Abstract
Background
Respiratory dysfunctions are an important cause of morbidity and death in cerebral palsy (CP) populations. Respiratory exercises in addition to conventional rehabilitation have been suggested to improve respiratory status in CP patients. The objective of this systematic review and meta-analysis was to verify the effects of the addition of respiratory exercises to conventional rehabilitation on pulmonary function, functional capacity, respiratory muscle strength, gross motor function and quality of life in children and adolescents with CP.
Methods
We searched for randomized controlled clinical trials in PubMed/Medline, Lilacs, SciELO, EMBASE and Physiotheraphy Evidence (PEDro) from their inception until July 2022 without language restrictions. Studies that included respiratory exercises (breathing exercise program; feedback respiratory training; incentive spirometer exercise; inspiratory muscle training; and combination of respiratory exercises + incentive spirometer exercise) in combination with conventional rehabilitation for children and adolescents with CP were evaluated by two independent reviewers. The mean difference (MD) and 95% confidence interval (CI) were estimated by random effect models.
Results
Ten studies met the eligibility criteria, including 324 children aged from 6 to 16 years. The meta-analysis showed an improvement in inspiratory muscle strength of 22.96 cmH2O (18.63–27.27, n = 55) and pulmonary function of 0.60 (0.38–0.82, n = 98) for forced vital capacity (L); 0.22 (0.06–0.39, n = 98) for forced expiratory volume at 1 second (L); and 0.50 (0.05–0.04, n = 98) for peak expiratory flow (L/min). Functional skills in daily living activities improved in the intervention group. Caregivers’ assistance of daily living activities, functional capacity, gross motor function and expiratory muscle strength showed a nonsignificant improvement. Social well-being and acceptance and functioning domains improved in only one study.
Conclusions
Emerging data show significant enhancements in inspiratory muscle strength and pulmonary function in CP patients after respiratory training in addition to conventional rehabilitation. There is no consensus on the frequency, type or intensity of respiratory exercises for children with and adolescents with CP.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12519-022-00642-1.
Keywords: Breathing exercises, Cerebral palsy, Inspiratory muscle training, Pediatrics, Rehabilitation, Respiratory exercise
Introduction
Cerebral palsy (CP) describes a set of permanent disorders related to mobility and postural development, and these disorders can affect respiratory status [1]. Recurrent aspiration, impaired airway clearance and lung function, spine and chest wall deformities, poor nutritional status and recurrent respiratory infections are the main causes of respiratory complications [2]. Alarmingly, respiratory dysfunctions are an important cause of morbidity and death in CP populations [2, 3].
Respiratory status is not directly affected by CP but due to secondary problems (e.g., decreased physical activity level, scoliosis) [3]. To better understand this context, Wang et al. [4] and Know and Lee [5] demonstrated that respiratory muscle strength in children with CP is lower than that in children with typical development and positively correlated with their capability levels of daily living, self-care and social function. In addition to respiratory muscle strength, another important concern is chest mobility. In contrast, children with CP commonly present restrictive pulmonary dysfunction due to reduced chest mobility, which can decrease lung expansion and compliance[6]. The above-mentioned disorders can modify the respiratory breathing pattern, which leads from irregular breathing to respiratory failure, directly affecting overall daily needs and quality of life [4]. These findings suggest that early initiation of respiratory exercises for children with CP should be considered, as it may improve and maintain chest mobility and respiratory function.
Pulmonary rehabilitation in children with chronic lung diseases has been extensively studied and points out protocols with respiratory exercises aimed at chest mobility and abdominal muscle strengthening, which lead to improved chest expansion and increased lower thoracic mobility, thus suggesting better diaphragmatic work and increased quality of life and exercise capacity [7–11]. Some of these exercises were also proposed for children with CP [12, 13]. However, available rehabilitation strategies to target the respiratory functioning of the population with CP are not addressed in the literature; protocols are not standardized, and there is no consensus on the optimal training modalities.
Rehabilitation strategies in CP can include aerobic and/or breathing exercises. Aerobic exercises are commonly performed over a long period of time with light to moderate intensity (e.g., running and cycling), limiting their application. Alternatively, respiratory exercises are increasingly regarded as an essential part of the overall physiotherapy in the management of CP and can be defined as techniques with or without the use of mechanical devices that encourage inspiration (e.g., incentive spirometer) and expiration [e.g., positive expiratory pressure (PEP)] [12, 13].
Respiratory training has been proven to be a safe and effective behavioral intervention for the prevention and rehabilitation of chronic conditions, in which, despite presenting different pathophysiology from CP, also have restrictive mechanisms [9–11]. However, evidence to date is scarce about the effectiveness of respiratory exercises in the population with CP [13]. In view of the above, this systematic review and meta-analysis aimed to analyze published randomized controlled clinical trials (RCTs) that investigated the effect of the addition of respiratory exercises to conventional rehabilitation on pulmonary function, functional capacity, respiratory muscle strength, gross motor function and quality of life in children and adolescents with CP.
Methods
This systematic review was completed in accordance with Cochrane Collaboration recommendations and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14, 15]. The protocol of this review was registered in the PROSPERO international prospective register of systematic reviews (reference number: 219302).
This systematic review included articles classified as randomized controlled trials that studied the effects of any form of respiratory exercise in children and adolescents with CP. All studies should explicitly report that at least one of the treatment groups received the addition of respiratory exercise to conventional rehabilitation as an intervention. We excluded studies that included children and adolescents presenting other cardiac and pulmonary diseases associated with CP or adults.
The main outcomes were pulmonary function [forced vital capacity (FVC); forced expiratory volume at one second (FEV1); peak expiratory flow (PEF)]; functional capacity [six-minute walk test (6MWT)]; respiratory muscle strength [(maximum inspiratory pressure (Pimax) and maximum expiratory pressure (Pemax)]; Gross motor function measure (GMFM), exercise capacity, daily living activities and quality of life were additional outcomes [Cerebral Palsy Quality of Life Questionnaire for Children (CP QOL-Child)].
We searched for literatures in the PubMed/Medline, Physiotherapy Evidence (PEDro), Scientific Electronic Library Online (SciELO), EMBASE, Latin American and Caribbean Literature in Health Sciences (Lilacs) databases from their inception until July 2022 without language restrictions. We used a standard protocol for literature search and controlled vocabulary whenever possible (DeCS/MeSH term for MEDLINE) [14]. We used three groups of keywords and their synonyms in the search strategy: study design, participants, and interventions.
The strategy developed by Higgins and Green [14] was used to identify RCTs in PUBMED. The search strategy for MEDLINE via PUBMED is presented in Supplementary Table 1. We adopted a search strategy using similar terms to identify the RCTs in the other databases. We checked the references of the articles included in this systematic review to identify other potentially eligible studies. The authors of ongoing studies were contacted by e-mail to confirm any data or obtain additional information.
Two authors independently evaluated the list of titles and abstracts from each database. If at least one of the reviewers considered one study eligible, the full text was obtained for complete assessment. Then, two reviewers independently assessed the full text of the selected studies to verify whether they met the eligibility criteria. Two authors independently extracted data from the studies using standard data extraction forms adapted from the Cochrane Collaboration [14].
Aspects of the study population, forms of respiratory exercises, follow-up period and rates of missing data, outcome measures, and results were verified. The quality of the included studies was scored by two authors using the PEDro scale, which is based on important criteria such as concealed allocation, intention-to-treat analysis, and the adequacy of follow-up [16, 17]. These characteristics make the PEDro scale a useful tool for assessing the quality of rehabilitation of RCTs. A third reviewer resolved any disagreements in rating the studies.
Pooled effect estimates were obtained by comparing the least square mean change from baseline to endpoint for each group and were expressed as the mean difference (MD) between groups. We converted the confidence interval (CI) to standard deviation (SD) when the SD of change was not available, as per Higgins and Green [14].
Only data closest to the end points of the exercise program were included. Size effects in crossover trials were only extracted at the first crossover point. Only one comparison was made: respiratory exercise combined with conventional rehabilitation versus conventional rehabilitation. Calculations were performed using a random effects model. Heterogeneity among studies was examined with Cochran’s Q and I2 statistics, in which values greater than 40% were considered indicative of high heterogeneity [18]. An α value ≤ 0.05 was considered statistically significant. Analyses were performed with Review Manager (Version 5.3) [19].
The quality of evidence for the outcomes pain and disability was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to interpret result findings and using GRADEpro GDT 2015 to import data from Review Manager to create a “Summary of findings table.” The assessment involved five items: risk of bias, imprecision, inconsistency, indirectness, and publication bias [14]. The quality of evidence was downgraded by one level for risk of bias when more than a quarter of the studies included in the meta-analysis were considered at high risk of bias (studies without allocation concealment, random allocation, and/or sample size calculation). The results were considered imprecise if the pooled sample size was < 300 for dichotomous or < 400 for continuous outcomes and inconsistent if the heterogeneity between RCTs was substantial (i.e., I2 > 40%). When possible, publication bias was assessed by visual inspection of funnel plots (scatterplot of the effect size from individual studies against its effect size) for the meta-analysis with 10 or more trials [14, 20]. Decisions to downgrade the quality of studies were justified using footnotes and making comments, when necessary, to aid readers’ understanding of the review.
Results
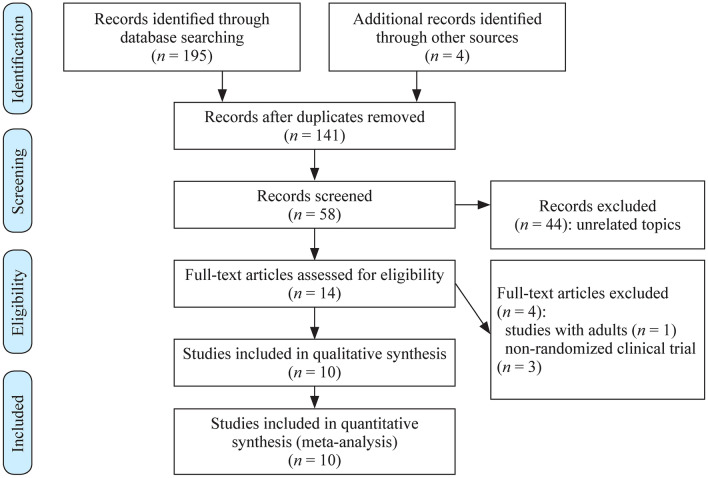
The initial research led to the identification of 195 studies, of which 181 studies were excluded based on the initial screening of the title and abstract, and 14 were considered potentially relevant and then retrieved for detailed analysis. Ten RCTs met the eligibility criteria and were included (Fig. 1) [21–30]. The ten randomized controlled trials were fully analyzed and approved by both reviewers, and the data were extracted. The mean quality of the studies was low-to-moderate (Table 1). After assessing methodological aspects with the PEDro scale tool, we found that all of the studies used random allocation, and only four studies performed concealed allocation [23, 24, 26, 29]. None of the studies blinded the patients or therapists, and only three studies blinded the assessors [23, 24, 29]. None of the studies used the principle of intention-to-treat analysis.
Fig. 1.
Flow diagram of included studies
Table 1.
Study quality on the PEDro scale
| Studies | Criteria | Total points | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Rothman et al. [21] | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Lee et al. [23] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Choi et al. [22] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Kelles et al. [24] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Kanna and Balabaskar [25] | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 4 |
| Varol-Kepenek et al. [26] | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Anand and Karthikbabu [27] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Atia and Tharwa [28] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| El-Refaey et al. [29] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Elseify et al. [30] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
1: eligibility criteria and source of participants; 2: random allocation; 3: concealed allocation; 4: baseline comparability; 5: blinded participants; 6: blinded therapists; 7: blind assessors; 8: adequate follow-up; 9: intention-to-treat analysis; 10: between-group comparisons; 11: point estimates and variability
Item 1 does not contribute to the total score. PEDro Physiotherapy Evidence
The characteristics of the randomized controlled trials are summarized in Table 2. The number of participants in the included studies was 324 children and adolescents. The age of participants ranged from 6 to 18 years [21–30]. Randomized clinical trials included children and adolescents of both sexes.
Table 2.
Details of included studies and interventions provided
| Studies | Patients (n, age) | Intervention types | Balance | Mobility | Pulmonary function/respiratory muscle strength | Quality of life | Key findings |
|---|---|---|---|---|---|---|---|
| Rothman et al. [21] |
N = 10 Age: 5–8 y |
IMT + CR (n = 5), CR (n = 5) | – | – | VC, FEV1 | – | After treatment, the experimental group obtained an average increase in vital capacity of 0.46 L compared to the control. In addition, the experimental group showed an average increase of 31% in relation to the pretest values, which was significant at the 0.005 probability level. The results of FEV1 between both groups showed no significant difference |
| Lee et al. [23] |
N = 20 Age: 6–12 y |
IMT + CR (n = 9), CR (n = 11) | – | – | FVC, FEV1, PEF, VC, IRV, ERV | – | FVC increased by 50% (P < 0.001) and FEV1 increased by 40% (P < 0.001) compared to the pre-intervention in the experimental group. There was no significant difference in PEF, VC, IRV, ERV between pre and post training |
| Choi et al. [22] |
N = 50, Age: 8–15 y |
ISE + CR (n = 25), CR (n = 23) | GMFCS: I to IV | FVC, FEV1, PEF | – | FVC (% predicted) increased from 84.25 to 92.18 (P < 0.001), FEV1 (% predicted) increased from 81.91 to 91.12 (P = 0.009) and PEF (L/min) increased from 191.60 to 217.60 (P = 0.003), after ISE in the experimental group, compared to baseline data | |
| Kelles et al. [24] |
N = 25 Age: 7–14 y |
IMT + CR (n = 13), Sham (n = 12) | GMFCS: I to II | 6MWT | FVC, FEV1, FEF, PEF, Pimax, Pemax | CP QOL-Child | CP QOL scores, in the domains of social well-being, acceptance, Pimax (22.85 cmH2O, 95% CI 12.84–32.86; P < 0.001); PEDI–FSS self-care (1.86, 95% CI 0.68–3.04; P = 0.007), PEDI–FSS mobility (2.78, 95% CI 1.72–3.84; P = 0.001) and social function (3.95, 95% CI 3.00–4.90; P = 0.015) and the distance covered during the 6MWT (57.1 m, 95% CI 37.58–6.62, P < 0.001) were significantly higher in the experimental group compared to the control group. However, there were no significant differences in Pemax and test scores |
| Kanna and Balabaskar [25] |
N = 30 Age: 6–14 y |
RE + CR (n = 15), CR (n = 15) | – | – | FVC, FEV1, FEV1/FVC, PEF | – | The results of the analysis between the groups on the improvement of FVC, FEV1, FEV1/FVC% and PEF showed a significant improvement by the value of the t test of the independent sample (P < 0.05) |
| Varol-Kepenek et al. [26] |
N = 30 Age: 7–16 y |
IMT + CR (n = 15), CR (n = 15) |
GMFCS: I to II |
6MWT | FVC, FEV1, FEV1/FVC, PEF, Pimax, Pemax | There were no significant changes in the FVC (82.07%), FEV1 (86.53%) and FEV1/FCV % values in both groups (P > 0.05), while Pimax, Pemax, PEF (85.6%, 85.6%, 69.21%) and 6MWT values were significantly increased (P < 0.001) after the training. The improvements in Pimax and Pemax values were significantly greater in the CPRP + IMT group compared with the CPRP group (P < 0.001) | |
| Anand and Karthikbabu [27] |
N = 40 Age: 8–15 y |
IMT + CR (n = 20), CR (n = 20) | GMFCS: I to III | – | FVC, FEV1 FEV1/FVC, maximal mid-expiratory flow | Significant improvements in FEV1, FVC and maximal mid-expiratory flow were found in the study group, but not in the control group | |
| Atia and Tharwa [28] |
N = 50 Age: 8–12 y |
ISE + CR (n = 30), CR (n = 20) | GMFCS: II to IV | 6MWT | FVC, FEV1, PEF, Pimax, Pemax | Time by group interaction showed no statistical significance between the groups in any outcome measures except for peak expiratory flow. The mean difference of 9.6 cmH2O (95% CI 2.3, 16.8) in the Pimax from baseline to 2-month follow-up supports the experimental intervention. Post-training, the between-group mean difference was 19.8 (95% CI −18.0, 57.6) meters in the 6MWT | |
| El-Refaey et al. [29] |
N = 26 Age: 6–12 y |
ISE + CR (n = 12), CR (n = 14) | GMFCS: III to IV | GMFM-88 | FVC, FEV1, PEF, Pimax, Pemax | PedsQL | After training, significant difference was observed between both groups in Pimax, Pemax, but in favor of study group (P = 0.001, 0.001, respectively), whereas there was a non-significant difference in the gross motor function, and health-related quality of life (P = 0.527, 0.876, respectively) |
| Elseify et al. [30] |
N = 50 Age: 6–18 y |
ISE + CR (n = 30), CR (n = 20) | GMFCS: II to IV | FVC, FEV1, FEV1/FVC, maximal mid-expiratory flow | The authors found significant improvements in FEV1%, FVC%, and maximal mid-expiratory flow in the study group, but not in the control group |
FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, PEF peak expiratory flow, VC vital capacity, FEF forced expiratory flow, Pimax maximal inspiratory pressure, Pemax maximal expiratory pressure, IRV inspiratory reserve volume, ERV expiratory reserve volume, GMFCS Gross Motor Function Classification Systems, GMFM Gross Motor Function Measure, TCMS Trunk Control Measurement Scale, PEDI Pediatric Evaluation of Disability Inventory, CPRP conventional physiotherapy rehabilitation program, CP QOL-Child Cerebral Palsy Quality of Life Questionnaire for Children, 6MWT six-minute walk test, IMT inspiratory muscle training, CR conventional rehabilitation, RE respiratory exercises, ISE incentive spirometry
The respiratory exercises differed between studies; the duration of programs ranged from four to eight weeks and were considered to be a combination of eight respiratory exercises called the “breathing exercise program”, feedback respiratory training, incentive spirometer exercise, inspiratory muscle training (IMT) with a threshold device, and a combination of respiratory exercises and incentive spirometer exercise [21–30].
The main outcomes analyzed in the included studies were pulmonary function (FVC, FEV1, and PEF); functional capacity (6MWT); respiratory muscle strength (Pimax and Pemax), GMFM, daily living activities and quality of life [CP QOL-Child, and Pediatric Quality of Life Inventory (PedsQL)].
The posology used was reported in most studies. There was a variation regarding the session time from 5–7 minutes [21] to 15 minutes [23, 25]. The frequency of the sessions ranged from two times a week in one study [22], three times a week in another study [23], five times a week in one study [25], and seven times a week in two studies [21, 24]. The characteristics of respiratory exercise intervention in the included studies are provided in Table 3.
Table 3.
Characteristics of respiratory exercises
| Studies | Outcome measures | Intervention types | Method of intervention intensity/volume | Frequency (per wk) | Time (min) | Length (wk) |
|---|---|---|---|---|---|---|
| Rothman et al. [21] |
Pulmonary function test (FVC, FEV1) |
IMT + CR (n = 5), CR (n = 5) |
Breathing Program consisting of eight exercises (1) Diaphragmatic breathing (2) Expiratory exercise utilizing abdominal musculature. The child was instructed while seated to blow a ping-pong ball across the table at different distances (3) Inspiration and expansion of the thorax. In the supine position, the child was instructed to inhale as he elevated his arms above his head and to exhale as he lowered his arms to his sides (4) The fourth exercise helped to stimulate inspiration. A belt was placed around the lower ribs and crossed in front of the child while seated. The child was instructed to exhale as the belt was tightened and to inhale as the belt was loosened (5) The child was supine and instructed to bring each knee to the chest while exhaling and to inhale as the knees were lowered (6) A straight sit-up with knees flexed and feet flat on the floor (7) Strengthening the lateral abdominal musculature, the external and internal obliques. This exercise was the same as exercise six except that the child was to bring each elbow toward the opposite knee (8) The eighth exercise was the same as exercise one except that a 5-pound weight was applied to the epigastric area to provide resistance to diaphragm motion The exercise schedule was set up with the exercises increasing in difficulty |
7 | 5–7 | 8 |
| Lee et al. [23] |
Pulmonary function test (FVC, FEV1, PEF, VC, IRV, ERV) |
IMT + CR (n = 9), CR (n = 11) |
15 min of feedback respiratory training using a feedback respiratory training device. This training device consists of a hand-held unit with a respiratory pouch and base station; it has a two-way piston valve connecting to a rebreathing bag 10 min of rest 30 min of conventional rehabilitation therapy The breathing frequency was chosen as the low frequency of 14–15 breaths/min to prevent fatigue or dizziness |
3 | 45 | 4 |
| Choi et al. [22] |
Gross Motor Function (GMFM-66) Pulmonary function test (FVC, FEV1, PEF) |
ISE + CR (n = 25), CR (n = 23) |
Using the Incentive Spirometer, they were instructed to hold their breath for as long as possible, or for at least 5 s, and then to breathe out slowly The flow rate progressively increased at 100 mL/s intervals from 100 mL/s to 600 mL/s, if the participants could maintain the balls lifted for at least 5 s BF: 10–15 per session/10 sessions per day |
2 | – | 4 |
| Kelles et al. [24] |
Trunk Control (TCMS) Pulmonary function test (FVC, FEV1, FEF25%–75%, PEF, Pimax, Pemax) Functionality (PEDI) Quality of life (CP QOL-Child) Functional Capacity (6MWT) |
IMT + CR (n = 13), Sham (n = 12) |
1 wk of familiarization to learn diaphragmatic breathing 15 min of IMT at 30% of Pimax (the training was adjusted weekly) BF: 10–15/min during 5–10 s for resting between breathings Twice a day |
7 | 30 | 8 |
| Kanna & Balabaskar [25] |
Pulmonary function test (FVC, FEV1, FEV1/FVC ratio, PEF) |
RE + CR (n = 15), CR (n = 15) |
30 min of neurodevelopmental treatment 15 min of respiratory exercises (breathing exercises, active shoulder/shoulder girdle ROM exercises; diaphragmatic breathing exercise; thoracic expansion exercise and incentive spirometry) |
5 | 45 | 6 |
| Varol-Kepenek et al. [26] |
Balance and postural stability (PST, LOST, TSIB) Pulmonary function test (FVC, FEV1, FEV1/FVC, PEF, Pimax, Pemax) Functional capacity (6MWT) |
IMT + CR (n = 15), CR (n = 15) |
45 min of conventional rehabilitation therapy 15 min of IMT at 30% of Pimax (the training was adjusted weekly) Twice a day |
7 | 15 | 8 |
| Anand and Karthikbabu [27] |
Pulmonary function test (FVC, FEV1, PEF, Pimax, Pemax) Functional capacity (6MWT) |
IMT + CR (n = 20), CR (n = 20) |
45 min of sensorimotor physical therapy 15 min of IMT at 30% of Pimax (the training was adjusted weekly by 5% of initial performance) |
3 | 15 | 6 |
| Atia and Tharwa [28] |
Pulmonary function test (FVC, FEV1, FEV1/FVC, maximal mid-expiratory flow) |
ISE + CR (n = 30), CR (n = 20) |
60 min of traditional physical therapy program 10 times of the patient to be motivated to achieve a preset volume by visual feedback. After each set of 10 breaths, the patient is asked to cough, so that mucus is cleared from the lungs Twice a day |
3 | 15 | 8 |
| El-Refaey et al. [29] |
Pulmonary function test (Pimax, Pemax) Gross Motor Function (GMFM-88) Quality of Life (PedsQL) |
ISE + CR (n = 12), CR (n = 14) |
60 min of traditional physical therapy program 5 times of mouth and slowly and maximally inhale through the spirometer to raise the ball in the cylinder as high as he/she can, and then hold the inspiration for at least 2–3 s before exhaling normally outside the mouthpiece |
5 | 30 | 4 |
| Elseify et al. [30] |
Pulmonary function test (FVC, FEV1, FEV1/FVC, maximal mid-expiratory flow) |
ISE + CR (n = 30), CR (n = 20) |
Traditional physical therapy program 10 times of the patient to be motivated to achieve a preset volume by visual feedback. After each set of 10 breaths, the patient is asked to cough, so that mucus is cleared from the lungs Twice a day |
7 | 15 | 4 |
FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, PEF peak expiratory flow, VC Vital capacity, FEF25–75% forced expiratory flow from 25 to 75%, Pimax maximal inspiratory pressure, Pemax maximal expiratory pressure, IRV inspiratory reserve volume, ERV expiratory reserve volume, GMFM-66 Gross Motor Function Measure–66 items, TCMS Trunk Control Measurement Scale, PEDI Pediatric Evaluation of Disability Inventory, CP QOL-Child Cerebral Palsy Quality of Life Questionnaire for Children, 6MWT 6-min walk test, IMT Inspiratory Muscle Training, CR conventional rehabilitation, RE respiratory exercises, ISE Incentive Spirometry Exercises, PST Postural Stability Test, LOST Limits of Stability Test, TSIB Test of Sensory Integration and Balance
Respiratory exercise plus conventional rehabilitation versus conventional rehabilitation
Pulmonary function
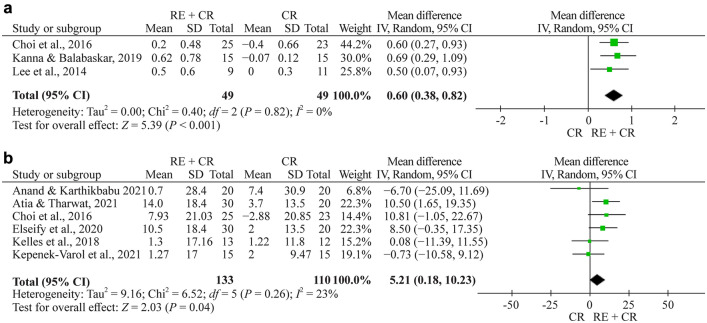
Totally eight studies evaluated FCV [22–28, 30]. Meta-analysis was performed by grouping studies that evaluated the results in liters and % in the assessment of FVC. The meta-analysis showed a pooled effect of 0.60 L (95% CI 0.38–0.82, n = 98, P < 0.001) and a significant effect of 5.2 (% of predicted, 95% CI 0.2–10.2, n = 243, P = 0.040) for respiratory exercise plus conventional rehabilitation group participants compared with the usual care group (Fig. 2a, b, respectively).
Fig. 2.
a Forest plot of FVC (forced vital capacity) (L) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. b Forest plot of FVC (%) (L) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. RE respiratory exercise, CR conventional rehabilitation, SD standard deviation, CI confidence interval
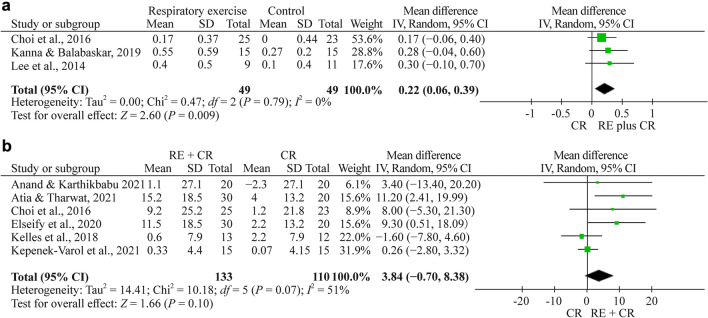
Totally eight studies evaluated FEV1 [22–28, 30]. Meta-analysis was performed by grouping studies that evaluated the results in liters and % in the assessment of FEV1. The meta-analysis showed a nonsignificant effect of 0.22 L (95% CI 0.06 to 0.39, n = 98, P < 0.009) and a nonsignificant effect of 3.84 (% of predicted, 95% CI −0.7 to 8.4, n = 243, P = 0.100) for the respiratory exercise plus conventional rehabilitation group participants compared with the conventional rehabilitation group (Fig. 3a, b, respectively).
Fig. 3.
a Forest plot of FEV1 (forced expiratory volume at 1 s) (L) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. b Forest plot of FEV1 (%) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. RE respiratory exercise, CR conventional rehabilitation, SD standard deviation, CI confidence interval
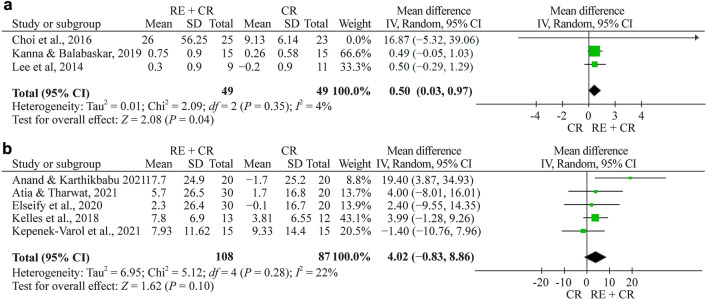
There are eight studies evaluating PEF [22–28, 30]. Meta-analysis was performed by grouping studies that evaluated the results in liters and % in the assessment of PEF. The meta-analysis showed a pooled effect of 0.50 (95% CI 0.05–0.04, n = 98, P = 0.030) in PEF (L/min) and a nonsignificant effect of 4.02 (% of predicted, 95% CI −0.8 to 8.9, n = 195) for the respiratory exercise plus conventional rehabilitation group participants compared with the conventional rehabilitation group (Fig. 4a, b, respectively).
Fig. 4.
a Forest plot of PEF (peak expiratory flow) (L/min) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. b Forest plot of PEF (%) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. RE respiratory exercise, CR conventional rehabilitation, SD standard deviation, CI confidence interval
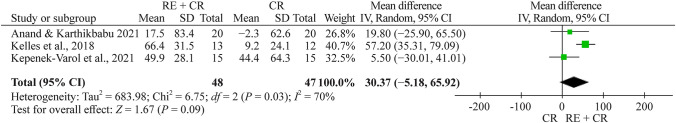
Functional capacity
Totally three studies evaluated functional capacity, all with the 6MWT [24, 26, 27]. The total number of children and adolescents in the respiratory exercise plus conventional rehabilitation group was 48, whereas 47 children and adolescents were included in the conventional rehabilitation group. The meta-analysis showed a nonsignificant effect of 30.4 m (95% CI −5.2 to 68.9, n = 95) for the respiratory exercise plus conventional rehabilitation group participants compared with the conventional rehabilitation group (Fig. 5).
Fig. 5.
Forest plot of functional capacity after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. RE respiratory exercise, CR conventional rehabilitation, SD standard deviation, CI confidence interval
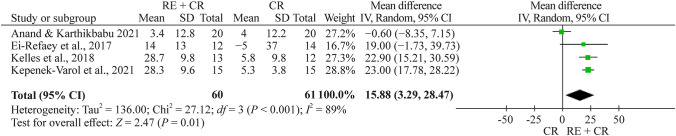
Respiratory muscle strength
Totally four studies evaluated Pimax using manovacuometry [24, 26, 27, 29]. The total number of children and adolescents in the respiratory exercise plus conventional rehabilitation group was 60, whereas 61 children and adolescents were included in the conventional rehabilitation group. The meta-analysis showed a significant effect of 15.9 cmH2O (95% CI 3.3 to 28.5, n = 121) for the respiratory exercise plus conventional rehabilitation group participants compared with the conventional rehabilitation group (Fig. 6).
Fig. 6.
Forest plot of Pimax (maximal inspiratory pressure) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. RE respiratory exercise, CR conventional rehabilitation, SD standard deviation, CI confidence interval
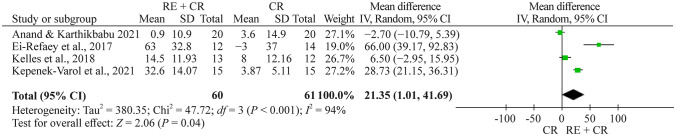
There are four studies evaluating Pemax using manovacuometry [24, 26, 27, 29]. The total number of children and adolescents in the respiratory exercise plus conventional rehabilitation group was 61, whereas 60 children and adolescents were included in the conventional rehabilitation group. The meta-analysis showed a significant effect of 21.4 cmH2O (95% CI 1.01–41.7, n = 121) for the respiratory exercise plus conventional rehabilitation group participants compared with the conventional rehabilitation group (Fig. 7).
Fig. 7.
Forest plot of Pemax (maximal expiratory pressure) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. RE respiratory exercise, CR conventional rehabilitation, SD standard deviation, CI confidence interval
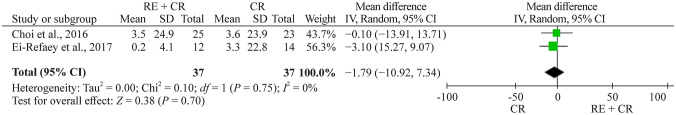
Gross motor function measure (GMFM)
Totally two studies evaluated GMFM [22, 29]. The meta-analysis showed a nonsignificant effect of –1.8 (95% CI –10.9 to 7.3, n = 74) for the respiratory exercise plus conventional rehabilitation group participants compared with the conventional rehabilitation group (Fig. 8).
Fig. 8.
Forest plot of GMFM (Gross Motor Function Measure) after respiratory exercise plus conventional rehabilitation versus conventional rehabilitation. RE respiratory exercise, CR conventional rehabilitation, SD standard deviation, CI confidence interval
Daily living activities
Kelles [24] assessed the effect of inspiratory muscle training on daily living activities. Daily living activities were assessed using the Pediatric Evaluation of Disability Inventory–Functional Skill Scale (PEDI–FSS) and Caregiver Assistance Scale (CAS). The PEDI–FSS self-care (1.86; 95% CI 0.68–3.04; P = 0.007), PEDI–FSS mobility (2.78; 95% CI 1.72–3.84; P = 0.001) and social function (3.95; 95% CI 3.00–4.90; P = 0.015) domain scores significantly improved in the treatment group compared with the control group. No difference was observed between the groups in the PEDI–CAS subscale scores.
Quality of life
Kelles [24] assessed the effect of inspiratory muscle training on the quality of life using CP QOL-Child. The CP QOL-Child social well-being and acceptance (13.73, 95% CI 12.86–14.60, P < 0.001) and functioning domain (5.09, 95% CI 2.09 to 8.09; P = 0.004) scores significantly improved in the treatment group compared with the control group. No difference was observed between the groups in the other domains of the CP QOL-Child.
El-Refaey et al. [29] assessed the effect of feedback respiratory training on the quality of life using the Pediatric Quality of Life Inventory (PedsQL). The comparison PedsQL revealed a nonsignificant difference between the groups.
Quality of evidence
The quality of evidence according to the GRADE system is presented in Supplementary Table 2. The quality of evidence for the functional outcomes was determined to be low for FEV1, PEF and Pimax and moderate for FVC, functional capacity and Pemax.
Discussion
Considering that breathing training in children with cerebral palsy can increase functionality, the findings of this study may contribute to clinical decision making. According to the meta-analysis results, respiratory exercises plus conventional rehabilitation were associated with improvements in pulmonary function and respiratory muscle strength when compared to conventional rehabilitation. Respiratory exercises have not been shown to be effective in improving gross motor function, and only one study demonstrated improvement in social well-being and acceptance and functioning domains of quality of life in individuals performing IMT. The quality of evidence for the FVC, FEV1 and quality of life outcomes were determined to be low to moderate.
Dystonia, spasticity, poor movement and lack of experience of certain postures can hinder the natural muscle development of children with CP, as well as disabling proper development of the chest and respiratory system [24]. Significant postural impairments and muscle disorders could lead to inadequate development of the rib cage during childhood with little use of the diaphragm, mobilizing small amounts of air during inspiration [25].
Thus, in thinking that hypoventilation negatively affects respiratory status, strategies to improve respiratory muscle strength and activation in people with CP should all be addressed [24]. Pulmonary rehabilitation in children and adolescents with asthma and chronic lung disease demonstrated that respiratory exercises of chest expansion increase thoracic mobility, suggesting better diaphragmatic contraction and increased quality of life and functional capacity [9–11]. Improvements in functional capacity were also noted in this review.
Lee et al. [23] related just a small increase in PEF compared to pretraining values. Keles [24] explained that the increase in PEF is probably related to the improvement in Pemax, since PEF is influenced by expiratory muscle strength. These results suggest that exercises that involve activating the expiratory muscles should be encouraged in pulmonary rehabilitation programs to better enhance PEF.
The samples in the studies included in this review consisted of children or adolescents between levels (I to IV) of the Gross Motor Function Classification System (GMFCS), while those with classification V who are totally dependent on daily life activities and locomotion were not tested. We observed that the studies whose samples were of children classified as I and II revealed minor changes in respiratory function or no differences compared to the control group. Know and Lee [31] investigated the difference in lung capacity and muscle strengthening related to respiration depending on the level of the GMFCS in children with CP through tests of respiratory function and respiratory pressure. Once both lung function and respiratory muscle strength are positively correlated with mobility, the findings indicated that a decrease in functional motor ability as classified by the GMFCS could be accompanied by respiratory dysfunction, reinforcing that the greater the severity is in the progression of GMFCS levels, the greater the energy expenditure and inefficient ventilatory behaviors are during breathing.
In addition, children have to present preserved cognitive and cooperative function for breathing exercises to be effective. These findings are relevant to clinical practice, as they discuss the need to develop protocols designed beyond overall development but also to focus on pulmonary function, respecting the condition of each patient and their specific needs of therapy. In general, the studies have a great variation in relation to the number of sessions and session time. We observed that the optimal level of intensity in intervention programs for CP is not clear [13].
The COVID-19 pandemic also emphasizes the importance of pulmonary functions among individuals with neurological disabilities, and further investigations into the prescription of breathing exercise variables (e.g., modality, load, frequency, duration) are recommended to enhance our understanding of the real positive effects of breathing exercises in children and adolescents with CP. Moreover, the relationship between posture and breathing has become an important topic for continuing education courses within the therapy community but is still little investigated [32–34]. Interest will likely increase as the physical therapy community focuses more on performance (what patients are doing within their living environments) than capacity (what patients demonstrate in the therapy session).
This study has limitations. One of them concerns the methodological quality of the study as observed in the PEDro scale. Although all studies affirmed allocation to the treatment groups, only three studies indicated a computerized allocation method [22–24] by researchers not involved with the project. Furthermore, only one study reported blind allocation and data collection [24]; both parents and raters in another study were blinded to group separation [23], and the rest of the studies presented no open reference to any blinding.
In conclusion, further investigations on respiratory exercises related to quality of life, functional and lung capacity, as well as the prescription of variables of these training protocols, are recommended to improve our understanding of the real positive results of respiratory exercises in children and adolescents with CP. Future well-controlled RCTs are required to reinforce the recommendation of respiratory exercise as an important rehabilitation treatment in people with cerebral palsy. Successful propositions of breathing exercises with other populations may provide a new direction for exercise prescription, such as lung volume recruitment [35]. In addition, it will be important to match exercise prescription to clinical/treatment characteristics of a patient subgroup or individual patient.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
LCTR, CTRL, DRB, AMDM contributed to conceptualization, data aquisition, formal analysis, writing–original draft. MPAF, NMG, SME contributed to conceptualization, writing–original draft, writing–review and editing. All authors read and approved the final manuscript.
Funding
The authors declare that no funding, exceptionally, or other support was received during the preparation of this manuscript.
Declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
Not needed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel DR, Neelakantan M, Pandher K, Merrick J. Cerebral palsy in children: a clinical overview. Transl Pediatr. 2020;9:125–135. doi: 10.21037/tp.2020.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gkaraveli M, Skordilis E, Grammatopoulou E, Karteroliotis K, Dania A, Morfis P, et al. The effect of inspiratory muscle training on respiratory pressure, pulmonary function and walking ability in preschool children with cerebral palsy. Ann Physiother Clin. 2019;2:1–8. [Google Scholar]
- 3.Boel L, Pernet K, Toussaint M, Ides K, Leemans G, Haan J, et al. Respiratory morbidity in children with cerebral palsy: an overview. Dev Med Child Neurol. 2019;61:646–653. doi: 10.1111/dmcn.14060. [DOI] [PubMed] [Google Scholar]
- 4.Wang HY, Chen CC, Hsiao SF. Relationships between respiratory muscle strength and daily living function in children with cerebral palsy. Res Dev Disabil. 2012;33:1176–1182. doi: 10.1016/j.ridd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Kwon YH, Lee HY. Differences in respiratory pressure and pulmonary function among children with spastic diplegic and hemiplegic cerebral palsy in comparison with normal controls. J Phys Ther Sci. 2015;27:401–403. doi: 10.1589/jpts.27.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ersöz M, Selçuk B, Gündüz R, Kurtaran A, Akyüz M. Decreased chest mobility in children with spastic cerebral palsy. Turk J Pediatr. 2006;48:344–350. [PubMed] [Google Scholar]
- 7.Sobrinho ASF, Scalassara PR, Dajer ME. Low-cost joystick for pediatric respiratory exercises. J Med Syst. 2020;44:186. doi: 10.1007/s10916-020-01655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hristara-Papadopoulou A, Tsanakas J, Diomou G, Papadopoulou O. Current devices of respiratory physiotherapy. Hippokratia. 2008;12:211–220. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Wang Q, Liu L, Yang W, Liu H. Effects of physical therapy on lung function in children with asthma: a systematic review and meta-analysis. Pediatr Res. 2021;89:1343–1351. doi: 10.1038/s41390-020-0874-x. [DOI] [PubMed] [Google Scholar]
- 10.Hilton N, Solis-Moya A. Respiratory muscle training for cystic fibrosis. Cochrane Database Syst Rev. 2018;24:CD006112. doi: 10.1002/14651858.CD006112.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson E, Pederson N, Rawson H, Daniel T. The effect of inspiratory muscle training on Duchenne muscular dystrophy: a meta-analysis. Pediatr Phys Ther. 2019;31:323–330. doi: 10.1097/PEP.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 12.Rutka M, Adamczyk WM, Linek P. Effects of physical therapist intervention on the pulmonary function in children with cerebral palsy: a systematic review and meta-analysis. Phys Ther. 2021;101:pzab129. doi: 10.1093/ptj/pzab129. [DOI] [PubMed] [Google Scholar]
- 13.El Banna EH, El Hadidy EI, Ali WM. Effect of respiratory therapy on pulmonary functions in children with cerebral palsy: a systematic review. Bull Fac Phys Ther. 2020;25:18. doi: 10.1186/s43161-020-00016-6. [DOI] [Google Scholar]
- 14.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). 2021. http://www.training.cochrane.org/handbook. Accessed 16 Jan 2022.
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivo SA, Macedo LG, Gadotti IN, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 17.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating of quality randomized controlled trials. PhysTher. 2003;83:713–722. [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Cochrane Collaboration. Review Manager (RevMan) Version 5.4. 2020. https://training.cochrane.org/online-learning/core-software/revman. Accessed 16 Jan 2022.
- 20.Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Rothman JG. Effects of respiratory exercises on vital capacity and force expiratory volume in children with cerebral palsy. Phys Ther. 1978;58:421–425. doi: 10.1093/ptj/58.4.421. [DOI] [PubMed] [Google Scholar]
- 22.Choi JY, Rha DW, Park ES. Change in pulmonary function after incentive spirometer exercise in children with spastic cerebral palsy: a randomized controlled study. Yonsei Med J. 2016;57:769–775. doi: 10.3349/ymj.2016.57.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Cha YJ, Kim K. The effect of feedback respiratory training on pulmonary function of children with cerebral palsy: a randomized controlled preliminary report. Clin Rehabil. 2014;28:965–971. doi: 10.1177/0269215513494876. [DOI] [PubMed] [Google Scholar]
- 24.Keles MN, Elbasan B, Apaydin U, Aribas Z, Bakirtas A, Kokturk N. Effects of inspiratory muscle training in children with cerebral palsy: a randomized controlled trial. Braz J Phys Ther. 2018;22:493–501. doi: 10.1016/j.bjpt.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanna BSS, Balabaskar K. A study on efficacy of respiratory exercises coupled with neuro developmental treatment on pulmonary function of children with spastic quadriplegic cerebral palsy. Biomed Pharmacol J. 2019;12:1519–1524. doi: 10.13005/bpj/1782. [DOI] [Google Scholar]
- 26.Varol-Kepenek B, Gurses NH, Içağasıoğlu DF. Effects of inspiratory muscle and balance training in children with hemiplegic cerebral palsy: a randomized controlled trial. Dev Neurorehabil. 2021;25:1–9. doi: 10.1080/17518423.2021.1905727. [DOI] [PubMed] [Google Scholar]
- 27.Anand B, Karthikbabu S. Effects of additional inspiratory muscle training on mobility capacity and respiratory strength for school-children and adolescents with cerebral palsy: a randomized controlled trial. Braz J Phys Ther. 2021;25:891–899. doi: 10.1016/j.bjpt.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atia DT, Tharwat M. Effect of incentive spirometer exercise combined with physical therapy on pulmonary functions in children with cerebral palsy. Int J Ther Rehabil. 2021;28:72. doi: 10.12968/ijtr.2020.0072. [DOI] [Google Scholar]
- 29.El-Refaey BH, Maksoud GMA, Ali OI. Efficacy of feedback respiratory training on respiratory muscle strength and quality of life in children with spastic cerebral palsy: randomized controlled trial. Bull Fac Phys Ther. 2017;22:46–52. [Google Scholar]
- 30.Elseify MY, Ramadan DA, Ishak SR. Effect of incentive spirometer exercise on pulmonary functions in children with spastic cerebral palsy. Egypt J Bronchol. 2019;13:716–721. doi: 10.4103/ejb.ejb_53_19. [DOI] [Google Scholar]
- 31.Kwon YH, Lee HY. Differences in respiratory function according to the level of gross motor function classification system in children with cerebral palsy. J Phys Ther Sci. 2014;26:389–391. doi: 10.1589/jpts.26.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivi E, Filippi M, Fornasari E, Mascia MT, Ferrari A, Costi S. Effectiveness of standing frame on constipation in children with cerebral palsy: a single-subject study. Occup Ther Int. 2014;21:115–123. doi: 10.1002/oti.1370. [DOI] [PubMed] [Google Scholar]
- 33.Littleton SR, Heriza CB, Mullens PA, Moerchen VA, Bjornson K. Effects of positioning on respiratory measures in individuals with cerebral palsy and severe scoliosis. Pediatr Phys Ther. 2011;23:159–169. doi: 10.1097/PEP.0b013e318218e306. [DOI] [PubMed] [Google Scholar]
- 34.Nwaobi OM, Smith PD. Effect of adaptive seating on the pulmonary function of children with cerebral palsy. Dev Med Child Neurol. 1986;28:351–354. doi: 10.1111/j.1469-8749.1986.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 35.Naughton PE, Sheers N, Berlowitz DJ, Howard ME, McKim DA, Katz SL. Objective measurement of lung volume recruitment therapy: laboratory and clinical validation. BMJ Open Respir Res. 2021;8:e000918. doi: 10.1136/bmjresp-2021-000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.