Abstract
Purpose:
To explore the efficacy and safety of TQB2450 combined with anlotinib in patients with locally advanced or metastatic soft-tissue sarcoma (LA/M STS).
Patients and Methods:
This was a single arm phase II study (TQB2450-Ib-02 study) performed at two hospitals in China to assess the potency of TQB2450 combined with anlotinib in patients with LA/M STS. Patients were previously unresponsive to at least one chemotherapy regimen. Anlotinib (12 mg every day) was administered orally from day 1 to day 14 every 3 weeks. TQB2450 was administered by intravenous infusion at 1,200 mg on day 1 every 3 weeks. The primary endpoint was the objective response rate (ORR). The secondary endpoints included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and safety.
Results:
Between January 2019 and June 2020, 30 patients were enrolled. The ORR was 36.67% and the DCR was 76.67%. The median PFS was 7.85 months [95% confidence interval (CI), 2.89–23.06] and the median OS was not reached [95% CI, 10.58–not estimable (NE)]. Among the patients with alveolar soft part sarcoma (ASPS; 12/30, 40%), the ORR was 75% and the median PFS was 23.06 months (95% CI, 8.97–NE). The most common treatment related adverse events were hypothyroidism (76.67%), hypertriglyceridemia (63.33%), hypercholesterolemia (60.00%), and elevated blood lactate dehydrogenase (53.33%).
Conclusions:
The study showed the promising activity in patients with ASPS, also indicating the trend of treatment efficacy in other sarcomas. The toxicity was tolerable. More studies with larger sample size and controlled arm were warranted.
Translational Relevance.
Current systemic treatment for unresectable, locally advanced, or metastatic soft-tissue sarcoma (LA/M STS) remains unsatisfactory. The multitarget tyrosine kinase inhibitor anlotinib had gained success in the second line treatment of LA/M STS. Immunotherapy could further improve outcomes for patients with advanced STS. TQB2450 is a humanized mAb against programmed death-ligand 1 (PD-L1). Treating patients with STS who failed first-line chemotherapy [for patients with alveolar soft part sarcoma (ASPS), previous systemic treatment naїve was allowed] with TQB2450 and anlotinib led to an objective response rate of 75.00% and 11.11% for ASPS and non-ASPS subtypes, respectively. The toxicity is tolerable. This treatment combination showed the promising activity in patients with ASPS and the trend of treatment efficacy in others sarcomas.
Introduction
Soft-tissue sarcoma (STS) is a rare heterogeneous malignant tumor that originates from the mesenchymal connective tissue. More than 50 different histologic subtypes of STS are currently recognized, accounting for 1% of all adult malignant tumors and 15% of pediatric malignant tumors (1, 2).
Surgery is the standard primary treatment for most patients with STS. However, a large proportion of patients present with locally advanced or metastatic disease (LA/M STS), which is unresectable and requires systemic treatment. Anthracycline-based chemotherapy is the standard first-line treatment. For patients who failed first-line treatment, antiangiogenesis therapy is an important approach (2). Anlotinib (AL3818) is a multitarget tyrosine kinase inhibitor (TKI) against both tumor angiogenesis and tumor cell proliferation that acts by targeting VEGFR, FGFR, platelet-derived growth factor receptor, and c-Kit simultaneously (3–5). In a single-arm phase II study and a randomized phase IIB study, anlotinib showed favorable efficacy and safety profiles in patients with LA/M STS who did not respond to first-line anthracycline-based chemotherapy (6). A multi-centered phase III trial comparing anlotinib to dacarbazine is currently in progress. The preliminary result was encouraging and may support anlotinib to become the first second-line treatment agent against STS with approval from FDA through a positive controlled study.
Immunotherapy is another possible strategy in the second-line treatment of LA/M STS. Early studies involving anti-programmed death 1 (PD-1) monotherapy indicated only moderate activity, except for patients with specific subtypes such as undifferentiated pleomorphic sarcoma (UPS) and alveolar soft part sarcoma (ASPS; ref. 7). The combination of PD-1 mAb and CTLA-4 mAb is also a meaningful attempt. Nivolumab combined with ipilimumab showed encouraging efficacy in certain sarcoma subtypes (8).
Immunotherapy combined with antiangiogenic therapy has a synergistic effect on the disease. Agents targeting VEGF/VEGFR can restore the function and enhance the infiltration of effector T cells, and reduce the number of immune cells that exert immunosuppressive function (9). At the same time, antiangiogenic therapy can normalize blood vessels in a short period, which in turn stabilizes endothelial cells, increases oxygen and drug delivery, reduces vascular permeability, and promotes the infiltration of T cells into the tumor (10, 11). Two exploratory studies of the combination of pembrolizumab plus axitinib and the combination of nivolumab plus sunitinib, respectively, demonstrated the feasibility of this strategy (12, 13). Anti–PD-ligand 1 (PD-L1) antibody also showed antitumor activity in LA/M STS. Coyne and colleagues (14) reported that atezolizumab helped achieve a partial response (PR; 42%) in patients with ASPS, demonstrating the therapeutic potential of PD-L1 inhibitors. However, as the study only included patients with the ASPS subtype, further exploration of the efficacy of PD-L1 mAb in STS is needed. More importantly, the potency of the combination of antiangiogenic agents and PD-L1 mAb is unknown.
TQB2450 is a novel humanized mAb against PD-L1, which has shown encouraging activity in combination with anlotinib in the treatment of multiple tumors (15–18). Therefore, this study aimed to explore the safety and preliminary effect of TQB2450 combined with anlotinib in patients with STS who were unresponsive to at least one chemotherapy regimen.
Patients and Methods
Study design and patients
TQB2450-Ib-02 was a single-arm phase II trial of TQB2450 combined with anlotinib in patients with advanced STS. This study was conducted at Peking University Cancer Hospital & Institute (Beijing, China) and Peking University People's Hospital (Beijing, China) in China. The study was conducted in accordance with the Declaration of Helsinki and approved by the Peking University Cancer Hospital & Institute ethics committee. All patients provided written informed consent.
Eligible patients were aged 18 to 70 years, had an Eastern Cooperative Oncology Group performance status of 0 to 2, life expectancy of at least 3 months, and were diagnosed with histologically confirmed LA/M STS, including synovial sarcoma, leiomyosarcoma, ASPS, UPS/malignant fibrous histiocytoma, fibrosarcoma, clear cell sarcoma, and epithelioid sarcoma subtypes. Patients needed to have experienced disease progression while on or after at least one line of anthracycline-based chemotherapy (except for patients with ASPS and clear cell sarcoma). Key exclusion criteria included prior treatment with antiangiogenic therapy or immunotherapy.
Treatment
Anlotinib (12 mg/day) was administered orally from day 1 to day 14 every 3 weeks until we observed disease progression, unacceptable toxicity, or until the patient withdrew consent. The anlotinib dose could be reduced to 10 mg or 8 mg at the discretion of the investigator. Dose reduction was generally recommended if grade 3 or grade 4 adverse events (AE) occurred. TQB2450 was administered by intravenous infusion at a dose of 1,200 mg on day 1 every 3 weeks for 60 ± 5 minutes. The treatment of TQB2450 was limited to a total of 24 months and the dose could not be adjusted during the study. Patients with progressive disease (PD) for the first time, identified according to the RECIST version 1.1, continued to receive the treatment, and were evaluated according to the immune RECIST (iRECIST) criteria.
Outcomes
The primary endpoint was the objective response rate (ORR), which was defined as the percentage of patients with complete or partial response. Tumor assessments were performed according to the RECIST version 1.1 every 6 weeks. Efficacy was further confirmed with the iRECIST; that is, patients who had disease progression according to the RECIST 1.1 were further examined to confirm the same using the iRECIST.
The secondary endpoints included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and AEs during the study based on the NCI Common Terminology Criteria for Adverse Events (NCI CTCAE) v5.0 criteria. PFS was defined as the time from the first date of drug administration to the time of disease progression according to RECIST 1.1 or death due to any cause, whichever occurred first. OS was measured from the first date of drug administration to the date of death from any cause. Patients who were event-free or were lost to follow-up were censored at the time of the last visit.
Statistical analysis
In the SARC028 study (7), the ORR of pembrolizumab for non-ASPS patients was 18% while the ORR of the atezolizumab for patients with ASPS was 42% (14, 19). Since both ASPS and non-ASPS patients can be enrolled in our study, we assumed that the target ORR should reach 30% and the minimum clinically meaningful ORR [lower limit of the 95% confidence interval (CI)] was 15%. So, assuming a targeted ORR of 30%, with a one-sided ± of 0.05, a sample size of 24 patients was required to declare the lower limit of 95% CI for ORR to exceed 15% based on the method of Confidence Intervals for One Proportion (19). With an expected dropout rate of 20%, a total of 30 patients would need to be enrolled.
All statistical analyses were performed with SAS version 9.4. Safety analysis was performed according to descriptive statistics. The incidence of AEs is expressed as percentage. ORR and DCR are expressed as percentages with their 95% CIs calculated by the Clopper–Pearson method. PFS and OS were estimated using the Kaplan–Meier survival method, and their 95% CIs were estimated by the Brookmeyer–Crowley method. For other variables such as demographic data, continuous variables are represented as mean ± SD or median and interquartile range, and categorical variables are represented as percentage.
Data availability
Data are available from the corresponding author upon reasonable request.
Results
Patient characteristics
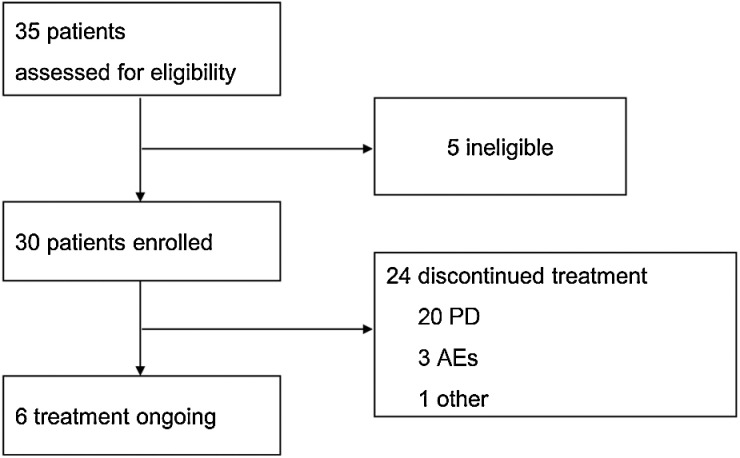
From January 2019 to June 2020, 35 patients were screened, and 30 patients (16 men and 14 women) were enrolled in the study (Fig. 1). The median age was 32.5 years. All patients had stage IV STS: 1 patient had brain metastases, 4 patients had liver metastases, 24 patients had lung metastases, and 9 patients had other metastases. A total of 16 patients received first-line treatment, 5 patients received second-line treatment, and 1 patient received third-line treatment. Notably, among the 30 patients, 12 had ASPS, 7 had synovial sarcoma, 5 had UPS, 4 had leiomyosarcoma, and 2 had other sarcoma subtypes. The baseline characteristics are summarized in Table 1.
Figure 1.
Patient flow.
Table 1.
Baseline characteristics.
| Characteristics | N = 30 |
|---|---|
| Age (years), median (range) | 32.5 (19.0–67.0) |
| Sex, n (%) | |
| Male | 16 (53.33) |
| Female | 14 (46.67) |
| ECOG performance status, n (%) | |
| 0 | 24 (80.00) |
| 1 | 6 (20.00) |
| Histology, n (%) | |
| ASPS | 12 (40.00) |
| Synovial sarcoma | 7 (23.33) |
| UPS | 5 (16.67) |
| Leiomyosarcoma | 4 (13.3) |
| Fibrosarcoma | 1 (3.33) |
| Epithelioid sarcoma | 1 (3.33) |
| Stage, n (%) | |
| IV | 30 (100.00) |
| Others | 0 |
| Brain metastasis, n (%) | |
| Yes | 1 (3.33) |
| No | 29 (96.67) |
| Liver metastasis, n (%) | |
| Yes | 4 (13.33) |
| No | 26 (86.67) |
| Lung metastasis, n (%) | |
| Yes | 24 (80.00) |
| No | 6 (20.00) |
| Another metastasis, n (%) | |
| Yes | 9 (30.00) |
| No | 21 (70.00) |
| Surgical history, n (%) | |
| Yes | 27 (90.00) |
| No | 3 (10.00) |
| Previous chemotherapy, n (%) | |
| Yes | 22 (73.33) |
| No | 8 (26.67) |
| Previous radiotherapy, n (%) | |
| Yes | 6 (20.00) |
| No | 24 (80.00) |
| Previous systemic therapy, n (%) | |
| First-line | 16 (53.33) |
| Second-line | 5 (16.6) |
| Third-line | 1 (3.33) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
The median duration of follow-up for PFS was 22.77 months. At the data cut-off date of October 8, 2021, 6 (20%) patients were still under treatment, and 24 patients discontinued treatment, including 20 (83%) with PD, 3 (13%) with grade 3–4 AEs, and 1 (13%) who withdrew consent (Fig. 1). The median duration of follow-up for OS was 24.74 months. At the data cut-off date, 11 patients had died.
Clinical efficacy
At the data cut-off date of October 8, 2021, 1 patient showed a complete response (CR), and 10 patients showed a partial response; thus, the study met its primary endpoint with an ORR of 36.67% (95% CI, 19.93–56.14; Table 2). In addition, 12 patients had stable disease (SD). The DCR was 76.67% (95% CI, 57.72–90.07; Table 2). Clinical benefit rate (CBR), which was defined as the percentage of patients who achieved CR, PR, and SD lasting at least 6 months, was 46.67%. The best percentage change for the sum of tumor diameters from baseline is shown in Fig. 2A. Rapid and long-term responses were observed. The median time to a tumor response was 2.96 months (95% CI, 1.41–6.24), and the duration of response was not reached (Fig. 2B; Supplementary Fig. S1).
Table 2.
Best objective response.
| Total | ASPS | Non-ASPS | SS | UPS | |
|---|---|---|---|---|---|
| N | 30 | 12 | 18 | 7 | 5 |
| PD | 4 (13.33) | — | 4 (22.22) | 3 (42.86) | 1 (20.00) |
| iUPD | 3 (10.00) | — | 3 (16.67) | — | 1 (20.00) |
| SD | 12 (40.00) | 3 (25.00) | 9 (50.00) | 4 (57.14) | 3 (60.00) |
| PR | 10 (33.33) | 8 (66.67) | 2 (11.11) | — | — |
| CR | 1 (3.33) | 1 (8.33) | — | — | — |
| ORR | 36.67 | 75.00 | 11.11 | 0 | 0 |
| 95% CI | 19.93–56.14 | 42.81–94.51 | 1.38–34.71 | — | — |
| DCR | 76.67 | 100 | 61.11 | 57.14 | 60.00 |
| 95% CI | 57.72–90.07 | 73.54–100 | 35.75–82.70 | 18.41–90.10 | 14.66–94.73 |
Abbreviations: SS, synovial sarcoma; iUPD, unconfirmed immune PD.
Figure 2.
A, Best percentage change for the sum of tumor diameters from baseline. B, Time to response and duration of study treatment. ES, epithelioid sarcoma; FS, fibrosarcoma; LMS, leiomyosarcoma; SS, synovial sarcoma; iUPD, unconfirmed immune PD.
The median follow-up was 24.74 months (95% CI, 22.14–25.82). At the data cut-off date, 20 (63%) patients had disease progression, and 11 (37%) patients died. The median PFS was 7.85 months (95% CI, 2.89–23.06; Fig. 3A), and the PFS at 6 months was 53.85%. The median OS was not reached (95% CI, 10.58–NE; Fig. 3B), and the 12-month OS rate was 69.14%.
Figure 3.
Kaplan–Meier estimates for PFS (A) and OS (B) in all patients. Anlo+TQB, Anlotinib+TQB2450.
Among patients with ASPS, the ORR was 75.00% (95% CI, 42.81–94.51), and the DCR was 100.00% (95% CI, 73.54–100.00; Table 2). The median PFS was 23.06 months, and no patient died (Supplementary Fig. S2). In non-ASPS patients, the ORR was 11.11% (95% CI, 1.38–34.71), and the DCR was 61.11% (95% CI, 35.75–82.70; Table 2). The median PFS of patients with other histologic types was 2.89 months (95% CI, 1.54–4.80), and the median OS was 10.58 months (95% CI, 7.16–NE). The median PFS of the 7 patients with synovial sarcoma was 2.07 months (95% CI, 1.35–5.95); however, their OS was greatly extended, with the median OS reaching 17.31 months. The median OS of the 5 patients with UPS was not reached (95% CI, 4.30–NE), and the median PFS was 4.80 months (95% CI, 2.89–4.80; Supplementary Table S1).
Notably, a patient who was initially positive for hepatitis B antigen turned negative after 14 cycles of treatment, which was also observed in the patients in other ongoing trials using the same drug combination.
Safety
All patients experienced treatment-related AEs (TRAE; incidence of 100%). The most common TRAEs of any grade were hypothyroidism (76.67%), hypertriglyceridemia (63.33%), hypercholesterolemia (60.00%), and elevated blood lactate dehydrogenase (53.33%; Supplementary Table S1). Grade 3–4 TRAEs were observed in 36.67% of patients, and the most common grade 3–4 TRAEs were hypertriglyceridemia (10.00%) and increased γ-glutamyltransferase (6.67%). No grade 5 TRAE was observed (Table 3).
Table 3.
TRAEs, occurring in e10%.
| Event | Any grade, n (%) | eGrade 3, n (%) |
|---|---|---|
| Hypothyroidism | 23 (76.67) | — |
| Hypertriglyceridemia | 19 (63.33) | 3 (10.00) |
| Hypercholesterolemia | 18 (60.00) | — |
| Elevated blood lactate dehydrogenase | 16 (53.33) | — |
| Elevated alanine aminotransferase | 15 (50.00) | 1 (3.33) |
| Hand-foot syndrome | 15 (50.00) | — |
| Elevatedγ glutamyltransferase | 13 (43.33) | 2 (6.67) |
| Elevated aspartate aminotransferase | 13 (43.33) | — |
| Increased blood bilirubin | 13 (43.33) | — |
| Hyperuricemia | 12 (40.00) | — |
| Hypertension | 9 (30.00) | 1 (3.33) |
| Decreased white blood cell count | 8 (26.67) | — |
| Urinary tract infection | 8 (26.67) | — |
| Decreased neutrophil count | 8 (26.67) | — |
| Proteinuria | 8 (26.67) | — |
| Diarrhea | 8 (26.67) | — |
| Hyperthyroidism | 7 (23.33) | — |
| Elevated alkaline phosphatase | 6 (20.00) | 1 (3.33) |
| Increased creatine phosphokinase | 5 (16.67) | 1 (3.33) |
| Occult blood | 5 (16.67) | — |
| Elevated thyroid-stimulating hormone | 4 (13.33) | — |
| Weight loss | 4 (13.33) | — |
| ECG T-wave abnormality | 4 (13.33) | — |
| Increased lipase | 4 (13.33) | — |
| Sinus tachycardia | 4 (13.33) | — |
| Laryngeal hemorrhage | 3 (10.00) | — |
| Anemia | 3 (10.00) | 1 (3.33) |
| Arrhythmia | 3 (10.00) | — |
| Swollen gums | 3 (10.00) | — |
| Sinus bradycardia | 3 (10.00) | — |
Five cases of treatment-related serious AEs (SAE) were observed, three of which were attributed to treatment with TQB2450 (immune-related liver damage, immune-related myocarditis, and diabetic ketoacidosis), and the other two were cases of pneumothorax that may have been caused by anlotinib.
Dose reduction of anlotinib was performed for 2 patients owing to grade 3 hypertriglyceridemia and grade 2 hemoptysis, respectively. TRAEs led to treatment termination of TQB2450 in 1 patient with immune-related myocarditis.
For the 3 patients who experienced immune-related SAEs (irSAE), 2 achieved a PR, and 1 had a SD. In addition, long-term OS benefits were observed in these patients, as all 3 patients were alive at the data cut-off date.
Discussion
The study showed the safety of this combination that the incidence of grade 3 or above AEs was relatively low, and no grade 5 AE occurred. Treatment regimen was efficacy for patients with ASPS that the ORR reached 75% and the median PFS was 23.06 months while the efficacy for patients with other sarcomas needed more investigation.
Previous studies provided some positive evidence for the combination of VEGFR TKIs and PD-1 inhibitors in patients with advanced STS. Martin-Broto and colleagues (13) subsequently reported the efficacy of nivolumab plus sunitinib in patients with advanced STS in a phase Ib/II trial; combining the STS cases of phase I and phase II trials, the ORR was 21%. Wilky and colleagues (12) also demonstrated the efficacy and safety of axitinib combined with pembrolizumab for STS in a phase II trial, with an ORR of 25%, and the ORR for ASPS and non-ASPS patients was 54.5% and 9.5%, respectively. Similarly, there is also a large gap in ORR between ASPS patients and non-ASPS patients in our study (75.00% vs. 11.11%).
ASPS is a rare STS subtype that is not sensitive to chemotherapy; however, the development of new VEGFR TKIs and immune checkpoint inhibitors has led to high response rates and prolonged survival among patients with ASPS in many clinical trials. In 2019, Judson and colleagues reported the efficacy of cediranib for ASPS in a multicenter, double-blind, placebo-controlled, randomized, phase II trial (20). The study met its primary endpoint that the median percentage change in sum of target marker lesion diameters for the evaluable population was −8·3% with cediranib versus 13·4% with placebo (P = 0·0010). However, no significant difference was observed in PFS (the median PFS was 10.1 vs. 4.9 months, P = 0.28).
In an anlotinib monotherapy phase IIB study, 1 out of 38 patients with ASPS showed a complete response, and 8 achieved a partial response, with an ORR of 23.7% and the median PFS of 18.23 months (3). Single-agent atezolizumab treatment also led to a 37.2% partial response, and the median treatment duration was 11.3 months (21). Compared with monotherapy, combination treatment with anti-angiogenesis TKIs and a PD-1 inhibitor seemed to have facilitated a better tumor response for this specific subtype. For the ASPS subgroup, analysis in the above-mentioned previous trials showed that the ORR of axitinib combined with pembrolizumab was 54.5% (12), and the ORR of nivolumab combined with sunitinib was 57% (13). The combination therapy in the current study also demonstrated the promising results in ASPS, suggesting that antiangiogenic drug combined with PD-L1 inhibitor might be another choice for the treatment of ASPS. Moreover, with a median follow-up of over 2 years, the median OS was still not reached for all patients as well as for patients with ASPS, and thus follow-up is currently in progress.
This study also included 18 non-ASPS patients, including 7 synovial sarcomas, 5 UPSs, and 6 other sarcomas. Synovial sarcoma is a rare form of sarcoma with a poor prognosis. Typically, synovial sarcomas are not sensitive to immunotherapy, with only a 10% response rate to immune checkpoint inhibitors (22). The current study included 7 patients with synovial sarcoma who had a median PFS of 2.07 months. In addition, our study enrolled 5 patients with UPS, which is a rare tumor that can occur in the dermis or subcutaneous soft tissue. Their median PFS was 2.89 months, and the median OS was not reached. These results provide some insights for future studies in these subtypes.
The safety profile of anlotinib in combination with TQB2450 was found to be good. The main TRAEs above grade 3 were hypertriglyceridemia (10.00%) and increased γ-glutamyltransferase (6.67%), and patients could resume treatment after adjusting medication and symptomatic treatment. Three patients experienced irSAEs, all of whom recovered after treatment; no study-related grade 5 AEs occurred. Only 1 patient withdrew from the study due to an irSAE but subsequently achieved long survival, indicating a possible correlation between irSAE and a better outcome, which should be further investigated.
This study had some limitations. The sample size was small and was also not conducive to exploring molecular markers. The patients included in the study are a heterogeneous population biased towards ASPS. New studies with larger sample sizes for non-ASPS need to be conducted to further explore the efficacy of combination therapy. In addition, this study was a single-arm study and thus could not compare the efficacy of anlotinib combined with TQB2450 to another treatment. Despite a median follow-up time of approximately 2 years, the median OS was not reached. This will be reported separately after longer-term survival data are obtained in the future.
Conclusion
The combination of anlotinib and TQB2450 showed promising activity in patients with ASPS, indicating the synergistic effects of PD-L1 mAbs and angiogenic agents. The trend of treatment benefit was also observed in patients with non-ASPS while more investigations with larger sample size and controlled arm were needed. The safety profile was favorable. A study to explore the potency of the treatment regime including VEGFR-TKI, immune checkpoint inhibitors, and chemotherapy against non-ASPS sarcomas is ongoing.
Supplementary Material
Acknowledgments
We are thankful to all the patients who participated in this clinical trial and their families. This study was funded by the Chia Tai TianQing Pharmaceutical Group Co., Ltd. and China International Medical Foundation (No. Z-2014–06–15331).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
J. Liu reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. T. Gao reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. Z. Tan reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. S. Li reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. J. Xu reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. R. Xue reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. L. Zhang reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. W. Guo reports grants from Chia Tai Tianqing Pharmaceutical Group Co., Ltd; and grants from China International Medical Foundation (No. Z-2014-06-15331) during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
J. Liu: Conceptualization, resources, supervision, investigation, methodology, writing–original draft, writing–review and editing. T. Gao: Resources, investigation, methodology, writing–review and editing. Z. Tan: Resources, data curation, formal analysis, investigation, methodology, writing–review and editing. S. Li: Resources, investigation, methodology, writing–review and editing. J. Xu: Resources, investigation, methodology, writing–review and editing. C. Bai: Resources, investigation, methodology, writing–review and editing. R. Xue: Resources, investigation, methodology, writing–review and editing. L. Xie: Resources, investigation, methodology, writing–review and editing. L. Zhang: Resources, investigation, methodology, writing–review and editing. Z. Fan: Conceptualization, resources, supervision, investigation, methodology, writing–original draft, writing–review and editing. W. Guo: Conceptualization, resources, supervision, investigation, methodology, writing–original draft, writing–review and editing.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020.CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S,et al. NCCN Guidelines Insights: Soft tissue sarcoma, version 1.2021.J Natl Compr Cancer Netw 2020;18:1604–12. [DOI] [PubMed] [Google Scholar]
- 3. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G,et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma.Clin Cancer Res 2018;24:5233–8. [DOI] [PubMed] [Google Scholar]
- 4. Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y,et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor.Cancer Sci 2018;109:1207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1.Gene 2018;654:77–86. [DOI] [PubMed] [Google Scholar]
- 6. Li S. Anlotinib: A novel targeted drug for bone and soft tissue sarcoma.Front Oncol 2021;11:664853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J,et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial.Lancet Oncol 2017;18:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN,et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials.Lancet Oncol 2018,Mar 19:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity.Front Immunol 2018;9:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity.Exp Mol Med 2020;52:1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, Ancukiewicz M,et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients.Proc Nat Acad Sci USA 2015;112:14325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D,et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial.Lancet Oncol 2019;20:837–48. [DOI] [PubMed] [Google Scholar]
- 13. Martin-Broto J, Hindi N, Grignani G, Martinez-Trufero J, Redondo A, Valverde C,et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: a multicenter, single-arm, phase Ib/II trial.J Immunother Cancer 2020;8:e001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coyne GO, Sharon E, Moore N,et al. Phase II study of atezolizumab in patients with alveolar soft part sarcoma [abstract]. In: Proceedings of the 2018 CTOS Annual Meeting;2018 Nov 14–17; Rome, Italy. Abstract nr. 021. [Google Scholar]
- 15. Zhou J, Gong J, Cao Y, Peng Z, Yuan J, Wang X,et al. Anlotinib plus TQB2450 in patients with advanced refractory biliary tract cancer (BTC): An open-label, dose-escalating, and dose-expansion cohort of phase Ib trial.J Clin Oncol 2021;39:292. [Google Scholar]
- 16. Cheng Y, Cui H, Wu C, Wang Y, Zhang T, Xin Y,et al. A phase Ib study of TQB2450 in combination with anlotinib in patients with advanced solid tumour [abstract]. In: Proceedings of the ESMO Virtual Congress 2020 Sept 19—21/Oct 16—18. Amsterdam (Netherlands): Elsevier; 2020. Abstract nr. 532MO. [Google Scholar]
- 17. Lan C, Zhao J, Yang F, Li R, Huang Y, Wang J,et al.Anlotinib in combination with TQB2450 in patients with platinum-resistant or platinum-refractory ovarian cancer (ACTION): a multicenter, single-arm, open-label, phase 1b trial. J Clin Oncol 39:15s, 2021 (suppl; abstr 5557). [Google Scholar]
- 18. Wang J, Xu B, Sun T, Ouyang Q, Han Y, Li Q,et al. A phase Ib study of TQB2450 plus anlotinib in patients with advanced triple-negative breast cancer.J Clin Oncol 2021;39:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd ed. Hoboken (NJ): Wiley;2003.
- 20. Judson I, Morden JP, Kilburn L, Leahy M, Benson C, Bhadri V,et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial.Lancet Oncol 2019;20:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naqash AR, O'sullivan Coyne GH, Moore N, Sharon E, Takebe N, Fino KK,et al. Phase II study of atezolizumab in advanced alveolar soft part sarcoma (ASPS).J Clin Oncol 2021;39:11519. [Google Scholar]
- 22. He M, Abro B, Kaushal M, Chen L, Chen T, Gondim M,et al. Tumor mutation burden and checkpoint immunotherapy markers in primary and metastatic synovial sarcoma.Hum Pathol 2020;100:15–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.