Abstract
Amplified hygiene and precautionary measures are of utmost importance to control the spread of COVID-19 and future infection; however, these changes in practice are projected to trigger a rise in the purchase, utilisation and hence, discharge of many disinfectants into the environment. While alcohol-based, hydrogen peroxide-based, and chlorine-based compounds have been used widely, quaternary ammonium compounds (QACs) based disinfectants are of significant concern due to their overuse during this pandemic. This review presents the classification of disinfectants and their mechanism of action, focusing on QACs. Most importantly, the occurrence, fate, toxicity and antimicrobial resistance due to QACs are covered in this paper. Here we collated evidence from multiple studies and found rising trends of concern, including an increase in the mass load of QACs at a wastewater treatment plant (WWTP) by 331% compared to before the COVID-19 pandemic, as well as an increases in the concentration of 62% in residential dust, resulting in high concentrations of QACs in human blood and breast milk and suggesting that these could be potential sources of persistent QACs in infants. In addition to increased toxicity to human and aquatic life, increased use of QACs and accelerated use of antibiotics and antimicrobials during the COVID-19 pandemic could multiply the threat to antimicrobial resistance.
Keywords: Disinfectants, Quaternary ammonium compounds, Sediment, Toxicity, Antimicrobial resistance
Graphical Abstract
1. Introduction
The COVID-19 pandemic is gripping the world today, with over 597 million people affected and more than 6.5 million dead by the end of September 2022 (https://coronavirus.jhu.edu/map.html). With the global economy weakening due to severe mobility restrictions and lockdowns, governments have been forced to relax restrictions in many parts of the world, which has only further increased the use of personal care products (PCPs) (Alygizakis et al., 2021, Kumar et al., 2021, Kumar and Jiang, 2022, Menon and Mohapatra, 2022, Mohapatra et al., 2021a, Mohapatra et al., 2022, Mohapatra and Menon, 2022). Center for Disease Control and Prevention (CDC) suggested that two critical activities can substantially lessen SARS-CoV-2 viral transmission risk: regular hand and surface sanitisation using environmental protection agency (EPA)-registered disinfectants and antimicrobials (MacGibeny and Wassef, 2021). For the disinfection of surfaces, the EPA published a list of antimicrobials against SARS-CoV-2 (MacGibeny and Wassef, 2021). Disinfectants meeting the EPA criteria often contain quaternary ammonium compounds (QACs) such as dialkyldimethylammonium compounds (DADMACs), alkyltrimethylammonium compounds (ATMACs) and benzalkonium compounds (BACs) (Table S1). Out of 594 EPA-listed disinfectants to use against SARS-CoV-2, QACs were the most active ingredients in 274 of the registered products. Around 51 products were tested effective against SARS-CoV-2 within 1 min of contact time (Table S2).
The production and sales of disinfectants have risen steeply during the pandemic, with a month’s production in 2020 equivalent to the entire years of production in 2019 (Lysol, 2022). Elevated usage over the pandemic through hand and surface disinfection could result in residues finding their way to wastewater treatment plants (WWTPs) and receiving waters such as surface or marine environments (Hora et al., 2020, Rizvi and Ahammad, 2022). The environmental fate and impact of chronic low doses of QACs could cause acute toxicity and lead to antimicrobial resistance. A commonly used quaternary ammonium salt, quaternium 15, is a well-known skin allergen that can cause dermatitis in humans when used in excess (Cahill and Nixon, 2005). In addition, this compound is known to persist in the environment and has even been reported to occur in marine ecosystems, where it caused reduced cellular viability and changes to the antioxidant mechanisms of the marine invertebrate, Mytilus galloprovincialis at sub-lethal concentrations (∼ 100 μg/L) (Faggio et al., 2016). Additionally, high residual levels of disinfectants may lead to the formation of N-nitrosodimethylamine (NDMA) in the environment, classified as a carcinogen and microbiocidal at a concentration range from 0.5 to 5 mg/L and 10–50 mg/L, respectively (Gerba, 2015). The development of antimicrobial resistance in a broad range of microorganisms is an additional concern, as reported elsewhere (Hegstad et al., 2010, Voumard et al., 2020a).
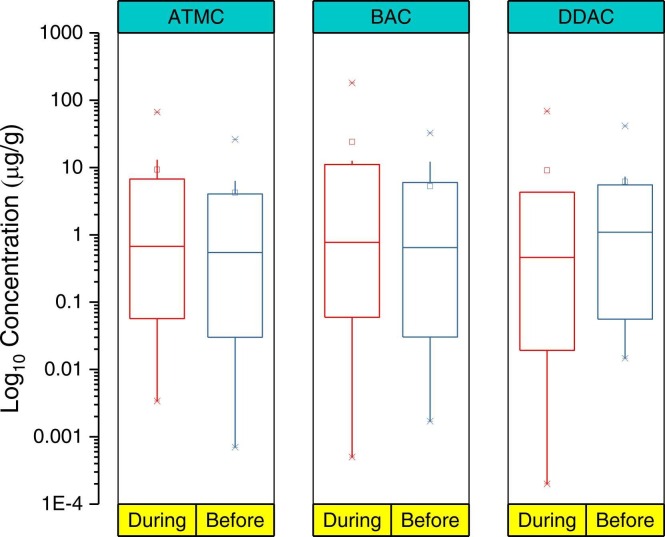
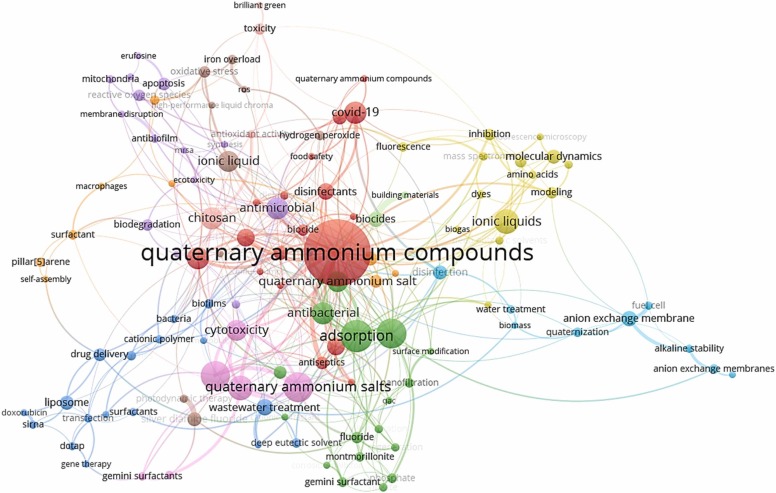
A recent study by Zheng et al. (2020) estimated a 62 % rise in the concentration of QACs in residential dust samples during COVID-19, with concentrations ranging from 1.95 to 531 μg/g (Table S3). The median concentration during the pandemic was 58.9 μg/g compared to 36.3 μg/g for the pre-pandemic period ( Fig. 1). Such a high concentration could increase the daily intake of these compounds in toddlers by ten times compared to adults staying in the same residential units (Zheng et al., 2020). An increase in the use of these QACs during the pandemic may also lead to increased loading in WWTPs, which, in turn, leads to increased discharge of such chemicals into the environment, where they can pose several chronic effects to aquatic and, eventually, human life. Increased use of household disinfection products having QACs is of additional concern during this pandemic as these chemicals are known to build up in human blood (Salamova et al., 2021). A recent study further reported the occurrence of 13 QACs in human breast milk, with total concentrations ranging from 0.33 to 7.4 ng/mL, indicating breastfeeding as one of the significant pathways QACs for infants. The most abundant QAC was dodecylbenzyldimethylammonium chloride (BAC C14), with a median concentration of 0.45 ng/mL (Zheng et al., 2022). Thus, it is crucial to recognize the concentration, fate and effects of disinfectants in the environments. Since, 2019 around 350 papers on QACs have been catalogued under “environmental science” category in the Scopus database. As shown in Fig. 2, a word cloud generated from recent literature (2019–2022) using VOSviewer (version 1.6.17) illustrates important keywords such as QACs and its co-occurrences with COVID-19, adsorption, wastewater treatment etc. Thus, this comprehensive review addresses various aspects of QACs, focusing on the occurrence, fate, toxicity and antimicrobial resistance development due to their widespread use. The broad objective of this paper is to collate knowledge on the distribution and fate of several QACs in various environmental matrices, such as wastewater, surface and groundwater, and sediments in the past ten years, with a particular focus on the pandemic. Several papers before 2011 were also referred to for an additional explanation. Hence, the main objectives of this paper are (i) To systematically conduct a thorough literature review on the occurrence and fate of QACs in the aquatic environment by reviewing papers published in the last few years with a special focus on the increased usage of QACs during the pandemic, (ii) To highlight research gaps on the frequent use of QACs and toxicity to the aquatic organism and human beings, (iii) To collate information on increased antimicrobial resistance due to the alarming use of antibiotics and QACs in various sectors, (iv) To critically review and discuss the increased use of QACs during this pandemic and future implications. In general, this study provides a comprehensive discussion on the classification of QACS, various sources of QACs, how humans and the aquatic environment are exposed, toxicity to aquatic organisms, and the resulting development of antimicrobial resistance (AMR).
Fig. 1.
Variation in the concentrations of QACs during and before the COVID19 pandemic (Reproduced with permission [Zheng et al., 2020]).
Fig. 2.
Network analysis of literature on QACs based on SCOPUS database.
2. Classification, mechanism of action and applications of QACs
2.1. Classification of QACs
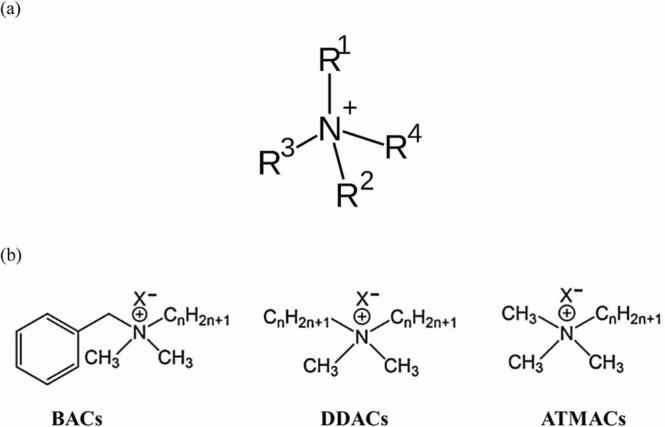
QACs are active components in over 200 disinfectants in the recently published list by EPA and are effective at inactivating enveloped viruses (Table S2 and S4) (Vereshchagin et al., 2021, Ying, 2006, Grigoras, 2021a, Grigoras, 2021b, Maillard, 2002). Their structure contains a positively charged nitrogen atom linked to at least one hydrophobic hydrocarbon chain, i.e., alkyl groups, which are usually short chain substituents such as methyl or benzyl groups ( Fig. 3a). The core molecular structure of QACs can have one (mono- QAC), two (bis-QAC), or more (multi-QAC, poly-QAC) charged nitrogen atoms, including many heterocyclic compounds such as piperidine, pyridine, and imidazole (Vereshchagin et al., 2021). Most of the QACs also contain chloride or bromide anions, form micelles, and the critical concentration of micelle formation decides the amphiphilic nature of these compounds. These structures make QACs easily adsorb negatively charged substances such as soil, sediments and sewage sludge (Ying, 2006). QACs are active ingredients in natural polymers such as cellulose, chitosan, dextran, starch, gum, poly(lactic acid) and synthetic polymers like poly(amidoamine), poly(urethane)s, poly(siloxane)s, poly(methacrylate)s and poly(ethylene) (Grigoras, 2021a, Grigoras, 2021b). BACs, ATMACs, DADMACs with alkyl chain lengths from C8 to C18 are the most common active QACs (Fig. 3b, Table S1) covered in this review.
Fig. 3.
General structure of (a) QACs and (b) BACs, DDACs and ATMACs (Zheng et al., 2022).
2.2. Mechanism of action of QACs
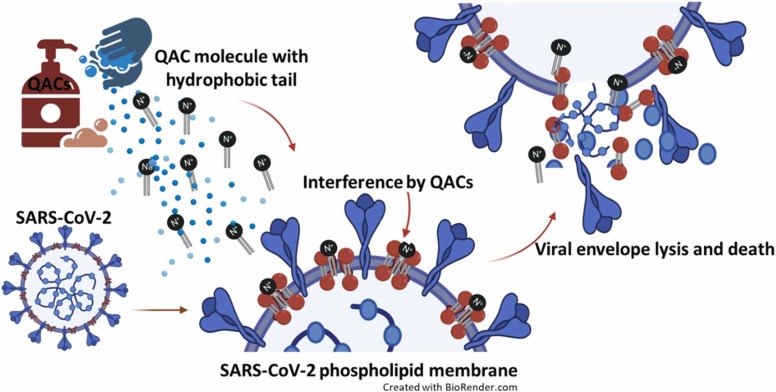
Knowing the mode of action of these compounds and understanding the molecular initiating event (MIE) is key to explaining QACs efficacy. The toxic action of QACs against the bacteria cell wall and membrane is because of the interaction between the cationic quaternary nitrogen with the head groups of the acidic phospholipids of the membrane and the negatively charged structural bacterial proteins (Maillard, 2002, Gilbert and Moore, 2005). The ionic interaction between QACs and bacteria cell membrane destabilises the cell membrane, causing leakage of intracellular low-molecular-weight material, proteins and nucleic acids, resulting in rapid cell lysis (Chapman, 2003). Like bacteria, QACs act on enveloped viruses by deactivating the protective lipid coating ( Fig. 4). Several studies have reported the effectiveness of QACs against influenza and SARS viruses (Schrank et al., 2020, Osimitz and Droege, 2021, Dellanno et al., 2009). These compounds can target intracellular genetic materials such as single-stranded RNA, in the case of SARS-CoV-2. Under normal circumstances, the hydrophobic regions of SARS-CoV-2, i.e., lipid, could help to moderate the proteins' functioning and stabilise it by divalent cations such as Ca2+ (Gilbert and Moore, 2005). Cationic QACs such as BACs could directly interact with the virus membrane by displacing divalent cations. Such intervention could decrease fluidity, raising the endocytic and lysosomal pH leading to cell leakage, lysis and cell death (Gilbert and Moore, 2005). The effectiveness of QACs is affected by their structure. Normally, alkyl chain lengths from C12 to C16 have greater inhibitory ability (Gerba, 2015). Some double-chained compounds are better at killing Gram-negative bacteria, but biscationic QACs are even better (Jennings et al., 2015). However, structural analogues of BAC, having amide and ester groups, could exhibit analogous activity and lower toxicity than BAC alone (Allen et al., 2017). The efficacy of various QACs against several viruses, including SARS-CoV, is presented in Table S4. Within 5–60 min of QAC exposure, more than 99.9% reduction could be achieved (Table S4). Furthermore, within 1 min contact time, several QACs have shown promising results against SARS-CoV-2 (Table S2).
Fig. 4.
Mode of action of QACs on the SARS-CoV-2 envelope.
2.3. Applications of QACs against SARS-CoV-2
QACs are the most common cationic surfactants used as the active components in fabric softeners, disinfectants, biocides, detergents, phase transfer agents, and PCPs, such as hair cleaning products. Besides these applications, QACs have also been used in veterinary products, including contraception formulations, diagnostic testing and vaccine production (Novo, 2020), and nasal sprays (Baker et al., 2020). Due to the effectiveness of ammonium chloride, cetylpyridinium chloride, and miramistin against a range of viruses, including murine coronavirus, hepatitis C, influenza, hepatitis B, poliovirus 1, HIV, herpes and SARS. Baker et al. (2020) suggested these cheap clinically approved products for mouthwash or nasal spray to reduce the point of entry of SARS-CoV-2 transmission.
Nowadays, due to their antibacterial and virucidal properties, these compounds have been extensively used in residential homes (Zheng et al., 2020), hospitals and quarantine centres (Barrios Andrés et al., 2021) and institutional settings (Table S2). These compounds are also found in antiviral coatings and surface wipes (Butot et al., 2021, Monge et al., 2020). It is also recommended to use didecyldimethylammonium chloride (DADMAC C10) and other QAC-based formulations in hospital settings, especially for patients with allergic contact dermatitis during this pandemic (Kreipe et al., 2021, Steinhauer et al., 2021). Ogilvie et al. (2021) found that among commercially available QACs and 0.2 % BAC solution, QACs could rapidly inactivate SARS-CoV-2 within 15 s of contact, even in the presence of a soil load or when diluted in hard water in hospital settings.
QACs such as BACs have been recently used in face mask filters as a biofunctional coating (Martí et al., 2021, Tuñón-Molina et al., 2021). It was found that BACs could effectively inactivate 99 % of SARS-CoV-2 particles in one minute of contact and methicillin-resistant Staphylococcus aureus and Staphylococcus epidermis (Martí et al., 2021, Tuñón-Molina et al., 2021), indicating their possible preventive use among healthcare workers and researchers. Surgical masks infused with QACs were also reported to inactivate a high dose of up to 1 × 105 pfu (plaque-forming units) of live SARS-CoV-2 within minutes (Selwyn et al., 2021).
With regard to personal care products (PCPs), these compounds have also been used as active ingredients in shampoos and body cleansers, and their effectiveness against human coronaviruses (HCoVs) HCoV-229E and SARS-CoV-2 have been assessed (Butot et al., 2021). Furthermore, Ijaz et al. (2021) confirmed their virucidal efficacies (≥ 3 to ≥ 6 log10 reduction) against SARS-CoV-2 and other emerging viruses within 30 s to 5 min of contact time, indicating their usefulness as surface and hand hygiene disinfectants. QACs are also used in mouthwash (Baker et al., 2020, Chen and Chang, 2021a, Muñoz-Basagoiti et al., 2021), with in vitro studies confirming the virucidal activity of oral care products containing a range of QACs (Muñoz-Basagoiti et al., 2021, Komine et al., 2021).
During this unprecedented time, safe consumption of food items has also further triggered researchers to find suitable QACs that could be used against SARS-CoV-2 and disinfect food items. A group of researchers at Tokyo University suggested using diluted QACs to disinfect food items, although their long-term toxic effects on humans have yet to be evaluated (Miyaoka et al., 2021). Such findings are of commercial interest, which could have triggered the general population to use QACs to disinfect vegetables and fruits. A critical review of the application of QACs in the food industry to control the SARS-CoV-2 transmission has been reported (Pedreira et al., 2021).
The ongoing pandemic has forced researchers to explore the virucidal ability of QACs for a wide range of applications, and with an expected market size growth from USD 1070.1 million in 2021 to USD 1548.1 million by 2027 i.e., compound annual growth (CAGR) rate of 5.4 % (Marketwatch, 2021), a large amount of QACs will end up at the WWTPs and, ultimately, the environment.
3. Environmental occurrence and fate of QACs
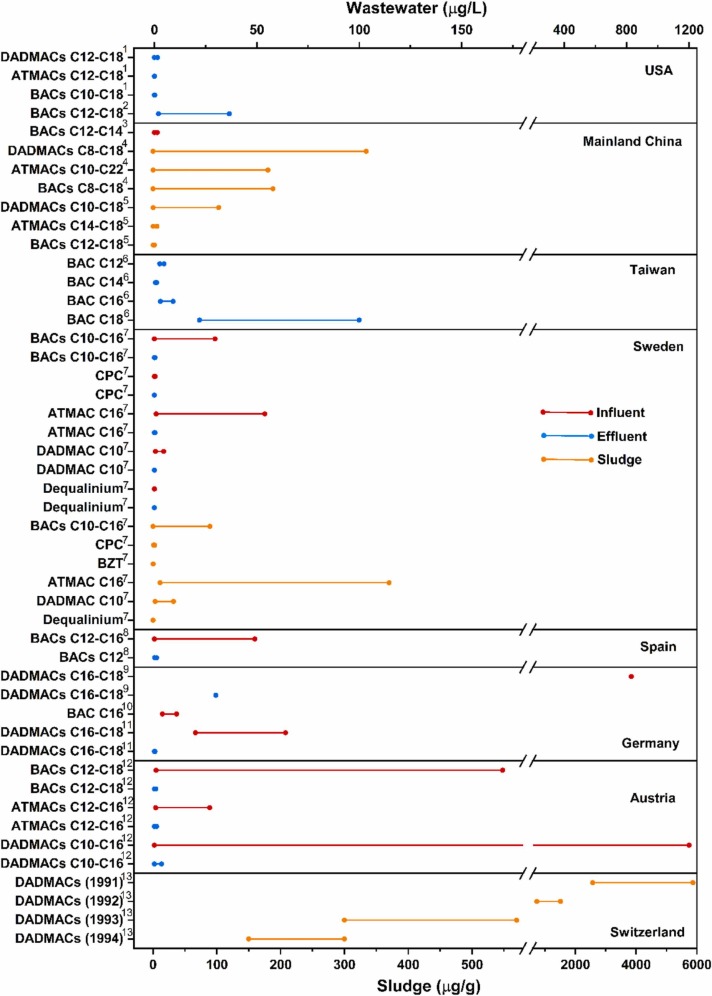
As previously discussed, the use of commercial antibacterial and personal cleaning products has been continually increasing during the widespread COVID-19 pandemic, and a large fraction of these products contains QACs. Some of these compounds enter WWTPs through the sewage collection system. However, QACs can pass through the WWTPs and be released into the aquatic environment. Study shows that about 75 % of QACs annually are released into WWTPs, while the remaining 25% of QACs are discharged directly into the environment (Li and Brownawell, 2010, Ruan et al., 2014). There are two main pathways to remove QACs: biodegradation and active sludge adsorption. Although the degradation of QACs usually happens on sludge solids, the biodegradation process accounts for only a small part of QAC degradation because sorption to solids is much faster than biodegradation. Thus, these compounds finally enter the environment through sewage discharge or surface flooding. The concentration of QACs detected worldwide in surface water and wastewater effluent range from less than 1 μg/L to approximately 100 μg/L, and their concentration could be ten times higher in raw wastewater (1200 μg/L) ( Fig. 5, Table S6). This section highlights the occurrence and fate of QACs in WWTPs, and surface water.
Fig. 5.
Concentration of QACs in different types of wastewater and sludge samples across different countries. 1 (Pati and Arnold, 2020), 2 (Ferrer and Furlong, 2001), 3 (Zhu et al., 2018), 4 (Ruan et al., 2014), 5 (Li et al., 2014), 6 (Ding and Liao, 2001), 7 (Alexandre et al., 2016), 8 (Merino et al., 2003), 9 (Gerike et al., 1994), 10 (Sütterlin, 2008), 11 (Radke et al., 1999), 12 (Clara et al., 2007), 13 (Fernández et al., 1996). (CPC: Cetylpyridinium chloride; BZT: Benzethonium chloride).
3.1. Wastewater and sludge
In an earlier study, Gerike et al. (1994) found high abundances of QACs in German raw sewage samples, with a mean concentration of 830 μg/L; subsequently, many studies across the globe have reported their concentration in wastewater (Fig. 5). Similarly, the concentrations of DADMACs, BACs and ATMACs in influent samples collected from WWTPs in Austria ranged from 0.17 to 1200 μg/L. In Taiwan, octadecylbenzyldimethylammonium chloride (BAC C18) was the most abundant BAC homologue, with high concentrations of 22–100 µg/L in industrial effluent samples. Among several QACs, dodecylbenzyldimethylammonium chloride (BAC C12) and tetradecylbenzyldimethylammonium chloride (BAC C14) were detected in the lower concentration range between 0.5 and 4.7 μg/L of effluent samples.
The concentration of QACs in wastewater and sludge samples at WWTPs might vary significantly between different years because of the prohibition of specific QACs. For example, after 1991, Europe replaced DADMACs with easily biodegradable diethyl ester dimethylammonium chloride; subsequently, the concentration range of DADMAC in sludge samples dropped from 2,570−5,870 μg/g in 1991 to 150 − 300 μg/g in 1994 (Fernández et al., 1996). In another study, relatively low levels of QACs were found in sludge samples collected from a WWTP in Guangzhou, with total concentrations of BACs, ATMACs and DADMACs up to 3.6, 8.3, and 156 μg/g, respectively (Li et al., 2014). Among BACs, BAC C12 is the most abundant QACs reported in both sewage and sludge samples based on the results obtained from ultrasonic extraction (USE) (Heyde et al., 2020). Through a non-targeted approach for the first time, Ruan et al. (2014) reported the presence of DADMAC C20 and mixed DADMAC C14:18, C18:20 in sewage sludge.
While most of these studies have reported QACs concentrations before the pandemic, only Pati and Arnold (2020) and Alygizakis et al., 2021 reported the occurrence of QACs in wastewater samples during the COVID-19 pandemic, at this time of writing. Notably, Pati and Arnold (2020) reported various homologs of BAC and DADMAC and 15 suspect QACs in at least one wastewater effluent sample with the concentration ranging from 0.4 μg/L to 6.6 μg/L, indicating the inability of the WWTPs to treat these contaminants. In the following year, Alygizakis et al. (2021), for the first time, reported an increase in the mass load of cationic QACs in raw wastewater by 331 %, i.e., 429 g/day in 2019 to 1897 g/day in 2020, highlighting their increase attributed to stay-at-home measures, working from home, social distancing, handwashing and surface-disinfection advisories during COVID19. Among the studied QACs, the mass loading for BAC C10, BAC C12, ATMAC C10, ATMAC C12, and dioctyldimethyl ammonium bromide (DADMAC C8:8) was increased by 322 %, 431 %, 173 %, 90 % and 181 %, respectively, during the pandemic.
3.2. Removal of QACs at WWTPs
The overall removal of QACs in different processes is summarised in Table S5 where several removal mechanisms including biodegradation, adsorption and advanced oxidations process have been highlighted. Bergero and Lucchesi (2018) studied the adsorption of cationic surfactants, tetradecyltrimethylammonium bromide (ATMAC C14), tetra- decylbenzyldimethylammonium chloride (BAC C14) and hexadecylbenzyldimethylammonium chloride (BAC C16) in activated sludge. It was found that within 2 h of achieving adsorption equilibrium, 81 %, 90 % and 98 % of ATMAC C14, BAC C14 and BAC C16 respectively, were completely adsorbed to the activated sludge. Similarly, Pseudomonas sp. was used to treat synthetic and industrial effluent containing BAC, in continuous up-flow biofilm aerobic reactors, and removal of up to 99.3 % was achieved in the lab (Fortunato et al., 2019). Similarly, by using an oxygen-based membrane biofilm reactor and increasing the solid recycling ratio up to 4 and the oxygen supply rate, the removal of QACs at WWTPs could be improved (Hajaya, 2021). Among biological WWTPs, cyclic-activated sludge systems are the most popular treatment units for removing organic compounds from wastewater (Zhu et al., 2018, Mohapatra et al., 2021b). Nevertheless, the biodegradation processes for QACs are highly dependent on several factors, including the concentration of QACs, the chemical structure of the compound, adsorption to biosolids, microorganisms consortia (e.g. QACs resistant or degrading microbes) and the presence of anionic surfactants (Brycki et al., 2014, García et al., 2001, Ying, 2006). Thus, an increased load of these compounds at the WWTPs may further hamper the overall removal process at biologically operated WWTPs by inhibiting microbial activity.
Several attempts were also made in an ultraviolet/persulfate (UV/PS) oxidation system for complete detoxification or mineralization of dodecyltrimethylammonium chloride (ATMAC C12) in wastewater matrixes, where HCO3− , Cl− and humic acid negatively affected ATMAC C12 elimination, whereas NO3− and SO4 2− had an insignificant effect on its removal (Lee et al., 2019). However, biodegradation coupled with the Fenton oxidation process can also enhance the degradation of these compounds by 85.5 % (Zhao et al., 2020).
3.3. Surface water
Several studies reported the concentrations of QACs in surface water, reservoir and marine water worldwide (Table S6) and all of these studies were conducted before the pandemic. For the first time, Radke et al. (1999) detected the concentration of DADMAC C16:16, DADMAC C16:C18, DADMAC C18:18 at 0.11 µg/L, 0.34 µg/L, and 0.41 µg/L, respectively, in Germany river water (Table S6). Similarly, the concentrations of most of the BACs in the surface waters of Taiwan were less than 1 µg/L, except for BAC C18. BAC C18 is the most abundant among the compounds with different chain lengths, with a concentration range from 2.6 to 55 µg/L, followed by BAC C16 in the range of 1.2–5.3 µg/L (Ding and Liao, 2001a). In contrast, the concentrations of ATMACs in a Taiwan river were much lower, with a maximum concentration of 0.66 µg/L (Ding and Tsai, 2003). The data indicate that wastewater in Taiwan contained high concentrations of BAC C18 that were directly discharged into the river. Although the removal rate of QACs at nontoxic levels is generally more than 90 % after biological treatment, continuous discharge of wastewater during the pandemic and, more specifically, untreated wastewater to surface waters could elevate their concentrations in surface water, particularly during the monsoon season. Due to their strong affinity to the soil, QACs tend to be adsorbed and concentrated on the soil surface (Martínez-Carballo et al., 2007a). Thus, rivers receiving surface runoff could potentially experience a high concentration of these compounds during the monsoon season, which was the case for high concentrations of QACs in Australian rivers (Martínez-Carballo et al., 2007a). Resuspension of sediment could further increase their aqueous concentration during the monsoon season and even during non-rainy seasons (Ruan et al., 2014). The highest BAC concentration (BAC C12 + BAC C14) up to 22,593 ± 7056 µg/L in roof runoff, was reported elsewhere (van De Voorde et al., 2012). Currently, no study during the pandemic has documented the concentrations of these compounds in surface waters. However, with an increased mass loading as high as 331 % (Alygizakis et al., 2021), QACs could potentially find their way into surface water and countries without proper sanitation or sewerage systems (Panth et al., 2021) may experience high concentrations.
3.4. Fate of QACs in surface water
In surface water, contaminants are simultaneously subjected to photodegradation, biodegradation and sorption (Eregowda and Mohapatra, 2020, Mohapatra et al., 2023). QACs, irrespective of structural variations, such as sidechain length and aromatic rings, are susceptible to indirect photodegradation, with OH· in natural waters with half-lives ranging from 12 to 94 days, implying slow abiotic degradation in water bodies (Hora and Arnold, 2020). Only BAC is reported to undergo direct photolysis. Although QACs are generally aerobically biodegradable in the environment, the adsorption of QACs to sediments or biosolids often outcompetes biodegradation in QACs with longer alkyl chain length and/or the substitution of a benzyl group for a methyl group (García et al., 2001, Li and Brownawell, 2010). As the removal of QACs in the environment relies mainly on microorganisms through biodegradation/biotransformation, microbial consortia play a crucial role. Pseudomonas, Xanthomonas sp. and Aeromonas hydrophila sp. are among the bacteria capable of degrading QACs (Brycki et al., 2014, Miura et al., 2008, Patrauchan and Oriel, 2003). However, the ability of these microorganisms to degrade QACs depends on various aspects, such as the concentration of QACs and types of QACs. Isolation of microbial consortia for biodegradation of QACs is challenging as QACs are biocides and toxic to microorganisms. Some QACs resistant microorganisms can survive but may not be involved in biodegradation, while those degrader microorganisms may not be resistant to QACs. As such, most studies showed the degradation of QACs through mixed cultures. The current advancement in next-generation sequencing allows more in-depth study into the microbial community through metagenomics sequencing. While critical micelle concentration (CMC) plays an important role in deciding the fate of QACs in environmental matrices, Mulder et al. (2020) postulated that the number of carboxyl groups and small molecular weights of dissolved organic matter could reduce the CMC of BAC C12 and BAC C16 at pH 6.
With a fundamental knowledge of the persistence and degradation rates of QACs, modelling would be a great tool to predict concentrations and assess the risks of QACs, particularly for environmental exposure of QACs during such an unprecedented time. The fate of QACs in the aquatic ecosystem with special attention to the sediments, is discussed in the next subsection.
3.5. Sediments
QACs can readily adsorb on soil or sediments due to their unique structure and strong affinity to organic and inorganic particles. High adsorption and recalcitrance to biodegradation lead to the frequent presence of QACs in sediments. Table 1 lists the concentrations of QACs in sediment samples in the world. In the urban estuarine sediment samples of the USA, the maximum concentration of ATMACs was 2820 ng/g and 6750 ng/g in 1998 and 2008, respectively (Lara-Martín et al., 2010). The concentrations of DADMACs C16-C18, ATMACs C16–18 and BACs C12–14 were higher in the sediment samples; for example, the concentration of BACs C12–14 varied from 40.7 to 672.8 ng/g and 5.3–730.1 ng/g, while the concentration of BACs C16–18 varied from 1.20 to 189.9 ng/g and 2.10–82 ng/g (Li et al., 2014). Among the different BAC homologues listed in the table, BAC- C12 was the most common compound, with an average percentage of 70%, similar to the composition of the industrial BAC mixture. For the ATMAC homologues, ATMAC C16 and ATMAC C18 were two major types. Among the DADMACs, DADMACs C16:C16, C16:C18, C18:C18 take up more than 90% of the total DADMACs detected in the sediments. In contrast, DADMAC only has an average percentage of 4% of the total DADMACs (Li and Brownawell, 2010). A similar conclusion was reached after analysing the sediment samples of seven other countries: For DADMACs and ATMACs, with longer alkyl chain length, such as DADMACs C16:18, and DADMACs C18:18, possessed the largest proportion in the sediment samples, while BAC C12 was still the most common type of BAC homologues (Kaj et al., 2014). The similar QACs average composition patterns and comparatively high concentrations indicate that QACs are persistent in sediments.
Table 1.
Concentration of QACs in different types of sediment samples (Alexandre et al., 2016, Dai et al., 2018, Heyde et al., 2021, Kaj et al., 2014, Lara-Mart́n et al., 2010, Li and Brownawell, 2010, Li et al., 2014, Martínez-Carballo et al., 2007b, Pintado-Herrera et al., 2017, Ruan et al., 2014, Zhang et al., 2015).
 |
Relatively high concentrations of BACs and DADMACs were reported in China and Japan in sediment samples, indicating high pollution levels in both countries (Dai et al., 2018). The vertical profiles from the sediment surface indicated a spike in BACs and DADMACs concentration during the 1970 s and 1980 s, but a significant drop in BAC concentration before the pandemic indicated a compositional change in commodities and the effectiveness of emission control strategies. The concentration of DADMAC remained relatively stable. In contrast, during this pandemic, a high concentration of BACs followed by ATMACs and DADMACs were reported in Mexican soil samples irrigated with sewage (Heyde et al., 2021). As expected, the concentrations of QACs increased linearly during the first few years of irrigation, but an exponential increase in the soil concentrations up to 155 µg/kg over time indicates strong adsorption and accumulation of these compounds in the soil. Environmental conditions, such as rainfall, will affect the precipitation of QACs because wet weather can increase the sedimentation ability of QACs. In Austria, the mean concentration of ATMAC and BAC in June was 35 ng/g and 1023 ng/g, respectively, while the mean concentration of ATMAC and BAC in August was only 19 ng/g and 351 ng/g, respectively (Martínez-Carballo et al., 2007a). Except for one study by Heyde et al. (2021), no additional studies on the occurrence of QACs in soil samples have been published during this pandemic.
Slower biodegradation of QACs has been observed in anoxic or anaerobic conditions in sludge and sediments (Lara-Mart́ńńn et al., 2010). Some studies investigated the temporal distribution of QACs concentration by analysing dated sediments with different depths of sediment cores (Pati and Arnold, 2020, Lara-Mart́n et al., 2010). In a study at Jamaica Bay, elevated QACs levels were found around the 1980 s, which was suspected to be associated with sewage sheds from upgrading works at the nearby wastewater treatment plant and drastic reduction after the upgrading works (Lara-Mart́ńńn et al., 2010). A similar finding was observed by Pati and Arnold (2020), where the authors suggest that the federally mandated industrial pre-treatment program initiated in 1981 could be one of the reasons for the decrease in QACs levels after the 1980 s. These studies further prove the high persistence and slow degradation of QACs once the compounds are transferred to anoxic/anaerobic compartments. Tezel et al. (2006) conducted a systematic study on the degradation of QACs in aerobic, anoxic and anaerobic conditions. Their studies revealed that QACs with shorter alkyl chain lengths and lower adsorption affinity cause a greater inhibitory effect on methanogenesis. Under methanogenic conditions, none of the QACs tested was biodegraded (Tezel et al., 2006). As QACs are biologically active compounds, several potential environmental impacts need to be considered due to their high usage during the pandemic. Thus, it is crucial to study their concentrations and consider the toxic impact of QAC residuals in sediments.
4. Toxicity of QACs to aquatic organisms and human
4.1. Toxicity to aquatic organisms
Exposure to QACs showed lethal effect or growth inhibition in aquatic organisms through oxidative stress, disruption of cell membrane or molecular defects causing apoptosis and endoplasmic reticulum stress (unfold or misfold of proteins) (Christen et al., 2017, Qian et al., 2022). In addition, a recent study demonstrated that exposure to QACs enhanced the release of cyanotoxin (i.e., microcystin MC-LR) via upregulation of synthesis proteins and damage to the cell membrane (Qian et al., 2022). Toxic effects were also observed in freshwater crustaceans at environmentally relevant concentrations of QACs (Lavorgna et al., 2016). As such, the increased use of QACs during the COVID-19 pandemic has raised concerns about their toxicity and potentially harmful effects on humans and aquatic organisms such as fish, algae, protozoa and other microorganisms (Qian et al., 2022, Chen et al., 2014, Zhu et al., 2010, Liang et al., 2013). Due to their amphiphilic nature, QACs are readily absorbed onto soil and sludge; thus, their concentration in wastewater at which they pose toxicity to receiving water bodies, has attracted much attention. The toxicity of QACs to organisms can be divided into two parts: (i) single QAC toxicity (Table S7) and (ii) QACs mixture toxicity (Table S8).
Different kinds of species have different sensitivities toward single QACs. For example, the 24 h median effective concentration values (EC50) of dodecyltrimethylammonium bromide (ATMAC C12), tetradecyltrimethylammonium bromide (ATMAC C14), hexadecyltrimethylammonium bromide (ATMAC C16) for Daphnia magna varied from 0.058 mg/L to 0.37 mg/L. Study shows that algae are more sensitive to QACs than other aquatic organisms such as fish and crustaceans (van Wijk et al., 2009). Due to their net positive charge, these compounds strongly bind with the negatively charged algal cell walls (Liang et al., 2013). The EC50 value of benzalkonium is 280 µg/L and 5.9 µg/L for fish and invertebrates, respectively (van de Voorde et al., 2012). Thus, BACs toxicity to other aquatic beings is usually much higher than microalgae and bacteria. Some species may have varying sensitivity to different QACs. The 48 h EC50 value of D. magna towards 5 kinds of single QACs varied from 6.6 µg/L to 0.3 µg/L. With a longer carbon chain, BACs are reported to show higher toxicities than the short carbon chained BACs. Thus, BAC C12 and BAC C14 have been reported to exhibit extremely high toxicity than stearyltrimethylammonium chloride (ATMAC C18), n-hexadecyltrimethylammonium chloride (ATMAC C16) and octyl-decyl dimethyl ammonium chloride (ODDAC).
There are instances where QACs are found combined with other chemical substances that may pose mixture toxicity (Table S8). In such a scenario, the toxicity of a single QAC could potentially differ from a mixture of QACs that are commonly encountered in the environment. The joint action of binary mixtures usually changes based on the concentration of individual components. For example, the binary mixture of ATMAC C16 and aromatic hydrocarbons (Ahs) has a synergistic toxicity effect on C. vulgaris when Ahs have low concentration; however, at a high concentration of Ahs, the joint action changes to an antagonistic effect because they will compete (Ge et al., 2010). Similarly, the joint action of ATMAC C16 and fluoranthene (Flu) also shifted from a synergetic toxic effect to an antagonistic effect when the levels of Flu grew from 50 µg/L to 200 µg/L (Yu et al., 2013). When the mixture of QACs is present in low concentrations in the environment, some hormetic effects are observed (Agathokleous et al., 2022, Mo et al., 2020). It may enhance the propagation of pathogenic microbes and increase the spread of antibiotic resistance.
4.2. The impact of QACs on animals models
The impact of QAC exposure has been mostly demonstrated in animal models. For example, few studies on mice showed a significant reduction in pregnancy (dropped by 50–60 %) when disinfectants containing n-alkyl dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DADMAC C10) were applied in the animal facility (Maher, 2008). In a long-term feed dosing study, results revealed a significant decrease in fertility rate, including a longer duration of the first litter, fewer pups, and a smaller number of pregnancies in the 120 mg ADBAC + DADMAC C10 disinfectant/kg/day exposure group compared to the control group (Melin et al., 2014). This study further demonstrated that exposure to the same set of disinfectants at the same dose in female mice decreased reproductive capability in terms of reduced ovulation and fewer estrus cycles, while male mice exhibited decreased sperm concentration and motility (Melin et al., 2016). Increasing placental abnormalities and late gestation fetal deaths were reported with feed dosing at the same concentration (Hrubec et al., 2017). Nevertheless, the exceptionally high dose in their toxicity studies was also criticised (Keating and Watkins, 2021, Hostetler, 2018).
Apart from higher doses in toxicity studies, the impacts of ADBAC and DADMAC C10 at lower concentrations or/and ambient levels (where disinfectant containing ADBAC and DADMAC C10 was used for cage cleaning) were also examined. Significant reductions in sperm concentration and motility were observed in male mice upon exposure to ambient levels of ADBAC + DADMAC C10 for 7 weeks or 8 days oral dosing at 7.5 mg/kg/day (Melin et al., 2016). Similarly, higher neural tube defects were found in the mice exposed to ambient levels of these compounds (Hrubec et al., 2017). However, the lack of information on the concentrations of ADBAC and DADMAC C10 at ambient levels hinders solid conclusions from the studies. According to regulatory guidelines, Hostetler et al. (2021) evaluated the prenatal toxicity potential of ADBAC and DADMAC C10 in rats and rabbits. The study reported that the no-observed-adverse-effect level (NOAEL) of ADBAC for maternal toxicity was 10 and 3 mg/kg/day in rats and rabbits, respectively, while prenatal toxicity was 100 and 9 mg/kg/day in rats and rabbits, respectively (Hostetler et al., 2021). For DADMAC C10, a NOAEL of 1 mg/kg/day was reported for maternal toxicity in both rats and rabbits, while 20 and 3 mg/kg/day was reported for prenatal toxicity in rats and rabbits, respectively (Hostetler et al., 2021). Furthermore, the degree of toxicity of BAC was found to be correlated with BAC concentration in blood and tissue and the route of exposure (Xue et al., 2004). Regarding fetal development in mice, there could be a risk of QACs disinfectants crossing the blood-placental barrier and embryonic blood-brain barrier, which subsequently alter sterol and lipid hemostasis that might contribute to neural tube defects, as demonstrated in a mouse neonatal brain study (Herron et al., 2019). While there is some evidence linking the impact of QAC exposure to fertility, developmental, and reproduction issues in animal models, it is largely unknown if these observations are translatable within a human context.
4.3. Human exposure to QACs and the potential health risk
Human exposure to QACs can occur through inhalation, dermal contact and oral ingestion. Application of ready-to-use QAC-related disinfectants is generally regarded as low risk with lower dosages of QAC compounds (Osimitz and Droege, 2021, EPA, 2022, EPA, 2017). However, a higher risk was found while handling concentrated QACs (EPA, 2022, EPA, 2017). Furthermore, when inhaled or ingested at a high concentration, QACs such as BAC C12-C16 and DADMAC C10 can disrupt mitochondrial functions leading to decreased ATP production and cell death (Osimitz and Droege, 2022). Increasing incidences of asthma have also been reported in healthcare workers exposed to disinfectants containing QACs (Migueres et al., 2021a, Gonzalez et al., 2014, Heederik and Heederik, 2014). The study by Migueres et al. indicated the sensitizing potential of QACs involving heterogeneous pathobiological pathways with a highly eosinophilic pattern of airway response to develop occupational asthma (Migueres et al., 2021). In addition to the debatable toxicity impact on prenatal and maternal animals and humans, a study also showed the potential penetration of QACs into bloodstreams, where QACs were detected in 80 % of 43 human volunteers (Hrubec et al., 2021). A similar study showed increasing detection frequency and significantly higher concentration of total QACs (15 QACs) in blood samples collected during the COVID-19 pandemic (median concentration = 6.04 ng/mL) compared to the samples collected before the pandemic (median concentration = 3.41 ng/mL), with an overall increase of 77 % (Zheng et al., 2021). Although no biochemical alterations in the body tissues were observed, the low clearance rate of QACs in the liver indicated their high bioaccumulation. Associations were also seen between the concentration of QACs in blood and inflammatory cytokines, mitochondrial function and cholesterol synthesis intermediaries or disruption of cholesterol homeostasis (Hrubec et al., 2021). Among the QACs, the study reported that benzalkonium chloride could inhibit the DHCR7 gene expression, which is responsible for providing instructions to 7-dehydrocholesterol reductase, responsible for cholesterol production.
Current industry practice assesses human health risks based on individual QAC compounds and/or the total risks attributed to each QAC compound. Possible synergetic effects of multiple QACs have also been suggested, but this hypothesis has not been established in animals or humans. More reviews on human health hazard assessment of QACs, particularly on unpublished studies submitted and reviewed by regulatory agencies, are presented by Luz et al. (2020) and Osimitz and Droege (2021). A recent paper by Agathokleous et al. (2022) further suggested including hormesis while evaluating the effects and risks of QACs as the linear-no-threshold (LNT), and threshold dose-response models are unable to identify or predict their toxic effects. The scientific evidence on enhanced metabolic stability, particularly in longer alkyl chain QACs, and the potential bioaccumulation of QACs in humans has dramatically raised additional concerns on the toxicity of QACs (Zheng et al., 2021, Seguin et al., 2019). Hence, there is a need for a proper evaluation of the safe usage of QACs, including epidemiological study and risk assessment, as well as alternatives to QACs for disinfecting applications.
5. Development of QAC and antimicrobial resistance phenotypes
Besides the toxicity of QACs, there is a growing concern that QACs can lead to increased antimicrobial resistance in bacteria (Hegstad et al., 2010, Mc Cay et al., 2010). The convergence of two public health issues, the COVID-19 pandemic and antimicrobial resistance, has raised concerns about the accelerated use of antibiotics and antimicrobials used to treat bacterial co-infections with SARS-CoV-2 (Bengoechea and Bamford, 2020, Hedberg et al., 2022, Mirzaei et al., 2020). One of the two key areas COVID-19 is anticipated to lead to the emergence of bacterial resistance is the use of QACs as disinfectants and biocides in hospitals and community settings due to their potent activity against a wide variety of pathogens (Chen and Chang, 2021b). Even prior to the pandemic, QACs were flagged as potential agents promoting antimicrobial resistance (Zhang et al., 2015, Pereira and Tagkopoulos, 2019, Tezel and Pavlostathis, 2011). This has raised interest in ecotoxicology studies and the development of risk assessments capturing dose-response models effects within the hormetic (low dose) zones to determine permissible levels of disinfectants in the environment (Agathokleous et al., 2019).
Certain Gram-negative bacterial species such as Pseudomonas aeruginosa display tolerance and intrinsic resistance to QACs and antibiotics by modifying cell membrane structures (e.g., fatty acid modification), using efflux pumps that extrude biocides or antibiotics out of the cell, or employing other adaptive strategies such as the formation of persister cells and biofilms (Mc Cay et al., 2010, Méchin et al., 1999, Pang et al., 2019). Mc Cay et al. (2010) showed that sublethal concentrations of QACs can induce antimicrobial resistance in several bacterial species, including P. aeruginosa strains where decreased sensitivity to QACs and increased resistance to antibiotics have been documented (Mc Cay et al., 2010). Studies on long and short term exposure of bacteria to sub-inhibitory concentrations of BACs (the most common types of QACs) have shown that bacteria can develop cross-resistance to more than one antibiotic or increased resistance to specific antibiotics through physiological adaptive mechanisms and genetic changes (Merchel Piovesan Pereira et al., 2020, Merchel Piovesan Pereira et al., 2021). Furthermore, an intrinsically QAC tolerant microorganism or an acquired QAC tolerant one is likely to exhibit cross-tolerance to other types of QACs upon exposure (Voumard et al., 2020b). Although it is thought that Gram-negatives are more resistant to QACs due to their innate defence and adaptive mechanisms mentioned above, phenotypic resistance in Gram-positives such as Staphylocci is increasingly being reported, particularly on surfaces that are treated with QACs (He et al., 2014).
QACs tolerance and antibiotic resistance have been described in BACs exposure experiments in Gram-negative and Gram-positive bacteria (Table S9). Collective studies show the minimum inhibitory concentration (MIC) values of Escherichia coli to BACs and antibiotics increased by 2 – 7 fold, and 1 – 116 fold, respectively, exhibiting increased cross-resistance to antibiotics of different classes. However, not all bacteria display the trend of the coupled increase of BACs and antibiotic resistance. For instance, other studies indicate that Gram-negative species such as Klebsiella spp. Chryseobacterium spp. and Enterobacter spp. show only increased tolerance to BACs, but not to other screened antibiotics (Table S9). Increasing antibiotic resistance has also been observed in Gram-positive QACs resistant strains (Akimitsu et al., 1999, Guérin et al., 2021a). Methicillin-resistant Staphylococcus aureus (MRSA) strains showed increasing resistance to β-lactam antibiotics with increased MICs values between 1 and 16 folds in BAC resistant strains compared to BAC susceptible strains (Akimitsu et al., 1999), while cross-resistance and increased antibiotic MICs were observed in BAC resistant Listeria monocytogenes isolated food-derived products (Yu et al., 2018) and environmental sources (Guérin et al., 2021b).
5.1. Intrinsic, adaptive, and acquired mechanisms of QAC tolerance and antibiotic resistance
5.1.1. Membrane permeability and physiological adaptive mechanisms
Bacteria use shared resistance mechanisms to evade biocides and antibiotics that can contribute to cross-resistance. The first line of defence, particularly in Gram-negatives, is the presence of the outer membrane composed of lipopolysaccharides layers and efflux pumps that deter QACs and other antagonists (e.g. drugs, toxins) from entering and disrupting the cell membrane’s physical and ionic stability (Wessels and Ingmer, 2013). Nonetheless, BACs can readily bind to and enter the cell membrane of bacteria by ionic and hydrophobic interactions, changing the properties and functions of the membrane, disrupting the integrity, ultimately resulting in leakage of essential intracellular constituents (Ferreira et al., 2011, Ortega Morente et al., 2013). Besides the disruption of cell membranes, QACs can strike an imbalance of membrane osmoregulation, inhibition of respiratory enzymes, dissipation of proton motive force and oxidative stress, which induce error-prone DNA replication and finally lead to mutations and gene transfers of bacteria (Blázquez et al., 2012, Ceragioli et al., 2010). For example, P. aeruginosa can modify the fatty acid structure (e.g. lauric, β-hydroxylauric, palmitic acids) in cell membranes to deter the penetration of biocides, and antibiotics impart intrinsic resistance (Méchin et al., 1999). Additionally, these species have a biofilm-forming capability providing a diffusion barrier to reduce accessibility to both antibiotics and QACs via the cell membrane (Mah and O’Toole, 2001, Drenkard, 2003). Studies have also demonstrated that multispecies biofilms show greater biocidal resistance than single species (Touzel et al., 2016). Other bacteria such as Bacillus and Clostridium form endospores, enabling them to enter a metabolically dormant state in the presence of a stressor, which allows them to resist chemical treatment and aid survival (Vijayakumar and Sandle, 2019).
5.1.2. Horizontal gene transfer (HGT) and its associated elements/genes
There are a variety of molecular mechanisms in play that contribute to increased biocide tolerance in bacteria (Mc Carlie et al., 2020). HGT within cells is mediated through MGEs such as transposons, insertion sequences, integrons and gene cassettes which can carry biocide, metal and antibiotic resistance genes (MBMRG, ARGs) (Mc Carlie et al., 2020, Pal et al., 2015, Partridge et al., 2018). Conjugative plasmids and integrative conjugative elements (ICE) are MGEs that carry conjugative elements that encode the machinery that allows the transfer of genes between cells (Partridge et al., 2018, Johnson and Grossman, 2015). For example, Kim et al. (2018a) found that BAC tolerance was mediated through an efflux pump associated with ICE in P. aeruginosa. In silico, data mining studies of genome and metagenomes showed co-occurrence patterns of ARG and BMRG signatures (Li et al., 2017). Metagenomic studies in the guts of pigs (Li et al., 2022), wastewaters (Zhang et al., 2021) and rivers (Thomas et al., 2020) have shown similar trends, which suggests that biocides, metals and antimicrobials present in the environment could select for these genes. In a study of bacteria isolated from seafood in supermarkets and fish markets using traditional culturing techniques, 7% of isolates were found to be resistant to multiple antibiotics and biocides, and a subset of antibiotic-resistant isolates was able to grow on media containing copper sulfate and zinc chloride with metal resistance genes pcoA/copA, pcoR, and chrB detected (Romero et al., 2017).
5.1.3. Efflux pumps and acquired resistance
Non-specific efflux pumps in microbes can transport a variety of substrates such as toxic substances, metabolites, quorum sensing molecules, biocides and antibiotics. There are five main categories of efflux pumps, (1) ATP (adenosine triphosphate)-binding cassette (ABC) family; (2) major facilitator superfamily (MFS), (3) resistance/nodulation/division (RND) family, (4) small multidrug resistance (SMR) family; and (5) multidrug and toxic compound extrusion (MATE) family (Wand, 2017). Biocidal resistance genes (BRGs) such as qacA/B, norA and smr have been found in opportunistic pathogens isolated from hospital environments which are hotspots with frequent QACs usage, implicating their importance in biocide resistance (Liu et al., 2015). These BRGs associated with efflux pumps have been identified in QAC resistant opportunistic pathogens, some of which exhibit resistance to antibiotics (Vijayakumar and Sandle, 2019).
The SMR family efflux pumps genes encoded by qac are the most notable BRG associated with efflux pumps that can conferresistance to QACs and are located on mobile genetic elements that can be horizontally transferred to other bacterial species by integrons carried by multidrug-resistant plasmids (Tezel and Pavlostathis, 2015). This mobile characteristic of qac genes and co-location on plasmids bearing antibiotic-resistant genes may result in dual resistance to QACs and antibiotics in recipient cells that acquire the plasmids.
In the context of QAC resistance, most efflux pumps in Gram-negatives tend to be chromosomally located (e.g. norA, smr, sugE, emrE), while Gram-positives have acquired plasmid encoding efflux pumps except for norA, which is chromosomally encoded in S. aureus (Poole, 2002; Buffet-Bataillon et al., 2012; Meade et al., 2021). Various global clinical studies have identified BRGs associated with efflux pumps (e.g. qacA/B, qacC, qacG, qacH, qacJ, qacE∆1, smr) in opportunistic pathogens, some of which exhibit resistance to both biocides and antibiotics. The highest occurrence of qacA/B genes was found in Gram-positive S. aureus and Enterococcus faecalis, while other Gram-negatives such as Klebisella and Pseudomonas carried qacE and cepA, on plasmids, the latter of which confers resistance to beta-lactam antibiotics (Vijayakumar and Sandle, 2019, Bush, 2018, Slipski et al., 2018). E. coli isolated from broiler chickens were also found carrying the qacE∆1 gene linked to class 1 integrons and multiple antibiotic resistance gene cassettes, with AmpC beta-lactamases, although phenotypic resistance was not established (Roedel et al., 2021). Other studies in the UK showed that 75 % of class 1 integrons (CL1) containing isolates from the environment that carried QAC resistant genes also carried AMR genes (Amos et al., 2018). Genes encoding for carbapenemases (e.g. bla VIM, bla IMP, bla NDM) associated with qac genes in species of BAC resistant Acintobacter have been isolated from hospital environments in China (Liu et al., 2017). Phenotypic evidence coupled with the occurrence of QAC and antibiotic resistance genes indicated that QACs at sub-MIC levels might offer selective pressure and promote the evolution and spread of AMR through horizontal gene transfer or/and efflux pump-mediated mechanisms of resistance (Gaze et al., 2011, Poole, 2005, Gillings et al., 2009).
5.1.4. Adaptive resistance through genetic mutations
Laboratory-based selective evolution experiments have characterised genetic changes in the genomes of bacteria to exhibit phenotypic resistance to biocides. For example, work by Merchel Piovesan Pereira et al. (2021) on E. coli showed that constant exposure to the same concentrations of biocides leads to a decreased susceptibility to medically relevant antibiotics, with a subset of cross-resistant strains having one or more mutated genes that are functional pathways implicated in resistance to antibiotics (i.e., porin regulators, multidrug efflux proteins, RNA polymerase subunits) (Merchel Piovesan Pereira et al., 2021). In another study of bacterial communities of river sediments, BAC-fed bioreactors were selected for P. aeruginosa mutants and had mutations in polymyxin resistance genes (pmrB) and induced expression of efflux pump genes, which resulted in increased phenotypic resistance to polymyxin, tetracycline and ciprofloxacin (Kim et al., 2018b). Mutants of Salmonella enterica Typhimurium exposed to biocides showed single nucleotide polymorphism (SNPs) and deletions in genes, and amino acid substitutions membrane proteins involved in resistance to antibiotics and detergents (Curiao et al., 2016). Earlier studies on laboratory-generated mutants of S. aureus showed that increased susceptibility to QACs was attributed to mutations in the chromosomal encoded norA gene (Furi et al., 2013).
Failed efficacy of biocides is suggested to be responsible for the rapid selection of disinfectant-tolerant bacterial mutants. In experiments conducted by Nordholt et al. (2021), BAC tolerant E.coli clones were shown to have faster growth rates and outcompeted their wild-type counterparts in the presence of sub-inhibitory concentrations of four antibiotics (Nordholt et al., 2021). From an evolutionary point of view, the increased propensity in the fitness of BAC tolerant and antibiotic resistance phenotypes (and genotypes) in laboratory-based studies suggests that these adapted strains may have a selective advantage for survival in environments contaminated with chemicals.
6. Consequences of the increased usage of QACs to the environment
From an environmental perspective, the recent COVID-19 pandemic has triggered a spike in usage of disinfectants (e.g., QACs), which has introduced unprecedented heavy loads of QACs residues to the environment, such as surface water, groundwater, soil and wastewater treatment plant, although the surge in these environments has yet to be comprehensively accessed. This is compounded by the prevalent use of antibiotics in the current pandemic (Reardon, 2020). The presence of high concentrations of QACs may inadvertently kill off indigenous communities of beneficial microbes found in these environments due to their high susceptibility to disinfectants compared to clinically related pathogens (Tezel and Pavlostathis, 2011, Yang and Wang, 2018), consequently affecting the aquatic ecosystem and posing a potential health risk to humans. A higher load of QACs could potentially affect the performance of WWTP, such as nitrification and denitrification process, thus, reducing nitrogen removal capacity (Zhang et al., 2015, Hajaya and Pavlostathis, 2012, Yang, 2007). Similarly, decreased efficiency of the anaerobic digestion system was observed with the introduction of a high concentration of QACs (Tezel and Pavlostathis, 2009). Methanogens which play a crucial role in the anaerobic digestion process, were inhibited with a high concentration of QACs (e.g., > 50 mg/L BAC), resulting in an accumulation of volatile fatty acids and lower removal efficiency of chemical oxygen demand (Tezel and Pavlostathis, 2009). Additionally, the concentrations of QACs at sub-MIC levels provide a selective pressure to promote the evolution and spread of AMR through genetic mutations, horizontal gene transfer or/and efflux pump-mediated mechanisms of resistance (Gaze et al., 2011, Poole, 2005, Gillings et al., 2009), although combinations of different resistance mechanisms have played a role in the overall survival and domination of different genotypes.
7. Conclusions
The mass load of QACs at a WWTP has increased by 331% during COVID-19 compared to before COVID-19. Due to their low degradation rate, many of these compounds are typically removed through sludge disposal from the WWTPs. In general, QACs containing twelve or more carbons in the side chain are more prevalent in sediment samples and possibly originate from municipal wastewater effluent and surface runoff. Their concentration is expected to rise in surface water due to resuspension of the river water during the wet/monsoon season and strong adsorption to suspended particles. The actual toxicity, particularly chronic toxicity to humans, remains unknown due to the knowledge gaps in clinical and exposure studies, including dose-response for multiple types of QACs. Although toxicity tests have been conducted extensively in industries and regulated in various legislations, the risks of exposure to multiple types of QAC are still fragmentary, likewise for long-term exposure in health effects. This is also the primary concern in environmental exposure, particularly to aquatic and benthic animals and humans. The contamination of QACs in the environment is a forefront challenge in mediating the spread of AMR, and hence there is an urgent need to conduct a robust risk assessment to evaluate both human and aquatic health risks associated with QACs and AMR. Additional efforts could be made on alternate disinfectant ingredients, including naturally available caprylic acid, citric acid, lactic acid and other active ingredients such as hydrogen peroxide, and alcohol (isopropyl alcohol or ethanol). With the emergence of new variants every year, the use of QACs will continue to rise, and environmental engineers, toxicologists and scientists should come together to study their occurrence, fate, toxicity, and antimicrobial potential.
Environmental Implication
Increased use of QACs during the COVID-19 pandemic has raised concerns about their toxicity and potentially harmful effects on humans and several aquatic organisms. Besides the toxicity of QACs, there is a growing concern that QACs can lead to increased AMR in bacteria. Thus, this comprehensive review addresses various aspects of QACs, focusing on the distribution and fate of several QACs in environmental matrices and during wastewater treatment. In general, this paper further provides a comprehensive discussion on the classification and sources of QACs, and the resulting toxicity and development of AMR.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research is supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme (Intra-CREATE Thematic Project NRF2019-THE001-0003).
Editor: Karina S.B. Miglioranza
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2022.130393.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
No data was used for the research described in the article.
References
- Agathokleous E., Anav A., Araminiene V., de Marco A., Domingos M., Kitao M., Koike T., Manning W.J., Paoletti E., Saitanis C.J. EPA’s proposed expansion of dose-response analysis is a positive step towards improving its ecological risk assessment. Environ. Pollut. 2019;246:566–570. doi: 10.1016/j.envpol.2018.12.046. [DOI] [PubMed] [Google Scholar]
- Agathokleous E., Barceló D., Iavicoli I., Tsatsakis A., Calabrese E.J. Disinfectant-induced hormesis: an unknown environmental threat of the application of disinfectants to prevent SARS-CoV-2 infection during the COVID-19 pandemic? Environ. Pollut. 2022;292 doi: 10.1016/j.envpol.2021.118429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimitsu N., Hamamoto H., Inoue R., Shoji M., Akamine A., Takemori K., Hamasaki N., Sekimizu K. Increase in resistance of methicillin-resistant Staphylococcus aureus to β-lactams caused by mutations conferring resistance to benzalkonium chloride, a disinfectant widely used in hospitals. Antimicrob. Agents Chemother. 1999;43:3042–3043. doi: 10.1128/aac.43.12.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre B., Barbara G., Laure W., Bruno D., Adriana G.-O., Emmanuelle V. Development of a multiple-class analytical method based on the use of synthetic matrices for the simultaneous determination of commonly used commercial surfactants in wastewater by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2016;1450:64–75. doi: 10.1016/j.chroma.2016.04.078. [DOI] [PubMed] [Google Scholar]
- Allen R.A., Jennings M.C., Mitchell M.A., Al-Khalifa S.E., Wuest W.M., Minbiole K.P.C. Ester- and amide-containing multiQACs: exploring multicationic soft antimicrobial agents. Bioorg. Med. Chem. Lett. 2017;27:2107–2112. doi: 10.1016/J.BMCL.2017.03.077. [DOI] [PubMed] [Google Scholar]
- Alygizakis N., Galani A., Rousis N.I., Aalizadeh R., Dimopoulos M.A., Thomaidis N.S. Change in the chemical content of untreated wastewater of Athens, Greece under COVID-19 pandemic. Sci. Total Environ. 2021;799 doi: 10.1016/J.SCITOTENV.2021.149230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos G.C.A., Ploumakis S., Zhang L., Hawkey P.M., Gaze W.H., Wellington E.M.H. The widespread dissemination of integrons throughout bacterial communities in a riverine system. ISME J. 2018;12:681–691. doi: 10.1038/s41396-017-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Williams A.J., Tropsha A., Ekins S. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm. Res. 2020;37:8–11. doi: 10.1007/s11095-020-02842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios Andrés J.L., Carriba Rodriguez M.J., Aranzamendi Zaldumbide M., Hernández Hernández J.M., Viciola García M. Evaluation of cleaning and disinfection protocols for SARS COV-2 on different hospital surfaces. Infect. Control Hosp. Epidemiol. 2021;2:1–2. doi: 10.1017/ice.2021.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengoechea J.A., Bamford C.G.G. SARS‐CoV‐2, bacterial co‐infections, and AMR: the deadly trio in COVID‐19? EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergero M.F., Lucchesi G.I. Degradation of cationic surfactants using immobilized bacteria: Its effect on adsorption to activated sludge. J. Biotechnol. 2018;272–273:1–6. doi: 10.1016/j.jbiotec.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Blázquez J., Couce A., Rodríguez-Beltrán J., Rodríguez-Rojas A. Antimicrobials as promoters of genetic variation. Curr. Opin. Microbiol. 2012;15:561–569. doi: 10.1016/j.mib.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Brycki B., Waligórska M., Szulc A. The biodegradation of monomeric and dimeric alkylammonium surfactants. J. Hazard. Mater. 2014;280:797–815. doi: 10.1016/j.jhazmat.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds - a critical review. Int. J. Antimicrob. Agents. 2012;39:381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Bush K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01076-18. e01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butot S., Baert L., Zubera A.S. Assessment of antiviral coatings for high-touch surfaces by using human coronaviruses Hcov-229e and Sars-Cov-2. Appl. Environ. Microbiol. 2021;87:1–8. doi: 10.1128/AEM.01098-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J., Nixon R. Allergic contact dermatitis to quaternium 15 in a moisturizing lotion. Australas. J. Dermatol. 2005;46:284–285. doi: 10.1111/J.1440-0960.2005.00210.X. [DOI] [PubMed] [Google Scholar]
- Ceragioli M., Mols M., Moezelaar R., Ghelardi E., Senesi S., Abee T. Comparative transcriptomic and phenotypic analysis of the responses of bacillus cereus to various disinfectant treatments. Appl. Environ. Microbiol. 2010;76:3352–3360. doi: 10.1128/AEM.03003-09/SUPPL_FILE/SUPPLEMENTAL_MATERIALS_MODIFIED.DOC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad. 2003;51:133–138. [Google Scholar]
- Chen M.H., Chang P.C. The effectiveness of mouthwash against SARS-CoV-2 infection: A review of scientific and clinical evidence. J. Formos. Med. Assoc. 2021:1–7. doi: 10.1016/j.jfma.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M.-H. Chen, P.-C. Chang, The effectiveness of mouthwash against SARS-CoV-2 infection: A review of scientific and clinical evidence, Journal of the Formosan Medical Association. (2021b). [DOI] [PMC free article] [PubMed]
- Chen Y., Geurts M., Sjollema S.B., Kramer N.I., Hermens J.L.M., Droge S.T.J. Acute toxicity of the cationic surfactant C12‐benzalkonium in different bioassays: How test design affects bioavailability and effect concentrations. Environ. Toxicol. Chem. 2014;33:606–615. doi: 10.1002/etc.2465. [DOI] [PubMed] [Google Scholar]
- Christen V., Faltermann S., Brun N.R., Kunz P.Y., Fent K. Cytotoxicity and molecular effects of biocidal disinfectants (quaternary ammonia, glutaraldehyde, poly (hexamethylene biguanide) hydrochloride PHMB) and their mixtures in vitro and in zebrafish eleuthero-embryos. Sci. Total Environ. 2017;586:1204–1218. doi: 10.1016/j.scitotenv.2017.02.114. [DOI] [PubMed] [Google Scholar]
- Clara M., Scharf S., Scheffknecht C., Gans O. Occurrence of selected surfactants in untreated and treated sewage. Water Res. 2007;41:4339–4348. doi: 10.1016/j.watres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Curiao T., Marchi E., Grandgirard D., León-Sampedro R., Viti C., Leib S.L., Baquero F., Oggioni M.R., Martinez J.L., Coque T.M. Multiple adaptive routes of Salmonella enterica Typhimurium to biocide and antibiotic exposure. BMC Genom. 2016;17:1–16. doi: 10.1186/s12864-016-2778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Wang C., Lam J.C.W., Yamashita N., Yamazaki E., Horii Y., Chen W., Li X. Accumulation of quaternary ammonium compounds as emerging contaminants in sediments collected from the Pearl River Estuary, China and Tokyo Bay, Japan. Mar. Pollut. Bull. 2018;136:276–281. doi: 10.1016/j.marpolbul.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Dellanno C., Vega Q., Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am. J. Infect. Control. 2009;37:649–652. doi: 10.1016/J.AJIC.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W.-H., Liao Y.-H. Determination of alkylbenzyldimethylammonium chlorides in river water and sewage effluent by solid-phase extraction and gas chromatography/mass spectrometry. Anal. Chem. 2001;73:36–40. doi: 10.1021/ac000655i. [DOI] [PubMed] [Google Scholar]
- Ding W.-H., Tsai P.-C. Determination of alkyltrimethylammonium chlorides in river water by gas chromatography/ion trap mass spectrometry with electron impact and chemical ionization. Anal. Chem. 2003;75:1792–1797. doi: 10.1021/ac020536y. [DOI] [PubMed] [Google Scholar]
- Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 2003;5:1213–1219. doi: 10.1016/J.MICINF.2003.08.009. [DOI] [PubMed] [Google Scholar]
- EPA, Didecyl Dimethyl Ammonium Chloride (DDAC) Final Work Plan (FWP) Registration Review: Initial Docket Case Number 3003, (2017). 〈https://www.regulations.gov/document/EPA-HQ-OPP-2015–0740-0004〉 (accessed October 31, 2022).
- EPA, Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC) Final Work Plan (FWP) Registration Review: Initial Docket Case Number 035, Environmental Protection Agency. (2017). https://www.regulations.gov/document/EPA-HQ-OPP-2015–0737-0004 (accessed October 31, 2022).
- Eregowda T., Mohapatra S. Resilience, Response, and Risk in Water Systems. Springer; Singapore: 2020. Fate of Micropollutants in Engineered and Natural Environment; pp. 283–301. [Google Scholar]
- Faggio C., Pagano M., Alampi R., Vazzana I., Felice M.R. Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat. Toxicol. 2016;180:258–265. doi: 10.1016/j.aquatox.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Fernández P., Alder A.C., Suter M.J.-F., Giger W. Determination of the quaternary ammonium surfactant ditallowdimethylammonium in digested sludges and marine sediments by supercritical fluid extraction and liquid chromatography with postcolumn ion-pair formation. Anal. Chem. 1996;68:921–929. doi: 10.1021/ac9505482. [DOI] [PubMed] [Google Scholar]
- Ferreira C., Pereira A.M., Pereira M.C., Melo L.F., Simões M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemother. 2011;66:1036–1043. doi: 10.1093/JAC/DKR028. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Furlong E.T. Identification of alkyl dimethylbenzylammonium surfactants in water samples by solid-phase extraction followed by ion trap LC/MS and LC/MS/MS. Environ. Sci. Technol. 2001;35:2583–2588. doi: 10.1021/ES001742V. [DOI] [PubMed] [Google Scholar]
- Fortunato M.S., Baroni S., González A.J., Álvarez Roncancio J.D., Storino A., Parise C., Planes E., Gallego A., Korol S.E. Biodegradation and Detoxification of Benzalkonium Chloride in Synthetic and Industrial Effluents in Upflow Biofilm Aerobic Reactors. Water Air Soil Pollut. 2019;230 doi: 10.1007/s11270-019-4126-9. [DOI] [Google Scholar]
- Furi L., Ciusa M.L., Knight D., di Lorenzo V., Tocci N., Cirasola D., Aragones L., Coelho J.R., Freitas A.T., Marchi E. Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory-generated mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 2013;57:3488–3497. doi: 10.1128/AAC.00498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García M.T., Ribosa I., Guindulain T., Sánchez-Leal J., Vives-Rego J. Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment. Environ. Pollut. 2001;111:169–175. doi: 10.1016/S0269-7491(99)00322-X. [DOI] [PubMed] [Google Scholar]
- Gaze W.H., Zhang L., Abdouslam N.A., Hawkey P.M., Calvo-Bado L., Royle J., Brown H., Davis S., Kay P., Boxall A. Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J. 2011;5:1253–1261. doi: 10.1038/ismej.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F., Xu Y., Zhu R., Yu F., Zhu M., Wong M. Joint action of binary mixtures of cetyltrimethyl ammonium chloride and aromatic hydrocarbons on Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2010;73:1689–1695. doi: 10.1016/j.ecoenv.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Gerba C.P. Quaternary ammonium biocides: efficacy in application. Appl. Environ. Microbiol. 2015;81:464–469. doi: 10.1128/AEM.02633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerike P., Klotz H., Kooijman J.G.A., Matthijs E., Waters J. The determination of dihardenedtallowdimethyl ammonium compounds (DHTDMAC) in environmental matrices using trace enrichment techniques and high performance liquid chromatography with conductometric detection. Water Res. 1994;28:147–154. [Google Scholar]
- Gilbert P., Moore L.E. Cationic antiseptics: diversity of action under a common epithet. J. Appl. Microbiol. 2005;99:703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- Gillings M.R., Holley M.P., Stokes H.W. Evidence for dynamic exchange of qac gene cassettes between class 1 integrons and other integrons in freshwater biofilms. FEMS Microbiol. Lett. 2009;296:282–288. doi: 10.1111/J.1574-6968.2009.01646.X. [DOI] [PubMed] [Google Scholar]
- Gonzalez M., Jégu J., Kopferschmitt M.C., Donnay C., Hedelin G., Matzinger F., Velten M., Guilloux L., Cantineau A., de Blay F. Asthma among workers in healthcare settings: role of disinfection with quaternary ammonium compounds. Clin. Exp. Allergy. 2014;44:393–406. doi: 10.1111/CEA.12215. [DOI] [PubMed] [Google Scholar]
- Grigoras, A.G., 2021a. Polym. Antimicrob. Quat. Ammonium Moieties doi: 10.1007/978-3-030-58259-3_5.
- Grigoras A.G. Natural and synthetic polymeric antimicrobials with quaternary ammonium moieties: a review. Environ. Chem. Lett. 2021;19:3009–3022. doi: 10.1007/s10311-021-01215-w. [DOI] [Google Scholar]
- Guérin A., Bridier A., le Grandois P., Sévellec Y., Palma F., Félix B., Roussel S., Soumet C., Karpíšková R., Pomelio F., Skjerdal T., Ricao M., Bojan P., Oevermann A., Wullings B., Bulawová H., Castro H., Lindström M., Korkeala H., Šteingolde Ž., Szymczak B., Gareis M., Amar C. Exposure to quaternary ammonium compounds selects resistance to ciprofloxacin in listeria monocytogenes. Pathogens. 2021;10:1–11. doi: 10.3390/PATHOGENS10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin A., Bridier A., le Grandois P., Sévellec Y., Palma F., Félix B., Group L.S., Roussel S., Soumet C. Exposure to quaternary ammonium compounds selects resistance to ciprofloxacin in Listeria monocytogenes. Pathogens. 2021;10:220. doi: 10.3390/pathogens10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajaya M. Mitigating the effects of quaternary ammonium compounds on biological wastewater treatment systems during the COVID-19 pandemic. Jordan J. Earth Environ. Sci. 2021;12:1–12. [Google Scholar]
- Hajaya M.G., Pavlostathis S.G. Fate and effect of benzalkonium chlorides in a continuous-flow biological nitrogen removal system treating poultry processing wastewater. Bioresour. Technol. 2012;118:73–81. doi: 10.1016/j.biortech.2012.05.050. [DOI] [PubMed] [Google Scholar]
- He G.X., Landry M., Chen H., Thorpe C., Walsh D., Varela M.F., Pan H. Detection of benzalkonium chloride resistance in community environmental isolates of staphylococci. J. Med. Microbiol. 2014;63:735–741. doi: 10.1099/JMM.0.073072-0/CITE/REFWORKS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg P., Johansson N., Ternhag A., Abdel-Halim L., Hedlund J., Nauclér P. Bacterial co-infections in community-acquired pneumonia caused by SARS-CoV-2, influenza virus and respiratory syncytial virus. BMC Infect. Dis. 2022;22:1–11. doi: 10.1186/S12879-022-07089-9/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heederik D., Heederik D. Cleaning agents and disinfectants: moving from recognition to action and prevention. Clin. Exp. Allergy. 2014;44:472–474. doi: 10.1111/CEA.12286. [DOI] [PubMed] [Google Scholar]
- Hegstad K., Langsrud S., Lunestad B.T., Scheie A.A., Sunde M., Yazdankhah S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010;16:91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- K. Hegstad, S. Langsrud, B.T. Lunestad, A.A. Scheie, M. Sunde, S.P. Yazdankhah, Does the Wide Use of Quaternary Ammonium Compounds Enhance the Selection and Spread of Antimicrobial Resistance and Thus Threaten Our Health?, 〈Https://Home.Liebertpub.Com/Mdr〉. 16 (2010) 91–104. 10.1089/MDR.2009.0120. [DOI] [PubMed]
- Herron J.M., Hines K.M., Tomita H., Seguin R.P., Cui J.Y., Xu L. Multiomics investigation reveals benzalkonium chloride disinfectants alter sterol and lipid homeostasis in the mouse neonatal brain. Toxicol. Sci. 2019;171:32–45. doi: 10.1093/toxsci/kfz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyde B.J., Barthel A., Siemens J., Mulder I. A fast and robust method for the extraction and analysis of quaternary alkyl ammonium compounds from soil and sewage sludge. PLoS One. 2020;15:1–16. doi: 10.1371/journal.pone.0237020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyde B.J., Anders A., Siebe C., Siemens J., Mulder I. Quaternary alkylammonium disinfectant concentrations in soils rise exponentially after long-term wastewater irrigation. Environ. Res. Lett. 2021;16 doi: 10.1088/1748-9326/abf0cf. [DOI] [Google Scholar]
- Hora P.I., Arnold W.A. Photochemical fate of quaternary ammonium compounds in river water. Environ. Sci. Process. Impacts. 2020;22:1368–1381. doi: 10.1039/d0em00086h. [DOI] [PubMed] [Google Scholar]
- Hora P.I., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ. Sci. Technol. Lett. 2020;7:622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Ambient and dosed exposure to quaternary ammonium disinfectants causes neural tube defects in rodents. Birth Defects Res. 2018;110:543–544. doi: 10.1002/BDR2.1194. [DOI] [PubMed] [Google Scholar]
- Hostetler K.A., Fisher L.C., Burruss B.L. Prenatal developmental toxicity of alkyl dimethyl benzyl ammonium chloride and didecyl dimethyl ammonium chloride in CD rats and New Zealand White rabbits. Birth Defects Res. 2021;113:925–944. doi: 10.1002/bdr2.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubec T.C., Melin V.E., Shea C.S., Ferguson E.E., Garofola C., Repine C.M., Chapman T.W., Patel H.R., Razvi R.M., Sugrue J.E. Ambient and dosed exposure to quaternary ammonium disinfectants causes neural tube defects in rodents. Birth Defects Res. 2017;109:1166–1178. doi: 10.1002/bdr2.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubec T.C., Seguin R.P., Xu L., Cortopassi G.A., Datta S., Hanlon A.L., Lozano A.J., McDonald V.A., Healy C.A., Anderson T.C. Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure. Toxicol. Rep. 2021;8:646–656. doi: 10.1016/j.toxrep.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M.K., Nims R.W., Zhou S.S., Whitehead K., Srinivasan V., Kapes T., Fanuel S., Epstein J.H., Daszak P., Rubino J.R., McKinney J. Microbicidal actives with virucidal efficacy against SARS-CoV-2 and other beta- and alpha-coronaviruses and implications for future emerging coronaviruses and other enveloped viruses. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-84842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M.C., Minbiole K.P.C., Wuest W.M. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015;1:288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- Johnson C.M., Grossman A.D. Integrative and conjugative elements (ICEs): what they do and how they work. Annu. Rev. Genet. 2015;49:577. doi: 10.1146/ANNUREV-GENET-112414-055018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L. Kaj, P. Wallberg, E. Brorström-Lundén, Quaternary ammonium compounds: analyses in a Nordic cooperation on screening, Nordic Council of Ministers, 2014.
- Keating A.F., Watkins A.J. Reproductive toxicology: Emerging toxicants and cellular targets. Reproduction. 2021;162:E5–E6. doi: 10.1530/REP-21-0373/VIDEO-1. [DOI] [PubMed] [Google Scholar]
- Kim M., Weigand M.R., Oh S., Hatt J.K., Krishnan R., Tezel U., Pavlostathis S.G., Konstantinidis K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018;84:e01201–e01218. doi: 10.1128/AEM.01201-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Hatt J.K., Weigand M.R., Krishnan R., Pavlostathis S.G., Konstantinidis K.T. Genomic and transcriptomic insights into how bacteria withstand high concentrations of benzalkonium chloride biocides. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00197-18. e00197-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine A., Yamaguchi E., Okamoto N., Yamamoto K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J. Oral Maxillofac. Surg. Med. Pathol. 2021;33:475–477. doi: 10.1016/J.AJOMS.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreipe K., Forkel S., Heinemann K.E., Amschler K., Fuchs T., Geier J., Buhl T. Contact sensitizations to disinfectants containing alcohols or quaternary ammonium compounds are rarely of clinical relevance. Contact Dermat. 2021;85:211–214. doi: 10.1111/cod.13844. [DOI] [PubMed] [Google Scholar]
- Kumar M., Jiang Guanging, et al. Lead time of early warning by wastewater surveillance for COVID-19: Geographical variations and impacting factors. Chemical Engineering Journal. 2022;441 doi: 10.1016/j.cej.2022.135936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Mazumder P., Mohapatra S., Kumar Thakur A., Dhangar K., Taki K., Mukherjee S., Kumar Patel A., Bhattacharya P., Mohapatra P., Rinklebe J., Kitajima M., Hai F.I., Khursheed A., Furumai H., Sonne C., Kuroda K., Thakur A.K., Dhangar K., Taki K., Mukherjee S., Patel A.K., Bhattacharya P., Mohapatra P., Rinklebe J., Kumar Thakur A., Dhangar K., Taki K., Mukherjee S., Kumar Patel A., Bhattacharya P., Mohapatra P., Rinklebe J., Kitajima M., Hai F.I., Khursheed A., Furumai H., Sonne C., Kuroda K. A chronicle of SARS-CoV-2: Seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J. Hazard. Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Mart́n P.A., Li X., Bopp R.F., Brownawell B.J. Occurrence of alkyltrimethylammonium compounds in urban estuarine sediments: Behentrimonium as a new emerging contaminant. Environ. Sci. Technol. 2010;44:7569–7575. doi: 10.1021/ES101169A/SUPPL_FILE/ES101169A_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Lavorgna M., Russo C., D’Abrosca B., Parrella A., Isidori M. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ. Pollut. 2016;210:34–39. doi: 10.1016/j.envpol.2015.11.042. [DOI] [PubMed] [Google Scholar]
- Lee M.Y., Wang W.L., Bin Xu Z., Ye B., Wu Q.Y., Hu H.Y. The application of UV/PS oxidation for removal of a quaternary ammonium compound of dodecyl trimethyl ammonium chloride (DTAC): the kinetics and mechanism. Sci. Total Environ. 2019;655:1261–1269. doi: 10.1016/j.scitotenv.2018.11.256. [DOI] [PubMed] [Google Scholar]
- Li L.-G., Xia Y., Zhang T. Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collection. ISME J. 2017;11:651–662. doi: 10.1038/ismej.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Brownawell B.J. Quaternary ammonium compounds in urban estuarine sediment environments-a class of contaminants in need of increased attention? Environ. Sci. Technol. 2010;44:7561–7568. doi: 10.1021/es1011669. [DOI] [PubMed] [Google Scholar]
- Li X., Luo X., Mai B., Liu J., Chen L., Lin S. Occurrence of quaternary ammonium compounds (QACs) and their application as a tracer for sewage derived pollution in urban estuarine sediments. Environ. Pollut. 2014;185:127–133. doi: 10.1016/j.envpol.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Li X., Rensing C., Vestergaard G., Arumugam M., Nesme J., Gupta S., Brejnrod A.D., Sørensen S.J. Metagenomic evidence for co-occurrence of antibiotic, biocide and metal resistance genes in pigs. Environ. Int. 2022;158 doi: 10.1016/j.envint.2021.106899. [DOI] [PubMed] [Google Scholar]
- Liang Z., Ge F., Zeng H., Xu Y., Peng F., Wong M. Influence of cetyltrimethyl ammonium bromide on nutrient uptake and cell responses of Chlorella vulgaris. Aquat. Toxicol. 2013;138–139:81–87. doi: 10.1016/J.AQUATOX.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhao H., Han L., Shu W., Wu Q., Ni Y. Frequency of biocide-resistant genes and susceptibility to chlorhexidine in high-level mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MuH MRSA) Diagn. Microbiol. Infect. Dis. 2015;82:278–283. doi: 10.1016/j.diagmicrobio.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Liu W.J., Fu L., Huang M., Zhang J.P., Wu Y., Zhou Y.S., Zeng J., Wang G.X. Frequency of antiseptic resistance genes and reduced susceptibility to biocides in carbapenem-resistant Acinetobacter baumannii. J. Med. Microbiol. 2017;66:13–17. doi: 10.1099/JMM.0.000403/CITE/REFWORKS. [DOI] [PubMed] [Google Scholar]
- Luz A., DeLeo P., Pechacek N., Freemantle M. Human health hazard assessment of quaternary ammonium compounds: didecyl dimethyl ammonium chloride and alkyl (C12–C16) dimethyl benzyl ammonium chloride. Regul. Toxicol. Pharmacol. 2020;116 doi: 10.1016/j.yrtph.2020.104717. [DOI] [PubMed] [Google Scholar]
- Lysol Maker Says Sales Are Surging - Bloomberg, (n.d.). 〈https://www.bloomberg.com/news/articles/2020–04-30/lysol-maker-reckitt-benckiser-raises-outlook-after-sales-surge#xj4y7vzkg〉 (accessed July 28, 2022).
- MacGibeny M.A., Wassef C. Preventing adverse cutaneous reactions from amplified hygiene practices during the COVID-19 pandemic: how dermatologists can help through anticipatory guidance. Arch. Dermatol. Res. 2021;313:501–503. doi: 10.1007/s00403-020-02086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah T.F.C., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Maher B. Lab disinfectant harms mouse fertility. Nature. 2008;453:964–965. doi: 10.1038/453964a. [DOI] [PubMed] [Google Scholar]
- Maillard J.Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002;92:16S–27S. [PubMed] [Google Scholar]
- Marketwatch, Quaternary Ammonium Compounds Market Size in 2021 Industry Demand, Market Share, Trend, Industry News, Business Growth, Top Key Players Update | CAGR of 5. 4 % | [ Reports Page No 109], (2021) 1–12.
- Martí M., Tuñón-Molina A., Aachmann F.L., Muramoto Y., Noda T., Takayama K., Serrano-Aroca Á. Protective face mask filter capable of inactivating SARS-CoV-2, and methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Polymers. 2021;13:1–12. doi: 10.3390/polym13020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Carballo E., Sitka A., González-Barreiro C., Kreuzinger N., Fürhacker M., Scharf S., Gans O. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part I. Application to surface, waste and indirect discharge water samples in Austria. Environ. Pollut. 2007;145:489–496. doi: 10.1016/j.envpol.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Martínez-Carballo E., González-Barreiro C., Sitka A., Kreuzinger N., Scharf S., Gans O. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part II. Application to sediment and sludge samples in Austria. Environ. Pollut. 2007;146:543–547. doi: 10.1016/J.ENVPOL.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Mc Carlie S., Boucher C.E., Bragg R.R. Molecular basis of bacterial disinfectant resistance. Drug Resist. Updates. 2020;48 doi: 10.1016/j.drup.2019.100672. [DOI] [PubMed] [Google Scholar]
- Mc Cay P.H., Ocampo-Sosa A.A., Fleming G.T.A. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology. 2010;156:30–38. doi: 10.1099/mic.0.029751-0. [DOI] [PubMed] [Google Scholar]
- Meade E., Slattery M.A., Garvey M. Biocidal resistance in clinically relevant microbial species: a major public health risk. Pathogens. 2021;Vol. 10:598. doi: 10.3390/PATHOGENS10050598. 598. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchin L., Dubois‐Brissonnet F., Heyd B., Leveau J.Y. Adaptation of Pseudomonas aeruginosa ATCC 15442 to didecyldimethylammonium bromide induces changes in membrane fatty acid composition and in resistance of cells. J. Appl. Microbiol. 1999;86:859–866. doi: 10.1046/j.1365-2672.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- Melin V.E., Potineni H., Hunt P., Griswold J., Siems B., Werre S.R., Hrubec T.C. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod. Toxicol. 2014;50:163–170. doi: 10.1016/j.reprotox.2014.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin V.E., Melin T.E., Dessify B.J., Nguyen C.T., Shea C.S., Hrubec T.C. Quaternary ammonium disinfectants cause subfertility in mice by targeting both male and female reproductive processes. Reprod. Toxicol. 2016;59:159–166. doi: 10.1016/j.reprotox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Menon N.G., Mohapatra S. The Covid19 pandemic: virus transmission and risk assessment. Curr. Opin. Environ. Sci. Health. 2022;28 doi: 10.1016/j.coesh.2022.100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchel Piovesan Pereira B., Wang X., Tagkopoulos I. Short-and long-term transcriptomic responses of Escherichia coli to biocides: a systems analysis. Appl. Environ. Microbiol. 2020;86:e00708–e00720. doi: 10.1128/AEM.00708-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchel Piovesan Pereira B., Wang X., Tagkopoulos I. Biocide-induced emergence of antibiotic resistance in Escherichia coli. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.640923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino F., Rubio S., Pérez-Bendito D. Mixed aggregate-based acid-induced cloud-point extraction and ion-trap liquid chromatography–mass spectrometry for the determination of cationic surfactants in sewage sludge. J. Chromatogr. A. 2003;998:143–154. doi: 10.1016/S0021-9673(03)00565-X. [DOI] [PubMed] [Google Scholar]
- Migueres N., Debaille C., Walusiak-Skorupa J., Lipińska-Ojrzanowska A., Munoz X., van Kampen V., Suojalehto H., Suuronen K., Seed M., Lee S. Occupational asthma caused by quaternary ammonium compounds: a multicenter cohort study. J. Allergy Clin. Immunol. Pract. 2021;9:3387–3395. doi: 10.1016/j.jaip.2021.04.041. [DOI] [PubMed] [Google Scholar]
- Mirzaei R., Goodarzi P., Asadi M., Soltani A., ali abraham Aljanabi H., Jeda A.S., Dashtbin S., Jalalifar S., Mohammadzadeh R., Teimoori A., Tari K., Salari M., Ghiasvand S., Kazemi S., Yousefimashouf R., Keyvani H., Karampoor S. Bacterial co-infections with SARS-CoV-2. IUBMB Life. 2020;72:2097–2111. doi: 10.1002/IUB.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Nishiyama N., Yamamoto A. Aquatic environmental monitoring of detergent surfactants. J. Oleo Sci. 2008;57:161–170. doi: 10.5650/JOS.57.161. [DOI] [PubMed] [Google Scholar]
- Miyaoka Y., Kabir M.H., Hasan M.A., Yamaguchi M., Shoham D., Murakami H., Takehara K. Establishment and utilization of an evaluation system for virucidal activity of disinfectants against a coronavirus with apparent applicability to SARS-CoV-2. J. Vet. Med. Sci. 2021;83:48–52. doi: 10.1292/jvms.20-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo L.Y., Liu Y.A., Zhu J., Qin L.T., Liang Y.P., Zeng H.H. Benefits from hazards, benefits from nothing, and benefits from benefits: the combined effects of five quaternary ammonium compounds to Vibrio qinghaiensis Q67. Environ. Sci. Eur. 2020;32:1–10. doi: 10.1186/S12302-020-00310-Z/FIGURES/4. [DOI] [Google Scholar]
- Mohapatra S., Menon N.G. Factors responsible for emergence of novel viruses: an emphasis on SARS-CoV-2. Curr. Opin. Environ. Sci. Health. 2022 doi: 10.1016/j.coesh.2022.100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S., Menon N.G., Mohapatra G., Pisharody L., Pattnaik A., Menon N.G., Bhukya P.L., Srivastava M., Singh M., Barman M.K. The novel SARS-CoV-2 pandemic:possible environmental transmission, detection, persistence and fate during wastewater and water treatment. Sci. Total Environ. 2021;765 doi: 10.1016/j.scitotenv.2020.142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S., Sharma N., Mohapatra G., Padhye L.P., Mukherji S. Seasonal variation in fluorescence characteristics of dissolved organic matter in wastewater and identification of proteins through HRLC-MS/MS. J. Hazard. Mater. 2021;413 doi: 10.1016/j.jhazmat.2021.125453. [DOI] [PubMed] [Google Scholar]
- Mohapatra S., Mrozik W., Acharya K., Menon N.G. Suspect and Nontarget Screening of Pharmaceuticals in Water and Wastewater Matrices. first ed. Springer; Cham, Neteherlands: 2022. [DOI] [Google Scholar]
- Mohapatra S., Snow D., Shea P., Gálvez-Rodríguez A., Kumar M., Padhye L.P., Mukherji S. Photodegradation of a mixture of five pharmaceuticals commonly found in wastewater: Experimental and computational analysis. Environmental Research. 2023;216(3) doi: 10.1016/j.envres.2022.114659. [DOI] [PubMed] [Google Scholar]
- Monge F.A., Jagadesan P., Bondu V., Donabedian P.L., Ista L., Chi E.Y., Schanze K.S., Whitten D.G., Kell A.M. Highly Effective Inactivation of SARS-CoV-2 by Conjugated Polymers and Oligomers. ACS Appl. Mater. Interfaces. 2020;12:55688–55695. doi: 10.1021/acsami.0c17445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder I., Siemens J., Sentek V., Amelung W., Smalla K., Jechalke S. Quaternary ammonium compounds in soil: implications for antibiotic resistance development. Rev. Environ. Sci. Biotechnol. 2018;17:159–185. doi: 10.1007/s11157-017-9457-7. [DOI] [Google Scholar]
- Mulder I., Schmittdiel M., Frei H., Hofmann L., Gerbig D., Siemens J. Soil water solutes reduce the critical micelle concentration of quaternary ammonium compounds. Environ. Sci. Pollut. Res. 2020;27:45311–45323. doi: 10.1007/s11356-020-10188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Basagoiti J., Perez-Zsolt D., León R., Blanc V., Raïch-Regué D., Cano-Sarabia M., Trinité B., Pradenas E., Blanco J., Gispert J., Clotet B., Izquierdo-Useros N. Mouthwashes with CPC Reduce the Infectivity of SARS-CoV-2 Variants In Vitro. J. Dent. Res. 2021;100:1265–1272. doi: 10.1177/00220345211029269. [DOI] [PubMed] [Google Scholar]
- Nordholt N., Kanaris O., Schmidt S.B.I., Schreiber F. Persistence against benzalkonium chloride promotes rapid evolution of tolerance during periodic disinfection. Nat. Commun. 2021;12:1–13. doi: 10.1038/s41467-021-27019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo Nordisk, Quats applications, (2020).
- Ogilvie B.H., Solis-Leal A., Lopez J.B., Poole B.D., Robison R.A., Berges B.K. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 2021;108:142–145. doi: 10.1016/j.jhin.2020.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega Morente E., Fernández-Fuentes M.A., Grande Burgos M.J., Abriouel H., Pérez Pulido R., Gálvez A. Biocide tolerance in bacteria. Int. J. Food Microbiol. 2013;162:13–25. doi: 10.1016/J.IJFOODMICRO.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Osimitz T.G., Droege W. Quaternary ammonium compounds: perspectives on benefits, hazards, and risk. Toxicol. Res. Appl. 2021;5 doi: 10.1177/23978473211049085. 239784732110490. [DOI] [Google Scholar]
- T.G. Osimitz, W. Droege, Adverse Outcome Pathway for Antimicrobial Quaternary Ammonium Compounds, 〈Https://Doi.Org/10.1080/15287394.2022.2037479〉. 85 (2022) 494–510. 10.1080/15287394.2022.2037479. [DOI] [PubMed]
- Pal C., Bengtsson-Palme J., Kristiansson E., Larsson D.G.J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom. 2015;16:1–14. doi: 10.1186/S12864-015-2153-5/FIGURES/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/J.BIOTECHADV.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Panth K., Acharya K., Mohapatra S., et al. Faecal pollution source tracking in the holy Bagmati River by portable 16S rRNA gene sequencing. npj Clean Water. 2021;4(12):1–10. doi: 10.1038/s41545-021-00099-1. [DOI] [Google Scholar]
- Partridge S.R., Kwong S.M., Firth N., Jensen S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018;31 doi: 10.1128/CMR.00088-17/ASSET/808BB10D–7E85-4717-AE02-F26B28D2DBE2/ASSETS/GRAPHIC/ZCM0041826370008.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S.G., Arnold W.A. Comprehensive screening of quaternary ammonium surfactants and ionic liquids in wastewater effluents and lake sediments. Environ. Sci. Process. Impacts. 2020;22:430–441. doi: 10.1039/c9em00554d. [DOI] [PubMed] [Google Scholar]
- Patrauchan M.A., Oriel P.J. Degradation of benzyldimethylalkylammonium chloride by Aeromonas hydrophila sp. K. J. Appl. Microbiol. 2003;94:266–272. doi: 10.1046/J.1365-2672.2003.01829.X. [DOI] [PubMed] [Google Scholar]
- Pedreira A., Taşkın Y., García M.R. A critical review of disinfection processes to control sars-cov-2 transmission in the food industry. Foods. 2021;10:1–16. doi: 10.3390/foods10020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira B.M.P., Tagkopoulos I. Benzalkonium chlorides: uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.00377-19/ASSET/B79481F3-869D-447A–9D41-322488926C3D/ASSETS/GRAPHIC/AEM.00377-19-F0001.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintado-Herrera M.G., Wang C., Lu J., Chang Y.P., Chen W., Li X., Lara-Martín P.A. Distribution, mass inventories, and ecological risk assessment of legacy and emerging contaminants in sediments from the Pearl River Estuary in China. J. Hazard. Mater. 2017;323:128–138. doi: 10.1016/J.JHAZMAT.2016.02.046. [DOI] [PubMed] [Google Scholar]
- Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002;92:55S–64S. [PubMed] [Google Scholar]
- Poole K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005;56:20–51. doi: 10.1093/JAC/DKI171. [DOI] [PubMed] [Google Scholar]
- Qian Y., He Y., Li H., Yi M., Zhang L., Zhang L., Liu L., Lu Z. Benzalkonium chlorides (C12) inhibits growth but motivates microcystins release of Microcystis aeruginosa revealed by morphological, physiological, and iTRAQ investigation. Environ. Pollut. 2022;292 doi: 10.1016/J.ENVPOL.2021.118305. [DOI] [PubMed] [Google Scholar]
- Radke M., Behrends T., Förster J., Herrmann R. Analysis of cationic surfactants by microbore high-performance liquid chromatography− electrospray mass spectrometry. Anal. Chem. 1999;71:5362–5366. doi: 10.1021/ac990453q. [DOI] [PubMed] [Google Scholar]
- Reardon S. Antibiotic treatment for COVID-19 complications could fuel resistant bacteria. Science. 2020 (1979) (1979) [Google Scholar]
- Rizvi S.G., Ahammad S.Z. COVID-19 and antimicrobial resistance: a cross-study. Sci. Total Environ. 2022;807 doi: 10.1016/J.SCITOTENV.2021.150873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roedel A., Vincze S., Projahn M., Roesler U., Robé C., Hammerl J.A., Noll M., Dahouk S. al, Dieckmann R. Genetic but no phenotypic associations between biocide tolerance and antibiotic resistance in Escherichia coli from German broiler fattening farms. Microorganisms. 2021;9:651. doi: 10.3390/microorganisms9030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J.L., Grande Burgos M.J., Pérez-Pulido R., Gálvez A., Lucas R. Resistance to antibiotics, biocides, preservatives and metals in bacteria isolated from seafoods: co-selection of strains resistant or tolerant to different classes of compounds. Front. Microbiol. 2017;8:1650. doi: 10.3389/fmicb.2017.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan T., Song S., Wang T., Liu R., Lin Y., Jiang G. Identification and composition of emerging quaternary ammonium compounds in municipal sewage sludge in China. Environ. Sci. Technol. 2014;48:4289–4297. doi: 10.1021/es4050314. [DOI] [PubMed] [Google Scholar]
- Salamova A., Zheng G., Webster T.F. Quaternary ammonium compounds: bioaccumulation potentials in humans and levels in blood before and during the covid-19 pandemic. Environ. Sci. Technol. 2021;55:14689–14698. doi: 10.1021/ACS.EST.1C01654/SUPPL_FILE/ES1C01654_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank C.L., Minbiole K.P.C., Wuest W.M. Are quaternary ammonium compounds, the workhorse disinfectants, effective against severe acute respiratory syndrome-coronavirus-2. ACS Infect. Dis. 2020;6:1553–1557. doi: 10.1021/acsinfecdis.0c00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin R.P., Herron J.M., Lopez V.A., Dempsey J.L., Xu L. Metabolism of benzalkonium chlorides by human hepatic cytochromes P450. Chem. Res. Toxicol. 2019;32:2466–2478. doi: 10.1021/ACS.CHEMRESTOX.9B00293/SUPPL_FILE/TX9B00293_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn G.S., Ye C., Bradfute S.B. Anti-sars-cov-2 activity of surgical masks infused with quaternary ammonium salts. Viruses. 2021;13:1–5. doi: 10.3390/v13060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipski C.J., Zhanel G.G., Bay D.C. Biocide selective TolC-independent efflux pumps in enterobacteriaceae. J. Membr. Biol. 2018;251:15–33. doi: 10.1007/S00232-017-9992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer K., Meister T.L., Todt D., Krawczyk A., Paßvogel L., Becker B., Paulmann D., Bischoff B., Eggers M., Pfaender S., Brill F.H.H., Steinmann E. Virucidal efficacy of different formulations for hand and surface disinfection targeting SARS CoV-2. J. Hosp. Infect. 2021;112:27–30. doi: 10.1016/j.jhin.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sütterlin H., Alexy R., Coker A., Kümmerer K. Mixtures of quaternary ammonium compounds and anionic organic compounds in the aquatic environment: elimination and biodegradability in the closed bottle test monitored by LC–MS/MS. Chemosphere. 2008;72:479–484. doi: 10.1016/J.CHEMOSPHERE.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Tezel U., Pavlostathis S.G. Transformation of benzalkonium chloride under nitrate reducing conditions. Environ. Sci. Technol. 2009;43:1342–1348. doi: 10.1021/es802177f. [DOI] [PubMed] [Google Scholar]
- Tezel U., Pavlostathis S.G. Role of quaternary ammonium compounds on antimicrobial resistance in the environment. Antimicrob. Resist. Environ. 2011:349–387. doi: 10.1002/9781118156247.CH20. [DOI] [Google Scholar]
- Tezel U., Pavlostathis S.G. Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015;33:296–304. doi: 10.1016/j.copbio.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Tezel U., Pierson J.A., Pavlostathis S.G. Fate and effect of quaternary ammonium compounds on a mixed methanogenic culture. Water Res. 2006;40:3660–3668. doi: 10.1016/j.watres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Thomas J.C., IV, Oladeinde A., Kieran T.J., Finger J.W., Jr, Bayona‐Vásquez N.J., Cartee J.C., Beasley J.C., Seaman J.C., McArthur J.V., Rhodes O.E., Jr Co‐occurrence of antibiotic, biocide, and heavy metal resistance genes in bacteria from metal and radionuclide contaminated soils at the Savannah River Site. Microb. Biotechnol. 2020;13:1179–1200. doi: 10.1111/1751-7915.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzel R.E., Sutton J.M., Wand M.E. Establishment of a multi-species biofilm model to evaluate chlorhexidine efficacy. J. Hosp. Infect. 2016;92:154–160. doi: 10.1016/j.jhin.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Tuñón-Molina A., Martí M., Muramoto Y., Noda T., Takayama K., Serrano-Aroca Á. Antimicrobial face shield: next generation of facial protective equipment against sars-cov-2 and multidrug-resistant bacteria. Int. J. Mol. Sci. 2021;22:1–14. doi: 10.3390/ijms22179518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Voorde A., Lorgeoux C., Gromaire M.-C., Chebbo G. Analysis of quaternary ammonium compounds in urban stormwater samples. Environ. Pollut. 2012;164:150–157. doi: 10.1016/j.envpol.2012.01.037. [DOI] [PubMed] [Google Scholar]
- van De Voorde A., Lorgeoux C., Gromaire M.C., Chebbo G. Analysis of quaternary ammonium compounds in urban stormwater samples. Environ. Pollut. 2012;164:150–157. doi: 10.1016/J.ENVPOL.2012.01.037. [DOI] [PubMed] [Google Scholar]
- van Wijk D., Gyimesi-van den Bos M., Garttener-Arends I., Geurts M., Kamstra J., Thomas P. Bioavailability and detoxification of cationics: I. Algal toxicity of alkyltrimethyl ammonium salts in the presence of suspended sediment and humic acid. Chemosphere. 2009;75:303–309. doi: 10.1016/j.chemosphere.2008.12.047. [DOI] [PubMed] [Google Scholar]
- Vereshchagin A.N., Frolov N.A., Egorova K.S., Seitkalieva M.M., Ananikov V.P. Quaternary ammonium compounds (Qacs) and ionic liquids (ils) as biocides: from simple antiseptics to tunable antimicrobials. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22136793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar R., Sandle T. A review on biocide reduced susceptibility due to plasmid-borne antiseptic-resistant genes—special notes on pharmaceutical environmental isolates. J. Appl. Microbiol. 2019;126:1011–1022. doi: 10.1111/JAM.14118. [DOI] [PubMed] [Google Scholar]
- Voumard M., Venturelli L., Borgatta M., Croxatto A., Kasas S., Dietler G., Breider F., Von Gunten U. Adaptation of Pseudomonas aeruginosa to constant sub-inhibitory concentrations of quaternary ammonium compounds †. Environ. Sci.: Water Res. Technol. 2020;6:1139. doi: 10.1039/c9ew01056d. [DOI] [Google Scholar]
- Voumard M., Venturelli L., Borgatta M., Croxatto A., Kasas S., Dietler G., Breider F., von Gunten U. Adaptation of Pseudomonas aeruginosa to constant sub-inhibitory concentrations of quaternary ammonium compounds. Environ. Sci. 2020;6:1139–1152. [Google Scholar]
- Wand M.E. Modeling the Transmission and Prevention of Infectious Disease. Springer; 2017. Bacterial resistance to hospital disinfection; pp. 19–54. [Google Scholar]
- Wessels S., Ingmer H. Modes of action of three disinfectant active substances: a review. Regul. Toxicol. Pharmacol. 2013;67:456–467. doi: 10.1016/j.yrtph.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Xue Y., Hieda Y., Saito Y., Nomura T., Fujihara J., Takayama K., Kimura K., Takeshita H. Distribution and disposition of benzalkonium chloride following various routes of administration in rats. Toxicol. Lett. 2004;148:113–123. doi: 10.1016/j.toxlet.2003.12.068. [DOI] [PubMed] [Google Scholar]
- Yang J. Georgia Institute of Technology; 2007. Fate and Effect of Alkyl Benzyl Dimethyl Ammonium Chloride in Mixed Aerobic and Nitrifying Cultures. [Google Scholar]
- Yang Y., Wang W. Benzyldimethyldodecyl ammonium chloride shifts the proliferation of functional genes and microbial community in natural water from eutrophic lake. Environ. Pollut. 2018;236:355–365. doi: 10.1016/j.envpol.2018.01.059. [DOI] [PubMed] [Google Scholar]
- Ying G.-G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 2006;32:417–431. doi: 10.1016/j.envint.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Yu F., Ge F., Zhou W., Tao N., Liang Z., Zhu L. Subcellular distribution of fluoranthene in Chlorella vulgaris with the presence of cetyltrimethylammonium chloride. Chemosphere. 2013;90:929–935. doi: 10.1016/j.chemosphere.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Yu T., Jiang X., Zhang Y., Ji S., Gao W., Shi L. Effect of benzalkonium chloride adaptation on sensitivity to antimicrobial agents and tolerance to environmental stresses in Listeria monocytogenes. Front. Microbiol. 2018;9:2906. doi: 10.3389/fmicb.2018.02906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Cui F., Zeng G. Ming, Jiang M., Yang Z. Zhu, Yu Z. Gang, Zhu M. Ying, Shen L. Qing. Quaternary ammonium compounds (QACs): a review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015;518–519:352–362. doi: 10.1016/j.scitotenv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang D., Peng Y., Chan C.-L., On H., Wai H.K.-F., Shekhawat S.S., Gupta A.B., Varshney A.K., Chuanchuen R., Zhou X. Metagenomic survey reveals more diverse and abundant antibiotic resistance genes in municipal wastewater than hospital wastewater. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.712843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Chen L., Ma H., Ma J., Gao D. Effective removal of polymer quaternary ammonium salt by biodegradation and a subsequent Fenton oxidation process. Ecotoxicol. Environ. Saf. 2020;188 doi: 10.1016/j.ecoenv.2019.109919. [DOI] [PubMed] [Google Scholar]
- Zheng G., Filippelli G.M., Salamova A. Increased indoor exposure to commonly used disinfectants during the COVID-19 pandemic. Environ. Sci. Technol. Lett. 2020;7:760–765. doi: 10.1021/acs.estlett.0c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Webster T.F., Salamova A. Quaternary ammonium compounds: bioaccumulation potentials in humans and levels in blood before and during the COVID-19 pandemic. Environ. Sci. Technol. 2021;55:14689–14698. doi: 10.1021/acs.est.1c01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Schreder E., Sathyanarayana S., Salamova A. The first detection of quaternary ammonium compounds in breast milk: Implications for early-life exposure. J. Expo Sci. Environ. Epidemiol. 2022:1–7. doi: 10.1038/s41370-022-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.J., Ma W.L., Xu T.F., Ding Y., Zhao X., Li W.L., Liu L.Y., Song W.W., Li Y.F., Zhang Z.F. Removal characteristic of surfactants in typical industrial and domestic wastewater treatment plants in Northeast China. Ecotoxicol. Environ. Saf. 2018;153:84–90. doi: 10.1016/j.ecoenv.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Zhu M., Ge F., Zhu R., Wang X., Zheng X., DFT-based A. QSAR study of the toxicity of quaternary ammonium compounds on Chlorella vulgaris. Chemosphere. 2010;80:46–52. doi: 10.1016/j.chemosphere.2010.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
No data was used for the research described in the article.