase assay experiments were performed in the presence of tempol to reduce ROS levels, which also prevented IL-6-mediated increases (0.81 ± 0.24, *P < 0.05 vs. IL-6). In addition, we saw no significant increase in MRE activity (Supplemental Fig. S1) following treatment with a lower IL-6 concentration (40 ng/mL) or with the “anti-inflammatory” cytokine, IL-10 (40 and 100 ng/mL), suggesting specificity for IL-6 and dosage. The dosage (100 ng/mL) found to increase MRE activation corresponds with previously published data, in which IL-6 induces activation of immune cells (42). Overall, these data reveal a novel mechanism of IL-6-mediated activation of the MR, via a Rac1- and ROS-mediated mechanism.
Reactive Oxygen Species Increases Mineralocorticoid Receptor Activation
As described previously, IL-6 increases in MRE activation may be through increased reactive oxygen species (ROS); however, we needed to confirm the ability of IL-6 to increase ROS in mDCT15 cells. Following IL-6 treatment (Fig. 4A), we observed significant increases in DHE staining (1,093 ± 62.3 vs. 373.8 ± 21.8 pcDNA3 control, ****P < 0.001, n = 4–12), which was similar to levels produced following aldosterone (1,183 ± 54.6) stimulation. Knowing the important role of Rac1 in ROS production, we used mDCT15 cell transfects with DN and CA Rac1 to determine if IL-6-mediated increases in ROS are Rac1-dependent. When mDCT15 cells were transfected with DN Rac1, IL-6 did not increase DHE staining (523.8 ± 37.6, n = 20). Of note, cells transfected with a constitutively active Rac1 (CA Rac1) alone showed DHE levels comparable to IL-6 (1321 ± 42.0, n = 8). No significant changes were seen in cells transfected with Wt Rac1 as compared with pcDNA3 alone, although levels were increased. We cannot rule out a response simply to increased levels of Rac1 expression in the cells.
Figure 4.

Interleukin 6 increases reactive oxygen species (ROS) via Rac1, and ROS can activate the mineralocorticoid response element (MRE) in mDCT15 cells. A: mDCT15 cells were transfected with pcDNA, Rac1 Wt, dominant negative (DN Rac1), or constitutively active (CA Rac1)-containing constructs. Cells were treated with IL-6 (100 ng/mL) for 30 min and stained for ROS levels (DHE). DHE fluorescence was measured and quantified. Representative images for each group are shown on left, with quantification expressed (right) as means ± SE, n = 4–20; ANOVA, *P < 0.05 and ****P < 0.0001. B: mDCT15 cells were transfected with a mineralocorticoid response element (MRE)-luciferase reporter vector. Cells were treated with vehicle, pyrogallol (50 μM), PG + spironolactone (10 nM), or PG + EHT-1864 (20 μM). Cell lysates were used for luciferase assay. Data are expressed as a fold change over MRE-luciferase-transfected cells only (MRE-only); means ± SE, n = 3–4. **P < 0.01; Wilcoxon signed-ranks.
In our MRE-luciferase experiments, we showed that IL-6-induced MRE activity is ROS-dependent (Fig. 3A). Here, we also investigated whether ROS can directly increase MRE activity. We used a spontaneous ROS generator, pyrogallol (PG), and observed a significant increase in MRE activity (3.054 ± 0.16, **P < 0.01, n = 4) that was prevented with Rac (0.274 ± 0.06, **P < 0.01, n = 4), suggesting that IL-6 increases in ROS via Rac1 activation (Fig. 4B). These experiments demonstrate the ability of IL-6 to increase ROS, in a Rac1-dependent manner, and that ROS can also directly increase MRE activity in a cell model of distal tubular cells (mDCT15). Together, Rac1 and ROS may both be needed for MRE activation.
Interleukin 6 Increases Activity of the Sodium Chloride Cotransporter
So far, we have shown that IL-6 can lead to nuclear MR translocation, as well as activation of downstream MR-sensitive genomic targets (MRE). However, to understand how cytokine activation can alter distal nephron Na+ uptake specifically in the aldosterone-sensitive distal nephron (ASDN), we treated mDCT15 cells with IL-6 (100 ng/mL) and used thiazide-sensitive 22Na+ uptake to measure sodium chloride cotransporter (NCC) activity (Fig. 5A) before and after IL-6 treatment (24, 34). mDCT15 cells treated with IL-6 (6 h) showed a significant increase in 22Na+ uptake, as compared with vehicle (1,913 ± 19.6 nmoL/mg/20 min vs. 1,443 ± 18.0 nmoL/mg/20 min vehicle, ****P < 0.001, n = 3). Similar to our MRE-luciferase studies, lower IL-6 levels (40 ng/mL) or IL-10 (100 ng/mL) did not increase thiazide-sensitive 22Na+ uptake.
Figure 5.
Interleukin 6 increases thiazide-sensitive 22Na+ uptake in mDCT15 cells. Thiazide-sensitive Na22-uptake studies performed on mDCT15 monolayers-scintillation counts obtained and normalized to protein levels. A: 22Na+-uptake studies were performed in mDCT15 cells treated (6 h) with either vehicle, IL-6 (100 ng/mL) or IL-10 (40 ng/mL or 100 ng/mL). B: 22Na+-uptake studies performed in the presence of (6 h): vehicle, IL-6 (100 ng/mL), or IL-6 + losartan (100 μM). Data are represented as means ± SE, n = 3, ****P < 0.0001 one-way ANOVA, Tukey’s post hoc.
Navar and colleagues (43) have shown that IL-6 increases hormonal protein expression in proximal tubular epithelial cells. We next investigated whether IL-6 increases distal nephron Ang II expression and/or can lead to activation of the AT1-receptor (AT1R) (Fig. 5B). Thiazide-sensitive 22Na+-uptake experiments were performed in the presence of an Ang II receptor blocker (ARB) [losartan (100 μM)]. Our data show no significant reductions in thiazide-sensitive 22Na+ uptake (n = 3, Fig. 5B) in mDCT15 cells treated with IL-6 and an ARB. These data strongly suggest that increases in NCC activity via IL-6 are mediated through the MR and not by increased DCT2 epithelium-produced Ang II, and subsequent activation of the AT1R.
Interleukin 6 Increases Activity of the Sodium Chloride Cotransporter via Rac1
We then performed a series of thiazide-sensitive 22Na+-uptake studies to better understand NCC-mediated activation by IL-6. Considering that our data have shown that IL-6 activates the MR via Rac1- and ROS-dependent mechanisms, we first used pyrogallol (PG), a spontaneous ROS generator, to investigate whether ROS can also directly increase thiazide-sensitive 22Na+ uptake. We showed (Fig. 6A) that NCC activity robustly increases with (25 min) ROS stimulation (1,834 ± 19.0 nmoL/mg/20 min vs. 1,506 ± 17.9 nmoL/mg/20 min, ****P < 0.001, n = 6). This increase was subsequently significantly reduced with administration of the ROS scavenger, tempol (15 min), following PG application (1,618 ± 26.8 nmoL/mg/20 min vs. 1,506 ± 17.9 nmoL/mg/20 min vehicle, ****P < 0.001, n = 6).
Figure 6.
Interleukin 6-mediated mineralocorticoid receptor (MR) activation increases thiazide-sensitive 22Na+ uptake in mDCT15 cells. Thiazide-sensitive Na22-uptake studies performed on mDCT15 monolayers-scintillation counts obtained and normalized to protein levels. A: 22Na+-uptake studies performed in the presence of vehicle, PG [pyrogallol, ROS generator; (50 μM), 25 min), or PG + Tempol [superoxide dismutase mimetic; (250 μM)], for last 15 min. B: 22Na+-uptake studies were performed in mDCT15 cells treated with either vehicle, IL-6 (100 ng/mL), IL-6 + Tempol (250 μM), IL-6 + EHT-1864 (20 μM), IL-6 + spironolactone (10 nM), and IL-6 + eplerenone (5 μM). All treatments were for 6 h, except a single acute IL-6 response (45 min). Data are represented as means ± SE, n = 4–8, *P < 0.05, **P < 0.01, ***P < 0.01, ****P < 0.0001 one-way ANOVA, Tukey’s post hoc.
Using two different MR antagonists, spironolactone and eplerenone (Fig. 6B), we also show that the IL-6-mediated increases in NCC activity are dependent upon MR activation (1,721 ± 25.3 nmoL/mg/20 min IL-6 + Spiro or 1,611 ± 17.3 nmoL/mg/20 min IL-6 + Eplerenone vs. 1,942 ± 27.4 nmoL/mg/20 min IL-6, ****P < 0.001, n = 3–8) at 6 h; MR antagonism did not completely prevent the IL-6-mediated increases in Na+ uptake. Furthermore, IL-6-mediated increases in 22Na+ uptake are inhibited (6 h) when experiments were performed with Rac inhibition (1,762 ± 33.2 nmoL/mg/20 min, ***P < 0.01, n = 4–8), as well as with the ROS scavenger (1,687 ± 38.4 nmoL/mg/20 min, ****P < 0.001, n = 4–8). We also assessed a rapid, 45 min response to IL-6 and found a significant increase in NCC activity (1,620 ± 13.9 nmoL/mg/20 min, *P < 0.05, n = 4). Together, these data imply a mechanism by which NCC is activated by IL-6 in an MR-specific manner, both acutely and chronically.
To better understand acute versus chronic IL-6 activation mechanisms, we next performed thiazide-sensitive 22Na+-uptake studies with both short-term (45 min) and long-term (6 h) incubations (Fig. 7) in the presence or absence of pharmacological inhibitors. Although not as robust, a single acute (45 min) treatment with IL-6 induces a significant increase in thiazide-sensitive 22Na+ uptake (1,579 ± 14.0 nmoL/mg/20 min vs. 1,461 ± 13.0 nmoL/mg/20 min vehicle, *P < 0.05, n = 6; Fig. 7A), which is significantly reduced with the MR antagonist (spiro, 1,503 ± 4.0 nmoL/mg/20 min, *P < 0.05 vs. IL-6, n = 3) and almost completely blocked by Rac inhibition (1,469 ± 16.0 nmoL/mg/20 min, ***P < 0.001 vs. IL-6, n = 3). However, reducing ROS levels (tempol) did not significantly affect this acute response, suggesting that acute increases in IL-6-mediated NCC activity are primarily via Rac1 and MR activation. In chronic/long-term studies (6 h, Fig. 7B), IL-6-mediated increases were blocked only when both ROS levels were reduced and Rac was inhibited (1,492 ± 6.3 nmoL/mg/20 min IL-6 + EHT+Tempol vs. 1,945 ± 23.0 nmoL/mg/20 min IL-6, ***P < 0.001 vs. 1,482 ± 5.5 nmoL/mg/20 min vehicle ***P < 0.0001, n = 3) or following ROS reduction and MR inhibition (1,522 ± 1.5 nmoL/mg/20 min, ****P < 0.0001, n = 3). This suggests that long-term stimulation with IL-6 may lead to increased ROS, revealing a time-specific mechanism for IL-6-mediated increases in NCC activity.
Figure 7.
Acute vs. chronic mechanisms for interleukin 6-mediated thiazide-sensitive 22Na+ uptake. A: acute treatment (45 min) 22Na+-uptake studies were performed in mDCT15 cells treated with either vehicle, IL-6 (100 ng/mL), EHT-1864 (20 μM), IL-6 + tempol (250 μM), or IL-6 + spironolactone (10 nM). B: chronic (6 h) 22Na+-uptake studies performed in the presence of vehicle or IL-6 (100 ng/mL), IL-6 + EHT-1864 (20 μM) + tempol (250 μM) or IL-6 + tempol (250 μM) + Spiro (10 nM). Data are represented as means ± SE, n = 3–6, *P < 0.05, ***P < 0.001, ****P < 0.0001, one-way ANOVA, Tukey’s post hoc.
Interleukin 6 Increases Activity of the Epithelial Sodium Channel via Rac1
The ASDN includes the late distal convoluted tubule (DCT2), the connecting tubule (CNT), and the cortical collecting duct (CCD) with all segments expressing the MR (14, 15). MR activation of epithelial sodium channel (ENaC) is usually thought to occur in the CCD, so to determine the ability of IL-6 to increase ENaC activity we used EVOM to measure monolayer voltage and resistance in a principal cell model. This allowed us to then calculate current. mpkCCD cells were plated on transwell inserts and measurements were taken at baseline and following IL-6 treatment (3 h) (Fig. 8A). We observed a significant increase in amiloride-sensitive current (7.39 ± 0.72 µA, ***P < 0.001, n = 6) over baseline (6.18 ± 0.67 µA). When we performed comparisons with the MR ligand, aldosterone, we found that IL-6 stimulation produced similar changes (Δ) in current (∼2 ΔµA n = 4–6) after 1 h (Fig. 8B).
Figure 8.
Interleukin 6 increases amiloride-sensitive current in mpkCCD cells. Transepithelial current was calculated using transepithelial electrical resistance and voltage measurements in mpkCCD cell monolayers plated on transwell inserts. mpkCCD cells were treated with IL-6 for 3 h [A, (30 ng/mL)], or 1 h [B, (100 ng/mL)]. mpkCCD cell current data were shown as either current (µA/cm2) (A) or change in (Δ) current from baseline (ΔµA) (B). Data are expressed as means ± SE; n = 6, ***P < 0.001 paired t test (A), n = 4–6, **P < 0.01, one-sample t test vs. baseline (B).
The DCT2 also expresses ENaC (14, 15). To assess whether IL-6-mediated MR activation also increases ENaC activity in the late distal nephron, we used EVOM to determine amiloride-sensitive current. Resistance and voltage measurements of mDCT15 cells plated on transwell inserts were obtained at baseline and then after aldosterone or IL-6 treatment (1 h) (Fig. 9). Interestingly, we even observed a trending higher current with IL-6 compared with aldosterone (10.00 ± 1.53 and 9.75 ± 2.83 ΔµA, n = 4, respectively).
Figure 9.
Interleukin 6 increases amiloride-sensitive current in mDCT15 via Rac1. Transepithelial current was calculated using transepithelial electrical resistance and voltage measurements in mDCT15 cell monolayers plated on transwell inserts. mDCT15 cells were transfected with pcDNA or DN Rac1 (2.5 µg total DNA) and plated on transwell inserts. Cells were treated with aldosterone (100 nM), IL-6 (100 ng/mL; 1 h) or IL-6 plus the gp130 inhibitor (2 µM). Changes in current were compared with baseline measurements (ΔµA). Data are expressed as means ± SE; n = 4–15, *P < 0.05, **P < 0.01, Kruskal–Wallis ANOVA.
Given the role we observed for Rac1 in IL-6-mediated activation of the MR, we assessed whether amiloride-sensitive current induced by IL-6 was similarly dependent on Rac1. mDCT15 cells were transfected with the same DN Rac1 construct and transepithelial resistance and voltage were measured (Fig. 9). We observed no IL-6-mediated increase in ENaC current (−1.17 ± 1.1 ΔµA, *P < 0.05, n = 6). In this experiment, we also added the gp-130 antagonist that would prevent IL-6-mediated signaling. We observed a complete inhibition of IL-6-induced ENaC current (−3.78 ± 1.5 vs. 10.0 ± 1.4 ΔµA, *P < 0.05, n = 4–12), suggesting specificity of this response to IL-6. Together, these data show that IL-6 is a strong stimulator of ENaC in cell culture models of both the DCT2 and in the CCD.
DISCUSSION
Many patients exhibit a low aldosterone-to-renin ratio (ARR), yet exhibit other signs of excessive MR activation and downstream activation of distal Na+ transporters (9, 21). Treatment with an MR blocker, such as eplerenone, reduces blood pressure as well as signs of end-organ damage such as proteinuria. This reduction can be independent of systemic aldosterone levels (44, 45). Though little investigated, these clinical observations strongly implicate an alternate mechanism of MR activation. Determining which physiological factors activate the MR, in the absence of ligand, is vital.
Increased levels of systemic cytokines have been implicated in the pathogenesis of HTN (4, 11, 46–48), and IL-17A has been implicated in chronic activation of Na+ transporters via an Ang II/SGK1 pathway (49). Here, we report a steroid-independent link between cytokines and distal Na+ transport through a novel MR activation pathway. Our in vitro studies investigating both MR-mediated activation and Na+ transport are in two cell lines that model the DCT2 (mDCT15) and CCD (mpkCCD). Both mDCT15 and mpkCCD cells endogenously express all the machinery needed for MR-mediated pathway activation and Na+ transporter activation (23, 24). It should be noted that we are aware of no other reports of distal nephron epithelial cells expressing the cognate receptor for IL-6 (50). However, Satou and colleagues (43) did find IL-6R expression in a proximal tubule cell line. Whether native ASDN expresses the IL-6R complex needs to be confirmed in vivo, but our data suggest that tubular epithelial cells (mDCT15, mpkCCD) may natively express membrane-bound IL-6Rα and IL-6Rβ, or gp130. The ramifications of renal expression of membrane IL-6R and/or soluble IL-6R may be important in resolving how IL-6 signals in the kidney, especially given the high expression of gp130 in our mDCT15 cell line. There is now evidence suggesting that IL-6Rα versus soluble IL-6Rα with gp130 transsignaling events can elicit very different responses (36, 51, 52). This may specify how IL-6 signals change in Na+ transport, with consequent changes in blood pressure, and how to reduce these responses (51–54). Using this in vitro approach, we have shown that IL-6 can activate the MR, resulting in stimulation of both NCC and ENaC. After activation, the MR is released from molecular chaperones and dimerizes before nuclear translocation—a first step in the MR-dependent signaling pathway (38, 39). Using mDCT15 cells transfected with eGFP-MR constructs, we observed comparable nuclear translocation of the MR with IL-6 treatment or with aldosterone treatment. Following nuclear translocation, the MR binds certain MR-sensitive nuclear targets, termed mineralocorticoid response elements (MRE). MRE activation increases transcription of multiple cell-signaling kinases, inducing the classical MR-dependent increases in Na+ transporter expression and activity (17, 21, 23, 44, 45, 55). Using a construct containing the MRE with a luciferase reporter, both aldosterone and IL-6 treatment produced significant increases in MRE activity. This increase was not seen with IL-10 treatment, suggesting “proinflammatory”-specificity for cytokine-mediated MR activation. The IL-6-mediated responses were not as robust as aldosterone; however, this might be expected as the activation of IL-6 is likely a secondary activation pathway (compared with aldosterone).
The previously identified cross talk between Rac1 and the MR suggested by Nagase and colleagues unlocked a new piece of MR signaling (40, 56). Increased ROS generation was linked to MR activation in cardiomyocytes (41). Interestingly, strong activation of Rac1 is also present in a DOCA-salt model of experimental HTN (57). However, little data has definitively demonstrated how Rac1 activation can occur in these models. Indeed, studies using Dahl SS rats suggest that increased dietary salt can activate this Rac1-MR pathway as shown by increased nuclear MR localization and increased Rac1 activity, all occurring with reduced serum aldosterone levels (57). Here, we propose that the increased proinflammatory milieu and increased levels of cytokines may be the upstream signal leading to increased ROS generation and Rac1 activation. Following confirmation of increased IL-6-mediated ROS generation in mDCT15 cells, we found that spontaneous ROS generation induces a strong increase in both MRE activity as well as increased thiazide-sensitive Na+ transport. We further demonstrated that the IL-6-mediated increases in ROS are dependent upon Rac1 activation. A DN Rac1 construct blocked the increases in ROS. We further show that IL-6-mediated increases in MRE activity are prevented when cotransfected with the DN Rac1 and pharmacological Rac inhibition, as well as with a reduction in ROS. Together, our data show that IL-6 can activate MR-specific events via a Rac1-ROS pathway (Fig. 10).
Figure 10.
Schematic for interleukin 6-mediated increases in sodium transport. Interleukin 6 (IL-6) is increased during hypertension, which activates the mineralocorticoid receptor (MR), via a Rac1 and reactive oxygen species (ROS)-dependent mechanism, leading to mineralocorticoid response element (MRE) activation. IL-6 then increases sodium current through the sodium chloride cotransporter (NCC) and the epithelial sodium channel (ENaC), in the late distal nephron. Image created with Biorender and published with permission.
MR activation leads to increases in distal Na+ transport; in the ASDN, those transporters are NCC and ENaC. The role of the MR in the regulation of Na+ uptake, and therefore blood pressure is undisputed. To implicate IL-6 in the pathway of MR-mediated increases in distal tubular Na+ transport, we assessed both NCC and ENaC responses to IL-6. Here, we show that IL-6 increases in thiazide-sensitive Na+ uptake via MR activation, both with an acute (45 min) and more chronic (6 h) incubation. Although both MR antagonists (spironolactone and eplerenone) prevented IL-6 increases in NCC activity, only eplerenone treatment reduced Na+ uptake to levels that were not significantly increased when compared with vehicle treatment. While examining mechanisms, we observed an interesting difference between acute (45 min) and chronic (6 h) IL-6 treatments. Acute IL-6 increases in NCC activity can be completely prevented with Rac inhibition, with a modest reduction when ROS levels were reduced. In contrast, chronic experiments showed that both Rac1-inhibition and a reduction in ROS are needed to completely prevent NCC-mediated increases in Na+ transport. Alternatively, blocking MR activity and ROS will also prevent this increase. We posit those immediate changes in Rac1 activation possibly through NADPH oxidase-associated increases in ROS generation. This burst, even at low levels, can stimulate NCC directly. With chronic activation, apparently, increases in Rac-NADPH oxidase expression occur, and inhibition of both systems is needed to prevent MR activation. Our study also suggests that eplerenone may be more efficient at blocking IL-6-mediated Rac1-ROS activation of the MR, perhaps through a more complete MR blockade; however, there is little published data to support this possibility one way or the other. Previously, it has been suggested that cytokines can increase Ang II levels, mostly in the proximal tubule (43, 58), but such an increase has not been definitively shown in the DCT2. Thus, we used losartan to confirm that our observed increases in Na+ uptake cannot be attributed to Ang II-mediated AT1R activation.
To investigate IL-6-mediated activation of ENaC, we used transepithelial resistance and voltage measurements in both mDCT15 (DCT2) and mpkCCD (CCD) cells. We observed an approximate 2 µA change in amiloride-sensitive current when mpkCCD cells were treated with IL-6 for 1 h or 3 h, which supports a single previous study by Dong and colleagues in M-1 cells (59). What we thought was most interesting, was the response observed in mDCT15 cells. There was a sizeable magnitude of current change, and of note, IL-6 responses were identical to aldosterone in both mpkCCD and mDCT15 cells. Furthermore, these responses (mDCT15 cells) seem to be completely mediated by the IL-6 receptor protein, gp-130, and Rac1. Previously, we have shown that NCC and ENaC can associate and comodulate responses (14). These studies may suggest an interesting possibility of synergized ENaC activity with NCC activation. Further studies will be needed to investigate this phenomenon. Even though the relative magnitude of MR activation by IL-6 is somewhat less than aldosterone, the ability of IL-6 to increase Na+ reabsorption is considerable and similar to aldosterone.
Despite the advancements in pharmacological agents to reduce HTN and end-organ effects, more than half of patients with hypertension do not have blood pressure under control. In addition, excess MR activation has numerous negative extrarenal consequences, increased ROS generation, and vascular smooth muscle contractility, as well as contributing to myocyte fibrosis (20, 45). Use of MR antagonists produces a variety of cardiovascular and renal benefits—all seemingly independent of Ang II (20). The implication of an alternative mechanism by which the MR can produce these events is profound. Identifying possible upstream targets, such as IL-6, opens new possibilities for therapeutic advances and personalized medicine.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.20164376.v1.
GRANTS
This study was funded by the National Institutes of Health (NIH) T-32 to Emory University DK007656, NIDDK K01 DK115660 (to B.M.W.), American Society of Nephrology Carl Gottschalk Research Scholars Award (to B.M.W.), Emory University SIRE program (to T.K.S./B.M.W.), NIDDK P01-DK-56788 (to B.K.), R01 DK110409 (to D.C.E.), and NIDDK R01 DK085097 (to R.S.H.), and the Department of Veteran’s Affairs MERIT Award I01BX002322-01 (to R.S.H). C.G-S. is supported by R01 HL144847 from the National Heart, Lung and Blood Institute, 1U54GM115428 from the National Institute of General Medical Sciences, and BX004681 from the Department of Veteran Affairs. This research project was also supported in part by the Emory University Integrated Cellular Imaging Microscopy Core.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M.W. conceived and designed research; B.M.W., T.K.S., H.C.M., H.J.v.E., A.S.M., G.H., O.P., O.A-K., B.K., and D.C.E. performed experiments; B.M.W., T.K.S., H.C.M., H.J.v.E., A.S.M., G.H., O.P., B.K., and D.C.E. analyzed data; B.M.W., T.K.S., H.C.M., H.J.v.E., G.H., O.P., O.A-K., C.G-S., D.C.E., and R.S.H., interpreted results of experiments; B.M.W., T.K.S., H.C.M., G.H., O.P., B.K., D.C.E. prepared figures; B.M.W. drafted manuscript; B.M.W., T.K.S., H.C.M., H.J.v.E., O.P., C.G-S., B.K., D.C.E., and R.S.H. edited and revised manuscript; B.M.W., T.K.S., H.C.M., H.J.v.E., A.S.M., G.H., O.P., O.A-K., C.G-S., B.K., D.C.E., R.S.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Masaaki Yoshigi, University of Utah, for help with this manuscript.
REFERENCES
- 1. Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med 17: 1402–1409, 2011. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 2. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 3. Itani HA, Harrison DG. Memories that last in hypertension. Am J Physiol Renal Physiol 308: F1197–F1199, 2015. doi: 10.1152/ajprenal.00633.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ 38: 20–24, 2014. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashmat S, Rudemiller NP, Lund H, Abais-Battad JM, Van Why SK, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F555–F561, 2016. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Itani HA, McMaster WG, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68: 123–132, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J. Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat Rev Nephrol 10: 625–643, 2014. doi: 10.1038/nrneph.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jansen PM, Frenkel WJ, van den Born B-JH, de Bruijne ELE, Deinum J, Kerstens MN, Arnoldus JHA, Woittiez AJ, Wijbenga JAM, Zietse R, Danser AHJ, van den Meiracker AH. Determinants of blood pressure reduction by eplerenone in uncontrolled hypertension. J Hypertens 31: 404–413, 2013. doi: 10.1097/HJH.0b013e32835b71d6. [DOI] [PubMed] [Google Scholar]
- 10. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 16: 448–457, 2015. [Erratum in Nat Immunol 18: 1271, 2017]. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 11. Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, Jeunemaitre X, Thomas A. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens 24: 1143–1148, 2011. doi: 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheung BMY, Ong KL, Tso AWK, Leung RYH, Cherny SS, Sham PC, Thomas GN, Lam TH, Lam KSL. Relationship of plasma interleukin-6 and its genetic variants with hypertension in Hong Kong Chinese. Am J Hypertens 24: 1331–1337, 2011. doi: 10.1038/ajh.2011.141. [DOI] [PubMed] [Google Scholar]
- 13. Lee DL, Sturgis LC, Labazi H, Osborne JB, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935–H940, 2006. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 14. Wynne BM, Mistry AC, Al-Khalili O, Mallick R, Theilig F, Eaton DC, Hoover RS. Aldosterone modulates the association between NCC and ENaC. Sci Rep 7: 4149, 2017. doi: 10.1038/s41598-017-03510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mistry AC, Wynne BM, Yu L, Tomilin V, Yue Q, Zhou Y, Al-Khalili O, Mallick R, Cai H, Alli AA, Ko B, Mattheyses A, Bao H-F, Pochynyuk O, Theilig F, Eaton DC, Hoover RS. The sodium chloride cotransporter (NCC) and epithelial sodium channel (ENaC) associate. Biochem J 473: 3237–3252, 2016. doi: 10.1042/BCJ20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loffing-Cueni D, Flores SY, Sauter D, Daidié D, Siegrist N, Meneton P, Staub O, Loffing J. Dietary sodium intake regulates the ubiquitin-protein ligase nedd4-2 in the renal collecting system. J Am Soc Nephrol 17: 1264–1274, 2006. doi: 10.1681/ASN.2005060659. [DOI] [PubMed] [Google Scholar]
- 17. Ong GS, Young MJ. Mineralocorticoid regulation of cell function: the role of rapid signalling and gene transcription pathways. J Mol Endocrinol 58: R33–R57, 2017. doi: 10.1530/JME-15-0318. [DOI] [PubMed] [Google Scholar]
- 18. Gomez-Sanchez EP. Third-generation mineralocorticoid receptor antagonists: why do we need a fourth? J Cardiovasc Pharmacol 67: 26–38, 2016. doi: 10.1097/FJC.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol 4: 965–994, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol 6: 261–273, 2010. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 21. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 116: 960–975, 2015. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 22.Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 202: 1028–1034, 1967. doi: 10.1001/jama.1967.03130240070013. [DOI] [PubMed] [Google Scholar]
- 23. Ko B, Mistry AC, Hanson L, Mallick R, Wynne BM, Thai TL, Bailey JL, Klein JD, Hoover RS. Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. Am J Physiol Renal Physiol 305: F645–F652, 2013. doi: 10.1152/ajprenal.00053.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ko B, Mistry AC, Hanson L, Mallick R, Cooke LL, Hack BK, Cunningham P, Hoover RS. A new model of the distal convoluted tubule. Am J Physiol Renal Physiol 303: F700–F710, 2012. doi: 10.1152/ajprenal.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ko B, Mistry A, Hanson L, Mallick R, Hoover RS. Mechanisms of angiotensin II stimulation of NCC are time-dependent in mDCT15 cells. Am J Physiol Renal Physiol 308: F720–F727, 2015. doi: 10.1152/ajprenal.00465.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 289: 11791–11806, 2014. doi: 10.1074/jbc.M113.531095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pavlov TS, Ilatovskaya DV, Levchenko V, Li L, Ecelbarger CM, Staruschenko A. Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct. FASEB J 27: 2723–2732, 2013. doi: 10.1096/fj.12-223792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol 304: C102–C111, 2013. doi: 10.1152/ajpcell.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012. doi: 10.1074/jbc.M111.298919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pavlov TS, Ilatovskaya DV, Levchenko V, Mattson DL, Roman RJ, Staruschenko A. Effects of cytochrome P-450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC). Am J Physiol Renal Physiol 301: F672–F681, 2011. doi: 10.1152/ajprenal.00597.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ilatovskaya DV, Pavlov TS, Levchenko V, Negulyaev YA, Staruschenko A. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J 25: 2688–2699, 2011. doi: 10.1096/fj.10-167262. [DOI] [PubMed] [Google Scholar]
- 32. Giry-Laterriere M, Cherpin O, Kim YS, Jensen J, Salmon P. Polyswitch lentivectors: “all-in-one” lentiviral vectors for drug-inducible gene expression, live selection, and recombination cloning. Hum Gene Ther 22: 1255–1267, 2011. doi: 10.1089/hum.2010.179. [DOI] [PubMed] [Google Scholar]
- 33. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci USA 104: 20120–20125, 2007. doi: 10.1073/pnas.0709506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deng S, Wang A, Chen X, Du Q, Wu Y, Chen G, Guo W, Li Y. HBD inhibits the development of colitis-associated cancer in mice via the IL-6R/STAT3 signaling pathway. Int J Mol Sci 20: 1069, 2019.doi: 10.3390/ijms20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolf J, Waetzig GH, Chalaris A, Reinheimer TM, Wege H, Rose-John S, Garbers C. Different soluble forms of the interleukin-6 family signal transducer gp130 fine-tune the blockade of interleukin-6 trans-signaling. J Biol Chem 291: 16186–16196, 2016. doi: 10.1074/jbc.M116.718551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grossmann C, Ruhs S, Langenbruch L, Mildenberger S, Strätz N, Schumann K, Gekle M. Nuclear shuttling precedes dimerization in mineralocorticoid receptor signaling. Chem Biol 19: 742–751, 2012. doi: 10.1016/j.chembiol.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 38. Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda) 26: 115–123, 2011. doi: 10.1152/physiol.00049.2010. [DOI] [PubMed] [Google Scholar]
- 39. Faresse N, Ruffieux-Daidie D, Salamin M, Gomez-Sanchez CE, Staub O. Mineralocorticoid receptor degradation is promoted by Hsp90 inhibition and the ubiquitin-protein ligase CHIP. Am J Physiol Renal Physiol 299: F1462–F1472, 2010. doi: 10.1152/ajprenal.00285.2010. [DOI] [PubMed] [Google Scholar]
- 40. Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 9: 86–98, 2013. doi: 10.1038/nrneph.2012.282. [DOI] [PubMed] [Google Scholar]
- 41. Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, Fujita T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension 59: 500–506, 2012. doi: 10.1161/HYPERTENSIONAHA.111.185520. [DOI] [PubMed] [Google Scholar]
- 42. Levy Y, Fermand JP, Brouet JC. Differential effects of low and high concentrations of interleukin 6 on human B cells. Eur J Immunol 20: 2389–2393, 1990. doi: 10.1002/eji.1830201105. [DOI] [PubMed] [Google Scholar]
- 43. Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, Kobori H. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol 295: F283–F289, 2008. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nishimoto M, Fujita T. Renal mechanisms of salt-sensitive hypertension: contribution of two steroid receptor-associated pathways. Am J Physiol Renal Physiol 308: F377–F387, 2015. doi: 10.1152/ajprenal.00477.2013. [DOI] [PubMed] [Google Scholar]
- 45. Ferrario CM, Schiffrin EL. Role of mineralocorticoid receptor antagonists in cardiovascular disease. Circ Res 116: 206–213, 2015. doi: 10.1161/CIRCRESAHA.116.302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harrison DG. The mosaic theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J Am Soc Hypertens 7: 68–74, 2013. doi: 10.1016/j.jash.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 68: 167–174, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Braun GS, Nagayama Y, Maruta Y, Heymann F, van Roeyen CR, Klinkhammer BM, Boor P, Villa L, Salant DJ, Raffetseder U, Rose-John S, Ostendorf T, Floege J. IL-6 trans-signaling drives murine crescentic GN. J Am Soc Nephrol 27: 132–142, 2016. doi: 10.1681/ASN.2014111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Montgomery A, Tam F, Gursche C, Cheneval C, Besler K, Enns W, Manku S, Rey K, Hanson PJ, Rose-John S, McManus BM, Choy JC. Overlapping and distinct biological effects of IL-6 classic and trans-signaling in vascular endothelial cells. Am J Physiol Cell Physiol 320: C554–C565, 2021. doi: 10.1152/ajpcell.00323.2020. [DOI] [PubMed] [Google Scholar]
- 53. Rose-John S. Therapeutic targeting of IL-6 trans-signaling. Cytokine 144: 155577, 2021. doi: 10.1016/j.cyto.2021.155577. [DOI] [PubMed] [Google Scholar]
- 54. Mitsuyama K, Matsumoto S, Rose-John S, Suzuki A, Hara T, Tomiyasu N, Handa K, Tsuruta O, Funabashi H, Scheller J, Toyonaga A, Sata M. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut 55: 1263–1269, 2006. doi: 10.1136/gut.2005.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McCormick JA, Bhalla V, Pao AC, Pearce D. SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology (Bethesda) 20: 134–139, 2005. doi: 10.1152/physiol.00053.2004. [DOI] [PubMed] [Google Scholar]
- 56. Kawarazaki H, Ando K, Shibata S, Muraoka K, Fujita M, Kawarasaki C, Fujita T. Mineralocorticoid receptor–Rac1 activation and oxidative stress play major roles in salt-induced hypertension and kidney injury in prepubertal rats. J Hypertens 30: 1977–1985, 2012. [DOI] [PubMed] [Google Scholar]
- 57. Shibata S, Mu SYu, Kawarazaki H, Muraoka K, Ishizawa K-I, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 121: 3233–3243, 2011. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, Navar LG, Kobori H. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol 311: 24–31, 2009. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, Dong Y. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Physiol Regul Integr Comp Physiol 299: R590–R595, 2010. doi: 10.1152/ajpregu.00207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.20164376.v1.