Abstract
Dysphagia evaluation and management has rapidly become the primary practice area of medical speech pathologists since its adoption in our field less than three decades ago. As a specialty, swallowing and swallowing disorders comprise the largest represented discipline with 10,059 specialty interest group members within the American Speech-Language-Hearing Association and 298 board-certified specialists in the American Speech Hearing Association. There are national and international organizations, such as the Dysphagia Research Society and its interdisciplinary journal Dysphagia, that provide continuing education for clinicians and a platform for dysphagia researchers. Despite this rapid growth, herein we identify some significant needs for improving the science and practice of dysphagia clinical care, including a deeper understanding of physiology and neurophysiology, standardization of evaluation, consensus on core sets of dysphagia parameters for clinical and research reporting, personalized algorithms for implementation of evidenced-based practice, metrics for therapy efficacy, and increased buy-in and funding from agencies. The goals of this article are to summarize the status quo of dysphagia research, evaluation, and treatment as well as to make predictions about the future. Medical trends that we speculate will influence dysphagia research and care in the future include, among others, imaging advances, personalized medicine, regenerative medicine, and telehealth.
Keywords: Dysphagia, swallowing, evaluation, management
As with any scientific and clinical care domain, rapidly advancing knowledge and technology in dysphagia evaluation and management has driven the field forward.1,2 Yet, as with many fields, we can also be slow to adapt. For example, fiberoptic endoscopic evaluation of swallowing (FEES) is empirically established as a valid, reliable, and valuable tool,3-9 but this instrument is still not widely adopted outside of major medical settings. Evaluation and treatment of dysphagia is one of the newer subspecialties in speech-language pathology, with required coursework starting as recently as the 1990s and American Speech-Language-Hearing Association (ASHA) standards published in 1997.10 Despite the relative youth of dysphagia clinical practice, we have seen exponential growth in our understanding of swallowing physiology and dysphagia etiology, evaluation, and treatment. There are national and international organizations, a dedicated interdisciplinary scientific journal, and rapid expansion in all areas of research including basic, clinical, and translational science in the last three decades. As of March 2016, a simple PubMed search of the term dysphagia yields 55,715 citations. It is important to recognize that dysphagia research, evaluation, and management is an interdisciplinary endeavor. As such, advances will certainly involve scientists and clinicians from a variety of fields, including but not limited to speech-language pathology, occupational therapy, nutrition, otolaryngology, gastroenterology, internal medicine, physiatry, radiology, oncology, plastic surgery, neurology, medical physics, neuroscience, epidemiology, physiology, nursing, education, social work, and psychology.
The purpose of this article is to discuss the status quo of the science and practice of dysphagia care as well as speculate what trends may influence the future. Some of the medical trends that we expect will influence dysphagia research and care in the future are imaging advances, personalized medicine, regenerative medicine, and telehealth. It is important to note that it is impossible to cover the vast array of contributions by the hundreds of clinician–scientists who have influenced this field and those currently on the cutting edge who will surely influence the future. Instead, we chose several areas of focus to illustrate some of the major tenets of this field including neurophysiology, diagnostics, therapies, and emerging technologies, such as analyses of large data sets, telehealth, and regenerative medicine. In addition, we acknowledge that, given adult focus of this issue, the focus of this article is primarily on adult dysphagia. We acknowledge remarkable progress in pediatric dysphagia practice and research, but much of this work was beyond the scope of this article. We apologize to those whose seminal work was not included. We hope to convince the reader that despite great progress, we still have a long way to go. Significant priorities for the immediate future include a deeper understanding of physiology and neurophysiology, standardization of evaluation, consensus on core sets of dysphagia parameters for clinical and research reporting, personalized algorithms for implementation of evidenced-based practice, metrics for therapy efficacy, and increased buy-in and funding from agencies.
SWALLOWING PHYSIOLOGY–NEUROPHYSIOLOGY
It is beyond the scope of a single article to review the advances made in our understanding of swallowing physiology as a whole. As such, we focus on neurophysiology in this article. We refer the reader to several seminal texts/articles that cover swallowing physiology, including neurophysiology, such as those from Miller,11 Martin-Harris et al,12 Martin et al,13-16 Hamdy et al,17 to name a few. To fully comprehend swallowing physiology and pathophysiology, a deep understanding of the peripheral and central neural mechanisms governing this vital function is crucial. Animal research in the 1950s, ’60s, and ’70s provided insights on the role of a brainstem-mediated central pattern generator in controlling swallowing function, providing the first indication that swallowing is a complex semiautomatic neurophysiological act mediated by brainstem nuclei.18-20 Further support of this complex peripheral control was provided by work including the use of intramuscular and surface electromyography (EMG) in humans.21-24 These seminal reports offered our first understanding of the neural control of swallowing and supported the notion that swallowing is regulated at the level of the brainstem. It was not until the late 1980s and early 1990s that evidence of the role of supramedullary areas in the regulation of swallowing neurophysiology emerged from clinical studies of patients with dysphagia after cortical or subcortical damage,25-29 as well as from neuroimaging research in healthy and patient populations.14,16,17,30-35 This large body of evidence identified a wide cortical network as active during swallowing, including bilaterally the lateral primary sensory and motor cortices, the supplementary motor area, the anterior cingulate cortex, the insula, the parietal lobules, and the prefrontal and inferior frontal areas (frontal operculum),14,16,17,30-35 with some studies also reporting activation in the basal ganglia, thalamus, and the cerebellum.17,32,34-36 Although the activation of this bilateral network in swallowing control is indisputable, many of the details are still unknown. Specifically, we are still unsure about the temporal sequence of these activations, the direction of the connectivity between these areas, as well as the exact roles of each area and each hemisphere in swallowing and how they are impacted by neurologic injury or disease.
Current and future work, including multimodal imaging approaches, will be essential to understand the complex supratentorial contribution to swallowing physiology. These details will be pivotal to help shift our clinical paradigm from symptomatic diagnosis and treatment to the development and refinement of novel modalities (behavioral, brain stimulation, and pharmaceutical) that will target neurophysiological underpinnings and not only dysphagia symptoms.
DIAGNOSTICS
Standardization and validation of our core set of clinical tools predominated the progress in dysphagia diagnostics in the past 30 years. Among these, the clinical swallow examination (commonly referred to as the bedside swallow evaluation), videofluoroscopic evaluation of swallow (commonly referred as modified barium swallow studies), and FEES are the workhorses in modern practice.
Clinical Swallowing Evaluation
Minimal standards for bedside swallow were introduced by Logemann and colleagues,37 with procedural details and critical parameters delineated. In daily practice, the bedside or clinical observation of a “natural” eating environment remains invaluable. Yet, as a tool, the bedside examination is often challenged because it lacks quantitative or objective benchmarks. For this reason, several swallow-specific tasks have been developed and validated in the last decade as quantitative metrics that can be obtained during a clinical or bedside (nonimaging) examination. Among others, these include the 3-ounce water screen,38 the Toronto bedside swallowing screening test,39 and the water swallow test,40 which seek to quantify or screen an individual’s capacity to swallow a large volume bolus challenge, as well as standardized clinical instruments, such as the Dysphagia Disorders Survey, used mostly in pediatrics.41 Normative ranges of performance on these measures allow clinicians a quantitative benchmark to contribute to their clinical decision making. The Mann Assessment of Swallowing Ability (MASA) was developed as an index measure weighting complementary features of the oral mechanism examination, neurocognitive abilities, functional status, and bolus trials to derive a summary score of the clinical assessment42; recent progress with the MASA includes validation of population-specific domains such as the MASA-C (cancer).43 As the field progresses, there is promise that affordable, noninvasive adjunctive measures such as cough strength testing,44-46 electrical impedance myography,47 swallowing frequency,48 or tongue pressure measures may serve as critical quantitative parameters that improve the yield of clinical examinations.49,50
Videofluoroscopy
Videofluoroscopy is arguably the most popular method of instrumental swallowing assessment among speech pathologists. Tremendous progress has been made to standardize the videofluoroscopic examination since its broad adoption into clinical practice more than two decades ago.51-56 As with any imaging procedure, diagnostic observations from videofluoroscopy can only be reliably quantified with adherence to a standard clinical protocol. Critical elements of standardization in videofluoroscopy include, among many, the concentration contrast agent(s), the bolus protocol, frame rate of image acquisition, and instructions provided to the patient during the examination. A uniform bolus protocol must be efficient to minimize radiation exposure (per As Low As Reasonably Achievable (ALARA) principle57) yet feature a sufficient range of consistencies to represent the complexity of oral intake. As a foundational step in the development of their standardized Modified Barium Swallow Impairment Profile (MBSImP), Martin-Harris and colleagues developed and statistically assessed a consensus-derived, optimal bolus protocol consisting of measured-volume and natural “cup sip” presentations of thin liquid barium (Varibar, Bracco Diagnostics Inc., Monroe Township, New Jersey), thick pudding, and a dry solid bolus.51 Yet, even this basic tenet of standardization is slowly adopted in clinical practice. Dozens of videofluoroscopic measures have been developed including bolus-based measures (such as the penetration-aspiration scale),58 Dynamic Imaging Grade of Swallowing Toxicity),59 physiologic ratings (such as MBSImP51), temporal scales (such as Oropharyngeal Swallow Efficiency)60, and kinematics (including numerous hyoid kinematic parameters61-63). As a field, we have yet to adopt a core set of quantitative videofluoroscopy parameters in clinical practice. This might represent a key goal for practice-oriented work in the next decade. Beyond that, semi-automated, pixel-based videofluoroscopic swallow metrics that leverage progress in image registration, autosegmentation, and deformation tracking of medical imaging procedures could make it a reality that clinicians in busy practices can integrate detailed quantitative analysis into their diagnostic paradigms to establish pathophysiology of dysphagia and degree of dysfunction (severity) necessary to personalize therapeutic strategies.61,63,64 As we look further to advancements in diagnostic modalities beyond fluoroscopy (such as high-resolution manometry, dynamic magnetic resonance imaging (MRI), and real-time computed tomography) and seek to integrate these modalities into routine practice, it is critical as a field to remember that procedural details must be clearly delineated but also replicable in standard practice to maximize utility of these procedures.
Fiberoptic Endoscopic Evaluation of Swallowing
Another valuable tool for assessing swallowing, airway protection, and palatal, pharyngeal, and laryngeal sensation is FEES. FEES was first introduced in the literature with Dr. Susan Langmore at the helm over 24 years ago.6 FEES allows an in-depth assessment of structure and function of the previously mentioned areas and has several advantages, including a direct view of pharyngeal and laryngeal tissues and secretions, increased equipment portability, and no radiation exposure, allowing for serial studies and even daily biofeedback programs.3,6,7,65-69 Although videofluoroscopy has been called the gold standard, this was in part because it was the first widely accepted tool. When FEES was compared with videofluoroscopy in terms of observing airway penetration and pharyngeal residue, FEES assessment revealed more severe gradings of both parameters and more reliable scores of penetration and aspiration.5,70-72 Like videofluoroscopy, FEES requires extensive training for reliable interpretation and the added skill set of passing an endoscope, which likely contributes to the reluctance of some clinicians to use this tool. Nevertheless, FEES is a critical tool in the clinician’s arsenal.
High-Resolution Manometry
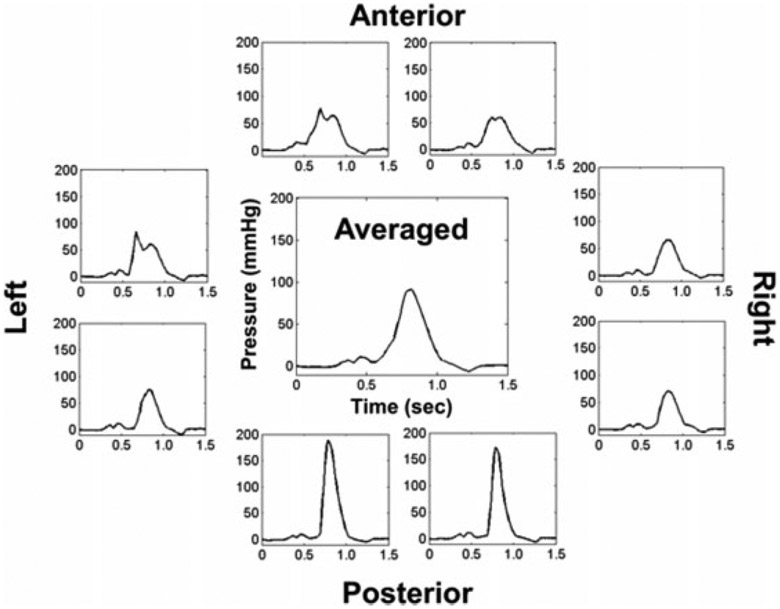
Safe and efficient swallowing requires coordination of muscle activations creating positive pressures at the tail of the bolus with simultaneous subatmospheric pressures below the head of the bolus in the upper esophageal sphincter (UES).73 Manometry directly measures these pressures to provide a real-time, quantitative output complementary to other instrumental techniques, such as videofluoroscopy and FEES. High-resolution manometry (HRM) improved on early manometric systems with the addition of up to 43 closely spaced (≤1 cm), solid-state, circumferential pressure sensors that record contact pressures at relatively high speeds (50 Hz).74 The addition of impedance monitoring to pharyngeal HRM provides data on bolus presence and residue, as well as UES distension during swallowing.75-84 Combinations of pharyngeal HRM, impedance, videofluoroscopy, and EMG in laboratory settings have recently started to improve understanding of swallowing mechanics, such as UES opening.81,85-87 Finally, new HRM technology allows for the separation of pressures circumferentially,88 affording more specific analysis of the origin of pressures in the asymmetric pharynx (Fig. 1). In select clinical centers, pharyngeal HRM is being used routinely by speech-language pathologists (SLPs) for evaluation and management of dysphagia.89 Clinical HRM studies have improved our understanding of how different disease processes impact swallowing pressures in pediatric and adult populations.90-100 Additionally, discriminant abilities of HRM are demonstrated by sophisticated algorithms that predict normal from disordered swallowing patterns as well as penetration or aspiration events.101-104 Obstacles to clinical adoption of pharyngeal HRM include high cost, training requirements for HRM catheter placement and pressure analysis, and an incomplete understanding of the best ways to meaningfully translate pressure data to dysphagia diagnostics and treatment planning. Applications of pharyngeal HRM for the evaluation and treatment of oropharyngeal dysphagia have exploded in the past 6 years. HRM undeniably will improve understanding of swallowing physiology and how swallowing pressures are impacted by age and disease processes in the near future. A key goal for implementation is the development of fast, reliable, and meaningful pressure analysis software in the coming decades.
Figure 1.
Three-dimensional high-resolution manometry of the tongue base region during a 10-mL water swallow in a healthy 26-year-old man. Averaged pressure wave (center) is a standard output from high-resolution manometry systems. Note differences in pressure wave shape, timing, and amplitude coming from different axial directions and the same rostral-caudal level in the pharynx.
Importantly, we now have the technology to complete simultaneous physiologic and diagnostic recordings, which can provide a more robust swallowing assessment (e.g., HRM and EMG, videofluoroscopic swallow study [VFSS] and FEES, and FEES and EMG). Unfortunately, with coding and billing limitations, this might not always be possible in the clinic, but perhaps represents a future objective to empower us to get the most relevant diagnostic and physiologic data to make hypothesis-driven treatment recommendations.
IMAGING
Advances in multimodal imaging approaches, including the combination of functional imaging techniques (task and resting functional magnetic resonance imaging [fMRI], positron emission tomography scanning, functional near-infrared spectroscopy, magnetoencephalography), structural connectivity, and perfusion imaging (diffusion tensor imaging, diffusion spectrum imaging, perfusion-weighted imaging, and so on) are emerging as the most comprehensive ways to fully understand central and peripheral neurophysiological mechanisms and swallowing kinematics and are slowly being implemented in our field.105-109 With the explosive advancement in imaging modalities, we believe that our understanding of human swallowing neurophysiology and physiology will exponentially increase as perhaps the primary advancement of the field in the immediate decades to come.
Diagnostically, in 2011, the first simultaneous dynamic MRI and interleaved fMRI sequence was reported that allowed us, for the first time, to use MRI to simultaneously evaluate oropharyngeal swallowing and brain activation.110 Since then, the temporal and spatial resolution of dynamic MRI has continuously increased, allowing for faster and better-quality image acquisitions.111 Although the clinical adoption of dynamic MRI as an alternative to videofluoroscopy is still limited, mainly because of cost limitations and the requirement for supine swallowing, future cost-effective MRI solutions may offer a high-quality noninvasive alternative to videofluoroscopic or videoendoscopic imaging.
Alternate imaging modalities like MRI are particularly attractive for some dysphagia populations such as cancer patients for whom dysphagia sequences, which, if validated (even as a screening), might be integrated with surveillance imaging. Adjunctive anatomic MRI kinetic data also provide superior insight into normal tissue pathology underlying dysphagia, offering the potential to examine these structure–function relationships in neuromuscular regions of interest. Similarly, the development and recent use of high-resolution three-dimensional computed tomography and three-dimensional dynamic MRI imaging can provide unique kinematic, bolus flow, and bolus clearance insights that are impossible to fully appreciate in the current typical two-dimensional imaging technology we use (VFSS, FEES).112-114 These techniques are mostly exploratory at this stage, but their continuous development and improvements are expected to significantly advance our diagnostic toolbox.
The use of high-resolution ultrasound offers another unique opportunity to noninvasively image the oropharyngeal swallowing mechanism. Ultrasound is primarily used in fetal and infant swallowing research,115,116 although efforts for its adoption in adult swallowing research have also been made.117,118 Compared with some of the aforementioned imaging modalities, ultrasound does not require radiation exposure, is economic, and allows for visualization of muscle contraction during movement.119 In addition, it has been used successfully as a biofeedback tool in speech intervention.120 Interpretation of ultrasound images of the head and neck has historically been difficult and training-intensive, which has limited its use and applicability to date. However, given recent advancements, this relatively cheap and noninvasive technology should be reexamined, as its use could potentially provide an alternative (to VFSS and FEES) diagnostic tool and a noninvasive adjunctive treatment modality.
THERAPIES/MANAGEMENT
Compensatory Strategies
In the past 30 to 35 years, our field has also made significant progress in the management of dysphagia. In the early 1980s, talented clinical researchers (with Jeri Logemann as a leader) began describing compensatory strategies for use in patients with swallowing disorders. Compensatory strategies are interventions designed to reduce, avoid, or bypass impaired anatomy or physiology and redirect bolus flow. These include head postures (e.g., chin tuck or head turns), laryngeal maneuvers (e.g., supraglottic swallow), dietary modifications, environmental adaptations, use of adaptive equipment, and sensory stimulation techniques.121-124 Although evidence for the effects of these strategies has been positive overall, their effects are temporary, lasting only as long as the strategy is applied, and poor patient adherence is a critical limitation.125,126
Thickeners
Thickeners are another hugely popular method of compensatory dysphagia management in clinical practice based on the notion that heavier bolus types are less likely aspirated in some disordered swallows. Simplicity and their ability to be applied in populations with coexisting neurocognitive abilities that preclude application of more complex compensations are relative advantages of thickened liquids. Yet, findings from protocol 201, one of the earliest multisite randomized dysphagia trials, highlighted potential risks and patient preferences against broad application of thickened liquids.122,127 Although blind prescriptions and lifelong recommendations for thickened liquids are generally discouraged, thickeners can be an extremely valuable resource to start or keep a patient swallowing and avoid immobilization of the pharynx when more normal textures are otherwise unsafe. Notable recent progress in this area led by Steele and others includes the International Dysphagia Diet Standardization Initiative that categorized a framework of thicknesses and delineated global labels and terminology of thickness.128,129 Adoption of this reference standard in routine clinical practice might represent a meaningful next step in the immediate future to improve dysphagia service delivery.
Exercise and Maneuvers
Dysphagia management also includes swallowing rehabilitative regimens (e.g., strengthening and range of motion exercises of the head and neck muscles) that aim to directly improve the underlying pathophysiological aspects of the disorder and have potentially more durable effects. Such approaches include lingual strengthening exercises,130-134 hyolaryngeal muscle strengthening,135-137 bolus-driven swallowing strengthening regimens such as the McNeil Dysphagia Treatment Program,138 and device-driven therapies such as expiratory muscle strength training and surface electromyographic biofeedback paradigms.139-142 Evidence on the efficacy of these paradigms is largely positive, but stems primarily from small case series or single-institution clinical trials with small or at best moderate effect sizes.
To date, there are few large-scale randomized clinical trials investigating the effects of compensatory or exercise protocols,122,143 urgently emphasizing an open area for future research. Obstacles to confirmatory clinical trials are considerable. Heterogeneity in the case mix, unclear fidelity of therapies, suboptimal adherence, and lack of consensus on what comprises “traditional therapy” for the control arm are chief among these. Novel trial designs such as Bayesian adaptive randomization strategies and registry trials may offer more flexibility and statistical power to test efficacy. A primary goal for researchers designing the next generation of clinical trials must start with highly focused eligibility that specifies the pathophysiology of dysphagia in case definition such that the planned therapy matches the target.
Neuromuscular Electrical Stimulation
In recent years, neuromuscular electrical stimulation (NMES) also emerged as a treatment modality that gained rapid popularity among clinicians and researchers. NMES has a long track record in rehabilitation medicine and physical therapy and has been shown to improve upper and lower extremity motor function.144,145 NMES works by transmitting low-voltage current through skin surface electrodes, triggering a nerve to fire and causing sensory responses and/or muscular contraction. A rather large body of literature on the use of NMES in dysphagia rehabilitation emerged in the past 10 to 15 years, including a few large-scale randomized clinical trials. In most of these studies, NMES was adjunctive to some form of “traditional” therapy, although a uniform definition of the term traditional therapy does not exist. Methodological differences between studies made it difficult for systematic reviews to reach consensus regarding the efficacy of NMES in dysphagia rehabilitation. Overall, results appear mixed; some studies reported positive effects of NMES,146-149 and others reported that NMES does not have an effect.143,150-152 Studies reporting positive effects suffer from small sample sizes, inadequate controls, marginal effect sizes, and methodological limitations. Despite the contradictory and limited positive evidence, NMES remains a popular treatment modality. Therefore, it is important to better understand ideal candidates, optimum settings, and potential benefits of NMES to guide clinical practice. Current and future studies using animal models and neuroimaging applications are expected to provide important dose–response and neurophysiological insights that will increase our understanding of this modality and its true rehabilitative potential.
Skill Training
Beyond strengthening, a more recent expansion in dysphagia rehabilitation is intensive skill training. Central to this therapy is the acknowledgment that not all patients with dysphagia have oropharyngeal weakness. Indeed, at times strength is adequate, but coordination, timing, and skill may be reduced. In pediatrics, skill acquisition is a continually advancing process and is more effective when principles of motor learning are consistently applied.153 In adult dysphagia, structured skill-based training programs have only recently emerged,142,154 aimed at improving timing and coordination aspects of the swallowing sequence. For instance, Martin-Harris and colleagues reported promising results of a phase II single arm clinical trial that integrated a novel biofeedback regimen to train an optimal respiratory phase-swallow pattern.154 Although this work is preliminary, the inclusion of motor learning approaches in dysphagia rehabilitation has the potential to open new therapeutic horizons and increase rehabilitation potential. Developing algorithms that help clinicians harmonize skill and strength training paradigms and select personalized therapies is a key objective for the next decade.
Individualized Therapy
As reported earlier, existing rehabilitative swallowing regimens typically entail broad exercise protocols involving the head and neck, typically performed in an intensive but often static manner.122,132,134-138,140 A “kitchen sink” and one-size-fits-all approach is status quo. That is, many providers report trying a wide variety of strategies in most patients (compensations, range of motion, strengthening, among others) and prescribe exercises on a daily, high-intensity schedule.155-157 Given the extreme complexity of swallowing, current exercise regimens, even when performed intensively, appear inadequate to rehabilitate the complex swallowing abnormalities typically seen in patients with moderate or severe dysphagia and are reported to have small to moderate effects.143 Furthermore, ever growing reimbursement reductions, caps, and the emerging focus on value-based health care threaten (rightfully so) extant practices where-in relatively similar therapies are applied for long treatment periods regardless of etiology or progress.
In response to often sobering results of “traditional” therapies, individualized, more intensive treatment approaches are emerging. Individualized or personalized intensive neuroplasticity driven approaches and “boot camp” approaches that couple functional skill training with progressive resistance exercise paradigms have started to surface.158,159 These approaches tailor or match interventions to the individual patient and their specific physiologic and pathophysiological profile, while systematically combining interventions in an evidence-based manner following neuroplasticity-driven paradigms. Although offering early evidence that a personalized model in dysphagia management is feasible and may be beneficial, more research in this direction is needed. In these applications of individualized therapy programs, interventions are delivered when patients are symptomatic and therefore referred for testing, and therapeutic strategies are selected based on clinical and physiologic swallowing data. Additional biomarkers such as genetic profiling, subclinical changes to swallowing function as a potential biomarker of disease, and disease progression benchmarks would further enhance this rehabilitation model. In our field, these areas (genetics and disease progression) are fully unexplored and should be targets of collaborative future research efforts.
Preventive Therapies
Dysphagia therapy is historically provided in a “reactive” model. Reactive therapy refers to a model in which the therapy begins after a symptomatic patient is referred to the speech pathologist. Strategies for preventive or early therapy greatly advanced in the last decade. In head and neck cancer populations, numerous randomized trials and observational studies now support a preventive swallowing therapy for patients receiving bilateral neck irradiation. Under a “use it or lose it” philosophy, preventive therapy encourages maximal use of the swallowing system during cancer treatment. Swallowing therapy includes targeted exercise and maintenance of oral intake throughout radiotherapy; early implementation of manual therapies may offer a new extension of this body of work in coming years.160-162 The next advancements in this area might include expansion of the preventive service delivery model to other dysphagia populations.
EMERGING APPROACHES
This is an exciting time for technological advances. There is rapid growth in understanding disease processes and treatments on cellular, molecular, and genetic levels. This means that targeted treatments based on individual characteristics will soon become a reality. Furthermore, as computing power and access to large data sets increases, our ability to understand the subtle differences in physiology, disease, disorder, and response to treatments also increases iteratively. Thus, instead of studies with small population samples that suffer from inherent bias, we have artificial intelligence,102,103,163 computer modeling,164,165 and big data statistical approaches that can “see” things in the data that were previously elusive to us as humans.79,166-168 Finally, our ability to use technology to connect patients and clinicians allows for clinical care under circumstances that were not previously possible. With these advances come growing pains. We need to be critical early adopters, but also reasonable consumers. This has always been a delicate balance in science and medicine.
Mobile Technology, Telehealth
One of the fastest growing areas in medicine is the area of telehealth. With the remarkable increase of Internet accessibility across the globe, the aim of telehealth is to enhance health care delivery and education in situations where face-to-face modalities or local experts are not available. For dysphagia diagnostics and treatment, the use of telehealth is in its infancy, but is anticipated to radically increase in the near future. With 22% of the U.S. population living in rural areas, and a relatively limited number of dysphagia-specialized SLPs globally, the development of telehealth is essential in underserved communities and among patients who have restricted mobility. Since the early 2000s, evidence has accumulated supporting both feasibility and reliability of teledynamic clinical swallowing assessments and telefluoroscopic swallow studies.169-177 Regarding dysphagia teletreatment, evidence is scarce including case studies or small-scale satisfaction surveys on the use of dysphagia apps.178-180
An additional promising area of telehealth includes wearable technology and devices. Wearable technology includes clothing and accessories that allow clinicians to remotely monitor physiologic events,181 control medication use,182 and monitor compliance. Their use could allow clinicians to remotely monitor exercise compliance, physiologic signs of distress during exercise or eating, as well as respiratory and cardiac physiology. This technology has the potential to change the face of dysphagia rehabilitation and constitutes another very exciting future endeavor.
With the growing evidence base and obvious need for improved access to quality dysphagia services, the American Telemedicine Association, ASHA, and state associations are actively advocating for changes in national and state health care policies supporting the use of telehealth for SLPs. It is our hope that current practice limitations (including reimbursement and state licensure) will soon be waived to allow for freer access to telehealth applications for dysphagia assessment and rehabilitation. It must be noted, however, that for the use of telehealth to be ethical and efficacious, no regulatory distinction should exist between a service delivered via telehealth and a service delivered in person. Therefore, it is clear that significant research efforts are needed before dysphagia telehealth can be widely adopted.
Noninvasive Brain Stimulation
Noninvasive brain stimulation techniques work by providing electrical stimulation to the brain without the use of surgically implanted or other invasive devices. Commonly used techniques are transcranial direct current stimulation (tDCS), which imparts low-intensity electrical current between two electrodes placed on the skull, and repetitive transcranial magnetic stimulation (rTMS), where the stimulation is produced by a high-current pulse sent through a copper coil that is placed on the skull.183 Applied to the motor cortex, tDCS and rTMS can either enhance or reduce the excitability of the stimulated region, thus facilitating or hindering the corresponding corticospinal or corticobulbar tracts and motor neurons.183 These techniques have been used for treatment of chronic pain,184 anxiety and depression,185,186 aphasia after stroke,187 and for motor rehabilitation,183 including swallowing treatment.188 Much of the noninvasive brain stimulation research is in the stroke population, and published work varies in terms of stimulation type, device placement (affected versus unaffected stimulation), stimulation parameters, stimulation schedule, outcome measures, and follow-up. A meta-analysis of seven randomized control trials investigating tDCS and rTMS in the rehabilitation of poststroke dysphagia revealed a moderate effect of noninvasive brain stimulation versus sham stimulation.188 With sufficient evidence, insights on neurophysiological bases of behavioral changes, and adequate clinical training, noninvasive brain stimulation may someday have a place in the clinician’s toolkit, even in populations beyond neurogenic dysphagia.
Alternative and Complementary Medicine
Most traditional dysphagia therapies are specifically targeted to changing local sensorimotor function in the upper aerodigestive tract and/or to changing relevant central control of the oropharyngeal swallow. As the field increases its focus on patient-centered outcomes, a more holistic approach to dysphagia management may be indicated. There is a very limited body of research evaluating the effects of acupuncture on dysphagia.189-191 Findings generally support an improvement of oropharyngeal dysphagia following acupuncture, but a more thorough understanding of the mechanisms of improvement following acupuncture are needed. Preliminary work of a joint-care therapy model coupling dysphagia treatment with counseling,192,193 suggests improved quality of life in patients undergoing chemoradiation for head and neck cancer. As dysphagia impacts much more than physiologic functioning,194-197 clinicians must recognize the need to treat the entire patient, not just the dysphagia.
CONCLUSION
This is an exciting time for the science and practice of swallowing and dysphagia. There have been significant advances in our knowledge of normal and disordered swallowing physiology, as well as evaluation and treatment. The success of these advances can be attributed to several factors. First and foremost, there is a considerable interdisciplinary effort across basic, clinical, and translational sciences. Many of our greatest contributors know how to navigate among these approaches and as such, we have comprehensive research programs, informed clinical care, good consumers of literature, and innovation. A close second is that science, technology, and communication is rapidly advancing. We should make an effort to be on the cutting edge while making evidence-based decisions. There is always risk and pushback with new information, but that cannot hold us back when there is much at stake for our patients.
Because dysphagia is a condition that results from a multitude of etiologies, diseases, disorders, injuries, and conditions, we do not have unified academic or medical departments, patient advocacy groups (although there is the newly formed National Foundation of Swallowing Disorders), or a federal funding agency that has designated swallowing and swallowing disorders as a major arm of its strategic plan. As a result, this condition that affects millions of people worldwide is understudied and underfunded.
Although there is undeniable room to grow as a field and provide more comprehensive evaluations and better-suited treatments to our patients, we should not be discouraged with our currently available tools. Clinicians should reasonably frame expectations on the possibilities of new technological advances. For example, big data sets and computer modeling will only be able to tell us so much about a patient’s likelihood to aspirate or how well a patient will do with a particular intervention. The clinician’s bedside acumen remains integral to patient buy-in and is an invaluable resource to the patient and family. Additionally, technology cannot replace clinical intuition and critical thinking! We must train clinicians to be creative thinkers who can maximize the utility of currently available tools, who can be a smart consumer of literature, and who can be an effective advocate with senior clinicians, physicians, and clinic administrators to acquire tools to improve their practice. As Kleim and Jones note “currently, learning is our best hope for remodeling the damaged brain,” in other words, we (as clinicians) are still the best hope for improving our patients’ rehabilitation potential and brain plasticity through learning and practice.198 As we move ahead to more exciting times, we should never forget this role.
Learning Outcomes:
As a result of this activity, the reader will be able to (1) list three currently used tools for dysphagia diagnosis; (2) state three new technologies that are on the horizon for evaluation and treatment of dysphagia; (3) identify that the control of swallowing involves a widespread neural network; (4) discuss the need for standardized research and clinical protocols for evaluating swallow function; and (5) compare strength versus skill training in dysphagia management.
Footnotes
Forecasting the Future: Expert Opinions on the Next Decade for Adult Speech and Language Disorders; Guest Editor, Audrey L. Holland, Ph.D.
REFERENCES
- 1.Association AS-L-H. ASHA SLP Health Care Survey 2015: Caseload characteristics. 2015. Available at http://www.asha.org/Research/memberdata/HealthcareSurvey/. Accessed March 30, 2016
- 2.Disorders ABoSaS. Find a specialist. 2016. Available at: http://www.swallowingdisorders.org/search/custom.asp?id=1177. Accessed March 30, 2016
- 3.Bastian RW. The videoendoscopic swallowing study: an alternative and partner to the videofluoroscopic swallowing study. Dysphagia 1993;8(4):359–367 [DOI] [PubMed] [Google Scholar]
- 4.Butler SG, Markley L, Sanders B, Stuart A. Reliability of the penetration aspiration scale with flexible endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol 2015;124(6):480–483 [DOI] [PubMed] [Google Scholar]
- 5.Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope 2007;117(10):1723–1727 [DOI] [PubMed] [Google Scholar]
- 6.Langmore SE, Schatz K, Olson N. Endoscopic and videofluoroscopic evaluations of swallowing and aspiration. Ann Otol Rhinol Laryngol 1991;100(8):678–681 [DOI] [PubMed] [Google Scholar]
- 7.Leder SB, Sasaki CT, Burrell MI. Fiberoptic endoscopic evaluation of dysphagia to identify silent aspiration. Dysphagia 1998;13(1):19–21 [DOI] [PubMed] [Google Scholar]
- 8.Suiter DM, Moorhead MK. Effects of flexible fiberoptic endoscopy on pharyngeal swallow physiology. Otolaryngol Head Neck Surg 2007;137(6):956–958 [DOI] [PubMed] [Google Scholar]
- 9.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 2011;55(1):204–215 [DOI] [PubMed] [Google Scholar]
- 10.ASHA Special Interest Division 13 SaSDD. Graduate curriculum on swallowing and swallowing disorders (adult and pediatric dysphagia). ASHA Desk Reference 1997;3:248a–248n [Google Scholar]
- 11.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev 2008;14(2):77–86 [DOI] [PubMed] [Google Scholar]
- 12.Martin-Harris B, Michel Y, Castell DO. Physiologic model of oropharyngeal swallowing revisited. Otolaryngol Head Neck Surg 2005;133(2):234–240 [DOI] [PubMed] [Google Scholar]
- 13.Martin R, Barr A, MacIntosh B, et al. Cerebral cortical processing of swallowing in older adults. Exp Brain Res 2007;176(1):12–22 [DOI] [PubMed] [Google Scholar]
- 14.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 2001;85(2):938–950 [DOI] [PubMed] [Google Scholar]
- 15.Sörös P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp 2009;30(8):2426–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin RE, MacIntosh BJ, Smith RC, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol 2004;92(4):2428–2443 [DOI] [PubMed] [Google Scholar]
- 17.Hamdy S, Mikulis DJ, Crawley A, et al. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol 1999;277(1 Pt 1):G219–G225 [DOI] [PubMed] [Google Scholar]
- 18.Doty RW. Influence of stimulus pattern on reflex deglutition. Am J Physiol 1951;166(1):142–158 [DOI] [PubMed] [Google Scholar]
- 19.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol 1956;19(1):44–60 [DOI] [PubMed] [Google Scholar]
- 20.Jean A, Car A, Roman C. Comparison of activity in pontine versus medullary neurones during swallowing. Exp Brain Res 1975;22(2):211–220 [DOI] [PubMed] [Google Scholar]
- 21.McCulloch TM, Perlman AL, Palmer PM, Van Daele DJ. Laryngeal activity during swallow, phonation, and the Valsalva maneuver: an electromyographic analysis. Laryngoscope 1996;106(11):1351–1358 [DOI] [PubMed] [Google Scholar]
- 22.Palmer PM, Luschei ES, Jaffe D, McCulloch TM. Contributions of individual muscles to the submental surface electromyogram during swallowing. J Speech Lang Hear Res 1999;42(6):1378–1391 [DOI] [PubMed] [Google Scholar]
- 23.Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol (1985) 1999;86(5):1663–1669 [DOI] [PubMed] [Google Scholar]
- 24.Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Laryngeal activity during upright vs. supine swallowing. J Appl Physiol (1985) 2002;93(2):740–745 [DOI] [PubMed] [Google Scholar]
- 25.Robbins J, Levin RL. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia 1988;3(1):11–17 [DOI] [PubMed] [Google Scholar]
- 26.Celifarco A, Gerard G, Faegenburg D, Burakoff R. Dysphagia as the sole manifestation of bilateral strokes. Am J Gastroenterol 1990;85(5):610–613 [PubMed] [Google Scholar]
- 27.Alberts MJ, Horner J, Gray L, Brazer SR. Aspiration after stroke: lesion analysis by brain MRI. Dysphagia 1992;7(3):170–173 [DOI] [PubMed] [Google Scholar]
- 28.Levine R, Robbins JA, Maser A. Periventricular white matter changes and oropharyngeal swallowing in normal individuals. Dysphagia 1992;7(3):142–147 [DOI] [PubMed] [Google Scholar]
- 29.Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia 1997;12(3):146–156 [DOI] [PubMed] [Google Scholar]
- 30.Humbert IA, Fitzgerald ME, McLaren DG, et al. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage 2009;44(3):982–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Chen Q, Yu B, et al. Structural and functional changes mapped in the brains of amyotrophic lateral sclerosis patients with/without dysphagia: a pilot study. Amyotroph Lateral Scler 2009;10(5–6):280–287 [DOI] [PubMed] [Google Scholar]
- 32.Kern M, Birn R, Jaradeh S, et al. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 2001;280(4):G531–G538 [DOI] [PubMed] [Google Scholar]
- 33.Li S, Luo C, Yu B, et al. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J Neurol Neurosurg Psychiatry 2009;80(12):1320–1329 [DOI] [PubMed] [Google Scholar]
- 34.Malandraki GA, Perlman AL, Karampinos DC, Sutton BP. Reduced somatosensory activations in swallowing with age. Hum Brain Mapp 2011;32(5):730–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp 2009;30(10):3209–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowell SY, Reynolds RC, Chen G, Horwitz B, Ludlow CL. Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Exp Brain Res 2012;219(1):85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logemann J. Evaluation and Treatment of Swallowing Disorders. 2nd ed. Austin, TX: Pro-Ed; 1998 [Google Scholar]
- 38.Suiter DM, Leder SB. Clinical utility of the 3-ounce water swallow test. Dysphagia 2008;23(3):244–250 [DOI] [PubMed] [Google Scholar]
- 39.Martino R, Silver F, Teasell R, et al. The Toronto Bedside Swallowing Screening Test (TOR-BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke 2009;40(2):555–561 [DOI] [PubMed] [Google Scholar]
- 40.Patterson JM, McColl E, Carding PN, Kelly C, Wilson JA. Swallowing performance in patients with head and neck cancer: a simple clinical test. Oral Oncol 2009;45(10):904–907 [DOI] [PubMed] [Google Scholar]
- 41.Sheppard JJ, Hochman R, Baer C. The dysphagia disorder survey: validation of an assessment for swallowing and feeding function in developmental disability. Res Dev Disabil 2014;35(5):929–942 [DOI] [PubMed] [Google Scholar]
- 42.Mann G. MASA: The Mann Assessment of Swallowing Ability. Clifton Park, NY: Singular; 2002 [Google Scholar]
- 43.Carnaby GD, Crary MA. Development and validation of a cancer-specific swallowing assessment tool: MASA-C. Support Care Cancer 2014;22(3):595–602 [DOI] [PubMed] [Google Scholar]
- 44.Wheeler Hegland K, Troche MS, Brandimore AE, Davenport PW, Okun MS. Comparison of voluntary and reflex cough effectiveness in Parkinson’s disease. Parkinsonism Relat Disord 2014;20(11):1226–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kallesen M, Psirides A, Huckabee ML. Comparison of cough reflex testing with videoendoscopy in recently extubated intensive care unit patients. J Crit Care 2016 [DOI] [PubMed] [Google Scholar]
- 46.Miles A, Moore S, McFarlane M, Lee F, Allen J, Huckabee ML. Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav 2013;118:25–31 [DOI] [PubMed] [Google Scholar]
- 47.Shellikeri S, Yunusova Y, Green JR, et al. Electrical impedance myography in the evaluation of the tongue musculature in amyotrophic lateral sclerosis. Muscle Nerve 2015;52(4):584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crary MA, Sura L, Carnaby G. Validation and demonstration of an isolated acoustic recording technique to estimate spontaneous swallow frequency. Dysphagia 2013;28(1):86–94 [DOI] [PubMed] [Google Scholar]
- 49.Yoshida M, Kikutani T, Tsuga K, Utanohara Y, Hayashi R, Akagawa Y. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia 2006;21(1):61–65 [DOI] [PubMed] [Google Scholar]
- 50.Butler SG, Stuart A, Leng X, et al. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol A Biol Sci Med Sci 2011;66(4):452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia 2008;23(4):392–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonilha HS, Humphries K, Blair J, et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia 2013;28(1):77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoeckli SJ, Huisman TA, Seifert B, Martin-Harris BJ. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia 2003;18(1):53–57 [DOI] [PubMed] [Google Scholar]
- 54.Stokely SL, Molfenter SM, Steele CM. Effects of barium concentration on oropharyngeal swallow timing measures. Dysphagia 2014;29(1):78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Logemann J. Manual for the Videofluoroscopic Study of Swallowing. London, UK: Taylor & Francis; 1986 [Google Scholar]
- 56.Lefton-Greif M, Carson K, Pinto J, McGrattan K, Wright J, Martin-Harris B. A novel tool for standardization of videofluoroscopic swallowing assessment in bottle-fed children. Paper presented at: 24th Annual Dysphagia Research Society Meeting; 2016; Tucson, AZ [Google Scholar]
- 57.Protection ICoR. Implications of commission recommendations that doses be kept as low as readily achievable. ICRP Report 1973;22:1–18 [Google Scholar]
- 58.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 1996;11(2):93–98 [DOI] [PubMed] [Google Scholar]
- 59.Hutcheson K, Barringer D, Knott J, et al. Dynamic Imaging Grade of Swallowing Toxicity (DIGEST): scale development and validation. Paper presented at: 24th Annual Dysphagia Research Society Meeting; February 24–26 2016; Tucson, AZ [Google Scholar]
- 60.Rademaker AW, Pauloski BR, Logemann JA, Shanahan TK. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res 1994;37(2):314–325 [DOI] [PubMed] [Google Scholar]
- 61.Kellen PM, Becker DL, Reinhardt JM, Van Daele DJ. Computer-assisted assessment of hyoid bone motion from videofluoroscopic swallow studies. Dysphagia 2010;25(4):298–306 [DOI] [PubMed] [Google Scholar]
- 62.Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res 2014;57(3):768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson TZ, Obeidin F, Davidoff AA, et al. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp 2014;(87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonard R, Belafsky PC, Rees CJ. Relationship between fluoroscopic and manometric measures of pharyngeal constriction: the pharyngeal constriction ratio. Ann Otol Rhinol Laryngol 2006;115(12):897–901 [DOI] [PubMed] [Google Scholar]
- 65.Kidder TM, Langmore SE, Martin BJ. Indications and techniques of endoscopy in evaluation of cervical dysphagia: comparison with radiographic techniques. Dysphagia 1994;9(4):256–261 [DOI] [PubMed] [Google Scholar]
- 66.Leder SB. Serial fiberoptic endoscopic swallowing evaluations in the management of patients with dysphagia. Arch Phys Med Rehabil 1998;79(10):1264–1269 [DOI] [PubMed] [Google Scholar]
- 67.Manor Y, Mootanah R, Freud D, Giladi N, Cohen JT. Video-assisted swallowing therapy for patients with Parkinson’s disease. Parkinsonism Relat Disord 2013;19(2):207–211 [DOI] [PubMed] [Google Scholar]
- 68.Hoppers P, Holm SE. The role of fiberoptic endoscopy in dysphagia rehabilitation. J Head Trauma Rehabil 1999;14(5):475–485 [DOI] [PubMed] [Google Scholar]
- 69.Denk DM, Kaider A. Videoendoscopic biofeedback: a simple method to improve the efficacy of swallowing rehabilitation of patients after head and neck surgery. ORL J Otorhinolaryngol Relat Spec 1997;59(2):100–105 [DOI] [PubMed] [Google Scholar]
- 70.da Silva AP, Lubianca Neto JF, Santoro PP. Comparison between videofluoroscopy and endoscopic evaluation of swallowing for the diagnosis of dysphagia in children. Otolaryngol Head Neck Surg 2010;143(2):204–209 [DOI] [PubMed] [Google Scholar]
- 71.Wu CH, Hsiao TY, Chen JC, Chang YC, Lee SY. Evaluation of swallowing safety with fiberoptic endoscope: comparison with videofluoroscopic technique. Laryngoscope 1997;107(3):396–401 [DOI] [PubMed] [Google Scholar]
- 72.Kelly AM, Leslie P, Beale T, Payten C, Drinnan MJ. Fibreoptic endoscopic evaluation of swallowing and videofluoroscopy: does examination type influence perception of pharyngeal residue severity? Clin Otolaryngol 2006;31(5):425–432 [DOI] [PubMed] [Google Scholar]
- 73.McConnel FMS. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope 1988;98(1):71–78 [DOI] [PubMed] [Google Scholar]
- 74.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut 2008;57(3):405–423 [DOI] [PubMed] [Google Scholar]
- 75.Lee TH, Lee JS, Hong SJ, et al. Impedance analysis using high-resolution impedance manometry facilitates assessment of pharyngeal residue in patients with oropharyngeal dysphagia. J Neurogastroenterol Motil 2014;20(3):362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee TH, Lee JS, Park JW, et al. High-resolution impedance manometry facilitates assessment of pharyngeal residue and oropharyngeal dysphagic mechanisms. Dis Esophagus 2014;27(3):220–229 [DOI] [PubMed] [Google Scholar]
- 77.Omari T, Kritas S, Cock C. New insights into pharyngo-esophageal bolus transport revealed by pressure-impedance measurement. Neurogastroenterol Motil 2012;24(11):e549–e556 [DOI] [PubMed] [Google Scholar]
- 78.Omari TI, Dejaeger E, Tack J, Vanbeckevoort D, Rommel N. An impedance-manometry based method for non-radiological detection of pharyngeal postswallow residue. Neurogastroenterol Motil 2012;24(7):e277–e284 [DOI] [PubMed] [Google Scholar]
- 79.Omari TI, Dejaeger E, Van Beckevoort D, et al. A novel method for the nonradiological assessment of ineffective swallowing. Am J Gastroenterol 2011;106(10):1796–1802 [DOI] [PubMed] [Google Scholar]
- 80.Omari TI, Ferris L, Dejaeger E, Tack J, Vanbeckevoort D, Rommel N. Upper esophageal sphincter impedance as a marker of sphincter opening diameter. Am J Physiol Gastrointest Liver Physiol 2012;302(9):G909–G913 [DOI] [PubMed] [Google Scholar]
- 81.Omari TI, Jones CA, Hammer MJ, et al. Predicting the activation states of the muscles governing upper esophageal sphincter relaxation and opening. Am J Physiol Gastrointest Liver Physiol 2016:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omari TI, Rommel N, Szczesniak MM, et al. Assessment of intraluminal impedance for the detection of pharyngeal bolus flow during swallowing in healthy adults. Am J Physiol Gastrointest Liver Physiol 2006;290(1):G183–G188 [DOI] [PubMed] [Google Scholar]
- 83.Szczesniak MM, Rommel N, Dinning PG, Fuentealba SE, Cook IJ, Omari TI. Intraluminal impedance detects failure of pharyngeal bolus clearance during swallowing: a validation study in adults with dysphagia. Neurogastroenterol Motil 2009;21(3):244–252 [DOI] [PubMed] [Google Scholar]
- 84.Szczesniak MM, Rommel N, Dinning PG, Fuentealba SE, Cook IJ, Omari TI. Optimal criteria for detecting bolus passage across the pharyngo-oesophageal segment during the normal swallow using intraluminal impedance recording. Neurogastroenterol Motil 2008;20(5):440–447 [DOI] [PubMed] [Google Scholar]
- 85.Jones CA, Ciucci MR, Hammer MJ, McCulloch TM. A multisensor approach to improve manometric analysis of the upper esophageal sphincter. Laryngoscope 2016;126(3):657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones CA, Hammer MJ, Hoffman MR, McCulloch TM. Quantifying contributions of the cricopharyngeus to upper esophageal sphincter pressure changes by means of intramuscular electromyography and high-resolution manometry. Ann Otol Rhinol Laryngol 2014;123(3):174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Omari TI, Wiklendt L, Dinning P, Costa M, Rommel N, Cock C. Upper esophageal sphincter mechanical states analysis: a novel methodology to describe UES relaxation and opening. Front Syst Neurosci 2014;8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyer JP, Jones CA, Walczak CC, McCulloch TM. Three-dimensional manometry of the upper esophageal sphincter in swallowing and nonswallowing tasks. Laryngoscope 2016. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knigge MA, Thibeault S, McCulloch TM. Implementation of high-resolution manometry in the clinical practice of speech language pathology. Dysphagia 2014;29(1):2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knigge MA, Thibeault S. Relationship between tongue base region pressures and vallecular clearance. Dysphagia 2016; ( Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 91.Jungheim M, Janhsen AM, Miller S, Ptok M. Impact of neuromuscular electrical stimulation on upper esophageal sphincter dynamics: a high-resolution manometry study. Ann Otol Rhinol Laryngol 2015;124(1):5–12 [DOI] [PubMed] [Google Scholar]
- 92.Kwiatek MA, Mirza F, Kahrilas PJ, Pandolfino JE. Hyperdynamic upper esophageal sphincter pressure: a manometric observation in patients reporting globus sensation. Am J Gastroenterol 2009;104(2):289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lan Y, Xu G, Dou Z, Wan G, Yu F, Lin T. Biomechanical changes in the pharynx and upper esophageal sphincter after modified balloon dilatation in brainstem stroke patients with dysphagia. Neurogastroenterol Motil 2013;25(12):e821–e829 [DOI] [PubMed] [Google Scholar]
- 94.Lee TH, Lee JS. High-resolution manometry for oropharyngeal dysphagia in a patient with large cervical osteophytes. J Neurogastroenterol Motil 2012;18(3):338–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nativ-Zeltzer N, Kahrilas PJ, Logemann JA. Manofluorography in the evaluation of oropharyngeal dysphagia. Dysphagia 2012;27(2):151–161 [DOI] [PubMed] [Google Scholar]
- 96.Park D, Oh Y, Ryu JS. Findings of abnormal videofluoroscopic swallowing study identified by high-resolution manometry parameters. Arch Phys Med Rehabil 2016;97(3):421–428 [DOI] [PubMed] [Google Scholar]
- 97.Rommel N, Omari T. The use of high resolution manometry for the assessment of swallowing in infants and young children. B-ENT 2008;4:27–39 [Google Scholar]
- 98.Takasaki K, Umeki H, Enatsu K, Kumagami H, Takahashi H. Evaluation of swallowing pressure in a patient with amyotrophic lateral sclerosis before and after cricopharyngeal myotomy using high-resolution manometry system. Auris Nasus Larynx 2010;37(5):644–647 [DOI] [PubMed] [Google Scholar]
- 99.Rommel N, Omari TI, Selleslagh M, et al. High-resolution manometry combined with impedance measurements discriminates the cause of dysphagia in children. Eur J Pediatr 2015;174(12):1629–1637 [DOI] [PubMed] [Google Scholar]
- 100.Cock C, Besanko L, Kritas S, et al. Maximum upper esophageal sphincter (UES) admittance: a non-specific marker of UES dysfunction. Neurogastroenterol Motil 2016;28(2):225–233 [DOI] [PubMed] [Google Scholar]
- 101.Hoffman MR, Jones CA, Geng Z, et al. Classification of high-resolution manometry data according to videofluoroscopic parameters using pattern recognition. Otolaryngol Head Neck Surg 2013;149(1):126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoffman MR, Mielens JD, Omari TI, Rommel N, Jiang JJ, McCulloch TM. Artificial neural network classification of pharyngeal high-resolution manometry with impedance data. Laryngoscope 2013;123(3):713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mielens JD, Hoffman MR, Ciucci MR, McCulloch TM, Jiang JJ. Application of classification models to pharyngeal high-resolution manometry. J Speech Lang Hear Res 2012;55(3):892–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kritas S, Dejaeger E, Tack J, Omari T, Rommel N. Objective prediction of pharyngeal swallow dysfunction in dysphagia through artificial neural network modeling. Neurogastroenterol Motil 2016;28(3):336–344 [DOI] [PubMed] [Google Scholar]
- 105.Li S, Ma Z, Tu S, et al. Altered resting-state functional and white matter tract connectivity in stroke patients with dysphagia. Neurorehabil Neural Repair 2014;28(3):260–272 [DOI] [PubMed] [Google Scholar]
- 106.Ye C, Murano E, Stone M, Prince JL. A Bayesian approach to distinguishing interdigitated tongue muscles from limited diffusion magnetic resonance imaging. Comput Med Imaging Graph 2015;45:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kober SE, Bauernfeind G, Woller C, et al. hemodynamic signal changes accompanying execution and imagery of swallowing in patients with dysphagia: a multiple single-case near-infrared spectroscopy study. Front Neurol 2015;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teismann IK, Steinstraeter O, Warnecke T, Ringelstein EB, Pantev C, Dziewas R. Measurement of pharyngeal sensory cortical processing: technique and physiologic implications. BMC Neurosci 2009;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harris ML, Julyan P, Kulkarni B, et al. Mapping metabolic brain activation during human volitional swallowing: a positron emission tomography study using [18F]fluorodeoxyglucose. J Cereb Blood Flow Metab 2005;25(4):520–526 [DOI] [PubMed] [Google Scholar]
- 110.Paine TL, Conway CA, Malandraki GA, Sutton BP. Simultaneous dynamic and functional MRI scanning (SimulScan) of natural swallows. Magn Reson Med 2011;65(5):1247–1252 [DOI] [PubMed] [Google Scholar]
- 111.Fu M, Zhao B, Carignan C, et al. High-resolution dynamic speech imaging with joint low-rank and sparsity constraints. Magn Reson Med 2015;73(5):1820–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fujii N, Inamoto Y, Saitoh E, et al. Evaluation of swallowing using 320-detector-row multislice CT. Part I: single- and multiphase volume scanning for three-dimensional morphological and kinematic analysis. Dysphagia 2011;26(2):99–107 [DOI] [PubMed] [Google Scholar]
- 113.Inamoto Y, Fujii N, Saitoh E, et al. Evaluation of swallowing using 320-detector-row multislice CT. Part II: kinematic analysis of laryngeal closure during normal swallowing. Dysphagia 2011;26(3):209–217 [DOI] [PubMed] [Google Scholar]
- 114.Ibragimov B, Prince JL, Murano EZ, et al. Segmentation of tongue muscles from super-resolution magnetic resonance images. Med Image Anal 2015;20(1):198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller JL, Kang SM. Preliminary ultrasound observation of lingual movement patterns during nutritive versus non-nutritive sucking in a premature infant. Dysphagia 2007;22(2):150–160 [DOI] [PubMed] [Google Scholar]
- 116.Miller JL, Sonies BC, Macedonia C. Emergence of oropharyngeal, laryngeal and swallowing activity in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Hum Dev 2003;71(1):61–87 [DOI] [PubMed] [Google Scholar]
- 117.Feng X, Cartwright MS, Walker FO, Bargoil JH, Hu Y, Butler SG. Ultrasonographic evaluation of geniohyoid muscle and hyoid bone during swallowing in young adults. Laryngoscope 2015;125(8):1886–1891 [DOI] [PubMed] [Google Scholar]
- 118.Sonies BC, Wang C, Sapper DJ. Evaluation of normal and abnormal hyoid bone movement during swallowing by use of ultrasound duplex-Doppler imaging. Ultrasound Med Biol 1996;22(9):1169–1175 [DOI] [PubMed] [Google Scholar]
- 119.Loram ID, Maganaris CN, Lakie M. Use of ultrasound to make noninvasive in vivo measurement of continuous changes in human muscle contractile length. J Appl Physiol (1985) 2006;100(4):1311–1323 [DOI] [PubMed] [Google Scholar]
- 120.Cleland J, Scobbie JM, Wrench AA. Using ultrasound visual biofeedback to treat persistent primary speech sound disorders. Clin Linguist Phon 2015;29(8–10):575–597 [DOI] [PubMed] [Google Scholar]
- 121.Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil 1989;70(10):767–771 [PubMed] [Google Scholar]
- 122.Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med 2008;148(7):509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steele CM, van Lieshout PH, Pelletier CA. The influence of stimulus taste and chemesthesis on tongue movement timing in swallowing. J Speech Lang Hear Res 2012;55(1):262–275 [DOI] [PubMed] [Google Scholar]
- 124.Rosenbek JC, Robbins J, Willford WO, et al. Comparing treatment intensities of tactile-thermal application. Dysphagia 1998;13(1):1–9 [DOI] [PubMed] [Google Scholar]
- 125.Colodny N. Dysphagic independent feeders’ justifications for noncompliance with recommendations by a speech-language pathologist. Am J Speech Lang Pathol 2005;14(1):61–70 [DOI] [PubMed] [Google Scholar]
- 126.Macqueen C, Taubert S, Cotter D, Stevens S, Frost G. Which commercial thickening agent do patients prefer? Dysphagia 2003;18(1):46–52 [DOI] [PubMed] [Google Scholar]
- 127.Logemann JA, Gensler G, Robbins J, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J Speech Lang Hear Res 2008;51(1):173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia 2015;30(1):2–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Initiative IDDS. IDDSI Framework. Available at: http://iddsi.org/resources/framework/. Accessed March 21, 2016
- 130.Clark HM, O’Brien K, Calleja A, Corrie SN. Effects of directional exercise on lingual strength. J Speech Lang Hear Res 2009;52(4):1034–1047 [DOI] [PubMed] [Google Scholar]
- 131.Robbins J, Kays SA, Gangnon RE, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil 2007;88(2):150–158 [DOI] [PubMed] [Google Scholar]
- 132.Lazarus CL, Husaini H, Falciglia D, et al. Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. Int J Oral Maxillofac Surg 2014;43(5):523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging 2008;3(4):735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rogus-Pulia N, Rusche N, Hind JA, et al. Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. J Am Geriatr Soc 2016;64(2):417–424 [DOI] [PubMed] [Google Scholar]
- 135.Shaker R, Kern M, Bardan E, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol 1997;272(6 Pt 1):G1518–G1522 [DOI] [PubMed] [Google Scholar]
- 136.Logemann JA, Rademaker A, Pauloski BR, et al. A randomized study comparing the Shaker exercise with traditional therapy: a preliminary study. Dysphagia 2009;24(4):403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JA, Crary MA. Effects of Mendelsohn maneuver on measures of swallowing duration post stroke. Top Stroke Rehabil 2012;19(3):234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crary MA, Carnaby GD, LaGorio LA, Carvajal PJ. Functional and physiological outcomes from an exercise-based dysphagia therapy: a pilot investigation of the McNeill Dysphagia Therapy Program. Arch Phys Med Rehabil 2012;93(7):1173–1178 [DOI] [PubMed] [Google Scholar]
- 139.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 2009;135(5):1301–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Troche MS, Okun MS, Rosenbek JC, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology 2010;75(21):1912–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crary MA, Carnaby Mann GD, Groher ME, Helseth E. Functional benefits of dysphagia therapy using adjunctive sEMG biofeedback. Dysphagia 2004;19(3):160–164 [DOI] [PubMed] [Google Scholar]
- 142.Athukorala RP, Jones RD, Sella O, Huckabee ML. Skill training for swallowing rehabilitation in patients with Parkinson’s disease. Arch Phys Med Rehabil 2014;95(7):1374–1382 [DOI] [PubMed] [Google Scholar]
- 143.Langmore SE, McCulloch TM, Krisciunas GP, et al. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: a randomized clinical trial. Head Neck 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke 2000;31(6):1360–1364 [DOI] [PubMed] [Google Scholar]
- 145.Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res 2004;154(4):450–460 [DOI] [PubMed] [Google Scholar]
- 146.Zhang M, Tao T, Zhang ZB, et al. Effectiveness of neuromuscular electrical stimulation on patients with dysphagia with medullary infarction. Arch Phys Med Rehabil 2016;97(3):355–362 [DOI] [PubMed] [Google Scholar]
- 147.Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care 2001;46(5):466–474 [PubMed] [Google Scholar]
- 148.Lim KB, Lee HJ, Lim SS, Choi YI. Neuromuscular electrical and thermal-tactile stimulation for dysphagia caused by stroke: a randomized controlled trial. J Rehabil Med 2009;41(3):174–178 [DOI] [PubMed] [Google Scholar]
- 149.Permsirivanich W, Tipchatyotin S, Wongchai M, et al. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: a randomized controlled study. J Med Assoc Thai 2009;92(2):259–265 [PubMed] [Google Scholar]
- 150.Bülow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia 2008;23(3):302–309 [DOI] [PubMed] [Google Scholar]
- 151.Christiaanse ME, Mabe B, Russell G, Simeone TL, Fortunato J, Rubin B. Neuromuscular electrical stimulation is no more effective than usual care for the treatment of primary dysphagia in children. Pediatr Pulmonol 2011;46(6):559–565 [DOI] [PubMed] [Google Scholar]
- 152.Heijnen BJ, Speyer R, Baijens LW, Bogaardt HC. Neuromuscular electrical stimulation versus traditional therapy in patients with Parkinson’s disease and oropharyngeal dysphagia: effects on quality of life. Dysphagia 2012;27(3):336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sheppard JJ. Using motor learning approaches for treating swallowing and feeding disorders: a review. Lang Speech Hear Serv Sch 2008;39(2):227–236 [DOI] [PubMed] [Google Scholar]
- 154.Martin-Harris B, McFarland D, Hill EG, et al. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil 2015;96(5):885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carnaby GD, Harenberg L. What is “usual care” in dysphagia rehabilitation: a survey of USA dysphagia practice patterns. Dysphagia 2013;28(4):567–574 [DOI] [PubMed] [Google Scholar]
- 156.Jones CA, Knigge MA, McCulloch TM. Speech pathologist practice patterns for evaluation and management of suspected cricopharyngeal dysfunction. Dysphagia 2014;29(3):332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krisciunas GP, Sokoloff W, Stepas K, Langmore SE. Survey of usual practice: dysphagia therapy in head and neck cancer patients. Dysphagia 2012;27(4):538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Malandraki GA, Rajappa A, Kantarcigil C, Wagner E, Ivey C, Youse K. The intensive dysphagia rehabilitation approach applied to patients with neurogenic dysphagia: a case series design study. Arch Phys Med Rehabil 2016;97(4):567–574 [DOI] [PubMed] [Google Scholar]
- 159.Hutcheson K, Kelly S, Barrow M, et al. Offering more for persistent dysphagia after head & neck cancer: The evolution of boot camp swallowing therapy. Paper presented at: Combined Otolaryngology Spring Meetings (COSM); April 22–26 2015; Boston, MA [Google Scholar]
- 160.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;83(1):210–219 [DOI] [PubMed] [Google Scholar]
- 161.Hutcheson KA, Bhayani MK, Beadle BM, et al. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngol Head Neck Surg 2013;139(11):1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krisciunas GP, Golan H, Marinko LN, Pearson W, Jalisi S, Langmore SE. A novel manual therapy program during radiation therapy for head and neck cancer—our clinical experience with 5 patients. Clin Otolaryngol 2015 [DOI] [PubMed] [Google Scholar]
- 163.Geng Z, Hoffman MR, Jones CA, McCulloch TM, Jiang JJ. Three-dimensional analysis of pharyngeal high-resolution manometry data. Laryngoscope 2013;123(7):1746–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hannam AG, Stavness IK, Lloyd JE, Fels SS, Miller AJ, Curtis DA. A comparison of simulated jaw dynamics in models of segmental mandibular resection versus resection with alloplastic reconstruction. J Prosthet Dent 2010;104(3):191–198 [DOI] [PubMed] [Google Scholar]
- 165.Curtis N. Craniofacial biomechanics: an overview of recent multibody modelling studies. J Anat 2011;218(1):16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngol Clin North Am 2013;46(6):957–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nicosia MA. A planar finite element model of bolus containment in the oral cavity. Comput Biol Med 2007;37(10):1472–1478 [DOI] [PubMed] [Google Scholar]
- 168.Snavely AC, Harrington DP, Li Y. A latent variable transformation model approach for exploring dysphagia. Stat Med 2014;33(25):4337–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burns CL, Ward EC, Hill AJ, Phillips N, Porter L. conducting real-time videofluoroscopic swallow study via telepractice: a preliminary feasibility and reliability study. Dysphagia 2016 [DOI] [PubMed] [Google Scholar]
- 170.Kantarcigil C, Sheppard J, Gordon A, Friel K, Malandraki G. A telehealth approach to conducting clinical swallowing evaluations in children with cerebral palsy. Res Dev Disabil 2016;55:207–217 [DOI] [PubMed] [Google Scholar]
- 171.Malandraki GA, McCullough G, He X, McWeeny E, Perlman AL. Teledynamic evaluation of oropharyngeal swallowing. J Speech Lang Hear Res 2011;54(6):1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malandraki GA, Markaki V, Georgopoulos VC, Bauer JL, Kalogeropoulos I, Nanas S. An international pilot study of asynchronous teleconsultation for oropharyngeal dysphagia. J Telemed Telecare 2013;19(2):75–79 [DOI] [PubMed] [Google Scholar]
- 173.Perlman AL, Witthawaskul W. Real-time remote telefluoroscopic assessment of patients with dysphagia. Dysphagia 2002;17(2):162–167 [DOI] [PubMed] [Google Scholar]
- 174.Sharma S, Ward EC, Burns C, Theodoros D, Russell T. Assessing swallowing disorders online: a pilot telerehabilitation study. Telemed J E Health 2011;17(9):688–695 [DOI] [PubMed] [Google Scholar]
- 175.Ward E, Crombie J, Trickey M, Hill A, Theodoros D, Russell T. Assessment of communication and swallowing post-laryngectomy: a telerehabilitation trial. J Telemed Telecare 2009;15(5):232–237 [DOI] [PubMed] [Google Scholar]
- 176.Ward EC, Sharma S, Burns C, Theodoros D, Russell T. Validity of conducting clinical dysphagia assessments for patients with normal to mild cognitive impairment via telerehabilitation. Dysphagia 2012;27(4):460–472 [DOI] [PubMed] [Google Scholar]
- 177.Ward EC, Burns CL, Theodoros DG, Russell TG. Evaluation of a clinical service model for dysphagia assessment via telerehabilitation. Int J Telemed Appl 2013;2013:918526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Malandraki GA, Roth M, Sheppard JJ. Telepractice for pediatric dysphagia: a case study. Int J Telerehabil 2014;6(1):3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wall LR, Ward EC, Cartmill B, Hill AJ, Porceddu SV. Examining user perceptions of SwallowIT: a pilot study of a new telepractice application for delivering intensive swallowing therapy to head and neck cancer patients. J Telemed Telecare 2015. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 180.Wall LR, Cartmill B, Ward EC, et al. “ScreenIT”: computerized screening of swallowing, nutrition and distress in head and neck cancer patients during (chemo)radiotherapy. Oral Oncol 2016;54:47–53 [DOI] [PubMed] [Google Scholar]
- 181.Fung E, Järvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Phys 2015;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee C, Kim H, Harburg D, et al. Biological lipid membranes for on-demand, wireless drug delivery from a thin, bioresorbable implant. NPG Asia Materials 2015;7(11):e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Klomjai W, Lackmy-Vallée A, Roche N, Pradat-Diehl P, Marchand-Pauvert V, Katz R. Repetitive transcranial magnetic stimulation and transcranial direct current stimulation in motor rehabilitation after stroke: an update. Ann Phys Rehabil Med 2015;58(4):220–224 [DOI] [PubMed] [Google Scholar]
- 184.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 2014;4:CD008208. [DOI] [PubMed] [Google Scholar]
- 185.Li H, Wang J, Li C, Xiao Z. Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults. Cochrane Database Syst Rev 2014;9:CD009083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.CADTH Rapid Response Reports. Transcranial Magnetic Stimulation for the Treatment of Adults with PTSD, GAD, or Depression: A Review of Clinical Effectiveness and Guidelines, Ottawa (ON): Canadian Agency for Drugs and Technologies in Health Copyright (c) 2014 Canadian Agency for Drugs and Technologies in Health. 2014. Available at http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0070405/. Accessed March 30, 2016 [PubMed] [Google Scholar]
- 187.Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving aphasia in patients with aphasia after stroke. Cochrane Database Syst Rev 2015;5:CD009760. [DOI] [PubMed] [Google Scholar]
- 188.Pisegna JM, Kaneoka A, Pearson WG Jr, Kumar S, Langmore SE. Effects of non-invasive brain stimulation on post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Clin Neurophysiol 2016;127(1):956–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cai H, Ma B, Gao X, Gao H. Tongue acupuncture in treatment of post-stroke dysphagia. Int J Clin Exp Med 2015;8(8):14090–14094 [PMC free article] [PubMed] [Google Scholar]
- 190.Xia W, Zheng C, Zhu S, Tang Z. Does the addition of specific acupuncture to standard swallowing training improve outcomes in patients with dysphagia after stroke? A randomized controlled trial. Clin Rehabil 2016;30(3):237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kikuchi A, Seki T, Takayama S, Iwasaki K, Ishizuka S, Yaegashi N. Effect of press needles on swallowing reflex in older adults with cerebrovascular disease: a randomized double-blind controlled trial. J Am Geriatr Soc 2014;62(12):2438–2440 [DOI] [PubMed] [Google Scholar]
- 192.van der Meulen IC, May AM, de Leeuw JR, et al. Long-term effect of a nurse-led psychosocial intervention on health-related quality of life in patients with head and neck cancer: a randomised controlled trial. Br J Cancer 2014;110(3):593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wall LR, Cartmill B, Ward EC, Hill AJ, Isenring E, Porceddu SV. Evaluation of a weekly speech pathology/dietetic service model for providing supportive care intervention to head and neck cancer patients and their carers during (chemo)radiotherapy. Support Care Cancer 2016;24(3):1227–1234 [DOI] [PubMed] [Google Scholar]
- 194.Carneiro D, das Graças Wanderley de Sales Coriolano M, Belo LR, de Marcos Rabelo AR, Asano AG, Lins OG. Quality of life related to swallowing in Parkinson’s disease. Dysphagia 2014;29(5):578–582 [DOI] [PubMed] [Google Scholar]
- 195.Chen PH, Golub JS, Hapner ER, Johns MM III. Prevalence of perceived dysphagia and quality-of-life impairment in a geriatric population. Dysphagia 2009;24(1):1–6 [DOI] [PubMed] [Google Scholar]
- 196.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope 2006;116(6):883–886 [DOI] [PubMed] [Google Scholar]
- 197.Plowman-Prine EK, Sapienza CM, Okun MS, et al. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord 2009;24(9):1352–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 2008;51(1):S225–S239 [DOI] [PubMed] [Google Scholar]