Abstract
In order to evaluate during experimental Trypanosoma brucei infections the potential role of tumor necrosis factor alpha (TNF-α) in the host-parasite interrelationship, C57BL/6 TNF-α knockout mice (TNF-α−/−) as well as C57BL/6 wild-type mice were infected with pleomorphic T. brucei AnTat 1.1 E parasites. In the TNF-α−/− mice, the peak levels of parasitemia were strongly increased compared to the peak levels recorded in wild-type mice. The increased parasite burden did not reflect differences in clearance efficacy or in production of T. brucei-specific immunoglobulin M (IgM) and IgG antibodies. Trypanosome-mediated immunopathological features, such as lymph node-associated immunosuppression and lipopolysaccharide hypersensitivity, were found to be greatly reduced in infected TNF-α−/− mice. These results demonstrate that, during trypanosome infections, TNF-α is a key mediator involved in both parasitemia control and infection-associated pathology.
African trypanosomes are extracellular parasitic protozoa transmitted by the bite of the tsetse fly (19). In order to complete their life cycle, trypanosomes require an obligatory developmental step in a mammalian host. Consequently, these parasites have to cope with the host’s immune system and establish a state of equilibrated growth regulation ensuring optimal survival and effective transmission. In the first place, African trypanosomes escape from immune recognition through the mechanism of antigenic variation of their variant-specific surface glycoproteins (VSG), the major surface antigen that acts as an ever-changing protective coat for the parasite (2, 15). Besides this active VSG-based defense mechanism, Trypanosoma brucei parasites may utilize host immunoregulatory molecules to regulate their development. For instance, both epidermal growth factor (5) and gamma interferon (IFN-γ) (14) were reported to exert growth-enhancing properties on T. brucei parasites. In contrast, tumor necrosis factor alpha (TNF-α) was documented (i) to be trypanolytic for T. brucei parasites in vitro (8, 10) and (ii) to reduce the parasite load in vivo (7, 9). Hence, African trypanosomes may parasitize the host’s cytokine network for their own benefit. However, chronic production of cytokines in turn influences the host in terms of immunopathology. In particular, the role of TNF-α in trypanosome-associated immunopathology was demonstrated in several studies documenting, for instance, (i) the enhanced expression of TNF-α mRNA in the brain of T. brucei-infected mice (6), (ii) the association between TNF-α production by monocytes and the severity of disease-associated anemia in trypanosome-infected cattle (17), (iii) the correlation between serum TNF-α levels and the severity of neuropathological symptoms in human sleeping sickness (13), and (iv) the involvement of TNF-α in trypanosome-elicited immunosuppression (3, 16). Taken together, the accumulated knowledge about trypanosome-elicited production of TNF-α indicates that this cytokine exerts dual effects during trypanosome infections, influencing both the parasite and the host. As such, the induction of TNF-α production during T. brucei infections could be either beneficial or devastating for the host. It was thus of importance to use TNF-α knockout (TNF-α−/−) mice (National Institute of Animal Health, Tsukuba City, Japan) (18) as a tool with which to reevaluate the role of TNF-α in the host-parasite interrelationship during trypanosome infections. The genes and gene expression for lymphotoxin-α and -β, as well as the gene for FasL, all belonging to the TNF gene family, were shown to be unaffected by the gene disruption strategy used to obtain the TNF-α−/− mice (18).
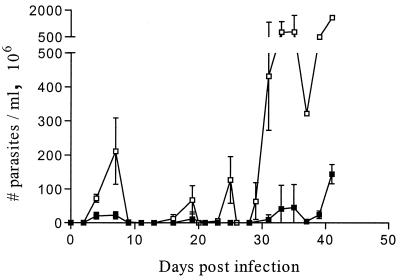
To evaluate the influence of endogenous TNF-α on T. brucei parasitemia, C57BL/6 TNF-α−/− mice and C57BL/6 wild-type mice (all between 6 and 8 weeks of age) were infected intraperitoneally with 5,000 pleomorphic T. brucei AnTat 1.1 E parasites. Parasitemia in both TNF-α−/− and wild-type mice was monitored at intervals of 2 or 3 days by enumeration of the number of parasites present in the blood. As shown in Fig. 1, TNF-α−/− mice exhibited significantly higher parasitemia peaks than did the wild-type mice. Despite these clear differences in peak levels, transient falls in parasitemia appear to occur at roughly the same time in TNF-α−/− and wild-type mice, indicating the presence of antiparasitic processes that are independent of TNF-α gene disruption. Since parasite clearance was documented to be antibody dependent (4), titers of antitrypanosome antibodies in serum were determined by enzyme-linked immunosorbent assay (ELISA) at different time points of infection (days 6, 7, 14, and 35). To this end, ELISA plates (Nunc, Roskilde, Denmark) were coated overnight with the flagellar pocket fraction (5 μg/ml of phosphate-buffered saline [PBS]), prepared from T. brucei AnTat 1.1 parasites via density gradient centrifugation as described previously (12). Subsequently, the plates were incubated with 1% bovine serum albumin–PBS for 1 h and incubated overnight with serial dilutions of sera isolated from naïve and T. brucei-infected TNF-α−/− and wild-type mice. Finally, plates were extensively washed and incubated with specific goat anti-mouse immunoglobulin M (IgM) (Promega, Madison, Wis.) or goat anti-mouse IgG (Sigma, St. Louis, Mo.) antibodies coupled to alkaline phosphatase. The ELISA was developed with 4-nitrophenyl phosphate substrate (Sigma), and the optical density at 450 nm (OD450) was measured. Mean OD values (±standard deviation) were plotted as a function of the reciprocal serum dilutions used. The results shown in Fig. 2 indicate that TNF-α−/− mice and wild-type mice produce similar humoral antitrypanosome responses during an experimental T. brucei infection. In both strains of mice, the levels of IgM and IgG antitrypanosome antibodies increased significantly after the first peak of infection and remained high until the animals died.
FIG. 1.
Parasitemia development of pleomorphic T. brucei AnTat 1.1 parasites in C57BL/6 wild-type (■) and TNF-α−/− (□) mice. Ten mice per group were infected at day 0 by intraperitoneal injection of 5,000 parasites. Results are expressed as means ± standard deviations.
FIG. 2.
Development of an antiflagellar pocket immune response during the experimental infection with T. brucei AnTat 1.1. Antibody titers were determined with the serum of both infected wild-type and TNF-α−/− C57BL/6 mice. Preimmune serum was used to determine aspecific binding. Serum samples were analyzed in triplicate (mean ± standard deviation) at day 6 (A), day 7 (B), day 14 (C), and day 35 (D), and serum antibody titers were checked for the wild-type IgM (●) and IgG (■) responses and the TNF-α−/− IgM (○) and IgG (□) responses.
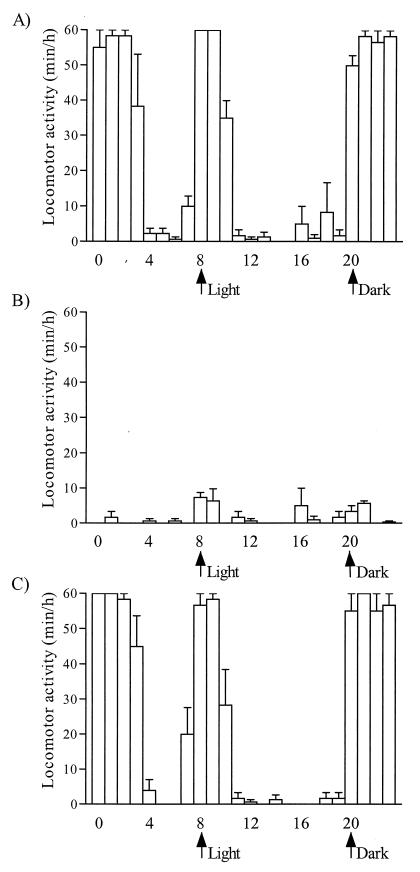
Despite a significantly higher parasite load, the average survival times of T. brucei-infected TNF-α−/− mice and wild-type mice were not significantly different (Fig. 3). Hereby it should be emphasized that, in TNF-α−/− mice, particularly during the late stage of infection, infection-related signs of morbidity were much less pronounced or even were absent compared to those in infected wild-type animals. The criteria used to quantify morbidity were lack of locomotor activity, anemia, and poor coat condition. The locomotor activity of control mice and T. brucei mice was recorded as the minutes per hour that the mice spent on spontaneous running around in the cage, eating, and drinking, as well as the time spent on cleaning their fur and nest. During the experimental setup, mice were kept in an animal facility with a regimen of 12 h of light and 12 h of dark per 24 h, starting with the first light cycle at 8 a.m. From day 8 postinfection, the time point that corresponded with the moment that numbers of parasites in the blood of both wild-type and TNF-α−/− mice dropped below detection limits, infected wild-type mice showed a severe reduction in locomotor activity. While noninfected mice normally have a long active period during the night and a short active period at the start of the light cycle (Fig. 4A), the data recorded showed that infected wild-type mice sit most of the time immobilized and spend only a limited time eating and drinking (Fig. 4B). The degree of impairment of locomotor activity of the infected wild-type mice persisted throughout the rest of the infection. In contrast to the infected wild-type mice, infected TNF-α−/− mice did not show any alterations in their locomotor activity on day 8 of the infection (Fig. 4C). Also during later stages of infection, these mice showed no change in locomotor activity. Together with the impairment in activity, a sudden drop in the numbers of erythrocytes present in the blood of the infected wild-type mice was recorded by microscopical blood analysis. Within 48 h after the first transient drop in parasitemia, the numbers of erythrocytes had dropped to 58% ± 12%. Anemia in the infected wild-type mice persisted throughout the rest of the infection, while no decrease in numbers of erythrocytes was recorded for the TNF-α−/− mice during the course of infection. The third parameter recorded to quantify trypanosomiasis-associated morbidity was the coat condition of the infected mice. Deterioration of the coat was observed in infected wild-type mice starting from day 8 postinfection, and a very poor coat condition was recorded toward the end of the infection (day 30), as shown in Fig. 5A. At the same stage of infection (day 30), no alterations in coat condition were observed in the infected TNF-α−/− mice (Fig. 5B), although very high numbers of parasites were present in the blood of these mice (Fig. 1).
FIG. 3.
Survival of T. brucei AnTat 1.1-infected C57BL/6 wild-type (■) and TNF-α−/− (□) mice. Ten mice per group were infected at day 0 by intraperitoneal injection of 5,000 parasites.
FIG. 4.
Locomotor activity was measured as the total time per hour spent by mice on running in their cage, eating, drinking, and cleaning their fur and nest. Locomotor activity was recorded during a 24-h period 8 days postinfection for noninfected wild-type mice (A), T. brucei AnTat 1.1-infected wild-type mice (B), and infected TNF-α−/− mice (C). Mice were kept in an animal facility with a 12-h-light–12-h-dark regimen. Three mice per experimental group were used, and the results are expressed as means ± standard deviation.
FIG. 5.
The coat condition of T. brucei AnTat 1.1-infected wild-type mice (A) was compared to the coat condition of infected TNF-α−/− mice (B). Both mice were photographed on day 30 of infection.
The lack of morbidity prompted a comparative analysis of T. brucei-induced lipopolysaccharide (LPS) hypersensitivity, reported to occur during late-stage experimental trypanosome infections (11). Noninfected and infected (14 days postinfection) TNF-α−/− mice, as well as noninfected and infected wild-type mice, were injected intraperitoneally with different doses of LPS from Escherichia coli 055:B5 (Difco Laboratories, Detroit, Mich.) resuspended in PBS (pH 7.2), and survival was recorded 48 h later. Figure 6A shows that, in infected wild-type mice, an LPS dose as low as 0.5 μg/mouse induced 100% mortality, while in noninfected controls, an LPS dose of 1 mg/mouse was needed to induce 100% mortality. In contrast, Fig. 6B shows that TNF-α−/− mice did not show any signs of LPS hypersensitivity upon infection with T. brucei parasites.
FIG. 6.
Survival after LPS challenge of noninfected (■) and T. brucei AnTat 1.1-infected (□) C57BL/6 wild-type mice (A) was compared to the survival after LPS challenge of noninfected (●) and infected (○) TNF-α−/− mice (B). Mice were infected at day 0 by intraperitoneal injection of 5,000 parasites, and at day 14 postinfection, LPS was administered via intraperitoneal injection. LPS sensitivity was analyzed in an LPS dose range from 0.01 μg/mouse up to 10 mg/mouse in both infected and noninfected mice, with five mice used per LPS data point.
Trypanosome-associated immunosuppression is another immunopathological feature that occurs during experimental and natural infections with African trypanosomes. Here, TNF-α was reported to play a role in trypanosome-mediated induction of suppressive macrophages (3). In particular, during the chronic phase of the infection, lymph node-associated suppressive macrophages were documented to require both TNF-α (as an inducer of IFN-γ) and IFN-γ (as an active mediator of suppressive activity). The involvement of TNF-α in T. brucei-elicited immunosuppression in the lymph nodes was thus reanalyzed in TNF-α−/− mice. Lymph node cells from T. brucei-infected wild-type or TNF-α−/− mice (LNCi), harvested during the chronic phase of infection (i.e., 35 days postinfection), were tested for their capacity to suppress the ex vivo concanavalin A (ConA)-induced blastogenesis of lymph node cells from noninfected wild-type mice (LNCn). Briefly, 2 × 105 LNCi (TNF-α−/− or wild type) were cocultured with 2 × 105 LNCn in 200 μl of RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 10% fetal calf serum (Boehringer Pharma, Mannheim, Germany), 50 U of penicillin-streptomycin per ml, 300 μg of l-glutamine per ml, and 5 × 10−5 M 2-mercaptoethanol. Cultures were stimulated with ConA (2.5 μg/ml) at 37°C for 48 h in a humidified atmosphere containing 5% CO2. Indomethacin (Sigma) (10 μg/ml) was added to all of the cultures in order to block any suppressive activity due to prostaglandins. Eighteen hours before harvesting, cultures were pulsed with 1 μCi of [3H]thymidine, and incorporation of the labeled material into the newly synthesized DNA was determined and compared to the proliferative response of LNCn. Table 1 shows that while LNCi from wild-type mice completely inhibit T-cell responsiveness of LNCn to ConA, LNCi from TNF-α−/− mice exhibit no suppressive activity. Since the suppressive activity of T. brucei-elicited suppressive macrophages in lymph nodes was documented to rely on a nitric oxide (NO)-independent, IFN-γ-dependent mechanism (1), induction of both immunomodulators was quantified in cocultures of LNCi and LNCn activated with ConA (Table 1). Compared to cocultures of LNCn and LNCi from wild-type mice, the absence of suppressive activity in cocultures containing LNCi from TNF-α−/− mice was shown to be associated with (i) a slight but not significant decrease of NO production (quantified as nitrite accumulation by Griess reaction) and (ii) a partial, although significant, reduction of IFN-γ secretion (P < 0.01), quantified by specific ELISA (Pharmingen, San Diego, Calif.). These data confirm previous results demonstrating than TNF-α contributes to the suppressive activity of LNCi via an upregulation of IFN-γ and that NO produced by LNCi is not involved in the inhibition of ConA-induced proliferation of LNCn (1). Furthermore, the fact that LNCi from TNF-α−/− mice still produce significantly higher levels of IFN-γ compared to LNCn indicates that IFN-γ may be required, yet is not sufficient, to cause suppressive activity, a finding that also is in agreement with previous results (3).
TABLE 1.
Involvement of TNF-α in the suppressive activity of LNCi
| Culturea | Proliferation (cpm)b | NO production (μM)c | IFN-γ production (U/ml)d |
|---|---|---|---|
| LNCn (WT) | 220,952 ± 1,752 | 2.5 ± 1 | 4 ± 0.7 |
| LNCn (TNF-α−/−) | 226,699 ± 3,111 | 2.2 ± 0.8 | 3.1 ± 0.8 |
| LNCn (WT) + LNCi (WT) | 831 ± 121 | 44.5 ± 5.0 | 511 ± 42 |
| LNCn (WT) + LNCi (TNF-α−/−) | 203,378 ± 18,740 | 31.5 ± 4.3 | 138 ± 11 |
LNC cultures at a concentration of 2 × 106/ml were stimulated with ConA. Cocultures of LNCn (2 × 106/ml) and LNCi (2 × 106/ml) were made in order to evaluate the suppressive activity of LNCi on the response of LNCn. The role of TNF-α in the suppressive activity was analyzed by using LNCi from wild-type (WT) mice as well as LNCi from TNF-α−/− mice.
Proliferation of cell cultures was measured by [3H]thymidine incorporation after 48 h of incubation in the presence of ConA. Representative results are shown as mean cpm ± standard deviation.
NO production was quantified by nitrite accumulation (Griess reaction) in the supernatants of cell cultures stimulated with ConA for 48 h. Results from triplicate measurements are shown as means ± standard deviations.
IFN-γ production was quantified by specific ELISA (Pharmingen) in the supernatants of cell cultures stimulated with ConA for 48 h. Results from triplicate measurements are shown as means ± standard deviations.
Collectively the results described herein point toward a crucial role of TNF-α during African trypanosome infections, influencing both the host and the parasite. Indeed, concerning the host, TNF-α participates in the apparent overall signs of morbidity as well as in immunopathological features of trypanosomiasis, such as LPS hypersensitivity and immunosuppressive activity in the lymph nodes. Of interest is the fact that, despite a reduction of immunopathology, the survival time of T. brucei-infected mice seems to be TNF-α independent. However, parasite development in T. brucei-infected TNF-α−/− mice reaches extremely high levels, corroborating the role of TNF-α in parasite control. It is possible that, during late-stage infections, the increased parasite burden is lethal to the host even in the absence of noxious cytokine side effects. Altogether, our results obtained with the present mouse model suggest that interference with TNF-α production during experimental African trypanosomiasis may be beneficial for both the host and parasite. Hence, the outcome of such intervention during natural T. brucei infections deserves further investigation.
Acknowledgments
This project has been funded by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, The Belgian National Fund for Scientific Research (NFWO—no. 6.0325.95), and the Flemish Government (Vlaams Actieprogramma Biotechnologie [VLAB]). This project was performed as part of an Interuniversity Attraction Pole Programme, financed by the Belgian state, “Diensten van de Eerste Minister—Federale diensten voor wetenschappelijke, technische en culturele aangelegenheden.” S.M. is a Postdoctoral Fellow of the Foundation of Scientific Research—Flanders (FWO).
REFERENCES
- 1.Beschin A, Brys L, Magez S, Radwanska M, De Baetselier P. Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. J Leukocyte Biol. 1998;63:429–439. doi: 10.1002/jlb.63.4.429. [DOI] [PubMed] [Google Scholar]
- 2.Cross G A M. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- 3.Darji A, Beschin A, Sileghem M, Heremans H, Brys L, De Baetselier P. In vitro simulation of immunosuppression caused by Trypanosoma brucei: active involvement of gamma interferon and tumor necrosis factor in the pathway of suppression. Infect Immun. 1996;64:1937–1943. doi: 10.1128/iai.64.6.1937-1943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dempsey W L, Mansfield J M. Lymphocyte function in experimental trypanosomiasis. V. Role of antibody and mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983;130:405–411. [PubMed] [Google Scholar]
- 5.Hide G, Gray A, Harrison C M, Tait A. Identification of an epidermal growth factor receptor in trypanosomes. Mol Biochem Parasitol. 1989;36:51–60. doi: 10.1016/0166-6851(89)90199-0. [DOI] [PubMed] [Google Scholar]
- 6.Hunter C A, Gow J W, Kennedy P G E, Jennings F W, Murray M. Immunopathology of experimental African sleeping sickness: detection of cytokine mRNA in the brains of Trypanosoma brucei brucei-infected mice. Infect Immun. 1991;59:4636–4640. doi: 10.1128/iai.59.12.4636-4640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas R, Magez S, Bajyana Songa E, Darji A, Hamers R, De Baetselier P. A role for TNF during African trypanosomiasis: involvement in parasite control, immunosuppression and pathology. 51st Forum in Immunology. Res Immunol. 1993;144:370–376. doi: 10.1016/s0923-2494(93)80082-a. [DOI] [PubMed] [Google Scholar]
- 8.Lucas R, Magez S, De Leys R, Fransen L, Scheerlinck J P, Rampelberg M, Sablon E, De Baetselier P. Mapping the lectin-like activity of tumor necrosis factor. Science (Washington, DC) 1994;263:814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- 9.Magez S, Lucas R, Darji A, Bajyana Songa E, Hamers R, De Baetselier P. Murine tumour necrosis factor plays a protective role during the initial phase of the experimental infection with Trypanosoma brucei brucei. Parasite Immunol. 1993;15:635–641. doi: 10.1111/j.1365-3024.1993.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 10.Magez S, Geuskens M, Beschin A, del Favero H, Verschueren H, Lucas R, Pays E, De Baetselier P. Specific uptake of tumor necrosis factor-α is involved in growth control of Trypanosoma brucei. J Cell Biol. 1997;137:715–727. doi: 10.1083/jcb.137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magez S, Stijlemans B, Radwanska M, Pays E, Ferguson M, De Baetselier P. The glycosyl-inositol-phosphate and dimyristoylglycerol moieties of the glycosylphosphatidylinositol anchor of the trypanosome variant-specific surface glycoprotein are distinct macrophage-activating factors. J Immunol. 1998;160:1949–1956. [PubMed] [Google Scholar]
- 12.Mkunza F, Olako W M, Powel C N. Partial protection against natural trypanosomiasis after vaccination with a flagellar pocket antigen from Trypanosoma brucei rhodesiense. Vaccine. 1995;13:151–154. doi: 10.1016/0264-410x(95)93128-v. [DOI] [PubMed] [Google Scholar]
- 13.Okomo-Assoumou M C, Daulouede S, Lemesre J L, N’Zila-Mouanda A, Vincendeau P. Correlation of high serum levels of tumor necrosis factor-alpha with disease severity in human African trypanosomiasis. Am J Trop Med Hyg. 1995;53:539–543. doi: 10.4269/ajtmh.1995.53.539. [DOI] [PubMed] [Google Scholar]
- 14.Olson T, Bakhiet M, Edlund C, Höjeberg B, Van der Meide P H, Kristensson K. Bidirectional activity signals between Trypanosoma brucei and CD8+ T cells: a trypanosome-released factor triggers interferon-γ production that stimulates parasite growth. Eur J Immunol. 1991;21:2447–2454. doi: 10.1002/eji.1830211022. [DOI] [PubMed] [Google Scholar]
- 15.Pays E, Vanhamme L, Berberof M. Genetic control for expression of surface antigens in African trypanosomes. Annu Rev Microbiol. 1994;48:25–52. doi: 10.1146/annurev.mi.48.100194.000325. [DOI] [PubMed] [Google Scholar]
- 16.Scheifer K W, Mansfield J M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 17.Sileghem M, Flynn J N, Logan-Henfrey L, Ellis J. Tumor necrosis factor production by monocytes from cattle infected with Trypanosoma (Duttonella) vivax and Trypanosoma (Nannomonas) congolense: possible association with severity of anaemia associated with the disease. Parasite Immunol. 1994;16:51–54. doi: 10.1111/j.1365-3024.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi T, Takata M, Ikeda A, Monmotani E, Sekikawa K. Failure of germinal center formation and impairment of response to endotoxin in tumor necrosis factor-α-deficient mice. Lab Investig. 1997;77:647–658. [PubMed] [Google Scholar]
- 19.Vickerman K, Tetley L, Hendry K A K, Turner C M R. Biology of African trypanosomes in the tsetse fly. Biol Cell. 1988;64:109–119. doi: 10.1016/0248-4900(88)90070-6. [DOI] [PubMed] [Google Scholar]