Abstract
Purpose:
Limited long-term data are available on immune checkpoint inhibitor use in patients with advanced esophageal squamous cell carcinoma (ESCC). We report 3-year follow-up data from our study of nivolumab versus chemotherapy (paclitaxel or docetaxel) in patients with previously treated ESCC.
Patients and Methods:
ATTRACTION-3 was a randomized, multicenter, open-label, phase III trial. Overall survival (OS), time from randomization to death from any cause, was the primary endpoint. An exploratory subanalysis assessed OS according to the best overall response (BOR) with and without landmark at 4 months.
Results:
Of the enrolled patients, 210 received nivolumab and 209 received chemotherapy. With a minimum follow-up of 36.0 months, OS was longer in the nivolumab versus the chemotherapy group (median, 10.9 vs. 8.5 months; HR, 0.79; P = 0.0264), with 3-year OS rates of 15.3% and 8.7%, respectively. The median OS was longer with nivolumab versus chemotherapy irrespective of the BOR (complete response/partial response: 19.9 vs. 15.4 months; stable disease: 17.4 vs. 8.8 months; and progressive disease: 7.6 vs. 4.2 months). Grade 3 or higher treatment-related adverse events were reported in 40 patients (19.1%) in the nivolumab group and 133 patients (63.9%) in the chemotherapy group.
Conclusions:
Nivolumab as second-line therapy demonstrated clinically meaningful long-term improvement in OS compared with chemotherapy in previously treated patients with advanced ESCC. The OS was consistently improved in the nivolumab group compared with the chemotherapy group regardless of BOR. Nivolumab was well tolerated over the 3-year follow-up.
Translational Relevance.
Patients with advanced refractory esophageal squamous cell carcinoma (ESCC) have limited treatment options after failure of first-line standard chemotherapies. Recently, immune checkpoint inhibitors have demonstrated improved overall survival in patients with advanced ESCC. These data represent the longest follow-up survival study conducted in patients with advanced ESCC treated with a checkpoint inhibitor in a phase III setting. After a minimum of 3 years of follow-up, nivolumab demonstrated significant improvement in overall survival versus chemotherapy. Additional exploratory analyses demonstrated improved survival benefit with nivolumab irrespective of best overall response. Nivolumab was well tolerated, and no new major late-onset select treatment-related emergent adverse events were observed. Nivolumab may play a significant role in the long-term management of patients with advanced ESCC.
Introduction
Esophageal cancer is one of the most aggressive gastrointestinal cancers and is the seventh most common type of cancer globally, with approximately 604,100 new cases in 2020 (1). Although a multidisciplinary approach including surgery, chemotherapy, and radiation is currently used to manage esophageal squamous cell carcinoma (ESCC), the prognosis remains poor. In Japan, the 5-year relative survival rate of distant metastatic esophageal cancer is less than 8% (2, 3). Many patients with advanced ESCC become refractory to first-line standard chemotherapy agents (cisplatin and 5-fluorouracil). The existing second-line chemotherapy treatments, mainly docetaxel and paclitaxel, are associated with poor long-term survival and with hematologic, gastrointestinal, and neurologic toxicities (4, 5). Recently, immune checkpoint inhibitors (ICI) have been approved as a new second-line treatment option in advanced ESCC (6–8). Furthermore ICIs in combination with chemotherapy or ipilimumab demonstrated statistically significant overall survival (OS) benefit compared with chemotherapy in untreated advanced ESCC (9, 10).
Nivolumab, an ICI, demonstrated survival benefits irrespective of programmed death ligand-1 (PD-L1) status for patients with unresectable advanced or recurrent ESCC refractory to or intolerant to one prior chemotherapy in the ATTRACTION-3 study (11). After a median follow-up of 17.6 months, the study reported a median OS of 10.9 months with nivolumab versus 8.4 months with chemotherapy [HR, 0.77; 95% confidence interval (CI), 0.62–0.96; P = 0.019]. The OS rates at 18 months were 31% and 21%, and the progression-free survival (PFS) rates at 12 months were 12% and 7% with nivolumab and chemotherapy, respectively (11). Nivolumab was accordingly approved as a second-line treatment in patients with unresectable advanced or recurrent or metastatic ESCC in Japan, South Korea, the United States, Taiwan, Brazil, Lebanon, the European Commission, and Australia as of March 2021 (12–17).
In another phase III randomized study (KEYNOTE-181), pembrolizumab as a second-line therapy demonstrated a clinically meaningful improvement in OS of 9.3 months with pembrolizumab versus 6.7 months with chemotherapy (HR, 0.69; 95% CI, 0.52–0.93; P = 0.0074) in patients with EC and those with a PD-L1 combined positive score (CPS) ≥10 (18). Accordingly, pembrolizumab was approved for patients with metastatic or locally advanced ESCC with a PD-L1 CPS of ≥10 in the United States, Japan, Taiwan, and China as of November 2020 (19). Furthermore, camrelizumab has recently been approved as a second-line treatment option for patients with advanced or metastatic ESCC in China (20).
Although the long-term survival benefits of nivolumab have been reported in other malignant diseases (21–23), there are limited long-term data from large randomized phase III trials on ESCC. We previously reported the 2-year follow-up data of nivolumab treatment for ESCC in the open-label, single-arm, multicenter, phase-II ATTRACTION-1 study (24). Here we present the results of patients with a minimum of 3 years of follow-up from the phase III ATTRACTION-3 study (11). Furthermore, we present the results of an exploratory analysis to assess the relationship between OS and the best overall response (BOR).
Patients and Methods
Study design and patients
ATTRACTION-3 was a randomized, open-label, multicenter, phase III study conducted in 90 sites across Denmark, Germany, Italy, Japan, South Korea, Taiwan, the United Kingdom, and the United States. Patients aged ≥20 years with unresectable advanced or recurrent ESCC refractory or intolerant to one prior fluoropyrimidine- and platinum-based chemotherapy, and a life expectancy of at least 3 months, were eligible for inclusion. The other key inclusion criteria included the presence of at least one measurable or nonmeasurable lesion per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. All patients provided written informed consent and the study protocol was approved by the institutional review board or an independent ethics committee at each site. The study was conducted according to the Good Clinical Practice guidelines by the international council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Randomization and assessments
In total, 419 patients were enrolled and randomly assigned (1:1) to receive either nivolumab (240 mg i.v. every 2 weeks; each cycle was 6 weeks) or chemotherapy according to the investigator's choice [100 mg/m2 of paclitaxel i.v. every week for 6 weeks, followed by 1 week off (each cycle was 7 weeks) or 75 mg/m2 of docetaxel i.v. every 3 weeks (each cycle was 3 weeks)]. The patients were stratified according to geographical region (Japan vs. the rest of the world), the number of organs with metastases (≤1 vs. ≥2), and PD-L1 expression (tumor proportion score, <1% and indeterminate vs. ≥1%) before randomization. Treatment was continued until disease progression or toxicity requiring treatment discontinuation. Patients could continue treatment with nivolumab beyond the initial disease progression based on the investigators’ judgment.
The tumor response was assessed using CT or MRI based on RECIST v1.1 at baseline, every 6 weeks for 1 year, and every 12 weeks thereafter until the initiation of poststudy treatment, disease progression, or recurrence. Complete or partial responses were confirmed by two or more successive scans, performed at least 4 weeks apart. Tumor cell PD-L1 expression was assessed on at least 100 viable tumor cells by a central laboratory using IHC (PD-L1 IHC 28–8 pharmDx assay; Dako, an Agilent Technologies). Adverse events (AE) were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 throughout the treatment period and 28 days after treatment discontinuation. Serious AEs and immune-mediated AEs were assessed throughout the study period and for 100 days after treatment discontinuation. Incidences of select treatment-related adverse events (TRAE) were also evaluated.
OS defined as the time from randomization to death from any cause, was the primary endpoint. The secondary endpoints included the proportion of patients with an objective response [objective response rate (ORR); the percentage of patients whose BOR was either a complete response (CR) or a partial response (PR)], the disease control rate [DCR; the percentage of patients with CR, PR, or stable disease (SD)], the BOR, PFS, the duration of response (DOR; time from the first response to the first documented tumor progression or death) and safety. In addition, an exploratory analysis of OS by BOR and a landmark analysis of OS for 4-month survivors were performed to assess the difference in Kaplan–Meier estimates of OS according to BOR [CR+PR, SD, and progressive disease (PD)]. In the subgroup of patients who survived for at least 3 years, information about the patients’ clinical status including response, disease progression, and subsequent treatment received, was also analyzed.
Statistical analysis
The sample size estimation and statistical analysis have been described previously (11). The cut-off date for the current follow-up was May 25, 2020 (3 years postenrollment of the last patient). OS and PFS were assessed in the intention-to-treat (ITT) population, which included all randomly assigned patients. On the other hand, ORR, DCR, and DOR were assessed in patients who had target lesion measurements at baseline. Safety was assessed in all patients who received at least one dose of the study drug.
The follow-up period was defined as the time from randomization of the last patient to clinical cut-off. Summary statistics were used to describe the baseline characteristics. The Kaplan–Meier method was used to estimate the OS and PFS rates, and the significance of the differences between the two groups was explored with a two-sided stratified log-rank test. A stratified Cox proportional-hazards model was used to calculate the HRs and the corresponding two-sided 95% CIs. For the exploratory analysis, the Kaplan–Meier curves of OS by BOR were generated for the subgroups of patients with and without landmarks at 4 months. All analyses were performed using SAS version 9.4 (SAS Institute, Inc.).
Data availability statement
The data generated in this study area available within the article and its Supplementary Data files. For individual patient-level data from clinical studies, requests can be made to Ono Pharma through the following website: https://www.clinicalstudydatarequest.com/. For more information on Ono Pharma's Policy for the Disclosure of Clinical Study Data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.
Results
Baseline characteristics
Between January 7, 2016 and May 25, 2017, 590 patients were assessed for eligibility and 419 were randomly assigned to treatment: 210 received nivolumab and 209 received chemotherapy. The response evaluable population comprised 329 patients (nivolumab, n = 171; chemotherapy, n = 158) and the safety population comprised 417 patients (nivolumab, n = 209; chemotherapy, n = 208). Further details of patient disposition are available in the primary publication (11). At the clinical cutoff (May 25, 2020), the minimum follow-up period was 36.0 months. The baseline characteristics of the overall population, response evaluable subpopulation, and the BOR subpopulations are presented in Supplementary Table S1A and S1B, respectively. There were no significant differences between the two groups in the response evaluable population and the BOR subpopulations as well as the overall population.
OS
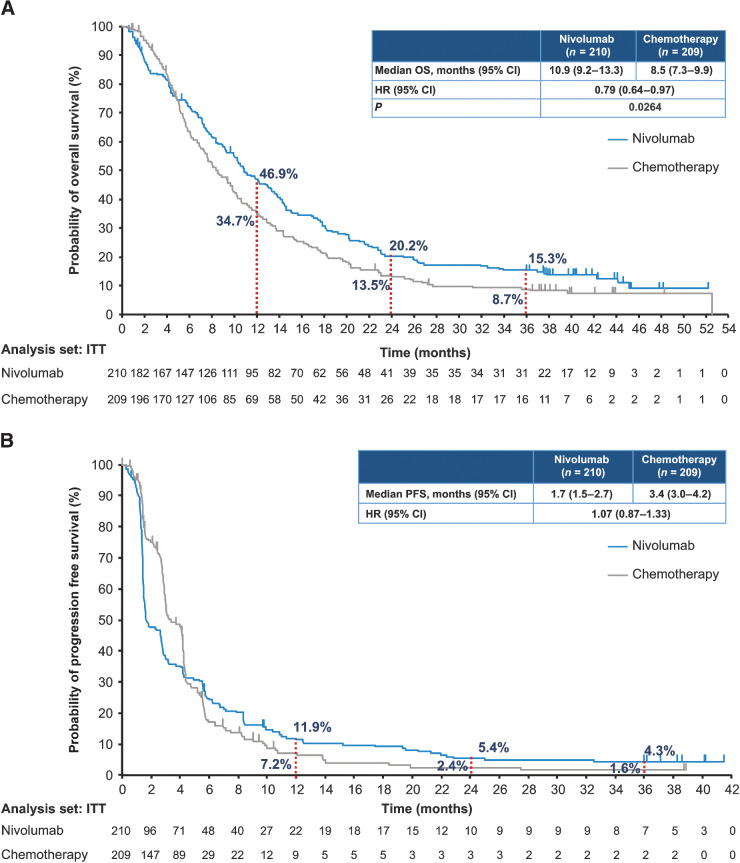
At the time of clinical cutoff (May 25, 2020), the median OS (95% Cl) in the nivolumab group versus the chemotherapy group was 10.9 (9.2–13.3) versus 8.5 (7.3–9.9) months, respectively. The risk of death was significantly lower in the nivolumab group than in the chemotherapy group (HR, 0.79; 95% CI, 0.64–0.97; P = 0.0264). Overall, a higher OS rate was observed in the nivolumab group compared with the chemotherapy group at the 1-year (46.9% vs. 34.7%), 2-year (20.2% vs. 13.5%), and 3-year (15.3% vs. 8.7%) follow-ups (Fig. 1A). The median OS in patients without measurable target lesions in the nivolumab group (n = 39) versus the chemotherapy group (n = 51) was 10.9 versus 12.0 months (HR, 1.11; 95% CI, 0.68‒1.83). On the other hand, the median OS in patients with measurable target lesions in the nivolumab group (n = 171) versus the chemotherapy group (n = 158) was 10.9 versus 7.7 months (HR, 0.74; 95% CI, 0.58–0.94). The median OS (95% CI) in patients with tumor PD-L1 expression <1% in the nivolumab group versus the chemotherapy group was 10.9 (8.4–13.9) versus 9.3 (7.2–12.0) months (HR, 0.85; 95% CI, 0.63–1.14). The corresponding values in patients with tumor PD-L1 expression ≥1% were 10.9 (8.0–14.2) versus 8.1 (6.0–9.9) months [HR, 0.70 (95% CI, 0.52–0.95); Supplementary Fig. S1].
Figure 1.
Kaplan–Meier curves for overall survival (A) and progression-free survival (B).
PFS
The median PFS (95% CI) in the nivolumab group compared with the chemotherapy group was 1.7 (1.5–2.7) versus 3.4 (3.0–4.2) months, respectively, with an HR of 1.07 (0.87–1.33; Fig. 1B). The PFS rate was higher in the nivolumab group compared with the chemotherapy group at the 1-year (11.9% vs. 7.2%), 2-year (5.4% vs. 2.4%), and 3-year (4.3% vs. 1.6%) follow-ups (Fig. 1B).
BOR
Thirty-three of 171 patients in the nivolumab group and 34 of 158 patients in the chemotherapy group achieved an objective response (Table 1). A patient on nivolumab newly achieved CR during the follow-up period. Overall, 31 patients survived for 3 years, including 23 in the nivolumab group and 8 in the chemotherapy group. The patients who survived for 3 years in the nivolumab group primarily included those with a BOR of SD or PD [14/23 (60.9%)]; while most of the patients who survived for 3 years in the chemotherapy group had a BOR of CR or PR [6/8 (75%)]. The DOR (95% CI) was 6.9 (5.4–11.1) months in the nivolumab group and 3.9 (2.8–4.2) months in the chemotherapy group. Three patients (3.2% of the 93 patients with PD) experienced suspected pseudo-progression, defined as tumor size reduction by ≥ 5% from baseline after an increase in tumor size of existing target lesions by ≥ 20% (refs. 25, 26; Supplementary Fig. S2).
Table 1.
Response and disease control in the overall population and patients who survived for 3 years.
| Overall populationa | Patients who survived for 3 yearsa | |||
|---|---|---|---|---|
| Nivolumab (n = 171) | Chemotherapy (n = 158) | Nivolumab (n = 23) | Chemotherapy (n = 8) | |
| BOR, n (%)b | ||||
| CR | 2 (1.2) | 2 (1.3) | 1 (4.3) | 2 (25.0) |
| PR | 31 (18.1) | 32 (20.3) | 8 (34.8) | 4 (50.0) |
| SD | 31 (18.1) | 65 (41.1) | 6 (26.1) | 1 (12.5) |
| PD | 93 (54.4) | 51 (32.3) | 8 (34.8) | 1 (12.5) |
| NE | 14 (8.2) | 8 (5.1) | 0 | 0 |
| ORR, n (%)b [95% CI] | 33 (19.3) [13.7–26.0] | 34 (21.5) [15.4–28.8] | 9 (39.1) [19.7–61.5] | 6 (75.0) [34.9–96.8] |
| DCR, n (%)b [95% CI] | 64 (37.4) [30.2–45.1] | 99 (62.7) [54.6–70.2] | 15 (65.2) [42.7–83.6] | 7 (87.5) [47.3–99.7] |
| Median DOR, months (95% CI) | 6.9 (5.4–11.1) | 3.9 (2.8–4.2) | - | - |
aRandomized patients who had target lesion measurements at baseline.
bPer investigator assessment based on RECIST, v 1.1.
OS by BOR
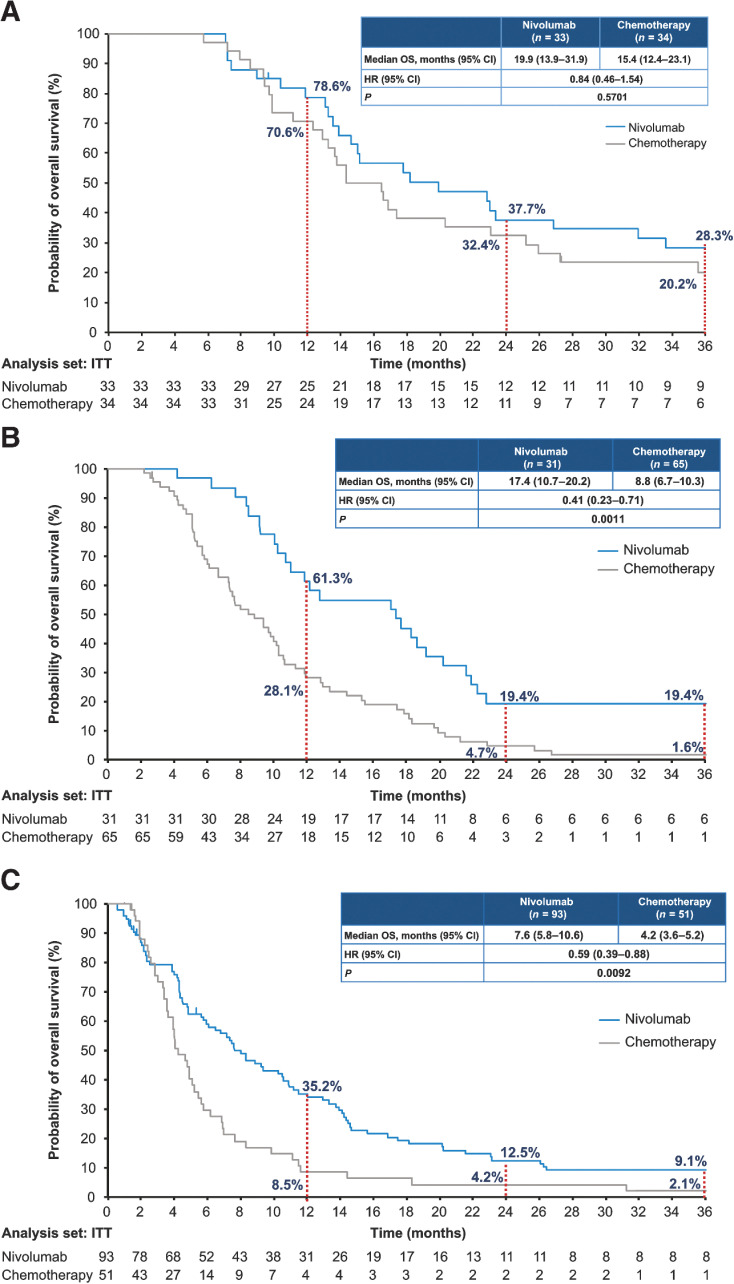
The median OS in patients with CR/PR, SD, and PD in the nivolumab group versus the chemotherapy group was 19.9 versus 15.4 months [HR (95% CI), 0.84 (0.46–1.54)], 17.4 versus 8.8 months [HR (95% CI), 0.41 (0.23–0.71)], and 7.6 versus 4.2 months [HR (95% CI), 0.59 (0.39–0.88)], respectively (Fig. 2). Similar results were observed in the subgroup of patients who survived for 4 months after randomization (Supplementary Fig. S3). Patients with CR/PR, and SD had a longer estimated median OS compared with patients with PD. However, the median OS in the nivolumab group was longer compared with the chemotherapy group, irrespective of BOR. In patients with PD, the median OS post a confirmed PD was 6.4 versus 2.5 months [HR (95% CI), 0.53 (0.35–0.80)] in the nivolumab group versus the chemotherapy group. In patients who received subsequent systemic therapy, nivolumab prolonged OS compared with chemotherapy, especially in the subgroup with BOR of PD (median, 11.8 vs. 5.4 months; HR, 0.53; 95% CI, 0.28–1.01; Supplementary Fig. S4). Univariate analysis showed none of the baseline characteristics (sex, age, race, BMI, ECOG PS, recurrent disease, lymph node/liver/lung/bone metastases, number of organs with metastases, prior surgery, prior radiotherapy, history of smoking, and tumor PD-L1 expression) significantly contributed to the OS prolongation or shortening (data not shown).
Figure 2.
Overall survival analysis by best overall response. Kaplan–Meier curves show OS by BOR in patients with CR or PR (A), in patients with SD (B), and in patients with PD (C).
Treatment and outcomes of 3-year survivors
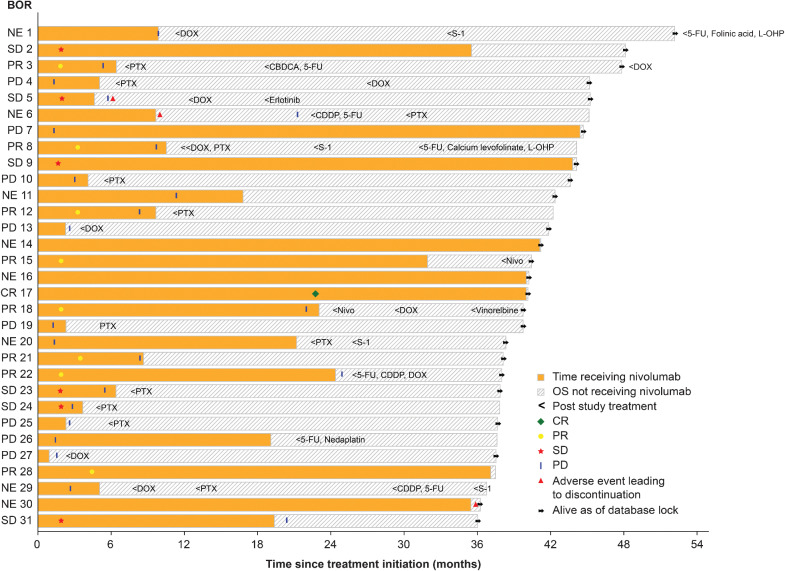
The disease course in patients who survived for a minimum of 3 years in the nivolumab group and the chemotherapy group is presented in Fig. 3 and Supplementary Fig. S5, respectively. Thirty-one patients survived for 3 years in the nivolumab group. In this group of long-term survivors, 20 patients discontinued nivolumab due to PD, while 6 patients received nivolumab as treatment postprogression. Nine patients received more than 30 months of therapy with nivolumab. Of note, one patient with PD early in the treatment (<3 months) continued nivolumab for more than 42 months and was alive at the time of clinical cut-off. Three patients within 3-year survivors discontinued nivolumab because of AEs. Twelve of the 3-year survivors received only nivolumab until the last follow-up while 19 patients received subsequent chemotherapy before the clinical cut-off. On the other hand, 16 patients survived for 3 years in the chemotherapy group and one patient received more than 30 months of chemotherapy.
Figure 3.
Treatment and outcomes of 3-year survivors in nivolumab group. Swimmer plot represents treatment-free period, discontinuations, BOR, and subsequent therapy of 3-year survivors (n = 31) in the nivolumab group. CDDP, cisplatin; CR, complete response; DOX, docetaxel; 5-FU, 5-fluorouracil; L-OHP, oxaliplatin; NE, not evaluable; Nivo, nivolumab; PTX, paclitaxel.
Subsequent therapy
At clinical cutoff, study treatment was permanently discontinued in 207 of 209 (99.0%) patients in the nivolumab group and 208 of 208 (100%) patients in the chemotherapy group; reasons for treatment discontinuation (nivolumab vs. chemotherapy) were the development of PD [140 (67.0%) vs. 138 (66.3%)], AEs [16 (7.7%) vs. 8 (3.8%)], physician's discretion [17 (8.1%) vs. 27 (13.0%)], and other reasons [21 (10.0%) vs. 19 (9.1%)]. In total, 127 (60.5%) of 210 patients in the nivolumab group and 118 (56.5%) of 209 patients in the chemotherapy groups received subsequent treatment. Most of the patients in the nivolumab and chemotherapy groups, received subsequent systemic therapy (56.2% vs. 49.8%). The most common subsequent treatments in both the nivolumab and chemotherapy groups were taxanes [106 (50.5%) of 210 patients in the nivolumab group and 45 (21.5%) of 209 patients in the chemotherapy group]. Three patients (1.4%) in the nivolumab group and 20 patients (9.6%) in the chemotherapy group received immunotherapy as a subsequent therapy (Supplementary Table S2). The proportions of patients with CR/PR, SD, and PD who received subsequent systemic therapy in the nivolumab versus chemotherapy groups were 57.6% versus 70.6%, 67.7% versus 47.7%, and 60.2% versus 35.3%, respectively.
Safety
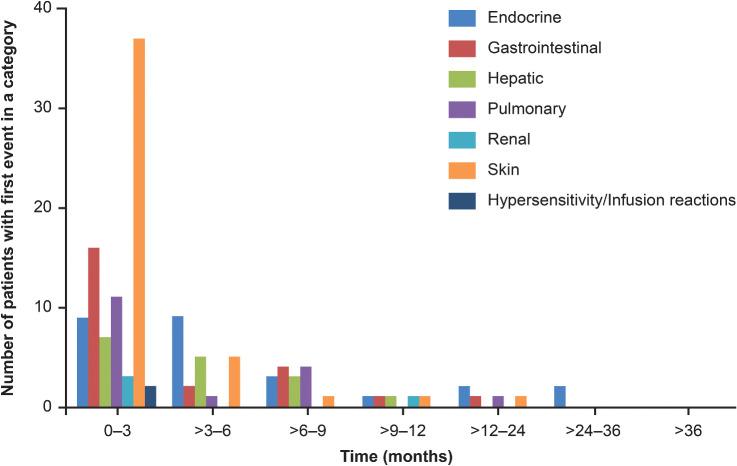
A total of 209 patients in the nivolumab group and 208 patients in the chemotherapy group were included in the safety analysis set. TRAEs of any grade were reported in 138 (66.0%) patients in the nivolumab group and 198 (95.2%) patients in the chemotherapy group, including 40 (19.1%) and 133 (63.9%) patients with ≥grade 3 TRAEs, respectively. Serious TRAEs were reported in 35 (16.7%) of 209 patients in the nivolumab group and 47 (22.6%) of 208 patients in the chemotherapy group (Supplementary Table S3). Most patients with select TRAEs experienced them within 3 months of starting nivolumab (Fig. 4). Skin and gastrointestinal TRAEs were the most commonly experienced AEs, and they tended to abate over time. No major late-onset select TRAEs were observed. The incidence rates of select TRAEs were comparable at 6–9 months and 1–3 years.
Figure 4.
Emergence of select TRAEs over time in the nivolumab group. The number of patients exhibiting the first event in a category within a certain time period is depicted.
Discussion
The current data, which were obtained after a minimum follow-up of 3 years, showed that nivolumab as a second-line therapy was beneficial in improving OS with a favorable safety profile versus chemotherapy regardless of tumor PD-L1 expression in patients with advanced ESCC. Other large-scale phase III clinical trials with ICIs as a second-line treatment for ESCC are ongoing, but long-term data are currently limited. This is the first phase III report of a 3-year follow-up of an ICI administered in patients with previously treated advanced ESCC.
The significant improvement in OS observed in the primary analysis was maintained and the proximity of the Kaplan–Meier curves between the nivolumab and chemotherapy groups observed in between 20 and 26 months in the primary analysis was improved during the 3-year follow-up, although no significant PFS benefit was observed between nivolumab and chemotherapy. The absence of PFS improvement in the presence of OS improvement was also observed with pembrolizumab in the KEYNOTE-181, suggesting that PFS might not indicate the survival benefit with PD-1 inhibitors as single-agent administration in patients with advanced ESCC. Overall, the improvements in long-term OS rates observed in this study are consistent with long-term studies of nivolumab in other tumor types (21, 22, 27–29).
Survival longer than 3 years was observed for 31 patients in the nivolumab group and 16 patients in the chemotherapy group. Among the 3-year survivors, the majority in the nivolumab group had a BOR of SD or PD [14/23 (60.9%)] while majority in the chemotherapy group had a BOR of CR or PR [6/8 (75%)]. This suggests that long-term survival can be expected, in nivolumab-treated patients with a BOR of SD or PD. Of note, 19 of the 31 long-term survivors in the nivolumab group received subsequent chemotherapy. Subsequent chemotherapy such as taxanes after nivolumab discontinuation might have contributed to the observed OS prolongation. Some retrospective studies in various types of tumors have demonstrated potential improvements in the ORR as a result of the high efficacy of chemotherapy after anti–PD-1 therapy (30–35). Osa and colleagues reported that nivolumab was detected on CD8 T cells more than 20 weeks after the last infusion of nivolumab in patients with non–small cell lung cancer (36). These results suggest that residual nivolumab after discontinuation might increase the efficacy of chemotherapy. Meanwhile, a distinctive feature of ICIs is a phenomenon called pseudo-progression (37–41): treatment with ICIs activates lymphocytes, which may accumulate in the tumor, resulting in an apparent enlargement in the tumor size (42). However, only three patients (3.2% of the 93 patients with PD) in the nivolumab group were suspected to experience pseudo-progression in tumors. It is unlikely that pseudo-progression greatly contributed to the prolonged OS in nivolumab-treated patients with a BOR of PD. Further studies are warranted to confirm this hypothesis that nivolumab may augment the efficacy of subsequent chemotherapy after its discontinuation.
Furthermore, the exploratory analysis showed that nivolumab offered a longer median OS compared with chemotherapy, regardless of the BOR. Responders with CR or PR in the nivolumab group tended to have a longer OS and a favorable 3-year survival rate, which reached up to 28.3% compared with 20.2% in the chemotherapy group. The OS rate in patients with SD in the nivolumab group plateaued and was overall higher than that achieved with chemotherapy (19.4% vs. 1.6%). Nivolumab offered a higher 3-year survival rate compared with chemotherapy in patients with PD (9.1% vs. 2.1%). Six patients among the 3-year survivors continued nivolumab after PD, suggesting that nivolumab could still be a viable treatment option in patients with PD as their BOR in the presence of overall clinical benefit. One possible reason for the improved OS with nivolumab in patients with PD is post-treatment with taxanes, 50.5% of patients received treatment with taxanes after nivolumab. This was greatly higher compared with 21.5% in the chemotherapy arm. The Kaplan–Meier curves of OS classified by BOR in the whole population were similar to those in the 4-month survivors.
The overall incidence of treatment-related events with longer follow-up was similar to the primary publication report (11) and the KEYNOTE-181 trial (18). The treatment discontinuation rate due to AEs with nivolumab was low, and no new safety signals were identified. This agrees with previous reports in patients with various types of cancer (23, 43–45). After analyzing the expression time of select TRAEs, we noted that most patients experienced their first TRAE within 3 months of starting nivolumab. Thereafter, the incidence of TRAEs was low and tended to abate over time as reported in the primary publication (11). This data suggests a favorable long-term tolerability profile of continued nivolumab therapy. However, monitoring of AEs is recommended to identify any potential late-onset events.
Recently, ICIs in combination with chemotherapy or ipilimumab demonstrated statistically significant benefits in OS compared with chemotherapy in patients with untreated advanced ESCC (9, 10). However, nivolumab monotherapy in the second- or later-line setting would still be a viable treatment option for many patients regardless of the evolving landscape with ICI combination therapy in the first-line setting because nivolumab monotherapy demonstrated a clinically meaningful durable response. Moreover, nivolumab has been approved in many countries as the second-line option in patients with advanced ESCC, while, in Europe, the use of pembrolizumab in combination with chemotherapy is currently restricted to subjects with CPS more than 10. Thus, ICI combination therapy may not be used in the first-line setting in certain patients. In addition, nivolumab monotherapy might also be used as a re-challenge in the third-line or later setting.
The results of this study should be interpreted considering certain limitations. Even though patients from different countries were enrolled, most of the patients included were Asians. The open-label study design might have introduced some bias related to adherence and toxicity assessment. However, the open-label design was acceptable because of the differences in the dosing regimens. The exploratory analysis of OS by BOR included a limited number of patients.
Conclusion
Nivolumab after a minimum of 3-year follow-up demonstrated a significant improvement in long-term OS compared with chemotherapy (paclitaxel or docetaxel) and a favorable safety profile in patients with advanced ESCC. Long-term survival with nivolumab compared with chemotherapy was observed regardless of the BOR. Nivolumab is a useful second-line treatment option for patients with advanced ESCC.
Supplementary Material
Acknowledgments
This study was funded by Ono Pharmaceutical Co., Ltd and Bristol-Myers Squibb. We thank the patients, their families, and the investigators. Editorial support, in the form of medical writing, assembling tables and creating high-resolution images based on authors’ detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Amit Garg of Enago Life Sciences, and was funded by Ono Pharmaceutical Co., Ltd., and Bristol-Myers Squibb.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
K. Kato reports other support from Ono Pharmaceutical during the conduct of the study as well as other support from MSD, BMS, Bayer, Shionogi, Taiho, AstraZeneca, Chugai, and Janssen outside the submitted work. B.C. Cho reports grants from Novartis, Bayer, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, AbbVie, Medpacto, GI Innovation, Eli Lilly, Blueprint Medicines, and Interpark Bio Convergence Corp.; personal fees from Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, BMS, Ono, Yuhan, Pfizer, Eli Lilly, Janssen, Takeda, MSD, Medpacto, Blueprint Medicines, TheraCanVac Inc, Gencurix Inc, Bridgebio Therapeutics, Kanaph Therapeutics Inc, Cyrus Therapeutics, Interpark Bio Convergence Corp., Guardant Health, Joseah BIO, and Champions Oncology; and other support from DAAN Biotherapeutics outside the submitted work. M. Takahashi reports grants from Ono Pharmaceutical Co. during the conduct of the study as well as grants and personal fees from Ono Pharmaceutical Co.; personal fees from Daiichi Sankyo Co., Bristol-Myers Squibb, and Taiho Pharmaceutical Co.; and grants from Chugai Pharmaceutical Co. outside the submitted work. K. Chin reports personal fees from Ono Pharmaceutical Co. Ltd. and Bristol-Myers Squibb during the conduct of the study as well as personal fees from Chugai Pharmaceutical Co., Ltd. and Taiho Pharma outside the submitted work. S. Kadowaki reports grants and personal fees from Ono Pharmaceutical and Bristol-Myers Squibb during the conduct of the study as well as grants and personal fees from Taiho Pharmaceutical, Eli Lilly, MSD, and Chugai Pharma; grants from Nobel Pharma; and personal fees from Bayer, Merck Serono, and Eisai outside the submitted work. M.-J. Ahn reports personal fees from AstraZeneca, Alpha Pharmaceuticals, Merck, Takeda, Lilly, Yuhan, MSD, and Ono outside the submitted work. Y. Hamamoto reports other support from Ono Pharmaceutical during the conduct of the study as well as other support from Ono Pharmaceutical outside the submitted work. Y. Doki reports personal fees from Ono Pharmaceutical during the conduct of the study. C.-C. Yen reports grants from Ono Pharmaceutical and Bristol-Myers Squibb during the conduct of the study as well as grants from TLC, EffPha, Athenex, Deciphera, Eisai, and BioAtla and other support from Deciphera, TTY Biopharm, CStone, Daiichi Sankyo, and Merck Sharp & Dohme outside the submitted work. S.-B. Kim reports research funding from Novartis, Sanofi-Aventis, and DongKook PharmCo.; has served on advisory boards for Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi-Sankyo; and owns shares in Genopeaks and NeogeneTC. C.-H. Hsu reports grants from Bristol-Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Roche, Ono Pharmaceutical, and EMD Serono and personal fees from Bristol-Myers Squibb and Ono Pharmaceutical during the conduct of the study as well as personal fees from Merck Sharp & Dohme, Roche, and EMD Serono and grants from BeiGene outside the submitted work. I. Xynos is an employee and stock owner of Bristol-Myers Squibb. Y. Matsumura reports other support from Ono Pharmaceutical during the conduct of the study. A. Takazawa reports personal fees from Ono Pharmaceutical outside the submitted work. Y. Kitagawa reports grants from Takeda Pharmaceutical, Yakult Honsha, Otsuka Pharmaceutical, Tsumura & Co., Kyouwa Hakkou Kirin, Dainippon Sumitomo Pharma, EA Pharma, Astellas Pharma, Toyama Chemical Co., Medicon, Eisai, Kaken Pharmaceutical, Teijin Pharma Limited, and Nihon Pharmaceutical; grants and personal fees from Chugai Pharmaceuticals, Taiho Pharmaceuticals, Asahi Kasei Pharma Corporation, Ono Pharmaceutical, Otsuka, and Nippon Covidien; and personal fees from Shionogi & Co., Ethicon, Olympus Corporation, Bristol-Myers Squibb K.K., AstraZeneca K.K., MSD K.K., and Smith & Nephew K.K. outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
M. Okada: Formal analysis, writing–original draft, writing–review and editing. K. Kato: Conceptualization, methodology, writing–original draft, writing–review and editing. B.C. Cho: Writing–original draft, writing–review and editing. M. Takahashi: Writing–original draft, writing–review and editing. C.-Y. Lin: Writing–original draft, writing–review and editing. K. Chin: Writing–original draft, writing–review and editing. S. Kadowaki: Writing–original draft, writing–review and editing. M.-J. Ahn: Writing–original draft, writing–review and editing. Y. Hamamoto: Conceptualization, methodology, writing–original draft, writing–review and editing. Y. Doki: Conceptualization, methodology, writing–original draft, writing–review and editing. C.-C. Yen: Writing–original draft, writing–review and editing. Y. Kubota: Writing–original draft, writing–review and editing. S.-B. Kim: Writing–original draft, writing–review and editing. C.-H. Hsu: Conceptualization, methodology, writing–original draft, writing–review and editing. E. Holtved: Writing–original draft, writing–review and editing. I. Xynos: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing. Y. Matsumura: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing. A. Takazawa: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing. Y. Kitagawa: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society. Survival rates for esophageal cancer by stage. Available from: https://cancerstatisticscenter.cancer.org/#!/statistics.
- 3. Cancer Information Service. Cancer statistics in Japan—survival. Available from: https://ganjoho.jp/en/professional/statistics/table_download.html.
- 4. Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 2004;15:955–9. [DOI] [PubMed] [Google Scholar]
- 5. Kato K, Tahara M, Hironaka S, Muro K, Takiuchi H, Hamamoto Y, et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol 2010;67:1265–72. [DOI] [PubMed] [Google Scholar]
- 6. Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus 2019;16:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, Version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:855–83. [DOI] [PubMed] [Google Scholar]
- 8. Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50–v7. [DOI] [PubMed] [Google Scholar]
- 9. Bristol Myers Squibb. Press release, April 08, 2021. Available from: https://news.bms.com/news/corporate-financial/2021/Bristol-Myers-Squibb-Announces-Opdivo-nivolumab-plus-Chemotherapy-and-Opdivo-plus-Yervoy-ipilimumab-Demonstrate-Superior-Survival-Benefit-Compared-to-Chemotherapy-in-Unresectable-Advanced-or-Metastatic-Esophageal-Squamous-Cell-Carcinoma/default.aspx.
- 10. Merck. Press release, August 19, 2020. Available from: https://www.merck.com/news/mercks-keytruda-pembrolizumab-in-combination-with-chemotherapy-significantly-improved-overall-survival-and-progression-free-survival-compared- with-chemotherapy-in-locally-advanced-or/.
- 11. Kato K, Cho BC, Takahashi M, Okada M, Lin C-Y, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506–17. [DOI] [PubMed] [Google Scholar]
- 12. ONO Pharmaceutical Co., Ltd. Press release, February 21, 2020. Available from: https://www.ono-pharma.com/sites/default/files/en/news/press/sm_cn200221_1. pdf.
- 13. ONO Pharmaceutical Co., Ltd. Press release, April 13, 2020. Available from: https://www.ono-pharma.com/sites/default/files/en/news/press/sm_cn200413_1. pdf.
- 14. ONO Pharmaceutical Co., Ltd. Press release, May 14, 2020. Available from: https://www.ono-pharma.com/sites/default/files/en/news/press/sm_cn200514_2. pdf.
- 15. ONO Pharmaceutical Co., Ltd. Press release, June 5, 2020. Available from: https://www.ono-pharma.com/sites/default/files/en/news/press/sm_cn200605_2. pdf.
- 16. Bristol Myers Squibb. Press release, November 24, 2020. Available from: https://news.bms.com/news/corporate-financial/2020/Bristol-Myers-Squibb-Receives-European-Commission-Approval-for-Opdivo-nivolumab-as-Second-Line-Treatment-for-Unresectable-Advanced-Recurrent-or-Metastatic-Esophageal-Squamous-Cell-Carcinoma/default.aspx
- 17. Bristol Myers Squibb. Press release, June 10, 2020. Available from: https://news.bms.com/news/corporate-financial/2020/US-Food-and-Drug-Administration-Approves-Opdivo-nivolumab-for-the-Treatment-of-Patients-with-Advanced-Esophageal-Squamous-Cell-Carcinoma-ESCC-After-Prior-Fluoropyrimidine–and-Platinum-based-Chemotherapy/default.aspx
- 18. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 19. Merck & Co. Press release, August 24, 2020. Available from: https://www.merck.com/news/mercks-keytruda-pembrolizumab-receives-two-new-approvals-in-japan/.
- 20. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832–42. [DOI] [PubMed] [Google Scholar]
- 21. Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959–65. [DOI] [PubMed] [Google Scholar]
- 22. Chen L-T, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastr Cancer 2020;23:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato K, Doki Y, Ura T, Hamamoto Y, Kojima T, Tsushima T, et al. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci 2020;111:1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastr Cancer 2021;24:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chvetzoff G, Tannock IF. Placebo effects in oncology. J Nat Cancer Inst 2003;95:19–29. [DOI] [PubMed] [Google Scholar]
- 27. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 Trial. JAMA Oncol 2019;5:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robert C, Long GV, Brady B, Dutriaux C, Di Giacomo AM, Mortier L, et al. Five-year outcomes with nivolumab in patients with wild-type BRAF advanced melanoma. J Clin Oncol 2020;38:3937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schvartsman G, Peng SA, Bis G, Lee JJ, Benveniste MFK, Zhang J, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017;112:90–5. [DOI] [PubMed] [Google Scholar]
- 31. Park SE, Lee SH, Ahn JS, Ahn M-J, Park K, Sun J-M. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non–small cell lung cancer. J Thorac Oncol 2018;13:106–11. [DOI] [PubMed] [Google Scholar]
- 32. Saleh K, Daste A, Martin N, Pons-Tostivint E, Auperin A, Herrera-Gomez RG, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer 2019;121:123–9. [DOI] [PubMed] [Google Scholar]
- 33. Greally M, Agarwal R, El Dika I, Shamseddine A, El-Olayan A, Haibe Y, et al. Maximizing response: a case report of salvage chemotherapy after immune checkpoint inhibition in a patient with previous chemo-refractory metastatic esophageal carcinoma. J Gastrointest Oncol 2019;10:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kankeu Fonkoua LA, Chakrabarti S, Sonbol MB, Kasi PM, Starr JS, Liu AJ, et al. Outcomes on anti-VEGFR-2/paclitaxel treatment after progression on immune checkpoint inhibition in patients with metastatic gastroesophageal adenocarcinoma. Int J Cancer 2021;149:378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sasaki A, Kawazoe A, Eto T, Okunaka M, Mishima S, Sawada K, et al. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open 2020;4:e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T, et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight 2018;3:e59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of response and progression to immunotherapy. J Clin Oncol 2018;38:169–78. [DOI] [PubMed] [Google Scholar]
- 38. Onesti CE, Frères P, Jerusalem G. Atypical patterns of response to immune checkpoint inhibitors: interpreting pseudoprogression and hyperprogression in decision making for patients' treatment. J Thorac Dis 2019;11:35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raimondi A, Randon G, Sepe P, Claps M, Verzoni E, de Braud F, et al. The evaluation of response to immunotherapy in metastatic renal cell carcinoma: open challenges in the clinical practice. Int J Mol Sci 2019;20:4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rini BI, McDermott DF, Hammers H, Bro W, Bukowski RM, Faba B, et al. Society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of renal cell carcinoma. J ImmunoTher Cancer 2016;4:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soria F, Beleni AI, D'Andrea D, Resch I, Gust KM, Gontero P, et al. Pseudoprogression and hyperprogression during immune checkpoint inhibitor therapy for urothelial and kidney cancer. World J Urol 2018;36:1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol 2018;58:125–35. [DOI] [PubMed] [Google Scholar]
- 43. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study area available within the article and its Supplementary Data files. For individual patient-level data from clinical studies, requests can be made to Ono Pharma through the following website: https://www.clinicalstudydatarequest.com/. For more information on Ono Pharma's Policy for the Disclosure of Clinical Study Data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.