Abstract
Purpose:
To conduct an updated exploratory analysis of overall survival (OS) with a longer median follow-up of 73.3 months and evaluate the prognostic value of molecular analysis by circulating tumor DNA (ctDNA).
Patients and Methods:
Patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2−) advanced breast cancer (ABC) were randomized 2:1 to receive palbociclib (125 mg orally/day; 3/1 week schedule) and fulvestrant (500 mg intramuscularly) or placebo and fulvestrant. This OS analysis was performed when 75% of enrolled patients died (393 events in 521 randomized patients). ctDNA analysis was performed among patients who provided consent.
Results:
At the data cutoff (August 17, 2020), 258 and 135 deaths occurred in the palbociclib and placebo groups, respectively. The median OS [95% confidence interval (CI)] was 34.8 months (28.8–39.9) in the palbociclib group and 28.0 months (23.5–33.8) in the placebo group (stratified hazard ratio, 0.81; 95% CI, 0.65–0.99). The 6-year OS rate (95% CI) was 19.1% (14.9–23.7) and 12.9% (8.0–19.1) in the palbociclib and placebo groups, respectively. Favorable OS with palbociclib plus fulvestrant compared with placebo plus fulvestrant was observed in most subgroups, particularly in patients with endocrine-sensitive disease, no prior chemotherapy for ABC and low circulating tumor fraction and regardless of ESR1, PIK3CA, or TP53 mutation status. No new safety signals were identified.
Conclusions:
The clinically meaningful improvement in OS associated with palbociclib plus fulvestrant was maintained with >6 years of follow-up in patients with HR+/HER2− ABC, supporting palbociclib plus fulvestrant as a standard of care in these patients.
Translational Relevance.
The long-term effect of cyclin-dependent kinase 4/6 inhibition plus endocrine therapy for advanced breast cancer (ABC) is an important area of research, and circulating tumor DNA (ctDNA) may provide valuable prognostic information. In PALOMA-3, palbociclib plus fulvestrant improved median overall survival (OS) versus placebo plus fulvestrant after 44.8 months of follow-up. In this updated exploratory analysis of the PALOMA-3 clinical trial, a continued improvement in median OS with palbociclib plus fulvestrant versus placebo plus fulvestrant was observed (34.8 vs. 28.0 months). Favorable OS in the palbociclib group was also observed across most subgroups regardless of ESR1, PIK3CA, or TP53 mutation status. These findings support the palbociclib plus fulvestrant regimen as a standard of care in patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative ABC and show that ctDNA analysis can provide prognostic information.
Introduction
Palbociclib is a first-in-class orally active cyclin-dependent kinase 4/6 (CDK4/6) inhibitor that is approved for the treatment of patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2−) advanced breast cancer (ABC; ref. 1). Findings from the PALOMA-3 study in women with HR+/HER2− ABC showed that palbociclib plus fulvestrant significantly prolonged progression-free survival (PFS) compared with placebo plus fulvestrant [11.2 vs. 4.6 months, respectively; hazard ratio, 0.50 (95% CI, 0.40–0.62]; one-sided P < 0.0001 (2, 3). At a median follow-up of 44.8 months, the final protocol-specified overall survival (OS) analysis demonstrated a longer OS with palbociclib plus fulvestrant compared with placebo plus fulvestrant that was not statistically significant [34.9 vs. 28.0 months, respectively; hazard ratio, 0.81 (95% CI, 0.64–1.03); one-sided P = 0.0429] (4). Moreover, sensitivity to prior endocrine therapy, nonvisceral disease, no prior chemotherapy for ABC, and an Eastern Cooperative Oncology Group performance status of 0 were identified as significant positive prognostic factors for OS. Patients without prior chemotherapy for ABC had longer median OS in the palbociclib arm versus placebo arm, while patients with prior chemotherapy for ABC had a similar OS among treatment arms (5).
With CDK4/6 inhibitors plus endocrine therapy now considered a standard of care for the treatment of HR+/HER2− ABC (6), a clinical need exists to identify patients who may be at risk of progressing early while receiving palbociclib plus endocrine therapy. Other than estrogen receptor and progesterone receptor expression, no clinical biomarkers presently exist for palbociclib treatment sensitivity or resistance (7). In addition, there is limited information regarding associations between genetic mutations and clinical outcome in ABC (8). However, circulating tumor DNA (ctDNA) is rapidly being incorporated in the clinical setting and during medication development as a preferred liquid biopsy diagnostic to analyze the genetic features of tumors (8–10).
This updated exploratory analysis reports OS results from PALOMA-3 with a longer median follow-up of 73.3 months and evaluated the prognostic value of genomic abnormalities identified by ctDNA.
Patients and Methods
Study design and patients
Detailed methodology of the PALOMA-3 clinical study has been described previously (11). Briefly, PALOMA-3 is a double-blind, multicenter, phase III study that included premenopausal or postmenopausal women with HR+/HER2− ABC whose disease had progressed on endocrine therapy. Patients were allowed up to one prior line of chemotherapy for ABC. Patients were excluded if they had active, uncontrolled, or symptomatic brain metastases or symptomatic visceral spread or were at risk for life-threatening complications. All patients provided written informed consent prior to enrollment. The study was approved by the Institutional Review Board at each study center, and conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki, with monitoring by an academic steering committee.
Randomization and masking
Patients were randomized 2:1 to receive palbociclib (125 mg orally per day for 3 weeks, followed by 1 week off) and fulvestrant (500 mg intramuscularly every 14 days for the first three injections followed by every 28 days) or matching placebo and fulvestrant. Patients were randomly assigned by the investigator or research staff via a centralized interactive web- and voice-based randomization system, which also generated the random allocation sequence. For each stratification level, random assignments to the treatments were made in a block size of six. Patients were assigned on the basis of three stratification factors: sensitivity to prior hormonal therapy, menopausal status, and presence of visceral metastases. The patients, investigators, and research staff were all blinded to treatment group assignment.
Outcomes
Investigator-assessed PFS was the primary endpoint of the study, and OS was a key secondary endpoint. OS was defined as the time from the date of randomization to the date of death due to any cause. In the absence of confirmation of death, survival time was censored to the last date the patient was known to be alive. Here we report the final unplanned exploratory OS analysis with 393 events in 521 randomized patients (75% of the total study population; data cut-off date: August 17, 2020) with a median follow-up of 73.3 months. The median OS was estimated using the Kaplan–Meier method; 95% CIs and hazard ratios were estimated using Cox proportional hazards models.
Plasma samples were collected and stored for ctDNA analyses at baseline and the end of treatment. A custom, amplicon error–corrected sequencing approach was used for ctDNA analyses, as reported previously (8, 12). The targeted panel of 17 targetable driver and CDK4/6-related genes included all exons (RB1, CDK4, CDK6, CDKN1A, CDKN1B, and NF1; exons 5–8 of TP53) and hotspots (ERBB2, PIK3CA, AKT1, ESR1, FGFR1, FGFR2, FGFR3, KRAS, HRAS, and NRAS). The allele fraction cutoff for calling gene mutations as positive was 0.5% allele frequency for whole genes and 0.3% for hotspots. PFS and OS rates were assessed among treatment arms in patients with circulating tumor fraction above or below a cutoff of 10% (high and low purity), as reported previously (8).
Adverse events (AE) were reported and severity was graded according to the NCI Common Terminology Criteria for Adverse Events, Version 4.0. Safety findings were summarized descriptively.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Results
A total of 521 patients were randomized into the study (347 patients in the palbociclib plus fulvestrant group and 174 patients in the placebo plus fulvestrant group). As reported previously (3), patient demographics and baseline clinical characteristics were generally similar between the treatment groups. As of the data cut-off date of August 17, 2020, 393 deaths had occurred; 258 deaths in the palbociclib plus fulvestrant group and 135 deaths in the placebo plus fulvestrant group. The median duration of follow-up was 73.3 months (95% CI, 73.0–74.0). A total of 18 patients remained on study treatment, including 15 patients (4.3%) in the palbociclib plus fulvestrant group and 3 patients (1.7%) in the placebo plus fulvestrant group (Supplementary Fig. S1).
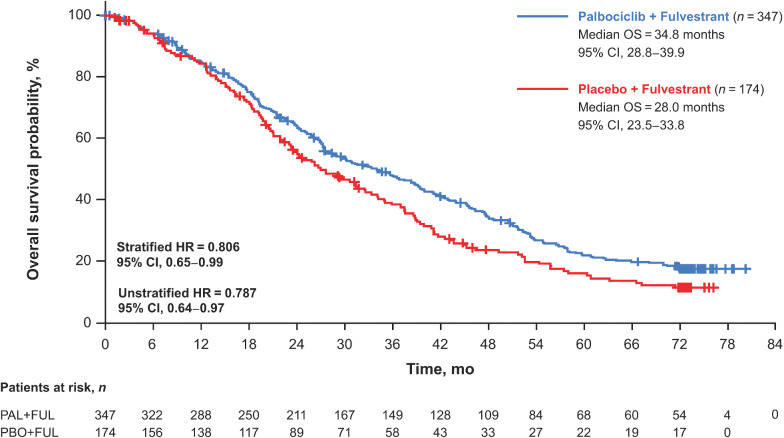
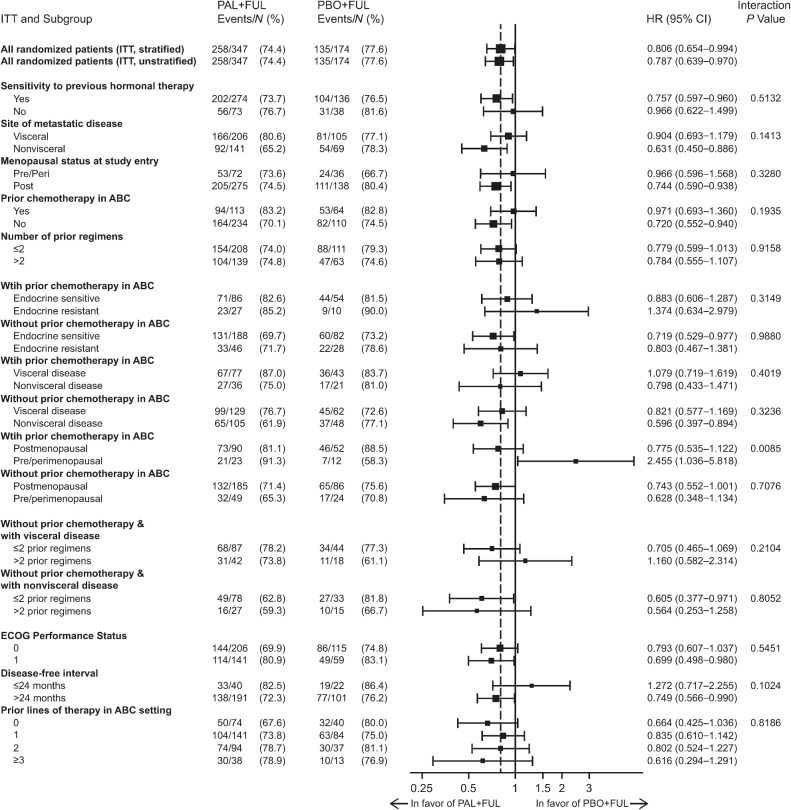
The median OS was 34.8 months (95% CI, 28.8–39.9) in the palbociclib plus fulvestrant group and 28.0 months (95% CI, 23.5–33.8) in the placebo plus fulvestrant group [stratified HR, 0.81 (95% CI, 0.65–0.99); Fig. 1]. The 5-year OS rate (95% CI) was 23.3% (18.7–28.2) in the palbociclib group versus 16.7% (11.2–23.3) in the placebo group; the 6-year OS rate was 19.1% (14.9–23.7) versus 12.9% (8.0–19.1) in the palbociclib and placebo groups, respectively. In the subgroup of patients who had not received prior chemotherapy for ABC (n = 344), the median OS was 39.3 months (95% CI, 34.5–44.4) in the palbociclib plus fulvestrant group and 29.7 months (95% CI, 23.8–35.5) in the placebo plus fulvestrant group [hazard ratio, 0.72 (95% CI, 0.55–0.94); Supplementary Fig. S2A]. In the subgroup of patients who had received prior chemotherapy for ABC (n = 177), the median OS was 24.6 months (95% CI, 21.3–30.0) in the palbociclib plus fulvestrant group and 24.3 months (95% CI, 18.9–36.3) in the placebo plus fulvestrant group [hazard ratio, 0.97 (95% CI, 0.69–1.36); Supplementary Fig. S2B]. Favorable OS with palbociclib plus fulvestrant compared with placebo plus fulvestrant was observed in most of the subgroups evaluated (Fig. 2).
Figure 1.
Kaplan–Meier curves of OS in all patients in the intent-to-treat population. FUL, fulvestrant; HR, hazard ratio; OS, overall survival; PAL, palbociclib; PBO, placebo.
Figure 2.
OS by subgroup. P value determined from a one-sided unstratified log-rank test; one-sided P value from log-rank test reflects the sign of the test statistic (z-Score). ABC, advanced breast cancer; ECOG, Eastern Cooperative Oncology Group; EOT, end of treatment; FUL, fulvestrant; ITT, intent-to-treat; PAL, palbociclib; PBO, placebo.
ESR1, PIK3CA, and TP53 gene mutation data by ctDNA analysis were available for 331 patients at day 1 and 195 patients at the end of treatment. Among the 331 patients at day 1, there were 160 of 331 (48.3%) patients with any detected mutation and 171 of 331 (51.7%) patients with no detected mutation. Similar to results previously reported in O'Leary and colleagues 2021 (8), ESR1, PIK3CA, and TP53 gene mutations were reported in 72 (21.8%), 55 (16.6%), and 51 (15.4%) patients, respectively, at day 1. At the end of treatment, mutations were reported in 61 (18.4%), 52 (15.7%), and 41 (12.4%) patients. At day 1 or the end of treatment, mutations were reported in 97 (29.3%), 71 (21.5%), and 57 (17.2%) patients. The most prevalent ESR1 mutation variants were D538G, Y537S, E380Q, and Y537N. Herein, data are presented for mutational status subgroups defined on the basis of the presence of a mutation at either day 1 or at the end of treatment.
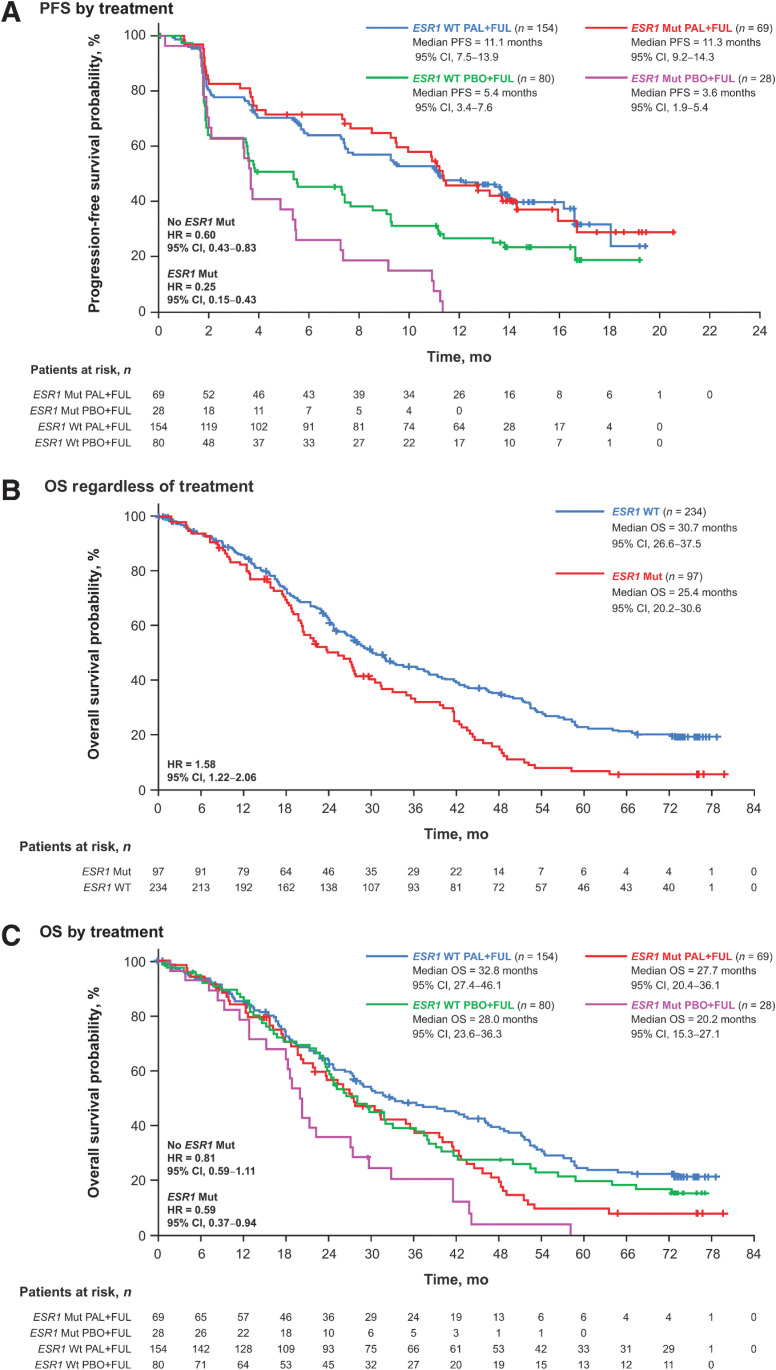
Palbociclib plus fulvestrant provided a PFS benefit regardless of ESR1 mutation status at day 1 or end of treatment (Fig. 3A). In patients without ESR1 mutations, the median PFS was 11.1 months (95% CI, 7.5–13.9) with palbociclib plus fulvestrant compared with 5.4 months (95% CI, 3.4–7.6) with placebo plus fulvestrant [HR, 0.60 (95% CI, 0.43–0.83)]. In patients with ESR1 mutations, the median PFS was 11.3 months (95% CI, 9.2–14.3) in patients receiving palbociclib plus fulvestrant versus 3.6 months (95% CI, 1.9–5.4) in patients receiving placebo plus fulvestrant [hazard ratio, 0.25 (95% CI, 0.15–0.43)].
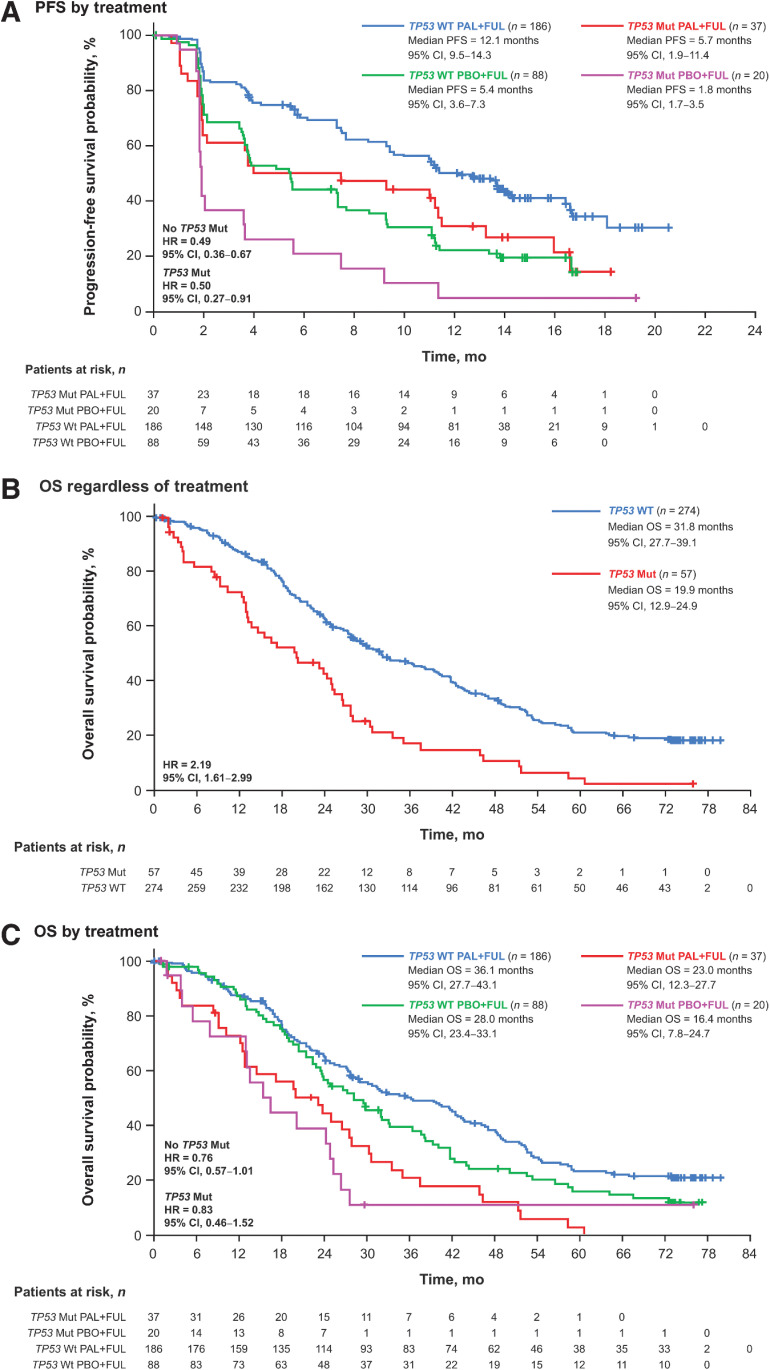
Figure 3.
Outcomes by ESR1 mutation status in ctDNA at day 1 or end of treatment. PFS by treatment (A), OS regardless of treatment (B), and OS by treatment (C). ctDNA, circulating tumor DNA; ESR1, estrogen receptor 1; FUL, fulvestrant; HR, hazard ratio; mut, mutation; OS, overall survival; PAL, palbociclib; PBO, placebo; PFS, progression-free survival; WT, wild type.
ESR1 mutations were prognostic for OS [hazard ratio, 1.58 (95% CI, 1.22–2.06); Fig. 3B] and were highly associated with shorter OS [<4 vs. ≥4 years; OR, 0.36 (95% CI, 0.19–0.68); Supplementary Table S1]. Regardless of ESR1 mutation status at day 1 or end of treatment, palbociclib plus fulvestrant provided OS benefit versus placebo plus fulvestrant (Fig. 3C). In patients without ESR1 mutations, the median OS was 32.8 months (95% CI, 27.4–46.1) with palbociclib plus fulvestrant and 28.0 months (95% CI, 23.6–36.3) with placebo plus fulvestrant [hazard ratio, 0.81 (95% CI, 0.59–1.11)]. In patients with ESR1 mutations, the median OS was 27.7 months (95% CI, 20.4–36.1) with palbociclib plus fulvestrant and 20.2 months (95% CI, 15.3–27.1) with placebo plus fulvestrant [hazard ratio, 0.59 (95% CI, 0.37–0.94)].
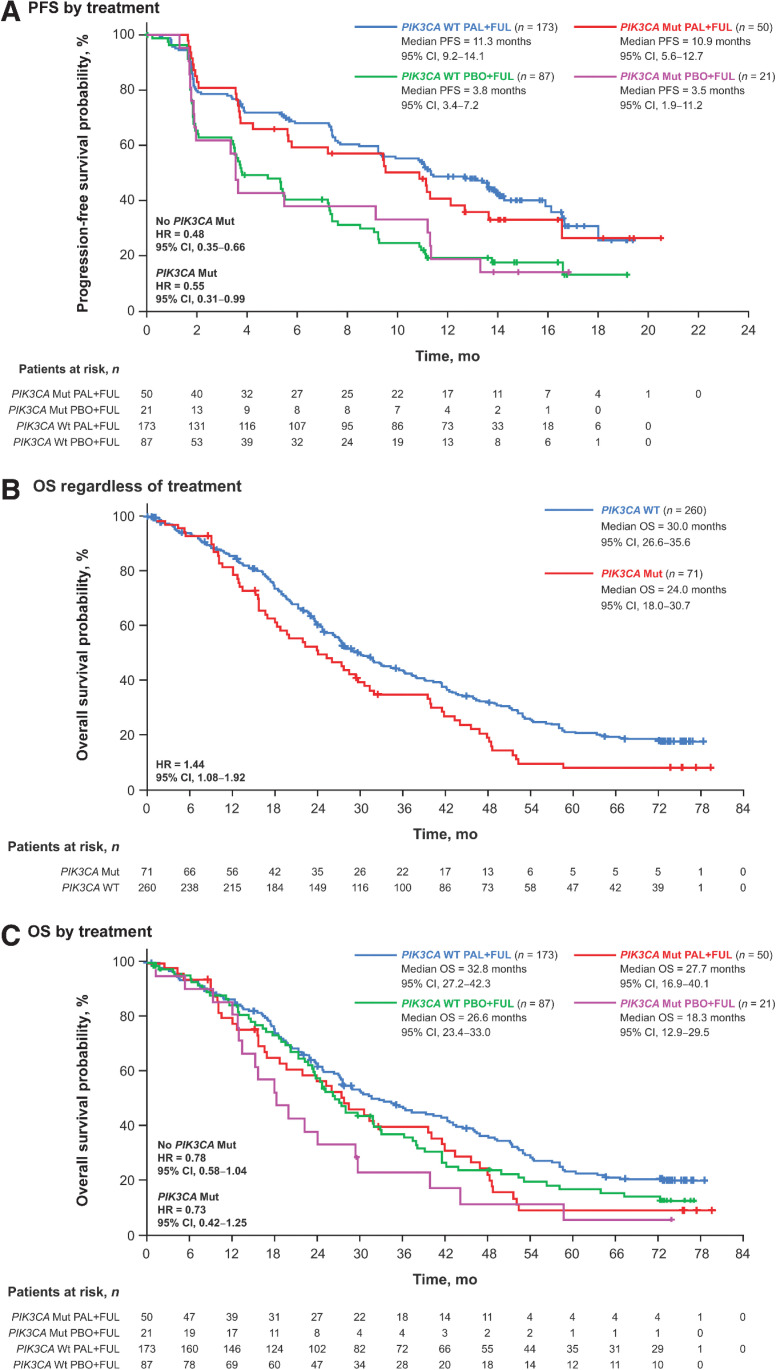
Palbociclib plus fulvestrant provided a PFS benefit compared with placebo plus fulvestrant regardless of PIK3CA status (Fig. 4A). In patients without PIK3CA mutations, the median PFS was 11.3 months (95% CI, 9.2–14.1) in patients receiving palbociclib plus fulvestrant and 3.8 months (95% CI, 3.4–7.2) in patients receiving placebo plus fulvestrant [HR, 0.48 (95% CI, 0.35–0.66)]. Palbociclib plus fulvestrant also improved median PFS compared with placebo plus fulvestrant in patients with PIK3CA mutations [10.9 months (95% CI, 5.6–12.7) vs. 3.5 months (95% CI, 1.9–11.2), respectively; hazard ratio, 0.55 (95% CI, 0.31–0.99)].
Figure 4.
Outcomes by PIK3CA mutation status in ctDNA at day 1 or end of treatment. PFS by treatment (A), OS regardless of treatment (B), and OS by treatment (C). ctDNA, circulating tumor DNA; FUL, fulvestrant; HR, hazard ratio; mut, mutation; OS, overall survival; PAL, palbociclib; PBO, placebo; PFS, progression-free survival; PIK3CA, phosphatidylinositol 3-kinase catalytic alpha polypeptide; WT, wild type.
In addition, PIK3CA mutations were prognostic for OS [hazard ratio, 1.44 (95% CI, 1.08–1.92); Fig. 4B] and were numerically associated with shorter OS [<4 vs. ≥4 years; OR, 0.55 (95% CI, 0.29–1.08); Supplementary Table S1]. Palbociclib plus fulvestrant provided an OS benefit compared with placebo plus fulvestrant regardless of PIK3CA mutation status (Fig. 4C). Among patients without PIK3CA mutations, the median OS was 32.8 months (95% CI, 27.2–42.3) in patients receiving palbociclib plus fulvestrant and 26.6 months (95% CI, 23.4–33.0) in patients receiving placebo plus fulvestrant [hazard ratio, 0.78 (95% CI, 0.58–1.04)]. In patients with PIK3CA mutations, the median OS was 27.7 months (95% CI, 16.9–40.1) with palbociclib plus fulvestrant and 18.3 months (95% CI, 12.9–29.5) with placebo plus fulvestrant [hazard ratio, 0.73 (95% CI, 0.42–1.25)].
Regardless of TP53 mutation status, palbociclib plus fulvestrant was associated with improvement in PFS compared with placebo plus fulvestrant (Fig. 5A). In patients without TP53 mutations, the median PFS was 12.1 months (95% CI, 9.5–14.3) in the palbociclib plus fulvestrant group versus 5.4 months (95% CI, 3.6–7.3) in the placebo plus fulvestrant group (hazard ratio, 0.49; 95% CI, 0.36–0.67). In patients with TP53 mutations, the median PFS was 5.7 months (95% CI, 1.9–11.4) with palbociclib plus fulvestrant compared with 1.8 months (95% CI, 1.7–3.5) with placebo plus fulvestrant [hazard ratio, 0.50 (95% CI, 0.27–0.91)].
Figure 5.
Outcomes by TP53 mutation status in ctDNA at day 1 or end of treatment. PFS by treatment (A), OS regardless of treatment (B), and OS by treatment (C). ctDNA, circulating tumor DNA; FUL, fulvestrant; HR, hazard ratio; mut, mutation; OS, overall survival; PAL, palbociclib; PBO, placebo; PFS, progression-free survival; TP53, tumor protein 53; WT, wild type.
TP53 mutations were prognostic for OS [hazard ratio, 2.19 (95% CI, 1.61–2.99); Fig. 5B] and were highly associated with shorter OS [<4 vs. ≥4 years; OR, 0.23 (95% CI, 0.09–0.60)] and prior chemotherapy in the metastatic setting [OR, 1.95 (95% CI, 1.09–3.49); Supplementary Table S1]. Palbociclib plus fulvestrant also provided an OS benefit versus placebo plus fulvestrant regardless of TP53 mutation status (Fig. 5C). In patients without TP53 mutation, the median OS was 36.1 months (95% CI, 27.7–43.1) with palbociclib plus fulvestrant and 28.0 months (95% CI, 23.4–33.1) with placebo plus fulvestrant [hazard ratio, 0.76 (95% CI, 0.57–1.00)]. In patients with TP53 mutation, the median OS was 23.0 months (95% CI, 12.3–27.7) with palbociclib plus fulvestrant and 16.4 months (95% CI, 7.8–24.7) with placebo plus fulvestrant [hazard ratio, 0.83 (95% CI, 0.46–1.52)]. TP53 mutations were more frequent among patients who received prior chemotherapy for ABC in both the palbociclib and placebo groups (24.3% and 23.3%, respectively) compared with patients who did not receive prior chemotherapy (13.1% and 15.4%, respectively). No difference in the frequency of PIK3CA and ESR1 mutations was observed in patients with or without prior chemotherapy in ABC.
Survival analyses by mutational burden demonstrated that a low-circulating tumor fraction (<10%) was associated with longer PFS in the palbociclib plus fulvestrant group compared with the placebo plus fulvestrant group [median PFS (95% CI), 13.6 months (11.3–16.6) vs. 5.5 months (3.7–9.1); hazard ratio, 0.46 (95% CI, 0.33–0.64); Supplementary Fig. S3A]. Similar results were observed with OS [44.5 months (35.6–51.6) vs. 28.0 months (23.4–36.3); hazard ratio, 0.61 (95% CI, 0.45–0.83); Supplementary Fig. S3B].
Neutropenia was the most frequently reported AE in the palbociclib plus fulvestrant group, as reported previously (palbociclib vs. placebo: any grade, 84.3% vs. 3.5%; grade 3, 57.7% vs. 0; grade 4, 11.9% vs. 0; Supplementary Table S2; refs. 4, 11). Other frequently reported AEs in the palbociclib plus fulvestrant group included leukopenia (any grade, 60.3%), infections (55.1%), fatigue (43.8%), nausea (36.2%), and anemia (32.2%). Febrile neutropenia was reported in 1.2% of patients in the palbociclib plus fulvestrant group and was not reported in the placebo plus fulvestrant group.
After discontinuing from study treatment, 267 patients (76.9%) in the palbociclib plus fulvestrant group and 144 patients (82.8%) in the placebo plus fulvestrant group received subsequent systemic anticancer therapy (Supplementary Table S3). Generally, patients in the palbociclib plus fulvestrant group received fewer subsequent chemotherapies, endocrine therapies, mTOR kinase inhibitors, or CDK4/6 inhibitors than patients in the placebo plus fulvestrant group.
Discussion
With over 6 years of median follow-up in the PALOMA-3 trial, this analysis demonstrated a clinically meaningful improvement in OS of 6.8 months with palbociclib plus fulvestrant versus placebo plus fulvestrant in patients with HR+/HER2− ABC who progressed on prior endocrine therapy. The prolonged OS benefit was particularly evident in patients with no prior chemotherapy in the advanced or metastatic disease setting. The lack of difference in OS among patients with prior chemotherapy may be due to patients with prior chemotherapy having tumors with a higher frequency of TP53 mutations and less endocrine sensitivity. In addition, PFS and OS were generally more favorable with palbociclib plus fulvestrant compared with placebo plus fulvestrant across subgroups, including patients with low circulating tumor fraction and regardless of ESR1, PIK3CA, or TP53 mutation status as detected by ctDNA. However, although a PFS and OS benefit was observed regardless of mutation status, patients without the mutations had a better prognosis and outcome. Moreover, no new safety signals were identified. These findings support the continued benefit of palbociclib plus fulvestrant as a standard of care in patients with HR+/HER2− ABC, regardless of ESR1, PIK3CA, or TP53 mutation status.
The current median OS of 34.8 months in the palbociclib plus fulvestrant group compared with 28.0 months in the placebo plus fulvestrant group [stratified hazard ratio, 0.81 (95% CI, 0.65–0.99)] are consistent with the results from the previous final protocol-specified OS analysis, which also demonstrated a trend toward improved OS with palbociclib plus fulvestrant versus placebo plus fulvestrant [34.9 vs. 28.0 months, respectively; hazard ratio, 0.81 (95% CI, 0.64–1.03)] (4). In patients with HR+/HER2− ABC, it is often difficult for treatments to demonstrate a significant OS improvement in clinical studies. Adequate power for the statistical analysis of OS is challenging to achieve when evaluating diseases associated with long postprogression survival (13). Moreover, clinical studies often allow patients in the placebo group to cross over to receive active treatments after disease progression, confounding the interpretation of OS results (14).
However, OS findings from abemaciclib (interim) and ribociclib phase III studies of patients with HR+/HER2− ABC who did not receive prior chemotherapy for ABC are available (15, 16). In the MONARCH 2 study, at a median follow-up duration of 47.7 months, abemaciclib plus fulvestrant improved median OS compared with placebo plus fulvestrant [46.7 vs. 37.3 months, respectively; hazard ratio, 0.76 (95% CI, 0.61–0.95)] (15). In the MONALEESA-3 study, at a median follow-up duration of 56.3 months, the median OS was 53.7 months with ribociclib plus fulvestrant and 41.5 months with placebo plus fulvestrant [hazard ratio, 0.73 (95% CI, 0.59–0.90)] (16). Each of these study results were statistically significant. Moreover, while some survival data were still immature, an FDA pooled analysis of CDK4/6 inhibitors for HER2− ABC demonstrated a hazard ratio of 0.77 (95% CI, 0.67–0.89) for OS between pooled CDK4/6 inhibitors plus fulvestrant versus placebo plus fulvestrant groups from PALOMA-3, MONARCH 2, and MONALEESA-3 (17). Together, these findings suggest CDK4/6 inhibitors provide a survival benefit for patients with HR+/HER2− ABC.
Previous analyses using ctDNA data from patients in the PALOMA-3 study have demonstrated that high-circulating tumor fraction of >10% is associated with a shorter median PFS compared with circulating tumor factor ≤10% in the palbociclib plus fulvestrant group [9.2 vs. 13.6 months; hazard ratio, 1.62 (95% CI, 1.17–2.24)] (8). The current analysis of PALOMA-3 further suggests a most significant benefit in patients with a low mutational burden (≤10%) who were treated with palbociclib plus fulvestrant compared with placebo plus fulvestrant. TP53 mutations and FGFR1 amplification were also associated with a shorter PFS [hazard ratio, 1.84 (95% CI, 1.27–2.65) and hazard ratio, 2.91 (95% CI, 1.61–5.25), respectively] (8). In addition, previous analyses have shown that approximately 31% of patients receiving palbociclib plus fulvestrant acquire mutations in growth factor receptors and signal transduction pathways, including 6% of patients acquiring PIK3CA mutations and 9% acquiring ESR1 mutations (12). However, the current analysis is the first CDK4/6 inhibitor study to our knowledge to evaluate the effect of tumor mutation profiles using ctDNA analyses on OS.
In the current analysis, regardless of mutation status, palbociclib plus fulvestrant prolonged OS compared with placebo plus fulvestrant. Moreover, palbociclib plus fulvestrant improved PFS versus placebo plus fulvestrant regardless of ESR1, PIK3CA, or TP53 mutation status. Overall, findings were similar whether mutations were analyzed at day 1, at end of treatment, or at day 1 and end of treatment combined. These findings are consistent with results from a previous analysis that demonstrated that palbociclib plus fulvestrant prolonged median PFS versus placebo plus fulvestrant both in patients with ESR1 mutations [9.4 vs. 3.6 months; hazard ratio, 0.43 (95% CI, 0.25–0.74)] and without ESR1 mutations [9.5 vs. 5.4 months; hazard ratio, 0.49 (95% CI, 0.35–0.70)] (18). Our findings are also consistent with results from the MONARCH 2 study in which abemaciclib plus fulvestrant improved PFS compared with placebo plus fulvestrant in patients regardless of ESR1 or PIK3CA mutation status (19). In the current analysis, patients with ESR1 mutations gained a greater benefit from palbociclib plus fulvestrant measured by both PFS and OS. Future research is warranted to further characterize genomic features that may affect the efficacy of palbociclib plus fulvestrant.
Limitations of this study include that it is an exploratory analysis. This study is also limited by the small numbers of patients included in some of the subgroups analyzed. Nevertheless, with >6 years of median follow-up in patients with HR+/HER2− ABC, palbociclib plus fulvestrant demonstrated longer OS compared with placebo plus fulvestrant, including in most of the subgroups assessed and regardless of ESR1, PIK3CA, or TP53 mutation status.
Conclusions
In patients with HR+/HER2− ABC who had progressed on prior endocrine therapy, the clinically meaningful improvement in OS with palbociclib plus fulvestrant was maintained with >6 years of follow-up, regardless of genomic alterations, and no new safety signals were identified. These findings support palbociclib plus fulvestrant as a standard of care in patients with HR+/HER2− ABC. Moreover, ESR1, PIK3CA, and TP53 mutational status can provide prognostic value in this clinical setting.
Supplementary Material
Acknowledgments
This study was sponsored by Pfizer Inc. Editorial/medical writing support was provided by Jill Shults, PhD, and Anny Wu, PharmD, of ICON (North Wales, PA) and was funded by Pfizer Inc.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
M. Cristofanilli reports personal fees from Lilly, Sermonix, Foundation Medicine, Guardant Health, Celcuity, Iylon, and Ellipses and grants and personal fees from Pfizer and Menarini during the conduct of the study. H.S. Rugo reports grants from Pfizer Inc., Merck, Novartis, Lilly, Roche, Daiichi, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Ayala, Astellas, and Gilead and personal fees from Puma Biotechnology, Samsung, and NAPO outside the submitted work. S.-A. Im reports grants, personal fees, and other support from AstraZeneca, Eisai, Pfizer Inc., Roche, and Daiichi-Sankyo; personal fees and other support from Amgen, Lilly, MSD, Novartis, and GSK; grants from Daewoong; other support from Hanmi; and grants and other support from Boryung Pharm outside the submitted work. D.J. Slamon reports other support from BioMarin, Pfizer Inc., Novartis, Eli Lilly, Amgen, and Seattle Genetics during the conduct of the study. N. Harbeck reports personal fees from Amgen, AstraZeneca, Daiichi-Sankyo, Exact Sciences, Gilead, Lilly, MSD, Novartis, Pierre Fabre, Pfizer Inc., Roche, Sandoz, and Seagen outside the submitted work. N. Masuda reports grants and personal fees from Pfizer Inc. during the conduct of the study; grants and personal fees from Chugai, AstraZeneca, Eli Lilly, and Eisai; grants from Daiichi-Sankyo, MSD, Novartis, Sanofi, Kyowa Kirin, and Nippon Kayaku outside the submitted work; and is executive board member of JBCRG (Japan Breast Cancer Research Group), unpaid. M. Colleoni reports grants from Roche outside the submitted work. A. DeMichele reports grants from Pfizer Inc., Genentech, Novartis, and Calithera outside the submitted work. S. Loi reports grants from research funding to their institution from Novartis, Bristol Meyers Squibb, Merck, Puma Biotechnology, Eli Lilly, Nektar Therapeutics, AstraZeneca, Roche-Genentech and Seattle Genetics; nonfinancial support from consultant (not compensated) from Seattle Genetics, Novartis, Bristol Meyers Squibb, Merck, AstraZeneca and Roche-Genentech; and other support from consultant (paid to institution) from Aduro Biotech, Novartis, GlaxoSmithKline, Roche-Genentech, AstraZeneca, Silverback Therapeutics, G1 Therapeutics, Puma Biotechnology, Pfizer Inc., Gilead Therapeutics, Seattle Genetics, Daiichi-Sankyo, Merck, Amunix, Tallac Therapeutics, Eli Lilly, and Bristol Meyers Squibb outside the submitted work. H. Iwata reports grants and personal fees from Pfizer Inc. during the conduct of the study, as well as grants and personal fees from Daiichi-Sankyo, Chugai, AstraZeneca, and Sanofi and personal fees from Lilly, MSD, and Novartis outside the submitted work. B. O'Leary reports grants from Pfizer Inc. during the conduct of the study. F. André reports grants from Roche, Lilly, Novartis, Pfizer Inc., Daiichi Sankyo, and AstraZeneca outside the submitted work. S. Loibl reports grants, nonfinancial support, and other support from Pfizer Inc. during the conduct of the study; grants and other support from AbbVie, AstraZeneca, and Celgene; other support from Amgen, Bayer, BMS, EirGenix, GSK, Lilly, Merck, Pierre Fabre, Prime/Medscape, Samsung, and Sanofi; grants, nonfinancial support, and other support from Gilead, Novartis, Roche, and Daiichi-Sankyo; nonfinancial support and other support from Puma Biotechnology and Seagen outside the submitted work; in addition, S. Loibl has a patent for EP14153692.0 pending, a patent for EP21152186.9 pending, a patent for EP15702464.7 issued, a patent for EP19808852.8 pending, and a patent for Digital Ki67 Evaluator issued and with royalties paid. E. Bananis reports other support from Pfizer Inc. during the conduct of the study and other support from Pfizer Inc. outside the submitted work; and E. Bananis is an employee of and stockholder in Pfizer Inc. Y. Liu is an employee of Pfizer Inc. and owns Pfizer Inc. stocks. X. Huang reports personal fees from Pfizer Inc. during the conduct of the study and outside the submitted work. S. Kim is an employee of Pfizer Inc. N.C. Turner has received advisory board honoraria from AstraZeneca, Bristol Myers Squibb, Lilly, Merck Sharpe & Dohme, Novartis, Pfizer Inc., Roche/Genentech, GlaxoSmithKline, Zentalis Pharmaceuticals, Repare Therapeutics, and Arvinas and research funding from AstraZeneca, Bio-Rad, Pfizer Inc., Roche/Genentech, Merck Sharpe & Dohme, Guardant Health, Invitae, Inivata, Personalis, and Natera. No disclosures were reported by the other authors.
Authors' Contributions
M. Cristofanilli: Data curation, formal analysis, validation, writing–original draft, writing–review and editing. H.S. Rugo: Data curation, formal analysis, writing–original draft, writing–review and editing. S.-A. Im: Data curation, formal analysis, writing–original draft, writing–review and editing. D.J. Slamon: Data curation, formal analysis, writing–original draft, writing–review and editing. N. Harbeck: Data curation, formal analysis, writing–original draft, writing–review and editing. I. Bondarenko: Data curation, formal analysis, writing–original draft, writing–review and editing. N. Masuda: Data curation, formal analysis, writing–original draft, writing–review and editing. M. Colleoni: Data curation, formal analysis, writing–original draft, writing–review and editing. A. DeMichele: Data curation, formal analysis, writing–original draft, writing–review and editing. S. Loi: Data curation, formal analysis, writing–original draft, writing–review and editing. H. Iwata: Data curation, formal analysis, writing–original draft, writing–review and editing. B. O'Leary: Data curation, formal analysis, writing–original draft, writing–review and editing. F. André: Data curation, formal analysis, writing–original draft, writing–review and editing. S. Loibl: Data curation, formal analysis, writing–original draft, writing–review and editing. E. Bananis: Conceptualization, data curation, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. Y. Liu: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, writing–review and editing. X. Huang: Conceptualization, data curation, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. S. Kim: Conceptualization, data curation, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. M.J. Lechuga Frean: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, writing–review and editing. N.C. Turner: Data curation, formal analysis, validation, writing–original draft, writing–review and editing.
References
- 1. Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 2015;21:4760–6. [DOI] [PubMed] [Google Scholar]
- 2. Cristofanilli M, DeMichele A, Giorgetti C, Turner NC, Slamon DJ, Im SA, et al. Predictors of prolonged benefit from palbociclib plus fulvestrant in women with endocrine-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in PALOMA-3. Eur J Cancer 2018;104:21–31. [DOI] [PubMed] [Google Scholar]
- 3. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. [DOI] [PubMed] [Google Scholar]
- 4. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–36. [DOI] [PubMed] [Google Scholar]
- 5. Rugo HS, Cristofanilli M, Loibl S, Harbeck N, DeMichele A, Iwata H, et al. Prognostic factors for overall survival in patients with hormone receptor-positive advanced breast cancer: analyses from PALOMA-3. Oncologist 2021;26:e1339–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO Guideline Update. J Clin Oncol 2021;39:3959–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoninger SF, Blain SW. The ongoing search for biomarkers of CDK4/6 inhibitor responsiveness in breast cancer. Mol Cancer Ther 2020;19:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Leary B, Cutts RJ, Huang X, Hrebien S, Liu Y, Andre F, et al. Circulating tumor DNA markers for early progression on fulvestrant with or without palbociclib in ER+ advanced breast cancer. J Natl Cancer Inst 2021;113:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Mattos-Arruda L, Caldas C. Cell-free circulating tumour DNA as a liquid biopsy in breast cancer. Mol Oncol 2016;10:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malapelle U, Sirera R, Jantus-Lewintre E, Reclusa P, Calabuig-Farinas S, Blasco A, et al. Profile of the Roche cobas® EGFR mutation test v2 for non-small cell lung cancer. Expert Rev Mol Diagn 2017;17:209–15. [DOI] [PubMed] [Google Scholar]
- 11. Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 2015;373:209–19. [DOI] [PubMed] [Google Scholar]
- 12. O'Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 2018;8:1390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hurvitz SA. Evolving options for the treatment of metastatic breast cancer: progression-free survival as an endpoint. Cancer Treat Rev 2011;37:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 2020;6:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol 2021;32:1015–24. [DOI] [PubMed] [Google Scholar]
- 17. Gao JJ, Cheng J, Prowell TM, Bloomquist E, Tang S, Wedam SB, et al. Overall survival in patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer treated with a cyclin-dependent kinase 4/6 inhibitor plus fulvestrant: a US Food and Drug Administration pooled analysis. Lancet Oncol 2021;22:1573–81. [DOI] [PubMed] [Google Scholar]
- 18. Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 2016;34:2961–8. [DOI] [PubMed] [Google Scholar]
- 19. Tolaney SM, Toi M, Neven P, Sohn J, Grischke EM, Llombart-Cussac A, et al. Clinical significance of PIK3CA and ESR1 mutations in circulating tumor DNA: analysis from the MONARCH 2 study of abemaciclib plus fulvestrant. Clin Cancer Res 2022;28:1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.