Summary
New evidence is emerging about the dynamics of interaction between circadian rhythms and brain waves, whose coordination occurs through the entrainment process. The so-called “oscillopathies” or dysfunctions in synchronization of neuronal oscillation in key brain networks lead to the onset of neurodegenerative diseases. A typical example of alteration is insomnia, a risk factor for the oscillopathies, increasingly widespread worldwide. Recently, synchronization of circadian rhythms in cell cultures has allowed an improvement in the physiological relevance of responses to stimuli. Furthermore, brain organoids and neurons cultured in microfluidic systems are the latest frontiers for in vitro reproduction of rhythmic electrical signals. In this review, the combination of these in vitro experimental approaches is proposed as suitable for a more direct investigation on the common mechanisms and neurophysiological substrates underlying brain waves and circadian oscillations, and useful to evaluate the effects of “oscillotherapeutic” drugs for personalized neuromedicine.
Subject area: Cellular physiology, Neuroscience, Cellular neuroscience
Graphical abstract
Cellular physiology; Neuroscience; Cellular neuroscience
Background
Circadian oscillations
The circadian rhythm in animals or humans is held by the neurons of the suprachiasmatic nuclei (SCN) of the hypothalamus, which temporally coordinates the entire organism in a rigorous cellular circadian synchrony. In SCN neurons, as well as in most of the other mammalian cells, the endogenous, intracellular clock mechanism is composed of a transcription–translation feedback loop (TTFL). This core loop or central circadian oscillator generates a self-sustaining rhythmicity of approximately 24 h under constant clock conditions and clock-controlled gene expression.1 As well summarized by Lee et al.,2 four sets of genes encode the proteins that form the central oscillatory feedback loop in mammalian circadian systems: brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1/brain and muscle ARNT-like 1 (Arntl/Bmal1), circadian locomotor output cycles kaput (Clock), cryptochromes (Cry1 and Cry2), and periods (Per1 and Per2). However, the core loop also retains a plasticity to temporally integrate a diverse set of cellular functions with environmental changes, allowing clock-controlled processes to be tuned to environmental conditions.1 In the morning, Bmal1 and Clock form heterodimers that bind to the E-boxes, which are abundant in the promoters of Per1 and 2 and Cry1 and 2, and lead to a gradual increase in the expression of Per and Cry genes during the day. As night falls, Per and Cry form a complex that is protected from further degradation and translocated into the nucleus. The nuclear Per-Cry complex then negatively regulates the expression of Per and Cry genes by disrupting the Bmal1/Clock dimer.2 This is achieved through the entrainment or synchronization, i.e., a process of alignment of period and phase between two interacting rhythms, driven by some external signals (called zeitgebers), such as light, which is the environmental parameter most used by all organisms, including humans, but also feeding/fasting, temperature, and arousal stimuli.1 On the other hand, the circadian rhythms of peripheral somatic cells are progressively dampened until desynchronization, unless neuronal central clock signals are provided for re-entrainment.3
Interestingly, the circadian clock also plays an important role in cell division and differentiation of stem and progenitor cells during physiological development and regeneration of tissues. This phenomenon is called circadian “gating” of the cell cycle,1,4 and the number of gates varies with the rate of cell division. Circadian clock can also influence cell division by synchronization or phase locking1: two oscillating systems are phase-locked when they oscillate at the same frequency and with a constant relationship between their phases; correspondingly, the circadian clock and the cell cycle are reciprocally coupled, but the ratio of coupling can be different from 1:1, and it can be altered with experimental conditions or cell differentiation in vitro.4 The biological significance of the interaction between the circadian clock and the cell cycles likely reflects the necessity to gate the cell cycle to specific times of the day. This connection between the cell cycle and the circadian clock in dividing cells raises questions about the nature of this interaction in differentiating cells, in terminally differentiated nondividing or senescent cells, and finally in the human-induced pluripotent stem cells (hiPSCs).5 Therefore, the circadian clock as autonomous timing system coordinates all processes from differentiation to death in cell lifetime, modulating gene expression, protein activity, and the structural and functional properties of intracellular organelles. Indeed, spatial compartmentalization is essential for highly specialized cellular functions, which are separated not only physically but also temporally as a further adaptive refinement. Consequently, it is intriguing that also cell fate can be influenced by the time of the day1; in addition, as further discussed in our review, it is interesting to evaluate how cell fate is also affected by a micropatterning-based geometric cell confinement.
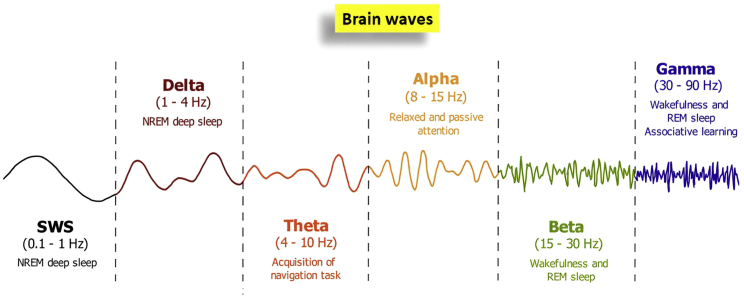
Brain waves
Synchronization and desynchronization of rhythms is also essential to the coordination of the activities of the CNS, where neurons communicate via electrochemical signals leading to flow of ionic currents through synaptic contacts. At the network level, the synchronization of the neuron’s electrical activity gives rise to rhythmic voltage fluctuations or repetitive patterns of neuronal activity traveling across brain regions, known as neuronal oscillations or brain waves.6 Brain waves (Figure 1) are oscillating electrical voltages of the extracellular field potential in the brain measuring just a few millionths of a volt that can be measured through recordings of local field potentials and through electroencephalography (EEG). In the human EEG, six major brain waves are recognized and distinguished by their different frequency ranges, amplitude, and meaning, i.e., alpha (8–15 Hz), beta (15–30 Hz), gamma (30–90 Hz), delta (1–4 Hz), theta (4–10 Hz), and slow (<1 Hz) waves,7 which underlie various physiological functions and correlate with different behavioral states.6 Neural oscillations can be modulated in space and time like circadian rhythms and are affected by the dynamic interplay between neuronal connectivity patterns, cellular membrane properties, intrinsic circuitry, speed of axonal conduction, and synaptic delays. In particular, slow oscillations, which take place during the behavioral quiescence of non-rapid eye movement (NREM) sleep or in anesthetized states, have been suggested as the default and spontaneously generated activity of the cortical neurons of the brain, which occur both in vitro and in vivo.8 At the cellular level, slow oscillations show spontaneous bistable fluctuations of the membrane potentials between two distinct UP-DOWN states, consisting of periods of membrane depolarization and sustained firing bursts of action potentials, known as active UP state, alternating with membrane hyperpolarization, known as quiescent DOWN state.8 Slow wave oscillations act for a low complexity state that integrates neuronal, synaptic, and connectivity properties of the cortex, providing a robust unifying paradigm for the study of a progressive complexity of cortical functions.9 Moreover, they are widely believed to play a crucial role in memory consolidation, as reported below, and their impairment is known to cause brain disorders.9 Interestingly, in the review of Buskila et al.,6 the contribution of astrocytes, a subtype of glia, has been highlighted in the formation and modulation of brain waves: this phenomenon is mainly ascribed to their close association with synapses that allows their bidirectional interaction with neurons, and their syncytium-like activity via gap junctions that enable the communication to distal brain regions through Ca2+ waves. Consequently, astrocytic alterations likely lead to aberrant modulation of both synaptic transmission and synchronization of network oscillations, which is also accompanied by changes in behavioral performance. Therefore, as well reviewed by Buskila et al.,6 astrocytes are increasingly assumed to play a key role in the propagation of signals between neurons and synchronization of coherently oscillating neuronal groups, but it is still unclear how this mechanism can control UP-DOWN states. Spontaneous activity resembling UP and DOWN states has been also found in cortical slices in vitro, in slabs and in vivo under extensive thalamic lesions, as reported by Jercog et al.,8 suggesting that the UP-DOWN transitions are effectively generated inside the local cortical networks. Such observations validate the importance to perform cultures of primary cortical neuron assemblies for the understanding of synchronous neuronal firing, as proposed in this review, precisely because of their intrinsic property to exhibit synchronous neuronal firings with alternating UP-DOWN states.
Figure 1.
The six major brain waves in the human electroencephalogram
The brain waves are distinguished by their different frequency ranges, amplitude and meaning, in alpha (8–12 Hz), beta (15–30 Hz), gamma (30–90 Hz), delta (1–4 Hz), theta (4–10 Hz), and slow (<1 Hz) waves, reporting the related physiological functions.
Architecture and disorders of sleep
Circadian rhythms and brain waves are in turn involved in sleep-wake processes, which are related to a neural circuitry consisting of the ascending wake-promoting pathway made of projections from various groups of cholinergic, noradrenergic, serotoninergic, dopaminergic, and histaminergic neurons located in the pedunculopontine and laterodorsal tegmental nucleus, locus coeruleus, dorsal and median raphe nucleus, and tuberomammillary nucleus, respectively.10,11 However, every 24 h, the arousal system is inhibited during sleep by sleep-active γ-aminobutyric acid (GABA)-ergic and galaninergic neurons of the ventrolateral preoptic nucleus (VLPO).10,11 The reciprocal inhibitory exchange between the major ascending monoaminergic arousal groups and the sleep-inducing VLPO acts as a feedback loop; when monoamine nuclei discharge intensively during wakefulness, they inhibit the VLPO, and when VLPO fire rapidly during sleep, block the discharge of the monoamine cell groups, functioning like an electrical “on-off” switch, enabling the body to maintain a stable state of wakefulness and sleep.10,11 Sleep consists of the alternation of two different physiological and behavioral states, each of them with distinct electrophysiological features: the rapid eye movement (REM) sleep or “cortical activation” and the NREM sleep or “cortical deactivation”. Specific characteristics of REM/NREM sleep phases depend on the complexity of the CNS; however, there are common shared traits among species, such as specific properties associated with the frequency spectrum of distinct sleep states. In particular, the EEG activity is characterized by two different patterns: slow oscillations in the delta frequency range (1–4 Hz) during the deep sleep (NREM), and rapid, low-voltage waves, such as beta (11–30 Hz) and gamma (30–120 Hz), during wakefulness and REM sleep (Figure 1). There are electrophysiological similarities between awaking states and REM sleep (also defined “paradoxical sleep”), which in an in vivo system are compensated by additional features (e.g., muscle atonia and metabolic/thermoregulatory alterations) that allow their distinction.12 Interesting papers by Gent et al.13,14 investigated the synchronized activity of the thalamo-cortical neuronal networks model of the sleep-wake cycle in mice, where the UP-DOWN states of vigilance, promoted by tonic firing, are alternated to quiescence or sleep, promoted by burst firing.8 During the transition from wakefulness to sleep, cortical neurons begin to undergo slow oscillations in their membrane potential; these oscillations are synchronized by thalamo-cortical circuits and reflected in the slow waves of EEG, characterizing the mammalian slow-wave sleep or delta sleep, following stage 2 of NREM deep sleep, and often propagating as waves traveling from anterior to posterior cortical areas.15 Precisely, these pathways of reciprocal connectivity between cortex and thalamus have been highlighted as responsible for the generation of rhythmic oscillations of the neuronal network involved both in the modulation of sleep and in the integration of sensorimotor information. This thalamic crosstalk between sleep and sensorimotor integration supports the involvement of sleep in fundamental functions such as memory consolidation, brain clearance and plasticity, and energy conservation. Noteworthy attention to the process of memory consolidation is found both in the review of Fields,16 focusing on the pivotal role of specific brain waves oscillating at different frequencies during the various stages of NREM and REM sleep for long-term memory retention, and the review of Gisabella et al.,17 pointing to the involvement of extracellular matrix molecules in synaptic regulation during sleep. In these processes, sleep is involved in changes in strength or efficacy of synapses formed during wakefulness for selective memories, i.e., experiences accumulated throughout the day are reactivated during slow-wave sleep in a process called systemic consolidation,17 where memories are deleted or sorted for short-term storage sites such as the hippocampus, and then transferred to long-term storage in neocortical areas to be integrated into existing knowledge schemas, which can mark them as potentially useful or unnecessary in the future.16,17 Memories are then strengthened in these long-term storage areas during REM sleep, in a process called synaptic consolidation, while the short-term storage memories are removed via synaptic pruning, greatly reducing the signal-to-noise ratio and restoring the capacity to form new synapses.17 To this aim, the activity of millions of neurons in many different regions of the brain must be linked to produce a coherent memory that intertwines emotions, sights, sounds, smells, sequences of events, and other stored experiences. In this way, several neurons are activated together; they send impulses at the same time and strengthen their synaptic connections, where learning takes place. However, the synapses alone store memories of only the most elementary impulses but learning and memory require the coupling of information from many different brain regions, and, consequently, the speed of transmission of impulses is crucial in the synchronization of brain waves.16 Besides, it is proposed that the strengthening of synapses in gray matter (synaptic plasticity) promotes changes in white matter modulating the thickness of myelin (myelin plasticity), an electrically insulating substance produced by oligodendrocytes, that enwraps axons and allows fast propagation of nerve pulses. This dynamic remodeling represents a new form of nervous system plasticity governed by the addition and subtraction of sheaths of myelin, which increases or decreases, respectively, the speed of information transmission through neural networks. Therefore, myelin plasticity becomes an essential partner to the synaptic plasticity in learning and memory consolidation because the proper conduction velocity is necessary to sustain oscillations that couple two regions of the brain at the same frequency.16 As an example of wave coherence in memory consolidation, brain wave activity during sleep couples between the hippocampus and the prefrontal cortex.16 Accordingly, mild disturbances of global sleep architecture or local sleep oscillations have dramatic consequences on behavioral sleep-wake instability and related disorders, of which insomnia is the most common, but obstructive sleep apnea, parasomnias, narcolepsy, restless leg syndrome, endocrine and metabolic homeostasis, as well as mood and cognitive performance are also included.14,18 At the same time, slight changes of network oscillations during sleep are increasingly recognized as being associated with early stages of neurological (e.g. Alzheimer and Parkinson diseases) and neuropsychiatric (e.g. major depression and schizophrenia) disorders.14 Besides, an overlap of dysregulation of circadian rhythms has also been well reported for all sleep, psychiatric, and neurodegenerative disorders.4 Consequently, the impairment of memory consolidation due to sleep deprivation could first involve the failure of synaptic plasticity, thus failing to reactivate or reproduce the wake-active neurons.17,19 A worsening of the circadian regulation of extracellular matrix molecules, as critical molecules in determining synaptic activity for memory consolidation during sleep, is probably involved.17 Subsequently, a weakening of myelin plasticity occurs, through the enlargement of the Ranvier nodes and thinning of the myelin, maybe thanks to impairment of the astrocytic peripheral processes moving and expanding close to the synapses in response to sleep loss and circadian rhythms dysfunction.20
Interactions between circadian and brain oscillations in sleep
Neuronal oscillations are used to coordinate brain activities in conjunction with the alternation of light and dark. In addition to the entrainment of SCN, the gene expression rhythms of the functions of most brain regions are controlled by local circadian clocks that integrate them into the daily rhythm of sleep and wakefulness. Moreover, the SCN coordinates these local clocks to ensure an optimum brain function, which otherwise can be compromised in the absence of its effective input.21 Circadian signals in vivo are known to affect the specific thalamic structures implicated in the generation of brain waves, although the exact mechanism remains to be clarified. The modulation of these healthy functional networks occurs over approximately 24 h and it is strong and repeatable.21 Focusing on such temporal periodicities may improve the physiological interpretation and use of functional connectivity analysis to investigate brain function in health and disease.22
Synchronization or phase locking between separate brain regions is measured by coherent oscillations among groups of neurons; coherence is a mathematical method useful to determine if two or more brain regions have similar neuronal oscillatory activity with each other. Coherence or synchronized neuronal oscillations has been proposed as a mechanism by which neurons can selectively communicate with one another, through waves traveling along the brain. As an example, successful memory encoding has been linked with theta coherence between hippocampus and medial prefrontal cortex and theta coherence between hippocampus and striatum has been correlated with acquisition of a navigation task; likewise, associative learning is correlated with gamma coherence over the parieto-temporo-occipital associative cortex.23 In this sense, the circadian modulation of coherence could represent the modulation of communication between neuronal populations (see Figure 2). This is consistent with several experimental observations, according to which entrainment by a specific synchronizer is not limited to a single neuronal network but is instead detected across a distributed network of brain structures.23 Therefore, a major challenge will therefore be to separate the coordinated link between SCN timing, local clock functions, and sleep/wakefulness. In this perspective, Herzog et al.21 proposed an intriguing outlook of the entire nervous system as a temporally resonant system, pre-tuned to oscillate on a circadian basis, with each brain region locked into a pattern that complements and supports those of other regions. As a result, all events affecting this circadian organization of neuronal networks are expected to disturb the normal brain function, in terms of sleep-dependent altered clearance of neurotoxic aggregates or in terms of psychiatric diseases.21 In this sense, the importance of the clock genes expression in regulation of homeostatic changes in NREM sleep time after sleep loss has been demonstrated in sleep-deprived, double knockout mice for Cry1/Cry2, which showed greater EEG delta power (tracking sleep need over time) together with a potentiation of Per1 and Per2 genes expression at the level of cortical neurons in comparison to the wild-type mice during recovery sleep.24 Otherwise, mutant mice for Per1/Per2 showed relatively subtle effects on post-sleep deprivation EEG delta power.24 Considering that Per2 expression in the brain responds to both sleep loss and time of day, and that a significant crosstalk between Per2 and delta power has been demonstrated,25 Per2 can be deemed the elective clock gene to keep track of and to anticipate homeostatic sleep need.25
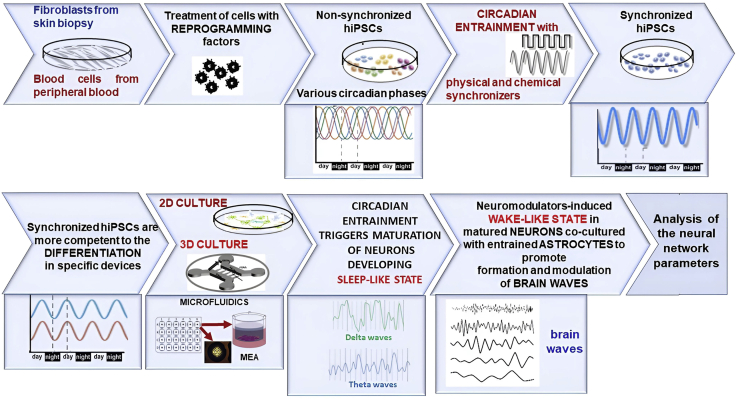
Figure 2.
Flow chart of the proposed relationship between circadian rhythms, sleep/wake cycle and brain waves in neural networks in vitro
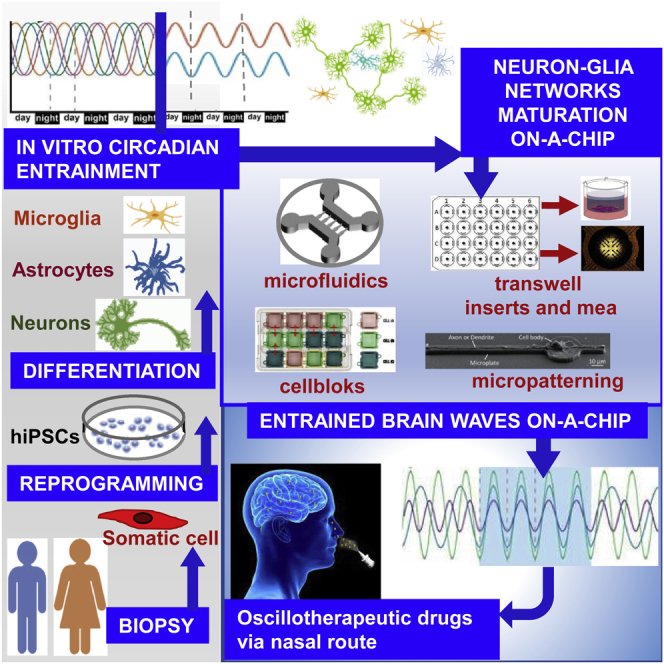
Proposed relationship was designed in 5 phases consisting of (i) fibroblasts or blood cells isolation from patient-derived skin biopsies or peripheral blood; (ii) appropriate reprogram of these human somatic cells into hiPSCs growing in culture at the non-synchronized circadian state; (iii) entrainment of the hiPSCs culture to the circadian synchronized state by treatment with physical (temperature cycles, mechanical stimuli) or chemical synchronizers (serum shock, forskolin, and glucocorticoids) as reviewed by Xie et al.4; (iv) differentiation of the hiPSCs into different cell types of the brain (neurons, astrocytes, microglia, and oligodendrocytes) grown in devices for 2D and 3D cultures; (v) circadian entrainment to trigger maturation of the neural networks. Development of the neural network involved neuromodulators, neurotransmitters, or electrical stimulation to induce sleep-like state switching wake-like state together with entrainment of co-cultured astrocytes to induce and modulate brain waves, such as cholinergically induced gamma oscillations.6,26,27 Synchronized measurement of neuronal activity from multiple electrodes at different locations across the neural co-culture on 3D devices allows the analysis of the functional connectivity in the neuronal network.
Topics of the review
Based on this background, this review focuses on the main experimental conditions that could reproduce in vitro the circadian regulation of brain waves, which could be measurable in entrained neural culture systems, that reflect the physiological response in vivo more closely. The process of entrainment of cerebral oscillations is also of growing interest as a therapeutic tool and/or target, hence various neurological and psychiatric disorders can indeed be conceptualized as specific “oscillopathies”,18 due to an altered synchronization of neuronal oscillation in key brain networks.5 In this regard, the modulation and restoration of cortical oscillations are also recently defined as “oscillotherapeutics”, that is network therapeutic,28 as an example by means of noninvasive neurostimulation technique, such as transcranial magnetic stimulation.29 For the oscillopathies, the patient-derived hiPSCs are becoming widespread preclinical models.5 Indeed, the ability to generate virtually any cell type by differentiating in vitro hiPSCs is particularly relevant in the field of neuroscience, due to the limited access to primary cells from the human CNS. This model could be also useful to elucidate the genetic basis of oscillopathies, allowing the integration between brain waves and gene expression both measured in the same patient. Indeed, patient-derived hiPSCs reprogrammed in specific neuronal networks could be placed on multi-electrode array (MEA) systems, a powerful electrophysiological tool for recording and stimulation of neural oscillations in response to certain stimuli or perturbations similar to electrocorticography (ECoG) and EEG,30 so that brain waves could be recorded immediately before the same neuronal networks are processed by technology of spatially resolved transcriptomics, to obtain general gradients of gene expression on intact neuronal network. A similar application has been proposed in brain slices deriving from neurosurgical resections,31 but we believe that the use of hiPSCs sourced from peripheral blood or skin fibroblasts and differentiated into neuronal networks or organoid cultures could first and foremost be less invasive for the patients, allowing a more direct patterning between brain waves and their underlying genes and providing an unlimited proliferation potential. Therefore, our proposal on relationship between circadian rhythms, sleep/wake cycle, and brain waves in neural networks in vitro is represented in Figure 2, where the entrainment of the circadian clock genes can be a valid tool for modulating synchronization between neuronal firing patterns, in principle through the induction of a coherent UP-DOWN state among the cortical neurons differentiated by the engineering of hiPSCs-derived brain cells in culture. This system may become a prototypic and predictive in vitro model for personalized and precision neuromedicine, to study the sleep-wake state-related dysfunctions, mainly represented by the insomnia, which can be the onset or indicator of neurodegenerative diseases.18
In vitro entrainment of rhythms and waves in 3D cell cultures
Compared to in vivo studies, the in vitro experiments are easier to control and relatively better in terms of duplicating results. As concerns the in vitro neuronal circuits, they are not same as the in vivo ones, but in vitro circuits also generate diverse activities, such as various synchronized bursting pattern dynamics or complex oscillatory behavior.32 Considering the circadian rhythms under typical culture conditions (obscurity, 37 ± 1°C, 5%–7% CO2), freshly explanted peripheral cells show chaotic or desynchronized expression of clock genes for 12–14 h and loose coordinated oscillating patterns of clock components.3 In particular, each cell follows its own rhythm, due to the absence of the appropriate synchronizer external signals usually acting as entrainments in vivo and consequently lacking endogenous humoral factors and or neuronal signals of SCN. Common methods used for in vitro synchronization of circadian rhythms have been extensively reported by Xie et al..4 Therefore, in cultured cells or tissue explants, synchronizers are external physicochemical stimuli, like thermic oscillations, exposure to high concentrations of serum for 2 h or to 1 μM dexamethasone for 1 h, or a single medium exchange, able to re-induce molecular oscillations of clock genes. Interestingly, due to the parallel increased expression of Per1 and Per2 genes reported in vitro by high concentrations of serum stimulation in cultured fibroblasts and immortalized cell lines33 or in vivo by light stimulation in SCN or by sleep deprivation in cortical neurons of cryptochrome-deficient mice,24,25 the serum shock may be a useful in vitro synchronizer to investigate the mechanisms underlying circadian clock gene expression in the sleep-deprived cultured cortical neurons. Centrifugal washing and serum starvation are used also to synchronize all the cultured cells in the same phase of the cell cycle.4 A synchronized circadian rhythm in a cell culture system can improve the physiological relevance of an appropriate in vitro method by reflecting more closely the biological in vivo situation.34 Somatic cells are known to remain entrainable by external cues in culture depending on their tissue origin and on the composition of the medium.3 However, it must be pointed out that a single application of the synchronizer can entrain the expression of clock genes in a cell population, but with a rapid dampening effect.3 The application of the synchronizer is supposed to be repeated with a circadian periodicity to entrain the oscillator system of cultured cells. The duration of application of a synchronizer depends on the entrainment capacity of the oscillator, i.e., its intrinsic period and distribution around 24 h and it is between 1 and 2 h after in vitro experiments on SCN neurons.3 Primary cultures of neurons derived from the SCN, on the contrary, retain in vitro the central circadian rhythm in constant conditions and in the absence of external entraining cues,3,21 as a prototypical example of a reciprocal addiction relationship between the molecular clockwork and rhythmic electrical activity.21 Indeed, the best characterized neuronal cell line is the immortalized SCN2.2 neurons of rat SCN for the central clock.3 Otherwise, cells of normal or cancerous phenotypes established in culture hold low levels of inactive peripheral clock genes until new synchronizer signals are repeated. In most of the experiments, non-neuronal cells are usually cultured without exposition to any neuronal signals, which can be supplied by means of a co-culture with SCN2.2 neurons to re-entrain circadian oscillations of clock components, as reported by Herzog et al..21
As regards the in vitro studies of brain waves in neuronal cells, most of them are aimed to the modeling of brain network disorders such as epilepsy.35 An interesting input for entrainment of brain waves has been performed by Odawara et al.,36 using a co-culture of hiPSC-derived neurons with astrocytes, where a convulsive firing enhancement of the high gamma and beta waves component was remarkably observed, confirming the prominent contribution of astrocytes in development and synchronization of brain waves also in vitro, as reviewed by Buskila et al.,6 and investigated by Makovkin et al.26 as cholinergically induced gamma oscillations by astrocytic modulation in a model hippocampal neural network. Moreover, Nieus et al.37 investigated the ability of rat hippocampal neural networks in vitro to process external stimuli in a state-dependent manner, by manipulating the cell cultures with the neurotransmitter norepinephrine, known to decrease the network synchrony both in vivo and in vitro, and MEA recordings. On the other hand, in vitro studies on brain waves in 2D neuronal cell cultures may be limited by the difficulty of reproducing the properties of the underlying brain wave network and key differences may result comparing cultures of dissociated cortical neurons with in vivo models or with neurons cultured in vitro with more preserved architectures.27 Indeed, 2D neuronal models are limited to side-by-side contact, lacking a 3D microenvironment and the relevant interactions between the cells and the surrounding extracellular matrix. However, pioneering studies carried out by the Tafti group38 and by Colombi et al.12,27 on spontaneous and pharmacologically manipulated 2D neuronal culture activities, in terms of brain waves linked to the sleep/wake cycle, will be discussed in the following section. These authors claimed to artificially “drive” the connectivity of neurons within the cultured network, as cell cultures are not necessarily random networks, but their versatile neuronal growth can be manipulated to develop networks with different topologies, such as a 3D cytoarchitecture. In this regard, an important step was taken by Trujillo et al.,39 demonstrating that in addition to synchronized firing, cortical organoids grown from hiPSCs also displayed delta and gamma waves on MEAs, as better described in the sections below.
In vitro experimental models recapitulating the essential features of sleep
The investigation of the neural network’s dynamical proprieties during sleep states is difficult while studying in vivo experimental systems. Interestingly, recent data from several different laboratories demonstrated that primary murine cortical neuronal cultures are usually characterized by a sleep-like state, i.e. synchronized, low-frequency firing patterns similar to the in vivo slow wave oscillations of NREM sleep, under spontaneous condition.12,38,40 In particular, since cortical cultures spontaneously lack sleep-wake rhythm that occurs in in vivo systems, Colombi et al.12 presented electrophysiological and genetic evidence that primary rat cortical cultures are able to encompass some essential features of sleep in a controllable way, mimicking wake-like states by the application of an agonist of acetylcholine receptor, carbachol, which caused a desynchronization of the spontaneous firing of the cultures. This study demonstrated that wake-like and sleep-like electrophysiological signatures can be dissociated in vitro and possibly studied independently within the same experimental model. Furthermore, they demonstrated that carbachol alters specific sleep-like signatures, such as delta and theta waves, more than beta and low gamma waves, typically present during wakefulness and REM sleep. Interestingly, to change the synchronized default sleep-like state into an awake-like firing state, Hinard et al.38 stimulated mouse embryonic primary cortical neurons with a cocktail of excitatory neurotransmitters, known to be upregulated with wakefulness, such as NMDA, AMPA, kainate, ibotenic acid, serotonin, histamine, dopamine and noradrenaline, carbachol, and orexin, proposing this in vitro model as an ideal approach in the pharmacology of sleep-wake states, whose cellular and molecular consequences can be investigated in a dish. Along this line, Colombi et al.27 proposed the addition of a cocktail of drugs, instead of carbachol alone, to their experimental design in order to better resemble the landscape of frequency oscillations observed in the awake human brain, together with a more intriguing possibility to artificially “drive” the connectivity of the cells within the cultured network. Rockstrom et al.41 highlighted this in vitro experimental approach reporting that co-cultures of dispersed neurons and glia spontaneously display a sleep-like default state as determined by the burstiness of neuron action potentials; following treatment with excitatory neurotransmitters or amino acids or electrical stimulation, a reversal to a more wake-like state has been observed. Moreover, these cultures develop the sleep-like state over the course of about 10 days as neurons make reciprocal connections and the properties of the emerging network become manifest; however, they revert to a more intense spontaneous sleep-like state the next day after a stimulus-induced wake state, such as a prolonged electrical stimulation, and thus indicating that sleep homeostasis also occurs in vitro.41,42 To this purpose, it must be considered that the current models for the cellular mechanisms in the primate cerebral cortex rely on extrapolating from a large body of work on rodents, primarily mice, cortical neurogenesis. However, the cortex of humans and other primates appears to follow different scaling rules than that of other mammals, including mice and rats, in terms of the relationship between cortical volume and cell number and overall body size. Human stem cell systems have been performed to study cerebral cortex neurogenesis in vitro, finding that the directed differentiation of human iPSCs to cerebral cortex progenitor cells strongly repeats the temporal order of cortical neurogenesis, including the production of the various progenitor cell types found in vivo.43 Since this mechanism for controlling cortical size is cell independently regulated, in vitro stem cell systems of cortical development provide experimental platforms to identify the relevant cellular mechanisms.43 However, it is known that maintaining long-term cell adhesion appears to be an experimental challenge in post-mitotic neuronal cultures, particularly for human neurons, which require significantly longer periods to differentiate, mature, and establish synaptic circuits in vitro in comparison to the animal primary neurons.44 Therefore, drawbacks as detachment and aggregation are common for human neurons requiring a long timeline in culture. Therefore, disadvantages such as detachment and aggregation are common for human neurons that require long timeline in culture. For this reason, in vitro 3D cell culture devices implemented with suitable extracellular matrix types, described below, can help counteract the above drawbacks. As an early example, plasma diaminopropane polymer treatment with a laminin-based coating of cell cultureware has been reported to provide the optimal microenvironment for long-term neuronal adhesion and functional maturation, improving models of human brain diseases frequently troubled by cell detachment for prolonged periods in culture.44
Our proposal is that the experimental conditions indicated so far can be merged to the nervous system model of Herzog et al.21 reported above, which is temporally resonant and oscillating on a circadian basis; in this way, a good in vitro model of sleep-wake cycles based on circadian entrainment of brain waves can be performed in isolated cortical neurons, murine12 and subsequently human, cultured in the devices listed below.
In vitro 3D cell culture devices: Transwell inserts, microfluidic systems, and micropatterning
Transwell inserts represent a versatile tool to combine cell types such as neurons and astrocytes in 3D co-culture approach (Table 1), separating an apical from a basolateral compartment.45 Usually, neurons are seeded in the lower or basolateral compartment of the multiwell transwell system, whereas astrocytes are cultured in the apical side of the insert. Therefore, the two cultures can be physically separated even if they shre the same medium, so that the impact of neurons, astrocytes, and soluble molecules can be separately analyzed, thus creating a powerful tool for neuron-astrocytes interaction studies,45 related to the role of astrocytes in synchronization of brain waves.6 As an alternative platform to study the bidirectional interaction of astrocytes with cortical neurons, the Circulatory Blocks of CELLBLOKS technology of Revivocell46 can be employed (Table 1), a multi-organ/cell type modular “plug and play” co-culture technology that emulates the organ microenvironments in a standard in vitro setting.46 We think that this system, firstly developed for the liver cells,46 may be suitable to address the formation of a tripartite synapse, consisting of the pre-synaptic terminal, the post-synaptic membrane, and the supporting astrocyte, as reported by Buskila et al.,6 which would allow the bidirectional interaction of astrocytes with cortical but also SCN neurons, cultured into different compartments, and to study how they interact and influence synapse formation for in vitro modeling of memory consolidation. As in Transwell inserts, in this system, cells communicate through media exchange and without physical interaction.
Table 1.
Current in vitro systems applicable to study the interaction between circadian rhythms and cerebral waves in the brain neural cells
| Physiological system to study | Type of in vitro system | Description of the in vitro system | Key features | Reference |
|---|---|---|---|---|
| Neuron-astrocytes bidirectional interactions: role of astrocytes in the synchronization of brain waves and synapses formation. | Transwell insert | Astrocytes are cultured into an insert (apical comp) and the neurons are seeded into the lower well (basolateral comp). | Astrocytes and neurons are in communication through the media exchange between the apical and basolateral comps without a physical interaction. | Buskila et al.6; Gottschling et al.45 |
| Circulatory Blocks™ CELLBLOKS® technology of Revivocell (Daresbury Lab, Cheshire, UK) | Multi-organ/cell type modular “plug and play” co-culture technology that emulates an organ microenvironment. Neurons and astrocytes are co-cultured in different comps. | Neurons and astrocytes form a tripartite synapse (pre-synaptic terminal, post-synaptic membrane, and cradling astrocytes) without a physical interaction. | Llabjani et al.46 | |
| Study and selective manipulation of an isolated neuronal networks. | Microfluidic systems | Multi-compartmentalized microfluidic device made of two parallel microfluidic comps separated by microgrooves. Neurons or NSCs are monocultured in the somato-dendritic comp. Cortical-cortical or cortical-thalamic neurons are co-cultured in different comps. |
Neurons grow and extend their axons though the microgroove regions, which allow to guide the axonal growth, into an adjacent, isolated axonal comp. | Paranjape et al.47 |
| The cortical region is the site of initiation of burst firing events, whereas thalamo-cortical connections are needed to maintain a prolonged synchronized bursting pattern in the cortical cells culture. | Kanagasabapathi et al.48; Kanagasabapathi et al.49 | |||
| Models of neuronal circuits, blood–brain barrier and neurovascular unit in microfluidic systems. | Advanced microfluidic technologies for neurological disease research. | Holloway et al.50 | ||
| Neuronal network communication and synchronization. | Micropatterning | Neurons are seeded on a micro-patterned device that can create a predefined neural network. | The geometry of the device induces a predefined neuronal connectivity and functional polarity, guiding the signal propagation among neurons population. The presence of astrocytes and extracellular matrix influence the excitability and synchronous activity of neurons. | Albers and Offenhäusser51 |
| Early neurodevelopment, from neuroepithelial formation to assembly of rudimentary network and study of brain waves. | Cerebral organoids | Brain spheroids composed of a mixed population of neurons and glial cells from iPSC-derived NSCs. | Organoids contain dozens of ventricles lined with radial glia/progenitors that differentiate into cortical and mature glia. Cytoarchitectonic development typical of a human brain with complex functional activity (coordinated electrical activity). | Guy et al.52; Govindan et al.53; Marton and Pașca54 |
| Human brain cortical organoids. | Dynamic changes in cell population and increases in electrical activity. Spontaneous formation of neuronal networks with periodic and regular oscillatory events (GABAergic signals). | Trujillo et al.39 | ||
| Co-culture of brain organoids with endothelial cells or organoids containing engineered cells with ETV2. | ETV-2-expressing population contributed to vascularization, leading to enhanced functional maturation with BBB characteristics. Uniform size, morphology, and synchronized differentiation are the main factors to be considered. | Kim et al.55 |
comp(s), compartment(s); NSC, Neural stem cell; iPSC, induced pluripotent stem cell; BBB, blood–brain barrier.
The use of microfluidic devices (Table 1) to compartmentalize cultured neurons is another method that has become standard in neuroscience.47 Multi-compartmentalized microfluidic devices consist of two parallel microfluidic compartments separated by microgrooves, which guide axonal growth, enabling measurements and manipulations of long-projection neurons with unique subcellular access. Neurons or neural stem cells are plated in the somato-dendritic compartment and then adhere to the bottom of the compartment surface after a few minutes. Differentiated neurons grow and extend their axons through the microgrooves region into an adjacent, isolated axonal compartment.47 In vitro co-culture of cortical and thalamic-dissociated neurons cultured in dual-compartment devices with microfluidic separation have recently been performed,48 providing sufficient flexibility in selectively manipulating an individual region of pharmacological interest.49 Indeed, unlike in vivo conditions, where multiple neuronal pathways influence any recorded region, isolated networks can be studied in a controlled and isolated environment. The interesting result of this research49 was that in microfluidics cortical-cortical co-cultures, a particular compartment acts as a network-wide burst leading compartment, and plays a major role in regulating the network bursting of both compartments, while in cortical-thalamic co-cultures, the compartment with cortical cells always acts as a network burst leading compartment, controlling the spread of network bursts to the thalamic compartment. These findings confirmed that the cortical region is the site of initiation of burst firing events, while reciprocal thalamo-cortical connections are required to maintain a prolonged synchronized bursting pattern in the cortical culture.48,49 A broad and specialized framework of current state of the art in microfluidic technologies for neurological disease research has been recently provided by Holloway et al..50 This latter review discussed challenges, limitations, and troubleshooting of microfluidic systems for early adopters, but also outlining the benefits and potential of integrating technologies, such as hiPSC-derived cortical neurons, which result highly similar to primary cell neurons and with extended time in culture.
Finally, it should be emphasized the micropatterning method as a further highly reproducible technique to devise neuronal networks in vitro with a predefined connectivity induced by the design of the gateway, producing neuronal networks with predefined connectivity and accompanied functional polarity, which is essential for the formation of these networks (Table 1). By this method, the direction of signal propagation among populations of neurons is guided, thus providing insights to network communication, and allowing to investigate more about learning processes by external manipulation of neural networks and signal cascades.51 In this method, it is remarkable that, independently from the orientation of signal propagation at the gateway, a critical number of cells are required for synchronized spontaneous activity, with a different cell density resulting in an unsynchronized firing pattern, which strongly impacts the signal propagation and excitation among populations of neurons.51 Moreover, directional neuronal outgrowth can be induced by the substrate-bound protein pattern geometry, the connectivity between adjacent populations of neurons, and the resulting direction of signal propagation among populations. Circadian rhythms and brain waves are modulated by extracellular interactions, including proteases and cell adhesion molecules. Astrocytes and the extracellular matrix are also likely to play key regulatory roles. Actually, the excitability and synchronous activity of neurons can be modulated by extracellular mechanisms, which affecting the ionic composition of the extracellular fluid, thus impact on the different brain rhythms.56 Unfortunately, the degree of overlap in the extracellular mechanisms of mammalian circadian and sleep-wake systems is not yet known and incoming studies on these dynamic extracellular processes are needed.56 Furthermore, choice of extracellular matrix used for neuronal cell culture in vitro is directly related not only to the degree of neuronal development but also neuronal function. Indeed, as an example, growth and distribution of neurons have been found different on Matrigel compared to poly-D-lysine, and this difference also alters their functionality in terms of firing threshold and sodium and potassium channel protein expression.57 Finally, micropatterning-based geometric cell confinement provides pioneering templates to investigate cellular function and fate, which are under entrainment of the circadian rhythms, as reported by Chaix et al.12
Cerebral organoids
Cerebral organoids are 3D models of in vitro brain spheroids, composed of a mixed population of neurons and glial cells, generated from human iPSC-derived neural stem cells, which have been widely reviewed by Guy et al.52 and Govindan et al.53 As also reported by Marton and Pașca,54 brain organoids can be summarized as a mixture of cell types, intercellular interactions, and microenvironments that mimic early neurodevelopment from neuroepithelial formation to assembly of rudimentary networks. Each organoid contains dozens of ventricles lined with radial glia/progenitors that differentiate into cortical neurons and mature glia. Therefore, since in vitro human cerebral organoids can recreate the cytoarchitectonic development typical of a human brain and manifest a complex functional activity, such as a coordinated electrical activity, they may be further developed and indicated for medical research and patient care in the sleep/wake cycle-associated disorders. In this purpose, we consider as a milestone of in vitro brain wave reproduction the research work of Trujillo et al.,39 which developed human brain cortical organoids showing dynamic changes in cell populations during their maturation in vitro together with consistent increases in electrical activity during several months in culture. The spontaneous formation of neuronal networks showed periodic and regular oscillatory events generated and sustained by glutamatergic and GABAergic signals. Noteworthy, the oscillatory activity switches to more irregular spatiotemporal patterns and the synchronous network events showed similar features to those observed in EEGs of human preterm newborns. However, it should be emphasized that a consistent limit of brain organoids is they do not contain functional vasculature, so a direct approach is to co-culture brain organoids with endothelial cells, or as an alternative, organoids containing engineered cells with transcription factor for endothelial lineage such as ETV2 could be controlled in an inducible manner. Notably, the ETV2-expressing population within organoids contributed to vascularization, leading to enhanced functional maturation with blood–brain barrier characteristics. Moreover, heterogeneous cellular responses to patterning signals might contribute to asynchronous differentiation, leading to organoid variability. Therefore, during organoid generation, various factors should be considered, such as uniform size and morphology, and synchronized differentiation.55
Milestone regulatory substances entraining neuronal excitability and sleep/wake cycle
As reviewed by Re et al.,58 melatonin is the main hormone integrating circadian rhythms and sleep/wake cycles, by directly regulating neuronal excitability. These authors address to the anticonvulsant activity of melatonin, highlighting the relationship between its mechanism of action through receptor-linked G protein-gated inwardly rectifying potassium (GIRK) channels and neuronal excitability related to seizures and epilepsy as sleep-related phenomena. In particular, melatonin receptor-linked GIRK channel activation, regulating resting membrane potential via hyperpolarization in neuronal populations of SCN and hippocampus, leads to a phase advance (early sleep), and both GIRK2 protein expression and current amplitude oscillate over the sleep/wake cycle. Melatonin has also been proved to be a synchronizer of protein synthesis oscillation in cell culture in vitro.59
Neuropeptide Y (NPY), released by thalamic neurons, is another important neurotransmitter that was found linking circadian rhythms and neuronal excitability, inducing phase advances in the SCN, that are accompanied by suppression of the clock gene PER2 and are mediated by long-term depression of neuronal excitability in a phase-specific manner.58 Cytosolic signaling by cAMP and intracellular calcium ([Ca2+]i), which are both outputs from and inputs to the intracellular TTFL, as well as a critical role played by the vasoactive intestinal peptide (VIP) signaling in synchronizing cellular clocks across the SCN have to be also considered.60 Synchronization by VIP in the SCN is paracrine, operating over an unconventionally long-time frame (i.e. 24 h) and wide spatial domain, mediated via the cytosolic pathways upstream of the TTFL.60 A primary purpose of cellular oscillators and intercellular waves of Ca2+ appears to be related to intercellular communication, and to increased cell synchrony. In this regard, the incorporation of this type of information could be of potential use in an area of the brain involved in timekeeping, and integration of astrocytes may lend greater stability to an oscillator based on rapidly responding neurons and astrocytes that show a slower response. Different regions of the brain appear to use similar physiological mechanisms, ion channels, and transmitters to perform different functions, in large part based on specific complex circuitry.61
At the end of this paragraph, we would pay attention to the reported somnogenic effect of the pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α) showed in cultured cortical neurons of mice,42 and the reverse signaling of transmembrane and soluble forms of TNF-α receptors inducing a wake-like state in vitro observed in the same neurons.62 In this regard, we would underline that sleep-wake cycle is also induced by an agent normally involved in inflammatory processes, thus leading us to think that sleep may in turn be an anti-inflammatory process, and that consequently insomnia could support neuroinflammation.
Conclusions and future directions
In this review, we aimed to highlight the potential in vitro models and methods for entrainment of ongoing neuronal activity in cell culture to the external circadian rhythmic inputs, to mimic what happens in sleep/wake rhythm in vivo. Citing also Lakatos et al.,63 which comprehensively reviewed the ubiquitous presence of entrainment in the brain and underlined the possibility to explore the neuronal entrainment by rhythmic neuromodulation, we suggest the goal of using entrainment as an experimental or therapeutic tool targeting several and so-called oscillopathies.63 Therefore, developing of in vitro neuronal cultures that incorporate key constituents of the CNS, such as specific types of micro-patterned extracellular matrices and supporting glial cells, such as astrocytes, is aimed to recapitulate in vivo morphology, physiology, and function more accurately, which may lead to more successful translation from bench to clinics. For example, we could hypothesize to build a valid predictive and preclinical model of personalized neuromedicine reproducing the sleep/wake rhythm in vitro, a microfluidic or Cellbloks system, where hiPSCs reprogrammed into SCN, thalamic, and cortical neurons are co-cultured in combination with astrocytes and treated with melatonin, NPY, TNF-α, and other neuromodulators proposed in the studies by Colombi et al..12,27 The in vitro neural systems, due to their simplicity, their state characterization, and their ability to control the intensity of emergent state properties, propose innovative experimental platforms that provide important “early” information on the mechanisms underlying sleep and wake states and, perhaps, they could even be applied to clarify the elusive issue of sleep function.
We conclude that sleep and wake states can be modulated in cultured in vitro networks, which share molecular regulatory mechanisms with local in vivo networks. In our view, the results of all in vitro studies reviewed here may take on translational clinical significance when implemented by nanoformulations, such as functionalized nanoparticles for both drug delivery and to lead to action potential activation in the CNS,64 targeting the suitable compounds tested in preclinical in vitro models for neuromodulation through the intranasal delivery route.65,66,67 The intranasal delivery of potential “oscillotherapeutic” drugs in CNS by means of nanoformulations ad hoc could circumvent or at least improve both the transcranial magnetic stimulation, a noninvasive therapeutic, but lacking targeting precision and adequate penetration when deep subcortical structures are concerned, and deep brain stimulation, a more potent but invasive surgical procedure that carries the potential risk of complications.68
Acknowledgments
Funding: this manuscript was funded by Fondo di Ateneo per la Ricerca (FAR) 2021 from the University of Ferrara, Italy.
Author contributions
B.P. conceived and wrote the first draft and G.B. and A.B. revised it for intellectual content. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Chaix A., Zarrinpar A., Panda S. The circadian coordination of cell biology. J. Cell Biol. 2016;215:15–25. doi: 10.1083/jcb.201603076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S., Nam H.G., Kim Y. The core circadian component, Bmal1, is maintained in the pineal gland of old killifish brain. iScience. 2021;24:101905. doi: 10.1016/j.isci.2020.101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaeffer B., Pardini L. Clock genes of mammalian cells: practical implications in tissue culture. In Vitro Cell. Dev. Biol. Anim. 2005;41:311–320. doi: 10.1007/s11626-005-0001-7. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y., Tang Q., Chen G., Xie M., Yu S., Zhao J., Chen L. New insights into the circadian rhythm and its related diseases. Front. Physiol. 2019;10:682. doi: 10.3389/fphys.2019.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran J., Anastacio H., Bardy C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. NPJ Parkinsons Dis. 2020;6:8. doi: 10.1038/s41531-020-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buskila Y., Bellot-Saez A., Morley J.W. Generating brain waves, the power of astrocytes. Front. Neurosci. 2019;13:1125. doi: 10.3389/fnins.2019.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byron N., Semenova A., Sakata S. Mutual interactions between brain states and Alzheimer's disease pathology: a focus on gamma and slow oscillations. Biology. 2021;10:707. doi: 10.3390/biology10080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jercog D., Roxin A., Barthó P., Luczak A., Compte A., de la Rocha J. UP-DOWN cortical dynamics reflect state transitions in a bistable network. Elife. 2017;6:e22425. doi: 10.7554/eLife.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Vives M.V., Massimini M., Mattia M. Shaping the default activity pattern of the cortical network. Neuron. 2017;94:993–1001. doi: 10.1016/j.neuron.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Eban-Rothschild A., Appelbaum L., de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–952. doi: 10.1038/npp.2017.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Egroo M., Koshmanova E., Vandewalle G., Jacobs H.I.L. Importance of the locus coeruleus-norepinephrine system in sleep-wake regulation: implications for aging and Alzheimer's disease. Sleep Med. Rev. 2022;62:101592. doi: 10.1016/j.smrv.2022.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombi I., Tinarelli F., Pasquale V., Tucci V., Chiappalone M. Simplified in vitro experimental model encompasses the essential features of sleep. Front. Neurosci. 2016;10:315. doi: 10.3389/fnins.2016.00315. Erratum in: (2016). 10, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gent T.C., Bandarabadi M., Herrera C.G., Adamantidis A.R. Thalamic dual control of sleep and wakefulness. Nat. Neurosci. 2018;21:974–984. doi: 10.1038/s41593-018-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gent T.C., Bassetti C.L., Adamantidis A.R. Sleep-wake control and the thalamus. Curr. Opin. Neurobiol. 2018;52:188–197. doi: 10.1016/j.conb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Timofeev I., Schoch S.F., LeBourgeois M.K., Huber R., Riedner B.A., Kurth S. Spatio-temporal properties of sleep slow waves and implications for development. Curr. Opin. Physiol. 2020;15:172–182. doi: 10.1016/j.cophys.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields R.D. The brain learns in unexpected ways: neuroscientists have discovered a set of unfamiliar cellular mechanisms for making fresh memories. Sci. Am. 2020;322:74–79. [PMC free article] [PubMed] [Google Scholar]
- 17.Gisabella B., Babu J., Valeri J., Rexrode L., Pantazopoulos H. Sleep and memory consolidation dysfunction in psychiatric disorders: evidence for the involvement of extracellular matrix molecules. Front. Neurosci. 2021;15:646678. doi: 10.3389/fnins.2021.646678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy J.F., Abbott S.M., Burgess H.J., Crowley S.J., Emens J.S., Epstein L.J., Gamble K.L., Hasler B.P., Kristo D.A., Malkani R.G., et al. Workshop report. Circadian rhythm sleep-wake disorders: gaps and opportunities. Sleep. 2021;44:zsaa281. doi: 10.1093/sleep/zsaa281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seibt J., Frank M.G. Primed to sleep: the dynamics of synaptic plasticity across brain states. Front. Syst. Neurosci. 2019;13:2. doi: 10.3389/fnsys.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vivo L., Bellesi M. The role of sleep and wakefulness in myelin plasticity. Glia. 2019;67:2142–2152. doi: 10.1002/glia.23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog E.D., Hermanstyne T., Smyllie N.J., Hastings M.H. Regulating the suprachiasmatic nucleus (SCN) circadian clockwork: interplay between cell-autonomous and circuit-level mechanisms. Cold Spring Harb. Perspect. Biol. 2017;9:a027706. doi: 10.1101/cshperspect.a027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith R.J., Alipourjeddi E., Garner C., Maser A.L., Shrey D.W., Lopour B.A. Infant functional networks are modulated by state of consciousness and circadian rhythm. Netw. Neurosci. 2021;5:614–630. doi: 10.1162/netn_a_00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munn R.G.K., Hardcastle K., Porter B., Bilkey D. Circadian-scale periodic bursts in theta and gamma-band coherence between hippocampus, cingulate and insular cortices. Neurobiol. Sleep Circadian Rhythms. 2017;3:26–37. doi: 10.1016/j.nbscr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolsius Y.G., Zurbriggen M.D., Kim J.K., Kas M.J., Meerlo P., Aton S.J., Havekes R. The role of clock genes in sleep, stress and memory. Biochem. Pharmacol. 2021;191:114493. doi: 10.1016/j.bcp.2021.114493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curie T., Mongrain V., Dorsaz S., Mang G.M., Emmenegger Y., Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36:311–323. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makovkin S., Kozinov E., Ivanchenko M., Gordleeva S. Controlling synchronization of gamma oscillations by astrocytic modulation in a model hippocampal neural network. Sci. Rep. 2022;12:6970. doi: 10.1038/s41598-022-10649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombi I., Nieus T., Massimini M., Chiappalone M. Spontaneous and perturbational complexity in cortical cultures. Brain Sci. 2021;11:1453. doi: 10.3390/brainsci11111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi Y., Berényi A. Oscillotherapeutics - time-targeted interventions in epilepsy and beyond. Neurosci. Res. 2020;152:87–107. doi: 10.1016/j.neures.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Pelkonen A., Pistono C., Klecki P., Gómez-Budia M., Dougalis A., Konttinen H., Stanová I., Fagerlund I., Leinonen V., Korhonen P., Malm T. Functional characterization of human pluripotent stem cell-derived models of the brain with microelectrode arrays. Cells. 2021;11:106. doi: 10.3390/cells11010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore H., Lega B.C., Konopka G. Riding brain "waves" to identify human memory genes. Curr. Opin. Cell Biol. 2022;78:102118. doi: 10.1016/j.ceb.2022.102118. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.H., Ryu J.R., Lee B., Chae U., Son J.W., Park B.H., Cho I.J., Sun W. Interpreting the entire connectivity of individual neurons in micropatterned neural culture with an integrated connectome analyzer of a neuronal network (iCANN) Front. Neuroanat. 2021;15:746057. doi: 10.3389/fnana.2021.746057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisor J.P., Pasumarthi R.K., Gerashchenko D., Thompson C.L., Pathak S., Sancar A., Franken P., Lein E.S., Kilduff T.S. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J. Neurosci. 2008;28:7193–7201. doi: 10.1523/JNEUROSCI.1150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ndikung J., Storm D., Violet N., Kramer A., Schönfelder G., Ertych N., Oelgeschläger M. Restoring circadian synchrony in vitro facilitates physiological responses to environmental chemicals. Environ. Int. 2020;134:105265. doi: 10.1016/j.envint.2019.105265. [DOI] [PubMed] [Google Scholar]
- 35.Saberi A., Aldenkamp A.P., Kurniawan N.A., Bouten C.V.C. In-vitro engineered human cerebral tissues mimic pathological circuit disturbances in 3D. Commun. Biol. 2022;5:254. doi: 10.1038/s42003-022-03203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odawara A., Matsuda N., Ishibashi Y., Yokoi R., Suzuki I. Toxicological evaluation of convulsant and anticonvulsant drugs in human induced pluripotent stem cell-derived cortical neuronal networks using an MEA system. Sci. Rep. 2018;8:10416. doi: 10.1038/s41598-018-28835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieus T., D'Andrea V., Amin H., Di Marco S., Safaai H., Maccione A., Berdondini L., Panzeri S. State-dependent representation of stimulus-evoked activity in high-density recordings of neural cultures. Sci. Rep. 2018;8:5578. doi: 10.1038/s41598-018-23853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinard V., Mikhail C., Pradervand S., Curie T., Houtkooper R.H., Auwerx J., Franken P., Tafti M. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J. Neurosci. 2012;32:12506–12517. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trujillo C.A., Gao R., Negraes P.D., Gu J., Buchanan J., Preissl S., Wang A., Wu W., Haddad G.G., Chaim I.A., et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell. 2019;25:558–569.e7. doi: 10.1016/j.stem.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krueger J.M., Nguyen J.T., Dykstra-Aiello C.J., Taishi P. Local sleep. Sleep Med. Rev. 2019;43:14–21. doi: 10.1016/j.smrv.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rockstrom M.D., Chen L., Taishi P., Nguyen J.T., Gibbons C.M., Veasey S.C., Krueger J.M. Tumor necrosis factor alpha in sleep regulation. Sleep Med. Rev. 2018;40:69–78. doi: 10.1016/j.smrv.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jewett K.A., Taishi P., Sengupta P., Roy S., Davis C.J., Krueger J.M. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. Eur. J. Neurosci. 2015;42:2078–2090. doi: 10.1111/ejn.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otani T., Marchetto M.C., Gage F.H., Simons B.D., Livesey F.J. 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell. 2016;18:467–480. doi: 10.1016/j.stem.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milky B., Zabolocki M., Al-Bataineh S.A., van den Hurk M., Greenberg Z., Turner L., Mazzachi P., Williams A., Illeperuma I., Adams R., et al. Long-term adherence of human brain cells in vitro is enhanced by charged amine-based plasma polymer coatings. Stem Cell Rep. 2022;17:489–506. doi: 10.1016/j.stemcr.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gottschling C., Dzyubenko E., Geissler M., Faissner A. The indirect neuron-astrocyte coculture assay: an in vitro set-up for the detailed investigation of neuron-glia interactions. J. Vis. Exp. 2016;117:54757. doi: 10.3791/54757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llabjani V., Siddique M.R., Makos A., Abozoid A., Hoti V., Martin F.L., Patel I.I., Raza A. Introducing CELLBLOKS®: a novel organ-on-a-chip platform allowing a plug-and-play approach towards building organotypic models. bioRxiv. 2022 doi: 10.1101/2022.04.05.4871. [DOI] [Google Scholar]
- 47.Paranjape S.R., Nagendran T., Poole V., Harris J., Taylor A.M. Compartmentalization of human stem cell-derived neurons within pre-assembled plastic microfluidic chips. J. Vis. Exp. 2019;147 doi: 10.3791/59250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanagasabapathi T.T., Massobrio P., Barone R.A., Tedesco M., Martinoia S., Wadman W.J., Decré M.M.J. Functional connectivity and dynamics of cortical-thalamic networks co-cultured in a dual compartment device. J. Neural. Eng. 2012;9:036010. doi: 10.1088/1741-2560/9/3/036010. [DOI] [PubMed] [Google Scholar]
- 49.Kanagasabapathi T.T., Franco M., Barone R.A., Martinoia S., Wadman W.J., Decré M.M.J. Selective pharmacological manipulation of cortical-thalamic co-cultures in a dual-compartment device. J. Neurosci. Methods. 2013;214:1–8. doi: 10.1016/j.jneumeth.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 50.Holloway P.M., Willaime-Morawek S., Siow R., Barber M., Owens R.M., Sharma A.D., Rowan W., Hill E., Zagnoni M. Advances in microfluidic in vitro systems for neurological disease modeling. J. Neurosci. Res. 2021;99:1276–1307. doi: 10.1002/jnr.24794. [DOI] [PubMed] [Google Scholar]
- 51.Albers J., Offenhäusser A. Signal propagation between neuronal populations controlled by micropatterning. Front. Bioeng. Biotechnol. 2016;4:46. doi: 10.3389/fbioe.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guy B., Zhang J.S., Duncan L.H., Johnston R.J., Jr. Human neural organoids: models for developmental neurobiology and disease. Dev. Biol. 2021;478:102–121. doi: 10.1016/j.ydbio.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govindan S., Batti L., Osterop S.F., Stoppini L., Roux A. Mass generation, neuron labeling, and 3D imaging of minibrains. Front. Bioeng. Biotechnol. 2021;8:1436. doi: 10.3389/fbioe.2020.582650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marton R.M., Pașca S.P. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol. 2020;30:133–143. doi: 10.1016/j.tcb.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Kim J., Sullivan G.J., Park I.H. How well do brain organoids capture your brain? iScience. 2021;24:102063. doi: 10.1016/j.isci.2021.102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper J.M., Halter K.A., Prosser R.A. Circadian rhythm and sleep-wake systems share the dynamic extracellular synaptic milieu. Neurobiol. Sleep Circadian Rhythms. 2018;5:15–36. doi: 10.1016/j.nbscr.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karahuseyinoglu S., Sekerdag E., Aria M.M., Cetin Tas Y., Nizamoglu S., Solaroglu I., Gürsoy-Özdemir Y. Three-dimensional neuron-astrocyte construction on matrigel enhances establishment of functional voltage-gated sodium channels. J. Neurochem. 2021;156:848–866. doi: 10.1111/jnc.15185. [DOI] [PubMed] [Google Scholar]
- 58.Re C.J., Batterman A.I., Gerstner J.R., Buono R.J., Ferraro T.N. The molecular genetic interaction between circadian rhythms and susceptibility to seizures and epilepsy. Front. Neurol. 2020;11:520. doi: 10.3389/fneur.2020.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brodsky V.Y., Zvezdina N.D. Melatonin as the most effective organizer of the rhythm of protein synthesis in hepatocytes in vitro and in vivo. Cell Biol. Int. 2010;34:1199–1204. doi: 10.1042/CBI20100036. [DOI] [PubMed] [Google Scholar]
- 60.Hastings M.H., Brancaccio M., Maywood E.S. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J. Neuroendocrinol. 2014;26:2–10. doi: 10.1111/jne.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colwell C.S., Ghiani C.A. Potential circadian rhythms in oligodendrocytes? Working together through time. Neurochem. Res. 2020;45:591–605. doi: 10.1007/s11064-019-02778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dykstra-Aiello C., Koh K.M.S., Nguyen J., Xue M., Roy S., Krueger J.M. A wake-like state in vitro induced by transmembrane TNF/soluble TNF receptor reverse signaling. Brain Behav. Immun. 2021;94:245–258. doi: 10.1016/j.bbi.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lakatos P., Gross J., Thut G. A new unifying account of the roles of neuronal entrainment. Curr. Biol. 2019;29:R890–R905. doi: 10.1016/j.cub.2019.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pampaloni N.P., Giugliano M., Scaini D., Ballerini L., Rauti R. Advances in nano neuroscience: from nanomaterials to nanotools. Front. Neurosci. 2018;12:953. doi: 10.3389/fnins.2018.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalpiaz A., Fogagnolo M., Ferraro L., Beggiato S., Hanuskova M., Maretti E., Sacchetti F., Leo E., Pavan B. Bile salt-coating modulates the macrophage uptake of nanocores constituted by a zidovudine prodrug and enhances its nose-to-brain delivery. Eur. J. Pharm. Biopharm. 2019;144:91–100. doi: 10.1016/j.ejpb.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 66.de Oliveira Junior E.R., Truzzi E., Ferraro L., Fogagnolo M., Pavan B., Beggiato S., Rustichelli C., Maretti E., Lima E.M., Leo E., Dalpiaz A. Nasal administration of nanoencapsulated geraniol/ursodeoxycholic acid conjugate: towards a new approach for the management of Parkinson's disease. J. Control. Release. 2020;321:540–552. doi: 10.1016/j.jconrel.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 67.Truzzi E., Rustichelli C., de Oliveira Junior E.R., Ferraro L., Maretti E., Graziani D., Botti G., Beggiato S., Iannuccelli V., Lima E.M., et al. Nasal biocompatible powder of Geraniol oil complexed with cyclodextrins for neurodegenerative diseases: physicochemical characterization and in vivo evidences of nose to brain delivery. J. Control. Release. 2021;335:191–202. doi: 10.1016/j.jconrel.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 68.Knoben R., Alosaimi F., Dominguez-Paredes D., Temel Y., Jahanshahi A. Nanomaterials in neuromodulation: what is the potential? Expert Rev. Neurother. 2022;22:287–290. doi: 10.1080/14737175.2022.2056447. [DOI] [PubMed] [Google Scholar]