Abstract
Epithelial cells and phagocytes contain antimicrobial polypeptides that participate in innate host defense. A recently cloned porcine β-defensin, PBD-1, was detected by Northern organ blots exclusively in the tongue epithelium. We generated recombinant PBD-1 peptide by using a baculovirus-insect cell expression system and obtained two forms (PBD-142 and PBD-138), which differed by N-terminal truncation. Only PBD-142 was found in scrapings of the surface of the dorsal tongue or the buccal mucosa. Immunohistochemical staining with antibody to PBD-142 revealed that PBD-1 was highly concentrated in an ∼0.1-mm-thick layer in the cornified tips of the filiform (but not fungiform) papillae of the dorsal tongue and in the superficial squamous cell layers of the buccal mucosa. By scraping, extraction, and semiquantitative Western blotting, the concentration of PBD-1 in the dorsal tongue surface and the buccal mucosa was estimated at 20 to 100 μg/ml. PBD-1 had antibacterial activity against Escherichia coli, Salmonella typhimurium, Listeria monocytogenes, and Candida albicans in 10 mM sodium phosphate buffer (pH 7.4). Added NaCl progressively inhibited the activity of PBD-1 against E. coli and C. albicans. In 10 mM sodium phosphate with 125 mM NaCl, the combinations of sublethal concentrations of PBD-1 and the porcine neutrophil peptide PG-3, PR-39, or PR-26 showed synergistic activity against E. coli or the multidrug-resistant S. typhimurium DT104. At its physiologic concentration, PBD-1 has antimicrobial effects under both low- and high-salt conditions encountered in the oral cavity and may contribute to the antimicrobial barrier properties of the dorsal tongue and oral epithelium.
Epithelial β-defensins were originally found in the bovine trachea and tongue and, more recently, on human epithelial surfaces (3, 4, 6, 20, 22). Studies of epithelial β-defensins in animal models have been hampered by the limited yield of peptides from natural sources and by the poor immunogenicity of many β-defensins. Among epithelial surfaces involved in innate immunity, the tongue is of special interest because of its high resistance to clinical infection despite its frequent exposure to trauma and microtrauma during mastication. We recently cloned a porcine cDNA that encodes a typical β-defensin (25), named porcine β-defensin 1 (PBD-1). Although the highly sensitive reverse transcription-PCR technique detected PBD-1 transcripts in many epithelia, amounts sufficient for detection by Northern blotting were found exclusively in the tongue epithelium.
In this study, we describe the preparation of recombinant PBD-1 and the corresponding rabbit antibody, the identification of the native PBD-1 forms in Western blots, the immunolocalization of native PBD-1 in the tongue and buccal epithelia, and the biological activity of the recombinant PBD-1 forms. The simple geometry of the tongue and buccal surface allowed us to estimate the local concentration of PBD-1. In areas of trauma or infection, neutrophils infiltrate into epithelia, and their microbicidal products supplement those made by the epithelial cells. Prominent among the secreted products are the cathelicidins (23), abundant antimicrobial peptides readily released by porcine and other mammalian neutrophils into inflammatory fluids (18). We explored the interaction of PBD-1 with two porcine neutrophil cathelicidins, protegrin 3 (PG-3) and the proline-arginine-rich peptide PR-39.
MATERIALS AND METHODS
Construction of recombinant PBD-1 baculovirus.
The construction of recombinant PBD-1 baculovirus was performed as described earlier for proprotegrin 3 and human β-defensin HBD-1 (15, 22). Flanking BamHI and EcoRI restriction sites (in boldface) for cloning of PBD-1 cDNA into the transfer vector were introduced by PCR mutagenesis with a sense primer (5′-GAAGGATCCTGGCCACCAGCATGAGACTCCACC-3′) and an antisense primer (5′-GAGCGAATTCTGAGCCATATCTGTGGGGTTG-3′). PBD-1 cDNA fragments that contained the translation start codon ATG, the entire coding region of PBD-1, a stop codon, and BamHI and EcoRI restriction sites at the 5′ and 3′ ends of the insert were cloned into the pBacPAK9 transfer vector (Clontech Laboratories, Inc., Palo Alto, Calif.) and transformed into Escherichia coli XL-1 Blue. Selection of PBD-1-containing clones was accomplished by hybridization of Southern blots with radiolabeled PBD-1 cDNA. The correct orientation and sequence of the PBD-1 insert were verified by dideoxynucleotide sequencing of both strands. After cotransfection of the BacPAK9-PBD-1 transfer plasmid and a defective baculovirus into insect Spodoptera frugiperda (Sf21) cells, we selected viable recombinant baculovirus clones that secreted a cationic protein detectable by acid-urea polyacrylamide gel electrophoresis (AU-PAGE) but not seen with control baculovirus.
Biosynthesis and purification of recombinant PBD-1.
Recombinant PBD-1 was generated from High-Five (Trichoplusia ni) insect cells infected with recombinant baculovirus. Recombinant peptides secreted after infection were purified from the culture media. These cationic peptides were adsorbed to a CM Macro-Prep ion-exchange resin (Bio-Rad, Hercules, Calif.), released from the resin with 10% acetic acid, and further purified by reverse-phase high-performance liquid chromatography (RP-HPLC) on a Vydac C18 column (1.6 by 250 mm) (Separation Group, Hesperia, Calif.).
Verification of recombinant PBD-1.
The major HPLC peptide peaks were individually collected and analyzed by AU-PAGE. Desired fractions were analyzed at the UCLA Center for Molecular and Medical Mass Spectrometry by the matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) method on the Voyager RP instrument (PerSeptive BioSystems, Framingham, Mass.) and by electrospray ionization mass spectrometry on Sciex API III (Perkin-Elmer Corp., Foster City, Calif.). NH2-terminal amino acid sequencing was performed at the UCLA Peptide Sequencing Facility.
Production of anti-PBD antibody.
Rabbit anti-PBD-1 serum was produced by immunization with the 42-amino-acid (aa) form of recombinant PBD-1. Purified PBD-1 was conjugated to ovalbumin by using a single-step cross-linking technique with glutaraldehyde (7). PBD-1–ovalbumin (300 μg) in complete Freund’s adjuvant was injected intradermally at multiple sites in 1.5- to 2-kg New Zealand White rabbits for the first immunization. Booster immunizations at 30-day intervals were similarly administered in incomplete Freund’s adjuvant. Prior to immunization and 10 days after each booster injection, blood was drawn by ear artery puncture, and serum was stored at −20°C.
Immunochemical identification of native PBD-1 peptide.
Immunohistochemical staining was performed as described earlier (22). Briefly, deparaffinized sections from tissues fixed with 10% formalin in Dulbecco’s phosphate-buffered saline were treated to inactivate endogenous peroxidase by incubation for 5 min in 0.1 M aqueous periodic acid and then for 2 min in 0.02% aqueous sodium borohydride and washed in Tris-buffered saline (TBS [500 mM NaCl, 20 mM Tris, pH 7.5]). Slides were subsequently incubated with rabbit anti-PBD-1 serum (1:1,000) or preimmune serum in a mixture of 1% gelatin (bovine skin 75 Bloom; Sigma Chemical Co., St. Louis, Mo.), 0.05% Tween 20 (Sigma), and 0.01% thimerosal in TBS for 18 h at room temperature. After three 20-min washes in 0.05% Tween 20 in TBS (TTBS), the slides were incubated with horseradish peroxidase-conjugated mouse anti-rabbit immunoglobulin G (Pierce, Rockford, Ill.) diluted 1:1,500 in 1% gelatin solution for 18 h at room temperature, washed in TTBS, and developed for 1 min in diaminobenzidine solution (30 mg of diaminobenzidine [Bio-Rad] dissolved in 50 ml of 50 mM Tris [pH 7.6] and filtered through a no. 4 filter, with 50 μl of 30% hydrogen peroxide added just before use). Slides were counterstained with Harris hematoxylin stain (Fisher Scientific).
Western blot analysis was performed as described earlier (18). Briefly, porcine tongue epithelial tissue (4.5 cm2) was scraped from freshly euthanized healthy pigs and extracted with 1 ml of 10% acetic acid at 4°C overnight. Alternatively, buccal mucosa of anesthetized pigs was gently scraped by a metal spatula to yield a mixture of epithelial fluid and desquamated cells. The cells were collected by centrifugation at 18,000 × g for 5 min, the supernatant was removed and replaced with an equal volume of 5% acetic acid, and the cells were extracted by three freeze-thaw cycles. Tissue debris was removed after a brief centrifugation, and 5 to 20 μl of the supernatant was subjected to AU-PAGE. After electrophoresis, proteins were electroblotted to an Immobilon-P membrane in 0.7% acetic acid, and the blots were probed with rabbit anti-PBD-1 antibody (1:1,000) and goat anti-rabbit immunoglobulin G–alkaline phosphatase conjugate (1:1,000) and then developed in a 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium solution. Dilutions of recombinant PBD-142 quantified by A205 relative to a bovine serum albumin standard (2 mg/ml; Pierce) were used as a positive control. On Western blots, estimates of PBD-1 concentrations in biological samples were made by visual comparison of the intensity of PBD-1 bands to concurrent PBD-1 standards.
Identification of the N terminus of the native PBD-1 peptide.
Scraped epithelial material from the tongue or buccal mucosa was analyzed by AU-PAGE and transferred to Immobilon PSQ membrane (Millipore Corp., Bedford, Mass.) as for Western blots. The membrane was briefly soaked in amido black stain (0.4% naphthol blue-black [Sigma], 25% isopropanol, 10% acetic acid) and destained with water, and the sole highly cathodal band was cut out and submitted for automated amino acid sequencing.
Microbes.
E. coli ATCC 35218 and Listeria monocytogenes EGD were laboratory strains. Salmonella typhimurium DT104 (swine origin) was a gift from Paula Fedorka-Cray at the U.S. Department of Agriculture, Athens, Ga. Candida albicans was a clinical strain from the UCLA Clinical Laboratories.
Peptides.
Recombinant PBD-1 was purified by RP-HPLC from the insect cell medium infected with recombinant PBD-1 baculovirus as described above. PG-3, PR-39, and PR-26 were synthetic peptides, based their natural sequences (8, 19).
CFU assay.
The antimicrobial activity of PBD-1 was evaluated by a CFU assay as described earlier (18). Briefly, overnight bacterial cultures were subcultured for 2 to 3 h at 37°C in a shaking water bath to obtain logarithmic-growth-phase microbes. C. albicans was cultured overnight in Trypticase soy broth and used in assays without subculture. Microbes and PBD-1 peptide were resuspended in 10 mM sodium phosphate buffer (low-salt medium [pH 7.4]) or 10 mM sodium phosphate buffer with 125 mM NaCl (normal-salt medium [pH 7.4]). In some experiments, as indicated, different pH and salt concentrations were used to study the effect of pH and salinity on the antibacterial activity of PBD-1. Microbes and the peptide were mixed in designated medium and incubated for 30 min or 3 h. After the incubation, the reaction was stopped by 1:100 dilution in ice-cold Hanks’ balanced salt solution. Microbes were spread on agar plates with a spiral plater (Spiral Biotech, Inc., Bethesda, Md.) that delivers a known volume per area and thus allows precise counts of the microbial colonies.
Synergistic effect of PBD-1 with PG-3, PR-39, and PR-26.
PBD-1 in combination with PG-3, PR-39, or PR-26 was further tested by the time-kill kinetic CFU assay for synergistic activity against E. coli ATCC 35218 or S. typhimurium DT-104 at 105 or 106 CFU/ml. The final antibacterial peptide concentrations in each tube were as follows: (i) no peptide, (ii) 30 μg of PBD-1 per ml, (iii) 2.5 μg of PG-3 per ml, (iv) 5 μg of PR-39 per ml, (v) 30 μg of PBD-1 per ml and 2.5 μg of PG-3 per ml, (vi) 30 μg of PBD-1 per ml and 5 μg of PR-39 per ml, and (vii) 30 μg of PBD-1 per ml and 5 μg of PR-26 per ml. Bacteria and peptide(s) were mixed in 1 ml of 10 mM sodium phosphate, 125 mM NaCl, and a 1:100 dilution of Trypticase soy broth. The tubes were incubated at 37°C in a shaking water bath, and 50-μl samples were serially diluted in saline at 45 min and 24 h, spread in triplicate onto Trypticase soy agar plates, and incubated at 37°C overnight. Synergy was defined as a >100-fold decline in the colony count with the peptide combination compared with PG-3, PR-39, or PR-26 alone (13).
RESULTS
Characterization of recombinant PBD-1.
The PBD-1 cDNA sequence encodes a 64-aa prepropeptide, MRLHRLLLVFLLM VLLPVPGLLK NIGNSVSCLRNKGVCMPG KCAPKMKQIGTCGMPQVKCCKRK (25). Two forms of recombinant PBD-1 peptide were isolated from media conditioned by insect cells that had been infected with recombinant baculovirus. They eluted from the C18 column at 27 and 28.5% acetonitrile during the RP-HPLC purification. Their sequences were determined by NH2-terminal amino acid sequencing and mass spectrometry and differed from each other only by NH2-terminal truncation (Fig. 1). The PBD-142 was the predominant (95%) form, consisting of 42 aa (KNIGNSVSCLRNKGVCMPGKCAPKMKQIGTCGMPQVKCCKRK). The earlier-eluting minor form, PBD-138, was also identified (NSVSCLRNKGVCMPGKCAPKMKQIGTCGMPQVKCCKRK). Both forms of PBD-1 were used for antibacterial activity studies. The predominant PBD-142 was used as an antigen to prepare polyclonal antibodies.
FIG. 1.
Verification of recombinant PBD-1 peptides and comparison with bovine epithelial β-defensins. (a) Verification of recombinant PBD-1 peptides. The molecular masses of recombinant PBD-1 peptides, measured by electrospray mass spectrometry, are shown as means ± standard deviations, based on four to six mass peak measurements. (b) Sequence comparison with bovine tracheal antimicrobial peptide (TAP [4]) and bovine lingual antimicrobial peptide (LAP [20]). The six cysteine residues conserved in all β-defensins are marked with asterisks. MS, mass spectrometry.
Characterization of the native PBD-1 form in the tongue.
Scrapings of the porcine tongue surface were analyzed by AU-PAGE, and after Coomassie staining, a single cathodal band migrating in a position characteristic of highly cationic defensins was seen. After transfer from a duplicate AU-PAGE gel, the band was cut out of amido black-stained Immobilon PSQ membrane. Its N-terminal amino acid sequence corresponded to PBD-142, and no shifted sequence that would be characteristic of somewhat shorter or longer N-terminal variants was detected. Subsequently, a similar analysis of buccal scrapings also identified the same peptide species.
Immunolocalization of PBD-1 in pig tongue epithelium.
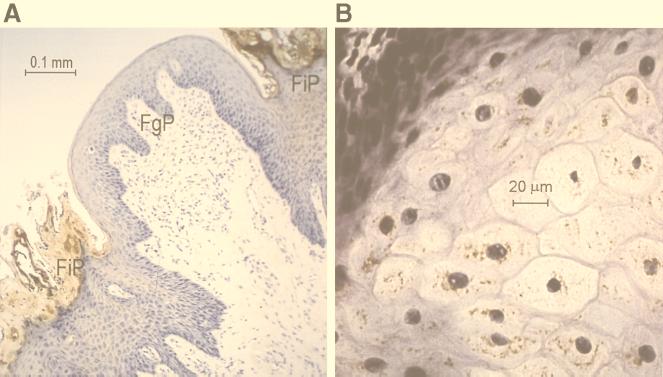
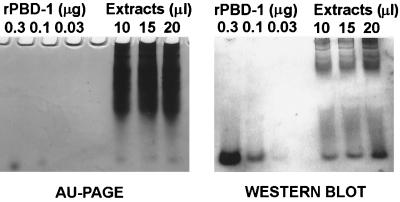
Serum from rabbits immunized with PBD-1–ovalbumin was collected after three consecutive immunizations at 1-month intervals. Dot blot analysis showed that as little as 10 ng of PBD-142 was detectable with a 1:2,000 dilution of the rabbit anti-PBD-1 serum. However, as much as 1 μg of PBD-138 was not detected by this rabbit anti-PBD-142 serum in the dot blot analysis (data not shown). This suggests that the antiserum is very specific for the predominant PBD-142 form. To identify the natural source of PBD-1 peptide, we performed immunohistochemical staining of porcine tongue, buccal mucosa, skin, trachea, and small intestine sections. PBD-1 was located on the tips of filiform papillae and in the relatively mature epithelial cells of the tongue, but not in the basal epithelial cells or fungiform papillae (Fig. 2). In buccal mucosal tissue sections stained with anti-PBD-1 serum, PBD-1 was seen predominantly in the superficial squamous cells (data not shown). Under the same conditions, there was no staining of the other tissues (data not shown). Rabbit preimmune serum did not show any reactivity with the tongue or buccal tissue (data not shown). In the AU-PAGE and Western blot analysis of tongue epithelial extracts, a cationic peptide with the same mobility as the recombinant PBD-1 on AU gel was detected by the anti-PBD-1 antibody (Fig. 3). By using semiquantitative Western blots with PBD-1 standards, we estimated that 4.5 cm2 of epithelium from a single tongue contained 5 μg of immunoreactive PBD-1. From the thickness of the layer staining for PBD-1 (∼0.1 mm as measured by micrometer) and the sampled area (4.5 cm2), we estimate that the volume occupied by PBD-1 was about 45 μl, yielding a concentration of PBD-1 of at least 100 μg/ml.
FIG. 2.
Immunochemical localization of PBD-1 in porcine tongue epithelial cells. (a) Low magnification. Dense brown immunostaining is seen in filiform papillae (FiP), but not in fungiform papillae (FuP). (b) High magnification. Granular immunostaining is visible in differentiating parabasal epithelial cells.
FIG. 3.
Determination of PBD-1 concentration in pig tongue epithelia. (Left panel) Porcine tongue epithelial extracts (10 to 20 μl) were subjected to AU-PAGE and stained with Coomassie blue. Recombinant PBD-142 (0.03 to 0.3 μg) was used as a standard. (Right panel) Proteins on an AU-PAGE with the same loading pattern were blotted to an Immobilon-P membrane; the blots were probed with rabbit anti-PBD-1 antibody and developed as described in Materials and Methods. Recombinant PBD-142 (0.03 to 0.3 μg) was used as a standard for the semiquantitative analysis of PBD-1 concentration.
To determine whether the PBD-1 peptide was predominantly contained in the cellular or the liquid layer of the tongue surface, we lightly scraped the tongue or buccal surfaces of anesthetized pigs with a metal spatula. The material was separated by centrifugation at 18,000 × g for 5 min into a cellular or particulate component and a liquid component, typically of about equal volume. For buccal scrapings, comparison of the liquid and cellular components by AU-PAGE and Western blotting showed that the cellular-particulate component contained nearly all of the PBD-1 in the sample (data not shown). Although similar analysis of scrapings from the tongue of anesthetized pigs was less conclusive due to very scant material, PBD-1 was detected only in the cellular fraction. Western analysis of the PBD-1 concentration in the cell pellet of buccal scrapings from live pigs yielded the estimate of 20 μg of PBD-1 per ml. In contrast to the measurements of tongue epithelium from euthanized pigs, the estimates from buccal scrapings of anesthetized pigs do not depend on morphometric data. However, these samples only contain the desquamating superficial layer of the epithelium.
PBD-1 peptides were active against both gram-positive and gram-negative bacteria.
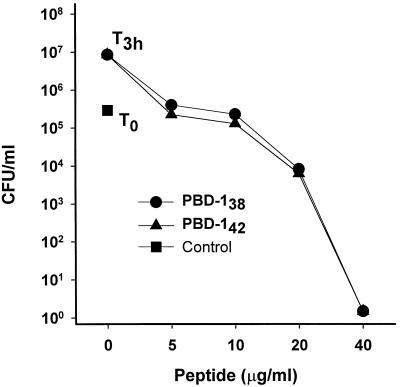
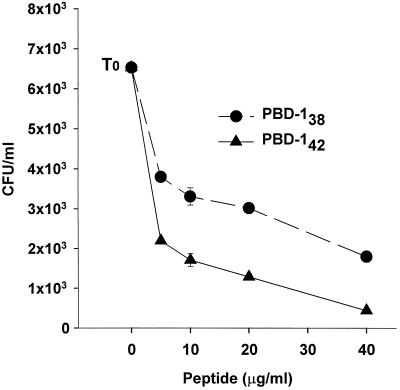
The antibacterial potency of PBD-1 peptides was determined in a CFU assay. Both PBD-142 and PBD-138 were highly active against E. coli in 10 mM sodium phosphate (pH 7.4) medium, with sterilization reached at 40 μg of PBD-1 per ml (Fig. 4, logarithmic CFU scale). In comparison, both peptides had weaker activity against L. monocytogenes (Fig. 5, linear CFU scale).
FIG. 4.
PBD-1 peptides were active against E. coli. The results of CFU assays with E. coli ATCC 35218 are shown. Controls: T0, initial bacterial inoculum; T3h, bacteria after 3 h at 37°C in 10 mM sodium phosphate. Bacteria were incubated with the indicated concentrations of PBD-142 or PBD-138 at 37°C for 3 h before being plated on Trypticase soy agar plates. CFU data are shown as the means and standard errors of triplicate determinations.
FIG. 5.
PBD-1 peptides were active against L. monocytogenes. The results of CFU assays with L. monocytogenes EGD are shown. Controls: T0, initial bacterial inoculum. Bacteria were incubated with the indicated concentrations of PBD-142 or PBD-138 at 37°C for 3 h in 10 mM sodium phosphate before being plated on Trypticase soy agar plates. CFU data are shown as the means and standard errors of triplicate determinations.
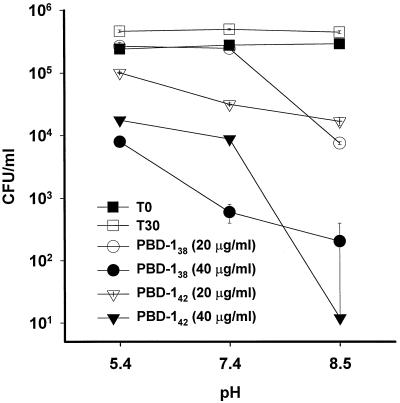
PBD-1 peptides were potentiated against E. coli by high-pH medium.
We tested the antibacterial potency of PBD-142 and PBD-138 in 10 mM sodium phosphate at different pH values (Fig. 6). Bacteria grew equally well in all of these media. However, the antibacterial activity of PBD-1 was significantly inhibited at pH 5.5 and enhanced at pH 8.5 in comparison with that at pH 7.5. The influences of pH on both forms of PBD-1 were similar at the two concentrations tested.
FIG. 6.
Effects of pH on the antibacterial activity of PBD-1 peptides. The results of CFU assays with E. coli ATCC 35218 are shown as the means and standard errors of triplicate determinations. Control: T0, initial bacterial inoculum; T30, bacteria incubated in 10 mM sodium phosphate at the indicated pH for 30 min at 37°C. Bacteria in the experimental groups were incubated with 20 or 40 μg of PBD-142 or PBD-138 per ml as indicated.
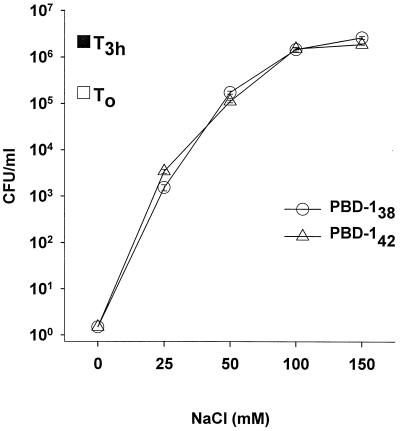
The antibacterial activity of PBD-1 was salt dependent.
The surface of the tongue is bathed in saliva whose electrolyte composition varies as a function of salivary stimulation and other environmental factors (11). The antimicrobial activity of many cationic antimicrobial peptides, including β-defensins (1, 2, 10), is greatly affected by salt concentration. To assess the influence of salinity on PBD-1, the antibacterial activity of both PBD-138 and PBD-142 in a wide range of salt concentrations was tested in a CFU assay. As shown in Fig. 7, their antibacterial activity was decreased when the salt concentration in the test media was increased. At 40 μg/ml in 10 mM sodium phosphate, both forms of PBD-1 peptide were bactericidal; however, when 100 to 150 mM NaCl was added to the medium, both peptides lost their activity against E. coli (Fig. 7) and L. monocytogenes (data not shown).
FIG. 7.
Effects of salinity on the antibacterial activity of PBD-1 peptides. The results of CFU assays with E. coli ATCC 35218 are shown as the means and standard errors of triplicate determinations. T0, initial bacterial inoculum; T3h, bacteria after 3 h at 37°C in 10 mM sodium phosphate. Bacteria in experimental groups were incubated with 40 μg of PBD-142 or PBD-138 per ml in 10 mM sodium phosphate and 0 to 150 mM NaCl.
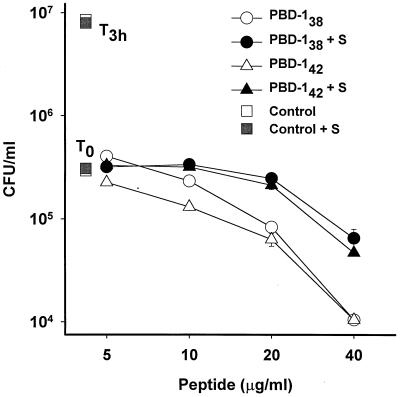
The antibacterial activity of PBD-1 peptides was partially inhibited by 2% porcine serum.
The E. coli bacteria used in this study were not sensitive to 2% fresh porcine serum, as indicated by the independence of their growth on the addition of serum to the medium (Fig. 8). However, when 2% fresh porcine serum was added to the test medium, the antibacterial activity of PBD-138 and PBD-142 at 20 and 40 μg/ml was partially inhibited. The bacteriostatic activity observed with 5 or 10 μg of PBD-1 per ml was unaffected by 2% serum.
FIG. 8.
Effects of serum on the antibacterial activity of PBD-1 peptides. The results of CFU assays with E. coli ATCC 35218 are shown as the means and standard errors of triplicate determinations. T0 and T3h are as in Fig. 7. Bacteria in experimental groups were incubated with different concentrations of PBD-142 (circles) or PBD-138 (triangles) in the presence (solid symbols) or absence (open symbols) of 2% serum (S).
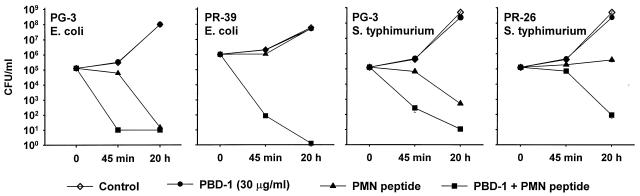
PBD-1 was synergistic with neutrophil-derived antibacterial peptides.
We examined the functional interactions of PBD-1 with the porcine neutrophil cathelicidins, PG-3 and PR-39. PBD-142 at 30 μg/ml was inactive against E. coli and S. typhimurium in normal-salt medium (10 mM sodium phosphate, 125 mM NaCl, 0.01× diluted Trypticase soy broth). Under the same conditions, PG-3 at 2.5 μg/ml or PR-39 or its variant PR-26 at 5 μg/ml was only bacteriostatic to E. coli and S. typhimurium. The addition of PBD-142 reduced E. coli CFU 104-fold after a 45-min incubation in comparison with PG-3 alone (Fig. 9). The addition of PBD-1 led to a 105- to 106-fold reduction in CFU per milliliter after 45 min and 20 h of incubation in comparison with PR-39 alone. Against the multidrug-resistant S. typhimurium strain DT104, the combination of PG-3 and PBD-142 lead to a 103-fold reduction in CFU per milliliter after 45 min and 20 h of incubation in comparison with a sublethal concentration of PG-3 alone. The combination of PR-26 and PBD-142 led to a 103-fold reduction in CFU per milliliter after 20 h of incubation in comparison with PR-26 alone. A similar result was also seen when PBD-1 was combined with PR-39 (data not shown).
FIG. 9.
Synergism of PBD-1 and porcine neutrophil peptides. The results of CFU antibacterial assays of combinations of PBD-1 and porcine neutrophil peptides PG-3, PR-39, and PR-26 against E. coli ATCC 35218 and S. typhimurium DT104 are shown. A combination of 10 mM sodium phosphate (pH 7.4) and 125 mM NaCl in 1% (vol/vol) Trypticase soy broth was used in the assay. CFU data are expressed as the means and standard errors of triplicate determinations.
The antifungal activity of PBD-1 for C. albicans.
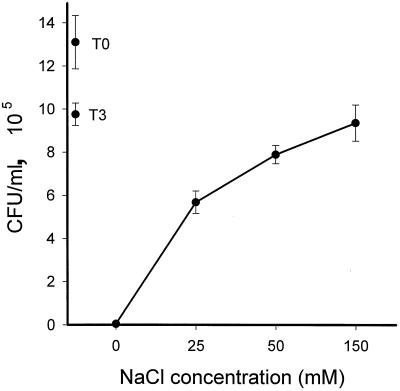
In 10 mM sodium phosphate medium (pH 7.4), PBD-142 (40 μg/ml) displayed marked fungicidal activity against C. albicans (Fig. 10). The activity was progressively inhibited by the addition of 25 to 150 mM NaCl to the incubation mixture.
FIG. 10.
Antifungal activity of PBD-1 against C. albicans. The CFU assays were performed with 10 mM sodium phosphate (pH 7.4) in 1% (vol/vol) Trypticase soy broth, with NaCl added to the indicated concentration. Means and standard errors of assays (n = 18) performed on 2 different days are shown.
DISCUSSION
We prepared PBD-1 by using the insect-baculovirus protein expression system, a well-established method for the generation of biologically active molecules, such as defensins and protegrins, that contain complex disulfide bonds (15, 22). One clone of transfected insect cells could produce two different forms of PBD-1 peptides, and both were stable and biologically active. Mass spectrometry and N-terminal sequencing analysis enabled us to confirm the sequences and disulfide formation. The cleavage site and the length of the predominant PBD-142 are identical to those of the bovine lingual antibacterial peptide (20), while the shorter form, PBD-138, is similar to the bovine tracheal antimicrobial peptide (4). Since both PBD-138 and PBD-142 are active and have counterparts in bovine epithelia, we speculated that both forms of PBD-1 peptides might exist in pigs. However, in tongue and buccal extracts analyzed by AU-PAGE, the predominant high-mobility Coomassie-stained band comigrated with PBD-142. The identification of PBD-142 as the predominant PBD-1 form expressed in the dorsal tongue and the buccal mucosa was confirmed by amino acid sequencing.
Immunoreactivity with anti-PBD-1 serum was seen exclusively in the mature epithelial cells of the tongue and the cheek mucosa. There appeared to be no immunostaining of basal cells, few staining granules in parabasal epithelial cells, and dense staining of cornified cells in filiform papillae and the superficial squamous cells in the cheek mucosa. What mechanisms might account for the formation of a highly concentrated barrier-like layer of PBD-1? We surmise that the synthesis of PBD-1 begins in the parabasal cells and that the intracellular PBD-1 concentration increases as the cells gradually migrate toward the surface, lose cytoplasmic volume, and become compacted. Thus, the PBD-1 content of cornified tips of the filiform papillae may be contributed by a large number of epithelial cells. A similar mechanism may account for the higher concentration of PBD-1 in superficial squamous cells of the cheek. PBD-1 expression was surprisingly restricted: neither fungiform papillae nor the ventral tongue epithelium reacted with our antibody. We speculate that the dorsal surface of the tongue and the exposed oral mucosa, most susceptible to trauma from mastication of food, may have evolved a more potent antimicrobial defense system than the less exposed ventral surface of the tongue. PBD-1 was also identified in the tongue and buccal epithelial extract by Western blot analysis. In preliminary experiments, we found no increase in PBD-1 immunostaining 24 h after the pig tongue was subjected to localized microinjury by a needle tip dipped in India ink. More extensive investigations will be required to characterize the regulation of PBD-1 peptide production in porcine tissues in healthy and diseased states.
The relatively simple geometry of the tongue and the highly concentrated distribution of PBD-1 allowed us to estimate, for the first time, the local concentrations of an epithelial defensin. At ∼20 to 100 μg/ml, the concentration of PBD-1 is sufficient to exert antimicrobial effects both at low and high salt concentrations that can develop in the oral cavity under diverse conditions. In comparison, the concentrations of human neutrophil defensins inside the phagocytic vacuoles of granulocytes have been estimated at >1 mg/ml (5). In which tissue compartment does PBD-1 exert its biological activity? The cell types that stained most intensely, the superficial squamous epithelial cells of the tongue and the buccal mucosa, are not known as secretory cells. Indeed, the fractionation of scrapings of the mucosal layers did not show substantial release of PBD-1 into the fluid layer on the tongue. The location of the defensin-rich layer in the superficial cells of the dorsal tongue and buccal mucosa suggests that it could function as a cellular antimicrobial barrier that prevents the penetration of oral bacteria into the interior layers of the tongue. The intracellular location of PBD-1 may also allow it to act against microbes that parasitize or lyse epithelial cells. Although lacking PBD-1, the fluid layer could also contribute to the host defense of the tongue and oral epithelium by its distinct assortment of salivary antimicrobial peptides and proteins.
The antibacterial activity of PBD-1 peptides was tested under different pH and salt concentrations to reflect the variations in pH and salinity in the oral cavity and on the tongue exposed to food and microbial stimuli. PBD-1 was most potently antimicrobial under low-salt conditions (10 mM sodium phosphate) and in a neutral-to-basic pH environment. Although very little is known about the composition of porcine saliva, studies have shown that the salivary apparatuses of humans and pigs are comparable in their anatomical, histological, and physiological aspects (21). In humans, the mean pH value of saliva is 7.32 and the pH increases with increasing salivary flow during eating (11). The mean concentrations of phosphate and chloride are 6.58 and 16.25 mM (11), indicating that the overall salt concentrations are much lower than those of plasma. If the pH and salinity of porcine saliva are similar to those of human saliva, the conditions on the tongue surface would be highly favorable to PBD-1 activity. It is not known how these conditions are modified in the superficial cellular compartment that contains the highest concentrations of PBD-1.
During inflammation or injury, serum proteins and neutrophil products reach the affected areas and are likely to interact with PBD-1. The partial inhibition of PBD-1 activity by serum is similar to that of other defensins (14) and could be due to the binding of defensins by specific serum proteins (16, 17). In contrast, our findings suggest that antimicrobial polypeptides from neutrophils could potentiate epithelial antimicrobial polypeptides. Previously, the most compelling evidence of antibacterial peptide synergy was observed with rabbit neutrophil bactericidal-permeability-inducing protein acting in concert with other protein constituents of the inflammatory fluid, including p15s and group II phospholipase A2 (12). Here, we provide direct evidence for synergistic interactions of PBD-1 with neutrophil-derived antimicrobial peptides. We previously demonstrated that protegrins are the predominant microbicidal peptides in porcine neutrophil secretions (18) and that the concentrations of PR-39 in serum is increased during Salmonella infection (24). The secretion and activation of protegrins and PR-39 by inflammatory neutrophils will enable these peptides to interact with epithelial peptides such as PBD-1. Such synergies may further amplify the microbicidal defenses in the inflamed porcine mucosal surfaces.
It remains to be seen whether the formation of a defensin-rich layer at the tips of the filiform papillae and the superficial layers of buccal mucosa is a common feature of higher animals or is a specialized adaptation in animals that ingest a diet rich in abrasive materials. It may not be coincidental that the human tongue is covered by a thick plaque of microorganisms, while only a few are found on the filiform papillae of the pig tongue (9).
ACKNOWLEDGMENTS
We thank Erika Valore, Thomas Kang, Alexander Cole, Christina Park, Lide Liu, Shawn McGill, Fernando Vinuela, Jr., and John Robert for their contributions to the manuscript.
This work was supported by United States Department of Agriculture National Research Initiative grants 98-35204-6594 (J. Shi) and 98-35204-6397 (F. Blecha and C. Ross) and by grant HL46809 from the National Institutes of Health (T. Ganz).
REFERENCES
- 1.Bals R, Goldman M J, Wilson J M. Mouse β-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66:1225–1232. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson J M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 4.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987;55:568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harder J, Bartels J, Christophers E, Schroeder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861–862. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 7.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. p. 80. [Google Scholar]
- 8.Kokryakov V N, Harwig S S, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 9.Kullaa-Mikkonen A, Hynynen M, Hyvonen P. Filiform papillae of human, rat and swine tongue. Acta Anat (Basel) 1987;130:280–284. doi: 10.1159/000146457. [DOI] [PubMed] [Google Scholar]
- 10.Lehrer R I, Ganz T. Endogenous vertebrate antibiotics. Defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann N Y Acad Sci. 1996;797:228–239. doi: 10.1111/j.1749-6632.1996.tb52963.x. [DOI] [PubMed] [Google Scholar]
- 11.Lentner C. Geigy scientific tables. Basel, Switzerland: Ciba-Geigy; 1981. pp. 114–122. [Google Scholar]
- 12.Levy O, Ooi C E, Weiss J, Lehrer R I, Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Investig. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moellering R C, Wennersten C, Weinberg A N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971;77:821–828. [PubMed] [Google Scholar]
- 14.Panyutich A, Ganz T. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol. 1991;5:101–106. doi: 10.1165/ajrcmb/5.2.101. [DOI] [PubMed] [Google Scholar]
- 15.Panyutich A, Shi J, Boutz P L, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun. 1997;65:978–985. doi: 10.1128/iai.65.3.978-985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panyutich A, Szold O, Poon P H, Tseng Y, Ganz T. Identification of defensin binding to C1-complement. FEBS Lett. 1994;356:169–173. doi: 10.1016/0014-5793(94)01261-x. [DOI] [PubMed] [Google Scholar]
- 17.Panyutich A V, Hiemstra P S, Van Wetering S, Ganz T. Human neutrophil defensins and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12:351–357. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 18.Shi J, Ganz T. The role of protegrins and other elastase-activated polypeptides in the bactericidal properties of porcine inflammatory fluids. Infect Immun. 1998;66:3611–3617. doi: 10.1128/iai.66.8.3611-3617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi J, Ross C R, Chengappa M M, Sylte M J, McVey D S, Blecha F. Antibacterial activity of a synthetic peptide (PR-26) derived from PR-39, a proline–arginine-rich neutrophil antimicrobial peptide. Antimicrob Agents Chemother. 1996;40:115–121. doi: 10.1128/aac.40.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolzenberg E D, Anderson G M, Ackermann M R, Whitlock R H, Zasloff M. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tryon A F, Bibby B G. Preliminary studies on pig saliva. Arch Oral Biol. 1966;11:527–532. doi: 10.1016/0003-9969(66)90159-2. [DOI] [PubMed] [Google Scholar]
- 22.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Investig. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, Ross C R, Dritz S S, Nietfeld J C, Blecha F. Salmonella infection increases porcine antibacterial peptide concentrations in serum. Clin Diagn Lab Immunol. 1997;4:774–777. doi: 10.1128/cdli.4.6.774-777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Wu H, Shi J, Ganz T, Ross C R, Blecha F. Molecular cloning and tissue expression of porcine beta-defensin-1. FEBS Lett. 1998;424:37–40. doi: 10.1016/s0014-5793(98)00134-3. [DOI] [PubMed] [Google Scholar]