Abstract
Plasmacytoid dendritic cells (pDCs) are crucial for corneal homeostasis through secretion of various anti-angiogenic molecules and growth factors. Due to its avascular nature, only a limited number of adoptively transferred cells home to the cornea, when administered systemically. In addition, local adoptive transfer of cells poses several challenges and the clinical application of commonly used techniques is limited. Herein, we detail a novel approach for local adoptive transfer of pDCs to the cornea for the treatment of corneal wounds. This approach utilizes a commonly used fibrin sealant as a means of transferring previously isolated cells locally on the cornea. The technique is simple, reproducible, and is accompanied with successful transfer and integration of a substantial number of the cells to the cornea. Application of this approach to transfer pDCs promotes corneal wound healing. Furthermore, this technique can be applied for adoptive transfer of any cell of interest to the cornea.
Keywords: Plasmacytoid dendritic cells, Cornea, Wound, Adoptive transfer, Healing
1. Introduction
Plasmacytoid dendritic cells (pDCs) are a subtype of immune cells, which are involved in immune responses, ranging from antiviral immunity to the induction of tolerance [1–4]. Recently, it has been demonstrated that pDCs exhibit anti-inflammatory properties in the cornea during both viral infections and sterile inflammation [5–7]. Further, pDCs play a critical role in maintaining corneal homeostasis by preserving corneal angiogenic privilege and maintaining corneal innervation [8, 9]. Moreover, pDCs secrete multiple growth factors necessary for tissue regeneration following corneal insults [9, 10].
A variety of approaches have previously been tested for adoptive transfer of cells of interest for the treatment of corneal diseases or for studying on the role of cells of interest in disease pathogenesis. The transferred cells have included stem cells, myeloid-derived suppressor cells, and subpopulations of T cells, such as γδ T cells, regulatory T cells, and in vitro-stimulated T-cell lines [11–17]. Systemic administration of the cells via intraperitoneal or intravenous injections is less favored [11–14], since considering the avascular nature of the cornea, systemic transfer of cells results in their distribution to multiple predominantly nonocular sites, such as the spleen and lymph nodes. In fact, via systemic approaches, only a minor fraction of transferred cells have been shown to home to the cornea [14–16]. This broad distribution of cells via systemic approaches increases the potential off-site adverse effects, and increases the number of cells required for adoptive transfer [14–16], making the transfer of rare cell types difficult and challenging the clinical feasibility of these methods. For local adoptive transfer of cells, subconjunctival and intrastromal injections have previously been employed [17, 18]. Considering that the conjunctiva contains a substantial density of lymphatic and blood vessels [19], subconjunctival transfer of cells may also lead to systemic distribution of cells [18]. The clinical application of intrastromal injections is limited due to its invasive nature and potential complications, such as corneal perforation [20–22].
Herein, we present a protocol of local adoptive transfer of pDCs to the cornea, utilizing commercially available fibrin sealants to promote corneal wound healing [7, 10, 23]. This approach has multiple advantages over other currently utilized techniques of adoptive transfer of cells, including its high efficacy, relatively simplicity, and high clinically feasibility. Further, this technique may overcome the above-mentioned shortcomings of comparable techniques, as the cells are locally transferred to the cornea and thus allow adoptive transfer of relatively low number of cells with superior efficacy of transfer. Notably, a significant fraction of the transferred cells can be observed within the cornea within hours and for up to at least 4 weeks after adoptive transfer. Furthermore, in addition to treatment of corneal wounds, this approach of direct topical adoptive transfer can be utilized to enrich corneas with any particular cells of interest to study their contribution in pathogenesis and/or to study their efficacy in treating a variety of corneal conditions, such as mechanical and chemical traumas, dry eye disease, infectious keratitis, neovascularization, transplantation, and nerve-mediated diseases. A potential limitation of this technique is the necessity of a temporary corneal epithelial curettage in order to allow the cells to integrate into the corneal stroma. Although for the purposes of adoptive transfer of cells, it is sufficient to establish a minimal epithelial debridement, which generally heals within 24–48 h in wild-type mice [24, 25], it may accompany corneal nerve damage that may alter or augment the pathophysiological processes underlying the aforementioned corneal diseases.
2. Materials
Unless otherwise noted, all buffers should be stored at 2–8 °C and kept on ice during the process. Prepare all buffers and solutions in advance, except for fibrin sealant, which should be prepared fresh at the time of use. Carefully follow all waste disposal regulations when disposing waste materials.
2.1. Donor Tissue Collection
2.2. Tissue Processing and Single Cell Preparation
An 8–10-week-old C57BL/6J wild-type mouse (see Notes 3 and 4).
Sterile 1-mL syringes for mechanical dissociation of spleen(s).
Conical tubes of 50 mL.
Cell strainers of 40 μm.
Sterile ice-cold PBS.
Ammonium-Chloride-Potassium (ACK) red blood cell (RBC) lysis buffer: To prepare 1 L of ACK RBC lysis buffer, add 800 mL of dH2O to a suitable beaker or flask. Add the following components: 8.29 g of NH4Cl (0.15 M), 1 g of KHCO3 (10 mM), and 37.2 mg of Na2EDTA (0.1 mM). Add a stir bar and place on a magnetic stir plate until completely dissolved. Adjust the pH of the solution to 7.2–7.4 with HCl or NaOH. Add dH2O to a final volume of 1 L. Filter the solution in a cell culture hood in a sterile fashion. Store the buffer at 2–8 °C (see Note 5).
2.3. Cell Staining and Sorting
Fluorescence-activated cell sorting (FACS) buffer: The following reagents should be added to 500 mL of calcium- and magnesium-free PBS to achieve the indicated concentrations: 146.1 mg of EDTA (1 mM) and 5 g of bovine serum albumin (1%). Sterile filter the solution and store at 2–8 °C (see Note 6).
Sterile FACS tubes.
Anti-mouse CD16/CD32 Fc block (for instance, BioXCell, West Lebanon, NH, USA or similar commercially available products by other companies); store at −20 °C or colder prior to thawing for the experiment.
A viability marker; store as recommended by the manufacturer (see Note 7).
Fluorophore-conjugated antibodies for staining pDCs (see Note 8) including anti-PDCA-1 (CD317, BST2), anti-CD45R/B220, and anti-Siglec-H, as well as their respective isotype controls (see Notes 9 and 10).
2.4. Cornea Wounding and Local Adoptive Transfer of Plasmacytoid Dendritic Cells
Wild-type C57BL/6J mice (see Note 11).
Institutional animal care and use committee (IACUC)-approved anesthetic (for instance ketamine and xylazine mixture) is required for anesthetizing the animals for corneal wounding and adoptive transfer of pDCs.
Proparacaine hydrochloride ophthalmic solution (0.5%; Alcon, Fort Worth, Texas, USA) to numb the cornea prior to epithelial debridement.
Ophthalmic artificial tears to prevent desiccation of the contralateral cornea; store at room temperature.
Neomycin, polymyxin B, and bacitracin triple antibiotic ophthalmic ointment; store at room temperature.
Surgical instruments for debridement of the corneal epithelium: a curved forceps, 2 mm trephine, and Algerbrush II corneal rust ring with 0.5 mm burr (The Alger Companies, Lago Vista, Texas, USA).
Fibrin sealant (e.g., TISSEEL® [Baxter International, Deer-field, IL, USA]) composed of (1) sealer protein solution and (2) thrombin solution for adoptive transfer of pDCs. Fibrin sealant should be stored at −20 °C or colder until immediately before use.
Heating lamp.
2.5. Outcome Assessments
IACUC-approved anesthetic (for instance, ketamine and xylazine mixture) for anesthetizing the animals for proper assessment of corneal wounds.
Slit-lamp biomicroscope equipped with a camera adaptor.
Fluorescein ophthalmic solution (0.25%; see Note 12).
Normal saline solution.
Cotton tip applicator.
Heating lamp.
3. Methods
3.1. Donor Tissue Collection
Add 3 mL of ice-cold PBS to one well of a six-well cell culture plate (see Note 13).
Euthanize an 8–10-week-old C57BL/6 wild-type mouse according to IACUC-approved procedure (see Notes 3 and 4).
Spray 70% ethanol on the skin of the abdomen and chest to disinfect the animal.
Place the animal on its right flank, exposing the left flank. Promptly incise the skin, fascia, and peritoneum of the left upper quadrant of the abdomen and left flank of the mouse to explore the spleen. By cutting the connective tissues, dissect and collect the spleen in a well of a six-well cell culture plate (containing 3 mL of ice-cold PBS, prepared in the step 1).
3.2. Tissue Processing and Single Cell Preparation
Place a cell strainer on a 50 mL conical tube and set the spleen on the cell restrainer. Gently dissociate the spleen mechanically using the round bottom of a plunger of a sterile 1 mL syringe.
Once the tissue is disrupted, wash the strainer with 5–7 mL of ice-cold PBS.
Discard the cell strainer and centrifuge the flow through at 1200 rpm for 5 min, at 2–8 °C.
Aspirate the supernatant and resuspend the pellet in 8 mL of ice-cold ACK RBC lysis buffer and incubate the samples in the lysis buffer for 1 min, at room temperature.
After incubation, add at least 12 mL of ice-cold PBS and centrifuge at 1200 rpm for 5 min, at 2–8 °C (see Note 14).
Aspirate the supernatant and repeat washing with 20 mL of ice-cold PBS if necessary (optional; see Note 15).
3.3. Cell Staining and Sorting
Prepare 1.5 mL of blocking buffer by adding 15 μL (1:100) of antimouse CD16/CD32 Fc block to the FACS buffer (see Note 16) and a viability marker per manufacturer’s instructions (see Note 7).
After completing centrifugation, aspirate the supernatant and resuspend the pellet in 1 mL of blocking buffer and incubate for 15 min, at 2–8 °C (see Note 17).
Divide the sample into two FACS tubes: (A) 100 μL of the sample for nonstained cell controls, as well as isotype controls, single stained controls, and fluorescence minus one controls for cell sorting, and (B) 900 μL of the sample for staining for pDCs with PDCA-1, CD45R/B220, and Siglec-H for actual sorting of pDCs.
- Stain the samples and controls with fluorophore-conjugated antibodies and their respective isotype controls (all 1:100) as follows:
- Add 700 μL of the blocking buffer to the cells in the control FACS tube (containing 100 μL of the original tube) and divide them into eight FACS tubes. Add 1 μL of fluorophore-conjugated antibodies or their isotype controls to prepare appropriate controls:
- Unstained cells.
- Isotype control for PDCA-1.
- Isotype control for CD45R/B220.
- Isotype control for Siglec-H.
- Anti-PDCA-1.
- Anti-CD45R/B220.
- Anti-Siglec-H.
- Anti-PDCA-1, anti-CD45R/B220, and Isotype control for Siglec-H.
- Add 9 μL of each fluorophore-conjugated antibody against PDCA-1, CD45R/B220, and Siglec-H to the FACS tube containing 900 μL of the original cells.
- Incubate the samples for 30–40 min, at 2–8 °C on a shaker with minimal exposure to light.
Add 3–4 mL of FACS buffer to each sample for washing, and centrifuge at 1200 rpm for 5 min, at 2–8 °C.
Resuspend the cells in at least 1 mL of FACS buffer.
Filter the cells through a 40 μm cell strainer (optional; see Note 18).
Sort pDCs as live PDCA-1+ CD45R/B220+ Siglec-H+ cells via a flow cytometric sorter (see Notes 19 and 20; Fig. 1).
Fig. 1.
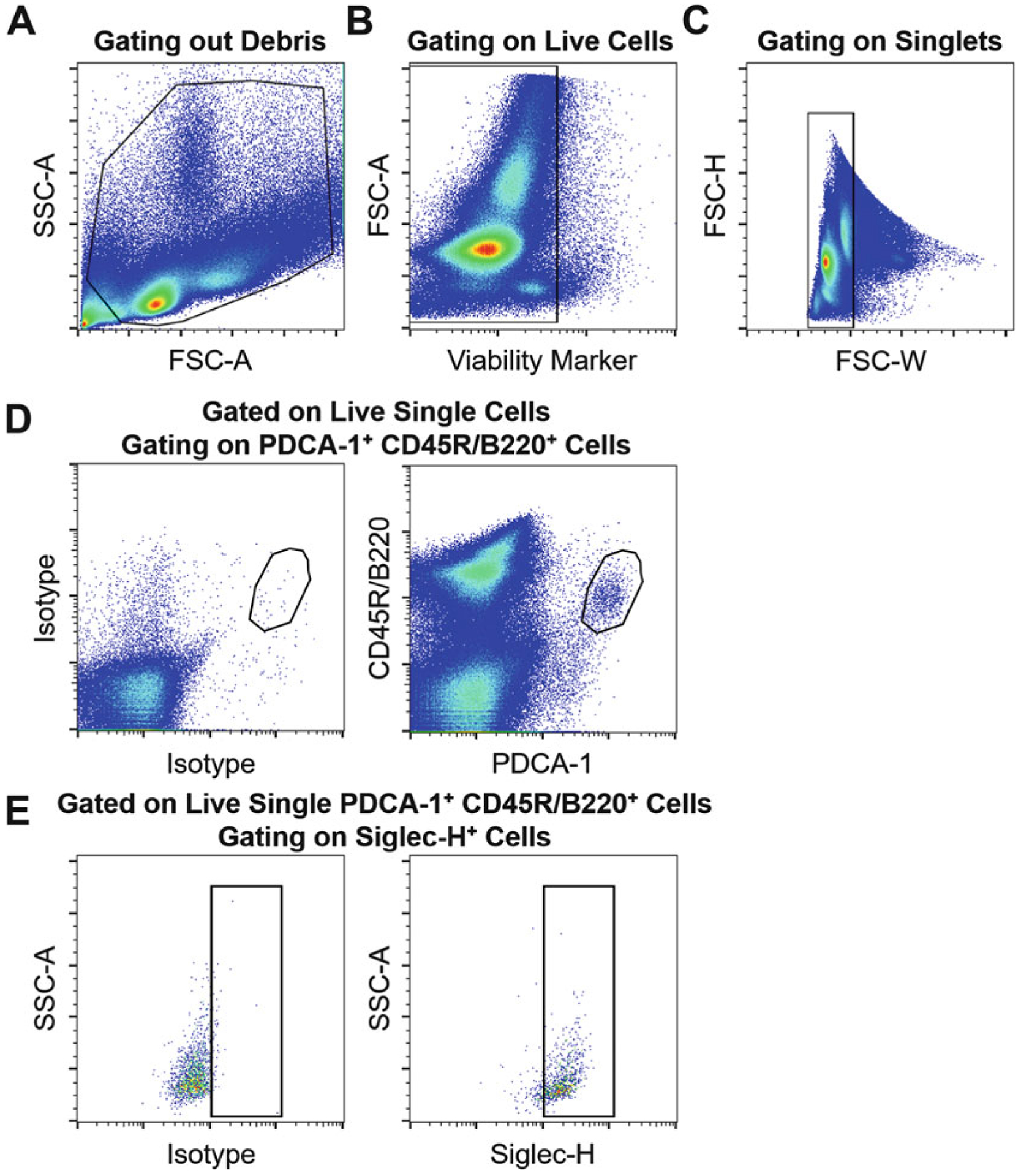
Sequential gating strategy for isolation of pDCs by flow cytometry. Flow cytometric dot plots representing gating out (a) debris, (b) dead cells, (c) doublets, as well as (d) gating on PDCA-1+ CD45R/B220+ cells, followed by (e) gating on Siglec-H+ cells using fluorescence minus one control (both panels in e are gated on live single PDCA-1+ CD45R/B220+ cells. In the left panel, an isotype for Siglec-H is used; however, in the right panel, an anti-Siglec-H antibody is applied). Through such sequential gating, live single PDCA-1+ CD45R/B220+ Siglec-H+ pDCs can be sorted
3.4. Cornea Wounding and Local Adoptive Transfer of Plasmacytoid Dendritic Cells
Wash the sorted pDCs with ice-cold PBS, centrifuge at 1200 rpm for 5 min, at 2–8 °C.
Thaw fibrin sealant TISSEEL® sealer protein solution and thrombin solution and warm both solutions to 37 °C, as instructed by the manufacturer (see Note 21).
After washing the cells, gently aspirate/decant the buffer completely, without disrupting the pellet.
Resuspend the cells in fibrin sealant sealer protein solution based on the number of cells sorted, such that a final cell concentration of 10,000 cells/μL is achieved (the volume of sealer protein solution depends on the number of sorted cells; see Note 22).
Anesthetize wild-type syngeneic (C57BL/6J) animals (see Note 11) per approved animal protocol. Confirm a deep state of anesthesia by toe pinch.
Apply a drop of 0.5% proparacaine ophthalmic solution on the cornea that will undergo wounding. On the contralateral cornea, apply ophthalmic artificial tears to prevent desiccation.
Under a dissecting microscope, stabilize the eyeball by a curved forceps and mark the central 2 mm of the cornea using a 2 mm trephine (Fig. 2; see Note 23).
After marking the cornea, use an Algerbrush II corneal rust ring to carefully remove the corneal epithelium (Fig. 2).
Apply 1 μL of the cell suspension (resuspended in sealer protein solution of fibrin sealant TISSEEL®) on the central cornea (see Note 24).
Quickly apply 1 μL of fibrin sealant thrombin solution (see Notes 25 and 26).
Wait for 5–10 min to ensure that fibrin sealant is solidified on the cornea (Fig. 2), and then apply triple antibiotic ointment on the cornea (see Note 27).
Different controls can be used for the adoptive transfer process. For a “sham” control, simply add 1 μL of sealer protein solution without the cells on the wounded cornea followed by quick addition of 1 μL of thrombin solution. Irrelevant cells can be used as additional controls.
Place the mouse in a regular cage and provide heat by a heating lamp (or other IACUC-approved approach). Monitor the mouse until recovered from anesthesia.
Repeat the process for remaining animals (see Notes 28 and 29).
Fig. 2.

Representative schematic and clinical corneal photograph illustrating the procedure. (a) Normal cornea prior to procedure for comparison. (b) Cornea following trephination using a 2 mm trephine to mark the border for epithelial debridement. (c) Cornea following mechanical debridement. (d) Typical solidified fibrin sealant on the cornea, approximately 10 min following adoptive transfer of pDCs
3.5. Outcome Assessment
At desired time points following corneal wounding and adoptive transfer (for instance 3, 6, 12, 24, 48, and 72 h), anesthetize the animal(s) according to the IACUC-approved protocol. Confirm a deep state of anesthesia by toe pinch.
Take a clinical photograph of the via slit-lamp biomicroscopy prior to fluorescein staining (optional).
Apply one drop of 0.25% fluorescein ophthalmic solution and leave it on the cornea for 2 min.
Wash the cornea using normal saline to remove excess fluorescein. Use a cotton tip applicator to avoid pouring of the excess fluorescein on the animal. Examine the cornea by slit-lamp biomicroscopy under the cobalt blue light.
Take photograph(s) of the cornea for visualizing the corneal fluorescein staining, hence to assess corneal epithelial defects (Fig. 3).
Place the mouse in a regular cage and provide heat by a heating lamp (or other IACUC-approved approach). Monitor the mouse until recovered from anesthesia.
Repeat the process for the remaining animals.
Measure the area with epithelial defect (which is stained with fluorescein) by ImageJ. In brief, open the acquired image in ImageJ. Using the “freehand selections” tool, select the stained area; on the “Analyze” tab, choose “Measure” to quantify the area with defect. Repeat similar measurements to quantify the area of the whole cornea. By dividing the defect area to the whole corneal area, the percentage of wounded area (to the total cornea) can be presented. Alternately, the corneal wound area can be measured over time and can be compared to the initial corneal wound measured at the baseline to express the percentage of wound closure (healing).
Fig. 3.
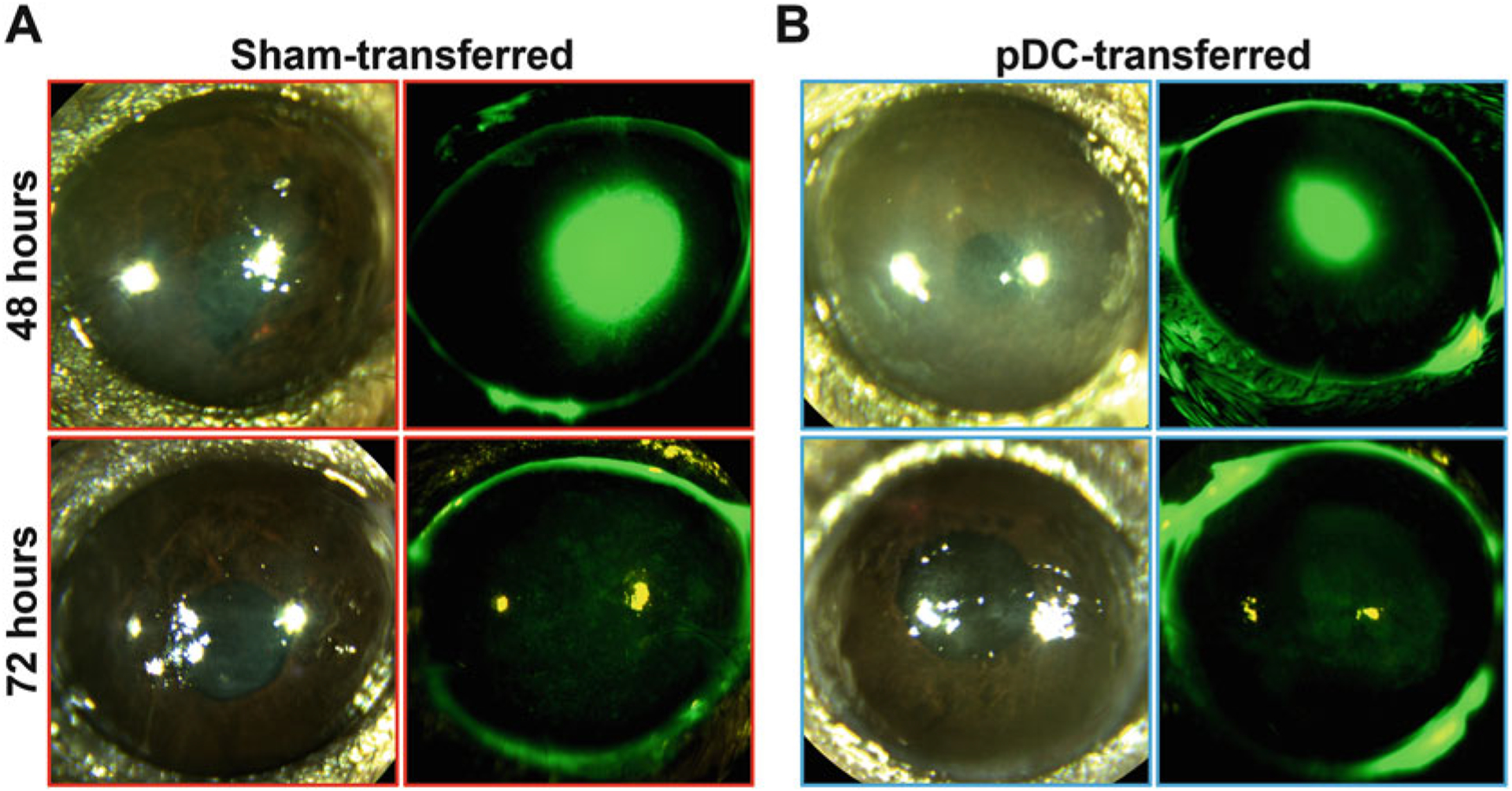
Representative images of the wounded cornea. Images represent clinical corneal photograph and corneal fluorescein staining 48 and 72 h after corneal wounding and (a) sham transfer and (b) pDCs’ adoptive transfer, showing smaller wound size in the cornea undergoing adoptive pDC transfer at 48 h following adoptive transfer
4. Notes
PBS free of calcium and magnesium is preferred to limit clumping, which may otherwise occur due to adhesion molecules.
One six-well cell culture plate can accommodate normal-sized spleens from multiple animals. However, a petri dish or a 12-well cell culture plate can also be used.
Although we suggest using wild-type C57BL/6J mice in this protocol, any strain of mice can be used as the source of cells. Similarly, any transgenic mice can also be used as the source of cells of interest.
The estimated number of donor animals needed depends on the total number of cells required for a particular experiment. From each naïve mouse spleen, the pDCs’ isolation yield is expected to approximate 100,000–200,000 cells (depending on the age and gender of the mouse, technical properties of the flow cytometry sorter, and experience with the process). Our recommendation is to transfer 10,000 pDCs to each cornea. Thus, if the experiment is planned for the adoptive transfer of pDCs to five mice, 5 × 10,000 = 50,000 pDCs are needed. However, if more than 10 mice are planned for adoptive transfer of pDCs, more donor mice may be needed. Alternatively, prior treatment of donor mice with FLT3 ligand (for instance 10 μg subcutaneously) daily for 7–10 days [26] or administration of tumor cells expressing the FLT3 ligand to the donor approximately 10–14 days prior to euthanizing the animal can enhance pDC density and thus isolation yield [27].
It is recommended to prepare ACK RBC lysis buffer in advance and store at 2–8 °C for up to 3–6 months.
It is recommended to prepare FACS buffer in advance and store at 2–8 °C for up to 3–6 months. Optionally, DNase I (25–50 μg/mL) can be added to the FACS buffer to further avoid cell clumping in the process of single cell preparation.
A variety of viability markers are available, such as 7-aminoactinomycin D (7-AAD), propidium iodide (PI), and amino-labeling viability dyes. For selecting one reagent, factors such as prior experience, technical specifications of flow cytometry sorter, potential spectral overlaps with fluorophore-conjugated antibodies, which may sophisticate the isolation process, and costs may be considered. Also, considering that sorted cells will be used for in vivo applications, it is vital to use nontoxic viability dyes dissolved in nontoxic solvents to minimize the effect of the marker on the viability and function of sorted cells.
In this protocol, we describe adoptive transfer of pDCs, a relatively rare population of immune cell type; however, the technique can be expanded to adoptive transfer of other cell types isolated by flow cytometry, commercially available isolation kits, or ex vivo generated or manipulated cells of interest.
Since murine pDCs harbor a unique cell surface repertoire, application of multiple cell markers is necessary to validly distinguish them; in this protocol, we present the minimum combination of markers to isolate pDCs (PDCA-1, CD45R/B220, and Siglec-H), to minimize the potential effect of the antibodies on the pDC function. However, if sources other than bone marrow, spleen, and lymph nodes are used to isolate pDCs, addition of anti-CD45 antibody, as a pan-leukocyte marker, is recommended. Further, addition of other markers such as CD11b, F4/80, CD3, and CD19 (in which pDCs lack) as well as CD11c, Ly6C, and Ly49Q (expressed by pDCs) might be necessary to differentiate pDCs.
Given that the various available cell sorting flow cytometers have different technical configurations and laser settings, we leave the choice of fluorophores and panel design to the readers. Importantly, panels should be designed carefully according to standard recommendations. In this regard, application of appropriate isotype controls, single stained controls, and fluorescence minus one controls are necessary for proper isolation of cells. In Fig. 1, we used PE-conjugated anti-PDCA-1, PE-Cy7-conjugated anti-CD45R/B220, and FITC-conjugated anti-Siglec-H.
Similar to Note 3, although in this protocol we suggest using wild-type C57BL/6J mice, any transgenic mouse and any strain of mice can be used, as long as syngeneic donors are used as the source of cells of interest (to avoid potential allogeneic immune response).
Other equivalent reagents can be used, such as fluorescein sodium strips, which can be placed in appropriate amounts of normal saline to yield 0.25% fluorescein solution.
If euthanizing multiple animals, multiple wells may be required. Each well of a six-well cell culture plate can fit 4–5 normal mouse spleens.
In order to decrease the duration of the procedure, it is recommended to continue with the first step of the next process (Subheading 3.3) while the sample is being centrifuged.
This second wash step is recommended to eliminate any clumping/debris that may arise from the preceding lysis step.
Blocking with FACS buffer containing 1:100 anti-CD16/CD32 Fc block is crucial to prevent nonspecific binding of fluorophore-conjugated antibodies (used in the next steps) to Fc receptors expressed on various immune cells.
In order to decrease the duration of the procedure, it is recommended to continue with preparation of the next steps (steps 3 and 4; labeling the tubes, taking the antibodies from the refrigerator, and arranging them on an ice-cold container) while the samples are in incubation.
This filtering step is recommended to remove any potential cell aggregates/clumps formed during the prior steps to prevent clogging the flow cytometry sorter.
Although only 10,000 pDCs are required for adoptive transfer to each mouse, considering the potential loss of cells during the following washing steps and potential loss of cells during the adoptive transfer by inexperienced users, we recommend to sort at least 150,000–200,000 pDCs, even if only one mouse is planned to receive the cells.
In order to decrease the duration of the procedure, it is recommended to continue with preparation of step 2 of the next section (Subheading 3.4; thawing fibrin sealant) while the pDCs are being sorted.
As mentioned by the manufacturer, this heating step to 37 °C is important for optimal enzymatic activity of the sealer protein solution and the thrombin solution.
For instance, if 200,000 pDCs are sorted, add 20 μL of fibrin sealant sealer protein solution. As another example, if 250,000 pDCs are sorted, add 25 μL of fibrin sealant sealer protein solution.
Trephines with a smaller diameter (for instance 1.5 mm) can also be used to mark the cornea and subsequently to create a smaller wound by debriding the epithelium. However, it should be considered that the smaller the wound, the faster it heals. Thus, if exploring the effect of another therapy (for instance, adoptive transfer of another cell type), which potentially has a small effect size, the power of the technique in detecting the differences between control and treatment groups will be limited. Also, it should be noted that if the aim of adoptive transfer is studying the role of cells of interest in pathogenesis or treatment of a corneal condition other than wound healing, generally, a small area of epithelial debridement is sufficient to allow transfer of cells.
Upon transferring the cells in the sealer protein solution of fibrin sealant to the cornea, the solution may slip from the central cornea. Ensuring proper depth of anesthesia, stabilizing the cornea with a curved forceps, or preventing potential hand shaking by supporting the pipet tip with the opposite hand can improve the process and success of the procedure.
When sealer protein solution and thrombin solution are mixed, the components will react to form a fibrin seal, securing the cells within the epithelial defect (Fig. 2).
Similar to sealer protein solution, the applied thrombin solution may slip from the cornea. As mentioned in Note 24, ensuring proper depth of anesthesia, stabilizing the cornea with a curved forceps, or preventing potential hand shaking by supporting the pipet tip with the opposite hand can improve the process and success of the procedure.
If more than one mouse is planned for adoptive transfer of pDCs, the procedure can be started on the next animal while the fibrin sealant is solidifying on the first mouse to accelerate the experiment.
It should be considered that the process of donor tissue collection, tissue processing and single cell preparation, cell staining, and sorting pDCs may take about 5 h. Thus, for performing corneal wounding and adoptive transfer of pDCs in multiple animals, it should be considered that the longer the process takes (the more the number of mice under the experiment), the more pDCs may undergo apoptosis, reducing the efficacy of the adoptive transfer of pDCs for corneal wound healing.
To assess if adoptive transfer of pDCs is successful, cells can be stained using a commercially available cell tracer prior to adoptive transfer and the corneas can be assessed at the desired time points to measure the density of the transferred cells and their longevity in the cornea. Nevertheless, considering that many of the commercially available cell tracers are toxic or are dissolved in toxic solvents, if pDCs are stained with such tracers, we do not recommend that cell function be studied after adoptive transfer, should tracers be used for verification of successful transfer. Alternatively, transgenic mice with fluorescently tagged pDCs such as DPE-GFP × RAG1−/− mice, in which pDCs are tagged with GFP [28], can be used for isolation of pDCs and tracking cells after adoptive transfer (Fig. 4).
Fig. 4.

Integration of adoptively transferred pDCs to corneal stroma. A DPE-GFP × RAG1−/− mouse with specifically GFP-tagged pDCs was used as the source of pDCs in this experiment. A representative corneal whole-mount micrograph, 48 h following adoptive transfer shows integration of GFP-tagged adoptively transferred pDCs to the recipient cornea
Acknowledgments
The authors would like to appreciate the technical assistance of Allen Parmelee and Stephen Kwok at Tufts University Flow Cytometry Core for their assistance in sorting the cells. This work was supported by the following grants to P.H.: NIH R01-EY022695, NIH R01-EY026963, NIH R01-EY029602, Massachusetts Lions Eye Research Fund, and Tufts Medical Center Institutional Support.
References
- 1.Swiecki M, Colonna M (2010) Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev 234(1):142–162. 10.1111/j.0105-2896.2009.00881.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Won HY, Lee JY, Ryu D et al. (2019) The role of plasmacytoid dendritic cells in gut health. Immune Network 19(1):e6. 10.4110/in.2019.19.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers NM, Isenberg JS, Thomson AW (2013) Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance? Am J Transplant 13(5):1125–1133. 10.1111/ajt.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Wu J, Zhu S et al. (2017) Disease-associated plasmacytoid dendritic cells. Front Immunol 8:1268. 10.3389/fimmu.2017.01268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sendra VG, Jamali A, Harris DL et al. (2014) Role of plasmacytoid dendritic cell in the immune regulation in sutured inflamed cornea. Invest Ophthalmol Vis Sci 55(13):1694–1694 [Google Scholar]
- 6.Sendra V, Jamali A, Harris DL et al. (2015) Plasmacytoid dendritic cells mediate adaptive immunity in acute herpes simplex virus keratitis. Invest Ophthalmol Vis Sci 56 (7):1856–1856 [Google Scholar]
- 7.Sendra VG, Jamali A, Lopez MJ et al. (2017) Plasmacytoid dendritic cells modulate corneal inflammation through transforming growth factor (TGF)-β1. Invest Ophthalmol Vis Sci 58(8):3618–3618 [Google Scholar]
- 8.Jamali A, Lopez MJ, Sendra V et al. (2016) Plasmacytoid dendritic cells maintain corneal heme-angiogenic privilege through secretion of anti-angiogenic molecules. Invest Ophthalmol Vis Sci 57(12):1430–1430 [Google Scholar]
- 9.Jamali A, Lopez MJ, Sendra V et al. (2015) Plasmacytoid dendritic cells demonstrate vital neuro-protective properties in the cornea and induce corneal nerve regeneration. Invest Ophthalmol Vis Sci 56(7):4355–4355 [Google Scholar]
- 10.Jamali A, Lopez MJ, Sendra VG et al. (2017) Local adoptive transfer of plasmacytoid dendritic cells as a novel therapeutic approach for corneal nerve regeneration. Invest Ophthalmol Vis Sci 58(8):993–993 [Google Scholar]
- 11.Verhagen C, Mor F, Kipp JB et al. (1999) Experimental autoimmune keratitis induced in rats by anti-cornea T-cell lines. Invest Ophthalmol Vis Sci 40(10):2191–2198 [PubMed] [Google Scholar]
- 12.He Y, Jie Y, Wang B et al. (2010) Adoptive transfer of donor corneal antigen-specific regulatory T cells can prolong mice corneal grafts survival. Cornea 29(Suppl 1):S25–S31. 10.1097/ICO.0b013e3181ea4999 [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick S, Lausch R, Barrington RA (2019) CCR6-positive gammadelta T cells provide protection against intracorneal HSV-1 infection. Invest Ophthalmol Vis Sci 60 (12):3952–3962. 10.1167/iovs.19-27810 [DOI] [PubMed] [Google Scholar]
- 14.Sarkar R, Mathew A, Sehrawat S (2019) Myeloid-derived suppressor cells confer infectious tolerance to dampen virus-induced tissue immunoinflammation. J Immunol 203 (5):1325–1337. 10.4049/jimmunol.1900142 [DOI] [PubMed] [Google Scholar]
- 15.Chauhan SK, Saban DR, Dohlman TH et al. (2014) CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol 192 (2):817–823. 10.4049/jimmunol.1203469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Jie Y, Ren D et al. (2012) In vitro-expanded CD4(+)CD25(high)Foxp3(+) regulatory T cells controls corneal allograft rejection. Hum Immunol 73(11):1061–1067. 10.1016/j.humimm.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Carrasco R, Sanchez-Abarca LI, Nieto-Gomez C et al. (2019) Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocular Surface 17(2):285–294. 10.1016/j.jtos.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Shao C, Chen Y, Nakao T et al. (2019) Local delivery of regulatory T cells promotes corneal allograft survival. Transplantation 103 (1):182–190. 10.1097/tp.0000000000002442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao S, Hafezi-Moghadam A, Ishibashi T (2012) Lymphatics and lymphangiogenesis in the eye. J Ophthalmol 2012:783163. 10.1155/2012/783163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendricks RL, Epstein RJ, Viana MA et al. (1991) A reproducible method for injecting the mouse corneal stroma. Invest Ophthalmol Vis Sci 32(2):366–370 [PubMed] [Google Scholar]
- 21.Epstein RJ, Stulting RD (1987) Corneal neo-vascularization induced by stimulated lymphocytes in inbred mice. Invest Ophthalmol Vis Sci 28(9):1505–1513 [PubMed] [Google Scholar]
- 22.Matthaei M, Meng H, Bhutto I et al. (2012) Systematic assessment of microneedle injection into the mouse cornea. Eur J Med Res 17:19. 10.1186/2047-783x-17-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamali A, Lopez MJ, Harris DL et al. (2019) Local adoptive transfer of plasmacytoid dendritic cells as a novel therapeutic approach for corneal neovascularization. Invest Ophthalmol Vis Sci 60(9):897–897 [Google Scholar]
- 24.Griffith GL, Kasus-Jacobi A, Lerner MR et al. (2014) Corneal wound healing, a newly identified function of CAP37, is mediated by protein kinase C delta (PKCdelta). Invest Ophthalmol Vis Sci 55(8):4886–4895. 10.1167/iovs.14-14461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Burns AR, Smith CW (2006) Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest Ophthalmol Vis Sci 47 (5):1947–1955. 10.1167/iovs.05-1193 [DOI] [PubMed] [Google Scholar]
- 26.Karsunky H, Merad M, Cozzio A et al. (2003) Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med 198(2):305–313. 10.1084/jem.20030323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mach N, Gillessen S, Wilson SB et al. (2000) Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res 60(12):3239–3246 [PubMed] [Google Scholar]
- 28.Iparraguirre A, Tobias JW, Hensley SE et al. (2008) Two distinct activation states of plasmacytoid dendritic cells induced by influenza virus and CpG 1826 oligonucleotide. J Leukoc Biol 83(3):610–620. 10.1189/jlb.0807511 [DOI] [PubMed] [Google Scholar]


