Abstract
Background
Extended‐duration thromboprophylaxis is used to decrease risk of venous thromboembolism (VTE) after surgery but may increase the risk of bleeding. The decision to complete a course of extended‐duration thromboprophylaxis can be challenging.
Objective
The objective of this study was to develop an acceptable patient decision aid (PtDA) to facilitate shared decision making for the use of extended‐duration thromboprophylaxis following major abdominal surgery.
Methods
An evidence‐based, risk‐stratified PtDA was created. The evidence on benefits and harms of a 28‐day postoperative course of low‐molecular‐weight heparin (LMWH) versus in‐hospital prophylaxis only were synthesized. Outcomes included minor bleeding, major bleeding, clinically significant VTE, and fatal VTE. Risks were calculated and reported by Caprini score. Alpha testing of the PtDA draft with various stakeholders was performed using a 10‐question survey to assess acceptability of the PtDA with patients, thrombosis experts, and surgeons. The primary outcome was the acceptability of the PtDA.
Results
Acceptability testing was performed with 11 patients, 11 thrombosis experts, and 11 surgeons. Most responders felt the language on the PtDA was easy to follow (28/33, 85%), and that the information was well balanced between management options (9/11 [82%] patients; 17/21 [80%] clinicians). Most patients (9/11, 82%) and clinicians (18/22, 82%) believed it would be a useful clinical tool, and were satisfied with the overall quality of the PtDA (27/33, 82%).
Conclusions
A risk‐stratified, evidence‐based PtDA was created to facilitate shared decision making for the use of extended‐duration LMWH following major abdominal surgery. This clinical tool was acceptable with patients and physicians and is available at https://decisionaid.ohri.ca/decaids.html.
Keywords: decision aid, decision support techniques, decision making, surgery, venous thromboembolism
Essentials.
A patient decision aid for extended use of blood thinners was created.
This decision aid was acceptable to patients and clinicians.
Eighty‐two percent of patients who used it recommend this decision aid for other patients.
Eighty‐two percent of clinicians plan to use this decision aid in their practice.
1. INTRODUCTION
Clinically detected venous thromboembolism (VTE) as a complication of surgery has been historically associated with a significant risk of mortality. 1 , 2 Asymptomatic deep vein thrombosis detected on screening ultrasound is often used as a surrogate outcome measure in VTE‐related research given the low incidence of symptomatic or fatal VTEs. 3 , 4 , 5 , 6 , 7 , 8 However, the clinical significance and patient importance of these asymptomatic VTEs remains uncertain.
The efficacy of thromboprophylaxis using low‐molecular‐weight heparin (LMWH) to prevent postoperative VTE events during hospitalization is common practice, but the optimal duration of thromboprophylaxis after surgery is largely unknown. 9 Extended‐duration thromboprophylaxis involves the administration of pharmacological thromboprophylaxis beyond hospital discharge using LMWH for 28 days after surgery. A Cochrane review on extended duration thromboprophylaxis pooled the results of four clinical trials that used asymptomatic VTE as the primary outcome. The results of this review led to guidelines from several professional societies supporting the use of extended‐duration thromboprophylaxis following major abdominal or pelvic surgery, including those from the American Society of Colorectal Surgeons, 10 the American Society of Clinical Oncology, 11 and the American Society of Hematology (ASH). 12 Despite these recommendations from clinical practice guidelines, an American study of Medicare beneficiaries reported only 1.5% of patients receive and fill a prescription for its use. 13
The risks and benefits of pharmacological thromboprophylaxis following major surgery vary among individuals based on patient, disease, and procedural factors. 14 Individualized VTE risk stratification using the 2005 Caprini score 15 is recommended to predict VTE risk and assist clinicians in identifying patients who should receive pharmacological thromboprophylaxis, including extended‐duration thromboprophylaxis for patients undergoing abdominopelvic surgery for cancer. 16 The Caprini risk assessment model uses patient‐level factors to estimate an individual's risk of VTE and has been validated in many settings and populations. 17 , 18 , 19 , 20 , 21 , 22 This risk stratification was used by Pannucci et al. 14 to describe the rates of postoperative VTE in four different groups of patients categorized by Caprini score: 3–4, 5–6, 7–8, and 9 or greater. The study by Pannucci et al. 14 found that the rate of VTE increases across each group and is highest in Caprini score 9 or greater.
The evidence supporting the use of extended‐duration thromboprophylaxis to prevent clinically meaningful and patient‐important VTEs is limited, leading to clinical equipoise regarding the risk‐to‐benefit ratio associated with its use following all major abdominal surgeries. 23 This results in challenging conversations between patients and physicians when reviewing the benefits and harms of extended‐duration thromboprophylaxis and likely leads to significant variability in clinical practice. Patient decision aids (PtDA) are one clinical tool that provides information in a patient‐centered way and helps facilitate shared decision making. A review of 105 PtDAs found that patients who used a decision aid had improved knowledge of their management options, were more informed, and had more accurate expectations of the benefits and harms of their options compared to patients who did not have access to a PtDA. 24 A subanalysis of 24 included studies with PtDAs based on the Ottawa Decision Support Framework showed similar outcomes. 25
Given the difficult decision patients and physicians face regarding the use of extended‐duration thromboprophylaxis after major abdominal surgery and the lack of high‐quality patient‐centered resources to inform this decision, we sought to develop an evidence‐based PtDA to promote shared decision making for patients undergoing major abdominal surgery, such as abdominal surgeries for resection of gastrointestinal malignancies or benign conditions, such as inflammatory bowel disease or diverticular disease. The objective of this study was to create and assess the acceptability of a novel PtDA developed to facilitate shared decision making between patients and health care providers deciding whether to use extended‐duration thromboprophylaxis following major abdominal surgery.
2. METHODS
Institutional ethics board approval was obtained for this study (OHSN‐REB 20200570‐01H). The Ottawa Decision Support Framework (ODSF) 26 and the International Patient Decision Aids Standards 27 (IPDAS) were used to guide the development of the PtDA. The structured process for developing a high‐quality PtDA described by McAlpine et al. 28 , 29 was followed.
2.1. Needs assessment
Many hospitals in the province of Ontario (Canada) have not systematically implemented extended‐duration thromboprophylaxis after all major abdominal surgeries. This is largely because the data supporting the practice is based on surrogate outcomes, and the cost of LMWH (approximately $300 for 30 days [Canadian funds]) is not always covered by provincial health insurance providers. 30 Furthermore, a survey of Canadian thrombosis experts demonstrated clinical equipoise among 80% of respondents regarding the benefits of pharmacological thromboprophylaxis after discharge following hospitalization for major abdominal surgery. 23 The clinical equipoise and lack of universal implementation of extended‐duration LMWH following major abdominal surgery results in many patients unaware that this is an option. A previous study highlighted a limited public awareness of VTE and its consequences and called for campaigns to increase public awareness of VTE. 31 Additionally, a randomized control trial investigating the effect of extended‐duration LMWH on disease‐free survival, postoperative bleeding, and VTE compared to the standard of care stopped recruitment early, as 35% declined participation because they were not interested in taking extended‐duration LMWH, with 10% specifically citing not wanting daily injections (R. Auer, personal communication, February 3, 2022). The potential benefits and harms of extended‐duration thromboprophylaxis depend on personal risk factors, further indicating the need to discuss this decision with patients using a tailored approach with best practices for risk communication. 32 These studies support the need for a PtDA to engage patients in understanding their personal risk of VTE, and the benefits and harms of extended‐duration thromboprophylaxis.
2.2. Creation of steering committee
A team of content and process experts was assembled to form the steering committee for the development of this PtDA. The steering committee was composed of a surgeon (RA); a thrombosis expert (MC); a medical student (VI); a surgical fellow (KM); and a world expert in the development, testing, and implementation of PtDAs (DS).
The steering committee discussed the various components of the PtDA including the intended patient population, the management options, and the clinically relevant and patient‐important outcomes.
2.3. Literature review for rates of benefits and harms
Once the steering committee determined the management options and the relevant benefits and harms to include on the PtDA, a thorough literature review was performed. PubMed and Ovid Medline databases were searched for the highest‐quality evidence on the risk of VTE with and without the use of extended‐duration thromboprophylaxis following major abdominal surgery. Literature that stratified risk of postoperative VTE based on the Caprini score was collected. The literature was reviewed by the steering committee, and the rates included for each outcome on the PtDA were agreed upon by consensus.
2.4. Formation of first draft
A prototype of the PtDA was created using the ODSF template (e‐training at https://www.decisionaid.ohri.ca). 25 Patient‐friendly language was used to highlight important background information including the descriptions of VTEs and extended‐duration thromboprophylaxis. A validated, patient‐administered Caprini score was included at the beginning of the decision aid with the intention of patients calculating their own Caprini score. The information on the rates of VTE and risk‐to‐benefit ratio of extended‐duration thromboprophylaxis was divided into four sections based on Caprini score. The four risk‐stratified sections included Caprini scores 3–4, 5–6, 7–8, and 9 or greater. Patients were instructed to proceed to the appropriate section of the document for them based on their Caprini score.
Knowledge questions were included to test patients' understanding of the information on the PtDA. A validated scoring tool used to assess decisional conflict was included. 33 A section of the PtDA also included an explicit values clarification exercise to allow patients to clarify and communicate their values and preferences regarding each of the management options.
2.5. Alpha testing
Clinicians and patients were invited to complete alpha testing. Clinician participants included surgeons and thrombosis experts who routinely see patients after major abdominal surgery and prescribe extended‐duration thromboprophylaxis. Patient participants included patients who previously underwent major abdominal surgery, defined as abdominal laparotomy or laparoscopy lasting more than 45 min, and faced the decision of receiving extended‐duration thromboprophylaxis or not (Table 1). Patients were contacted via email after discharge from the hospital inviting them to participate. The number of clinicians and patient participants who were included in this study was based on previous studies assessing the acceptability testing of a PtDA in a similar setting. 28 , 34 , 35 , 36 , 37 Alpha testing involved participants reviewing the PtDA draft, and then completing a 10‐question online survey that was based on a validated acceptability instrument. 38 The results of the alpha testing were reviewed by the steering committee and used to update the PtDA into a finalized format.
TABLE 1.
Patient demographics
| Variable | Total cohort of patients (n = 11) |
|---|---|
| Age, years, median (range) | 64 (39–78) |
| Sex, n (%) | |
| Female | 7 (64) |
| Male | 4 (36) |
| Marital status, n (%) | |
| Single | 1 (9) |
| Married | 9 (82) |
| Not reported | 1 (9) |
| Preferred language, n (%) | |
| English | 9 (82) |
| French | 2 (18) |
3. RESULTS
A novel, evidence‐based PtDA was created to facilitate shared decision making regarding the choice to receive extended‐duration pharmacological thromboprophylaxis using LMWH or not after major abdominal surgery. The ODSF and IPDAS were followed to guide the systematic development of this decision aid.
3.1. Results of steering committee
The steering committee determined that a risk‐stratified PtDA was necessary to present accurate risks and benefits to an individual patient. The Caprini score was agreed upon as the tool that would be included to allow for postoperative VTE risk stratification. Risks and benefits of LMWH were grouped into four risk categories: Caprini scores 3–4, 5–6, 7–8, and 9 or greater.
The steering committee decided that the important outcomes to present on the PtDA included the rates of symptomatic nonfatal VTEs, fatal VTEs, major bleeding, and clinically relevant nonmajor bleeding following major abdominal surgery. The impact of completing a course of extended‐duration thromboprophylaxis or not on the clinical outcomes was also agreed upon as an important aspect of the information that should be provided to patients on the PtDA.
3.2. Synthesis of the literature
The results of the literature review highlighted the limited data available on the risk‐stratified rates of clinically relevant outcomes following major abdominal surgery. A summary of the evidence available is shown in Table 2. Two systematic reviews and meta‐analyses provided the highest quality of evidence to inform the outcomes on the PtDA. 14 , 39 One meta‐analysis pooled the results of four randomized controlled trials on the risk ratio for VTE without pharmacological thromboprophylaxis. 39 The second meta‐analysis reported the benefits and harms of thromboprophylaxis among surgical patients stratified by Caprini scores. 14 In addition to these meta‐analyses, one observational study and one clinical guideline (ASH, 2021) used to inform the rates quoted on the PtDA. 12 , 40 We used the data reported in these studies to extrapolate the benefit and harm rates to inform the PtDA due to the limited risk‐stratified data.
TABLE 2.
Summary of evidence
| Study characteristics | Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Study Type | Population | Rate of major bleeding with LMWH | Rate of major bleeding without LMWH | Rate of minor bleeding with LMWH | Rate of minor bleeding without LMWH | Rate of VTE with LMWH | Rate of VTE without LMWH | Rate of fatal VTE without LMWH | Rate of fatal VTE with LMWH | Comments |
| Rausa 39 | 2018 | Meta‐analysis |
Postoperative patients n = 1525, 649 extended‐duration thromboprophylaxis, 641 conventional |
1.37 (RR) | — | 1.42 (RR) | 1.2 (RR) | 0.47 (RR) | — | — | — | — |
| Pannucci 14 | 2017 | Meta‐analysis | 11 studies, n = 14,776 contained risk‐stratified VTE data, 8 studies | — | — | — | — | — |
3–4: 0.7% 5–6: 1.8% 7–8: 4.0% >8: 10.7% |
— | — | VTE rate stratified by Caprini score |
| Douketis 40 | 2007 | Prospective cohort | 2052 patients who recently discontinued anticoagulation | — | — | — | — | — | — | 0.002% | — | — |
| Lyman 12 | 2021 | American Society of Hematology 2021 guidelines | — | — | 1.0 (RR) | — | — | — | — | — | — | — |
Abbreviation: RR, Risk Ratio.
Once the summary‐of‐evidence table was created, the steering committee reviewed the data available and reached consensus on the rates for each benefit and harm to include on the PtDA. The benefits included preventing symptomatic nonfatal VTE and preventing death as a result of VTE. The harms included clinically relevant minor bleeding and major bleeding events requiring transfusion, repeat surgery, or death. The rates for each benefit and risk were stratified into four groups on the PtDA for each management strategy: Caprini scores 3–4, 5–6, 7–8, and 9 or greater.
3.3. Patient decision aid prototype
The PtDA prototype was 11 pages total. There were two common pages, two risk‐tailored pages for each risk strata, plus a final page indicating references and authors. Including all aspects essential to high‐quality PtDAs (Table 3), the PtDA met all six IPDAS qualifying criteria, all six certification criteria, and 19 of 23 quality criteria (Table 3). 41 Patient‐friendly language was used, and the Flesch–Kincaid score for readability level was 8.7, indicating an eighth‐grade reading level. The PtDA used both descriptive text and numbers to represent the data in a patient‐centered manner. An example of how the outcomes were reported on the PtDA is shown in Figure 1.
TABLE 3.
International patient decision aid standards criteria met by patient decision aid
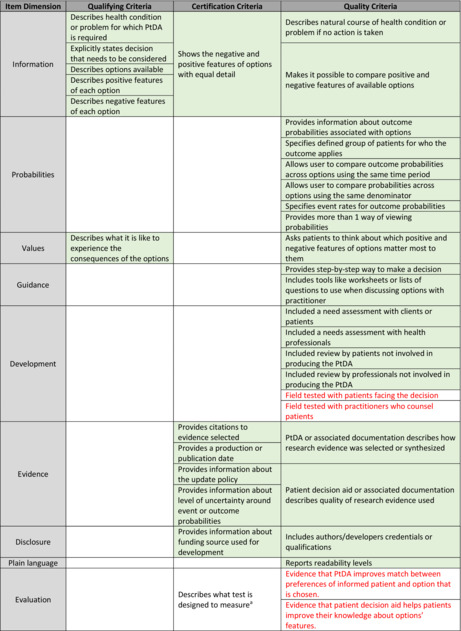
Note: Red text: Criteria that will be met once beta testing is completed. Green shaded boxes: PtDA meets these criteria.
Applicable only for decision aids designed to facilitate decisions regarding tests.
FIGURE 1.
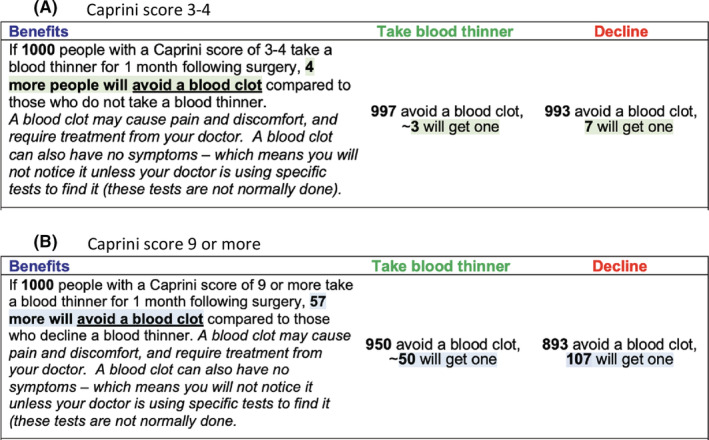
Example of outcome presentation on patient decision aid
3.4. Results of alpha testing
Seventeen patients who had previously made the decision to take or decline extended‐duration LMWH following their major abdominal surgery at one academic center were contacted by email via their surgeon if they met inclusion criteria. Eleven patients responded and completed alpha testing, giving a response rate of 65%. Eleven thrombosis experts and 11 surgeons also completed alpha testing. All respondents completed the full 10‐question survey with the exception of one clinician who missed one question (Table 4).
TABLE 4.
Patient and clinician survey results
| Question | Patients (n = 11) | Clinicians (n = 22) |
|---|---|---|
| Language in decision aid, n (%) | ||
| Appropriate | 9 (82) | 19 (86) |
| Difficult to read | 2 (18) | 1 (4) |
| Neutral | 2 (9) | |
| Amount of Information provided, n (%) | ||
| About right | 6 (54) | 10 (45) |
| More than desired | 3 (27) | 10 (45) |
| Less than desired | 2 (18) | 2 (9) |
| Length of decision aid, n (%) | ||
| Appropriate | 9 (82) | 12 (55) |
| Too long | 2 (18) | 10 (45) |
| Harms and benefits included, n (%) | ||
| Well‐balanced | 82% (9) | 17 (80) a |
| Biased toward taking LMWH | 18% (2) | 4 (20) |
| Overall quality of decision aid, n (%) | ||
| Satisfied | 73% (8) | 19 (86) |
| Not satisfied | 27% (3) | 1 (4) |
| Neutral | 2 (9) | |
| Benefits and harms easy to follow, n (%) | ||
| Agree | 9 (82) | N/A |
| Disagree | 2 (18) | |
| PtDA would be helpful during decision making, n (%) | ||
| Helpful | 9 (82) | N/A |
| Neutral | 1 (9) | |
| Not helpful | 1 (9) | |
| Would recommend this tool for future patients, n (%) | ||
| Yes | 9 (82) | N/A |
| No | 2 (18) | |
| Agree with benefits and harms reported, n (%) | ||
| Agree | N/A | 19 (86) |
| Disagree | 1 (4) | |
| Neutral | 1 (4) | |
| Believed this is a useful tool for counseling, n (%) | ||
| Yes | N/A | 18 (82) |
| No | 3 (14) | |
| Neutral | 1 (4) | |
| Anticipate using PtDA in practice (n, %) | ||
| Agree | N/A | 10 (45) |
| Disagree | 4 (18) | |
| Neutral | 8 (36) | |
Abbreviations: N/A, not applicable; PtDA, patient decision aid.
n = 21.
The language of the PtDA was felt to be easy to follow by 28 of 33 (85%) of all responders (95% confidence interval [CI], 0.68–0.95; 82% [9/11] patients [95% CI, 0.48–0.98]; and 86% [19/22] clinicians [95% CI, 0.65–0.97]). The information provided on the management options was reported to be well balanced by 9 of 11 (82%) patients (95% CI, 0.68–0.95), and 17 of 21 (80%) of clinicians (95% CI, 0.58–0.95). Most clinicians (19/22 [86%; 95% CI, 0.65–0.97]) and patients (8/11 [73%; 95% CI, 0.39–0.94]) were satisfied with the overall quality of the decision aid. The length of the decision aid was felt to be appropriate by 9 of 11 (82%) of patients (95% CI, 0.48–0.98) and 12/22 (54%) of clinicians (95% CI, 0.32–0.75).
Most patients felt that the PtDA would have been helpful in their decision making regarding extended‐duration thromboprophylaxis following major abdominal surgery (9/11; 82% [95% CI, 0.48–0.98]), and most clinicians believed the PtDA would be a useful tool when counseling a new patient on the use of extended duration thromboprophylaxis in the future (18/22; 82% [95% CI, 0.60–0.95]).
Narrative feedback from responders consistently commented that the PtDA was easy to follow and clearly written. Strengths identified by patients included the PtDA's explanation of why thromboprophylaxis is used after surgery and the ability of the PtDA to facilitate informed decision. Patients also appreciated the ability to improve their knowledge of VTEs and their associated risks. Strengths identified by clinician participants included the use of a risk‐stratified presentation of outcomes and the attention to pharmacological thromboprophylaxis in the setting of postsurgical care. Suggested areas for improvements from both clinicians and patients largely focused on the instructions and use of calculating the Caprini score. This was described as a potential area for confusion, and concern was raised that many future patients would not be able to accurately calculate their personal score.
3.5. Final PtDA
The results of the alpha testing were reviewed in detail by the steering committee and used to update the PtDA. Based on the alpha‐testing feedback, the PtDA was adjusted such that a member of the health care team would assist patients in calculating their Caprini score, and then patients would proceed to complete the appropriate risk‐stratified section of the PtDA independently. The final version is freely available on the Ottawa Hospital Research Institute's A to Z Inventory of PtDAs (https://decisionaid.ohri.ca/decaids.html) and is presented in Appendix S1.
4. DISCUSSION
The decision of whether to complete a course of extended‐duration thromboprophylaxis with LMWH following major abdominal surgery is a challenge and is faced routinely by patients and clinicians. To facilitate shared decision making we created a novel, evidence‐based, risk‐stratified PtDA following best practices to create high‐quality PtDAs. 26 , 41 Our PtDA was found to be acceptable among patients, surgeons, and thrombosis experts. Most surgeons and thrombosis experts plan to use this tool in their clinical practice, and most patients responded that they would recommend this PtDA to future patients facing this decision. This indicates that the tool is acceptable among important stakeholders and fulfills a previously unmet need.
The decision to use extended‐duration thromboprophylaxis or not following major abdominal surgery is complex given the limited evidence available on its effect on symptomatic VTE and bleeding rates. There are many factors that influence patients' risk of VTE following surgery, and it remains a challenge for clinicians to stratify patients and identify high‐risk populations. While several professional societies recommend extended‐duration thromboprophylaxis for high‐risk populations, there is no consensus on how to identify these patients. 10 , 11 , 12 This leads to inconsistent practice between hospitals and clinicians based on their preferences and previous experience with the use of extended‐duration thromboprophylaxis. 42 The Caprini risk score is one tool that clinicians can use to help identify who is at high risk of developing VTE. While there are additional factors that may influence one's risk of VTE such as specific procedure or type of procedure, the Caprini score remains a validated tool that can help clinicians identify which patients may be at higher risk of developing VTE. Of concern is when patients are excluded from the decision‐making process because the decision is complex and somewhat controversial. There are effective interventions such as PtDAs that can support them to participate actively in decision making.
A PtDA to facilitate shared decision making regarding the use of extended‐duration thromboprophylaxis after major abdominal surgery aims to support patients facing this decision. Patients are provided information on the important outcomes and the potential risks and benefits of extended‐duration thromboprophylaxis to ensure that they are informed and adequately understanding of their options. This includes having a better understanding of their personal risk of VTE. Use of this PtDA will also allow clinicians to gain a better understanding of patients' values and preferences with respect to extended‐duration LMWH. Patients at high risk of VTE may be more interested in accepting the risks of LMWH, while patients at low risk of VTE may be less inclined; however, this largely depends on the value each individual patient places on the potential benefits and harms of extended‐duration thromboprophylaxis. We anticipate that this PtDA will be used by patients as part of their discharge planning following their major abdominal surgery. The clinician could introduce the decision to be made and provide the patient with our PtDA, allowing them time to review the information on options and clarify their values and preferences. Once the patient has completed the PtDA, the clinician could use a shared decision‐making approach to verify patients' understanding, answer their questions, elicit their values and preferences, and come to agreement on whether or not to prescribe extended‐duration thromboprophylaxis.
Risk‐stratified outcomes within our PtDA provides for a personalized approach to the decision‐making process for patients trying to decide whether they want to receive extended‐duration thromboprophylaxis of not after surgery. 43 The process of assessing an individual patient's risk of VTE compared to the risk of bleeding is challenging and often confusing, which makes it time consuming to review with patients. This PtDA uses the Caprini score to highlight patients' risk of VTE based on their personal circumstances and provides risk‐stratified data to patients and clinicians in a patient‐friendly manner. The information in the PtDA allows patients to understand the risks and benefits of extended‐duration thromboprophylaxis in a personalized way and allows them to make a truly informed decision based on their values and preferences. Patients who reviewed the PtDA during alpha testing recommend its use for future patients, highlighting that this is a useful and patient‐friendly tool.
Patient decision aids educate and inform patients about their management options including the associated evidence‐based outcomes to support shared decision‐making. 44 A systematic review examining patient‐reported barriers and facilitators to shared decision making found that many patients believe they are unable to be involved in medical decision making. 45 The information presented in a PtDA is designed to facilitate shared decision making by helping patients clarify and communicate their values and preferences and to more completely understand the benefits and harms associated with their options. This process can help patients personalize the information, understand their role in the decision, and appreciate the scientific uncertainty despite the best available evidence. 46
This PtDA is novel, and to the best of our knowledge, there has never been a PtDA created to facilitate the shared decision making for the use of extended‐duration thromboprophylaxis after major abdominal surgery before. Despite the novelty of this tool, there are some limitations of this study. First, the benefits and harms included on the PtDA represent the best available evidence at this time. As new evidence becomes available, the rates of these outcomes will need to be modified to reflect the most accurate information available. Second, beta testing has not yet been performed. Beta testing the final PtDA can be done by comparing patients' knowledge scores or decisional conflict scores before and after using the PtDA in clinical practice. 29 , 41 Previous articles that outline the PtDA development process suggest that beta testing is not required prior to implementation when a validated process is used for development. 29 , 47 Future studies may also assess whether this PtDA would lead to better patients outcomes, including the experience and results of the decision‐making process. Third, we did not include surgeons or thrombosis experts who practice at community hospitals in the alpha testing for this PtDA. It is possible that their practice varies from an academic practice in a way that may mean this PtDA is not as useful. Given that the cost of LMWH is a factor that may influence some patients' decisions, it is a relevant source of information to include, but we chose not to include it given that costs of thromboprophylaxis medications vary by country. Information on participant education level, race, ethnicity, and socioeconomic status were not available for this study. This may limit the results of the study, as the population may not represent the target audience. Finally, we used the Caprini score to stratify patients' risk of VTE for this PtDA; however, other factors such as specific procedure or length of procedure may impact patients' risk of VTE and may have been missed, as they are not included in the Caprini score. 48 , 49
5. CONCLUSION
We used a systematic approach to develop a novel PtDA to facilitate shared decision making for the use of extended‐duration thromboprophylaxis following major abdominal surgery. This PtDA was acceptable to patients, thrombosis experts, and surgeons and meets a gap in resources available for patients on this topic. The PtDA is freely available for international use at https://decisionaid.ohri.ca/decaids.html.
AUTHOR CONTRIBUTIONS
VI contributed to study design and data and statistical analysis and wrote the manuscript. KM contributed to the study design and data analysis and provided key revisions to the manuscript. ED and DS contributed to study design and provided key revisions to the manuscript. MC and RA were responsible for study conception and planning and provided key revisions to the manuscript.
FUNDING INFORMATION
This study was funded by a grant from the Ottawa Hospital Academic Medical Organization, Canadian Institute of Health Research, and the Ottawa Hospital Foundation. DS holds a University of Ottawa Research Chair in Knowledge Translation to Patients. MC holds a Tier 1 Research Chair in Cancer & Thrombosis from the Faculty and Department of Medicine of the University of Ottawa. RA holds a Tier 1 Research Chair in Perioperative Cancer Therapeutics from the Faculty of Medicine of the University of Ottawa, partially funded by the Department of Surgery and Division of General Surgery.
RELATIONSHIP DISCLOSURE
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: VI, KM, ED, DS, and RA do not have any relevant conflicts to disclose. MC has received research funding from BMS, Pfizer, and Leo Pharma. He has also received honoraria from Bayer, Sanofi, Servier, BMS, Pfizer, and Leo Pharma.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Ivankovic V, McAlpine K, Delic E, Carrier M, Stacey D, Auer RC. Extended‐duration thromboprophylaxis for abdominopelvic surgery: Development and evaluation of a risk‐stratified patient decision aid to facilitate shared decision making. Res Pract Thromb Haemost. 2022;6:e12831. doi: 10.1002/rth2.12831
Name of Institution where work was carried out: The Ottawa Hospital, Ottawa, ON, Canada
Handling Editor: Dr Suzanne Cannegieter
REFERENCES
- 1. Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611‐1617. doi: 10.1111/j.1538-7836.2005.01415.x [DOI] [PubMed] [Google Scholar]
- 2. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:335‐342. doi: 10.1016/j.jvs.2006.10.034 [DOI] [PubMed] [Google Scholar]
- 3. Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975‐980. doi: 10.1056/nejmoa012385 [DOI] [PubMed] [Google Scholar]
- 4. Kakkar VV, Balibrea JL, Martínez‐González J, Prandoni P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223‐1229. doi: 10.1111/j.1538-7836.2010.03892.x [DOI] [PubMed] [Google Scholar]
- 5. Lausen I, Jensen R, Jorgensen LN, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: randomised controlled study of prolonged thromboprophylaxis. Eur J Surg. 1998;164:657‐663. doi: 10.1080/110241598750005534 [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen MS, Jorgensen LN, Wille‐Jørgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboeembolic complications. Thromb Haemostasis. 2006;4:2384‐2390. [DOI] [PubMed] [Google Scholar]
- 7. Sakon M, Kobayashi T, Shimazui T. Efficacy and safety of enoxaparin in Japanese patients undergoing curative abdominal or pelvic cancer surgery: results from a multicenter, randomized, open‐label study. Thromb Res. 2010;125:e65‐e70. doi: 10.1016/j.thromres.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 8. Vedovati MC, Becattini C, Rondelli F, et al. A randomized study on 1‐week versus 4‐week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Ann Surg. 2014;259:665‐669. doi: 10.1097/SLA.0000000000000340 [DOI] [PubMed] [Google Scholar]
- 9. Felder S, Rasmussen MS, King R, et al. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2019;3:CD004318. doi: 10.1002/14651858.CD004318.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleming F, Gaertner W, Ternent CA, et al. The American Society of Colon and Rectal Surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14‐20. doi: 10.1097/DCR.0000000000000982 [DOI] [PubMed] [Google Scholar]
- 11. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496‐520. doi: 10.1200/JCO.19.01461 [DOI] [PubMed] [Google Scholar]
- 12. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927‐974. doi: 10.1182/bloodadvances.2020003442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merkow RP, Bilimoria KY, McCarter MD, et al. Post‐discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg. 2011;254:131‐137. doi: 10.1097/SLA.0b013e31821b98da [DOI] [PubMed] [Google Scholar]
- 14. Pannucci CJ, Swistun L, MacDonald JK, Henke PK, Brooke BS. Individualized venous thromboembolism risk stratification using the 2005 caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta‐analysis. Ann Surg. 2017;265:1094‐1103. doi: 10.1097/SLA.0000000000002126 [DOI] [PubMed] [Google Scholar]
- 15. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70‐78. doi: 10.1016/j.disamonth.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 16. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e227S‐e277S. doi: 10.1378/chest.11-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuentes H, Paz L, Al‐Ogaili A, et al. Validation of a patient‐completed Caprini risk score for venous thromboembolism risk assessment. TH Open. 2017;1:e106‐e112. doi: 10.1055/s-0037-1607339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941‐948. doi: 10.1001/jamasurg.2015.1841 [DOI] [PubMed] [Google Scholar]
- 19. Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251:344‐350. doi: 10.1097/SLA.0b013e3181b7fca6 [DOI] [PubMed] [Google Scholar]
- 20. Pannucci CJ, Bailey SH, Dreszer G, et al. Validation of the caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212:105‐112. doi: 10.1016/j.jamcollsurg.2010.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krauss ES, Segal A, Cronin MA, et al. Implementation and validation of the 2013 Caprini score for risk stratification of arthroplasty patients in the prevention of venous thrombosis. Clin Appl Thromb. 2019;25:107602961983806. doi: 10.1177/1076029619838066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shuman AG, Hu HM, Pannucci CJ, Jackson CR, Bradford CR, Bahl V. Stratifying the risk of venous thromboembolism in otolaryngology. Otolaryngol Head Neck Surg. 2012;146:719‐724. doi: 10.1177/0194599811434383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al Rawahi B, Le Gal G, Auer R, Carrier M. A survey of thrombosis experts evaluating practices and opinions regarding venous thromboprophylaxis in patients post major abdominal surgery. Thromb J. 2017;15:2. doi: 10.1186/s12959-016-0126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;2017:CD001431. doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoefel L, O'Connor AM, Lewis KB, et al. 20th anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555‐581. doi: 10.1177/0272989X20936209 [DOI] [PubMed] [Google Scholar]
- 26. Légaré F, O’Connor AM, Graham ID, Wells GA, Tremblay S. Impact of the ottawa decision support framework on the agreement and the difference between patients’ and physicians’ decisional conflict. Med Decis Mak. 2006;26:373‐390. doi: 10.1177/0272989X06290492 [DOI] [PubMed] [Google Scholar]
- 27. Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Br Med J. 2006;333:417‐419. doi: 10.1136/bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McAlpine K, Breau RH, Stacey D, et al. Shared decision‐making for the management of small renal masses — development and acceptability testing of a novel patient decision aid. Can Urol Assoc J. 2020;14:385‐391. doi: 10.5489/CUAJ.6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, Van Der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13:S2. doi: 10.1186/1472-6947-13-S2-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laureano M, Ebraheem M, Crowther M. Extended venous thromboembolism prophylaxis after abdominopelvic cancer surgery: a retrospective review. Curr Oncol. 2019;26:106‐110. doi: 10.3747/co.26.4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wendelboe AM, McCumber M, Hylek EM, Buller H, Weitz JI, Raskob G. Global public awareness of venous thromboembolism. J Thromb Haemost. 2015;13:1365‐1371. doi: 10.1111/jth.13031 [DOI] [PubMed] [Google Scholar]
- 32. Bonner C, Trevena LJ, Gaissmaier W, et al. Current best practice for presenting probabilities in patient decision aids: fundamental principles. Med Decis Making. 2021;41:821‐833. doi: 10.1177/0272989X21996328 [DOI] [PubMed] [Google Scholar]
- 33. Légaré F, Kearing S, Clay K, et al. Are you SURE? Assessing patient decisional conflict with a 4‐item screening test. Can Fam Physician. 2010;56:e308‐e314. [PMC free article] [PubMed] [Google Scholar]
- 34. McAlpine K, Breau RH, Stacey D, et al. Development and acceptability testing of a patient decision aid for individuals with localized renal masses considering surgical removal with partial or radical nephrectomy. Urol Oncol Semin Orig Investig. 2019;37:811.e1‐811.e7. doi: 10.1016/j.urolonc.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 35. McAlpine K, Lavallée LT, Stacey D, et al. Development and acceptability testing of a patient decision aid for urinary diversion with radical cystectomy. J Urol. 2019;202:1001‐1007. doi: 10.1097/JU.0000000000000341 [DOI] [PubMed] [Google Scholar]
- 36. Bouhadana D, Nguyen DD, Raizenne B, et al. Evaluating the acceptability of an online patient decision aid for the surgical management of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Can Urol Assoc J. 2021;15:247‐254. doi: 10.5489/CUAJ.7492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bigelow EO, Windon MJ, Fakhry C, Kiess AP, Seiwert T, D'Souza G. Development of a web‐based, patient‐centered decision aid for oropharyngeal cancer treatment. Oral Oncol. 2021;123:105618. doi: 10.1016/j.oraloncology.2021.105618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Connor AM, Cranney A. User Manual – Acceptability. Ottawa Hospital Research Institute; 1996. Cited June 2, 2020. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Acceptability.pdf [Google Scholar]
- 39. Rausa E, Kelly ME, Asti E, et al. Extended versus conventional thromboprophylaxis after major abdominal and pelvic surgery: systematic review and meta‐analysis of randomized clinical trials. Surgery. 2018;164:1234‐1240. doi: 10.1016/j.surg.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 40. Douketis JD, Gu CS, Schulman S, Ghirarduzzi A, Pengo V, Prandoni P. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med. 2007;147:766. doi: 10.7326/0003-4819-147-11-200712040-00007 [DOI] [PubMed] [Google Scholar]
- 41. Joseph‐Williams N, Newcombe R, Politi M, et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Making. 2014;34:699‐710. doi: 10.1177/0272989X13501721 [DOI] [PubMed] [Google Scholar]
- 42. McAlpine K, Breau RH, Carrier M, et al. Thromboprophylaxis practice patterns and beliefs among physicians treating patients with abdominopelvic cancers at a Canadian Centre. Can J Surg. 2021;63:E562‐E568. doi: 10.1503/CJS.015219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Légaré F, Härter M, Stiggelbout A, Thomson R, Stacey D. Choosing treatments and the role of shared decision‐making. In: Nolte E, Merkur S, Anell A, eds. Achieving Person‐Centred Health Systems. 1st ed. Cambridge University Press; 2020:283‐317. [Google Scholar]
- 44. Woolf SH, Chan ECY, Harris R, et al. Promoting informed choice: transforming health care to dispense knowledge for decision making. Ann Intern Med. 2005;143:293‐300. doi: 10.7326/0003-4819-143-4-200508160-00010 [DOI] [PubMed] [Google Scholar]
- 45. Joseph‐Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient‐reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94:291‐309. doi: 10.1016/j.pec.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 46. O'Connor AM, Llewellyn‐Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Aff. 2004;23:VAR‐63‐VAR‐72. doi: 10.1377/hlthaff.var.63 [DOI] [PubMed] [Google Scholar]
- 47. Stacey D, Ludwig C, Archambault P, et al. Feasibility of rapidly developing and widely disseminating patient decision aids to respond to urgent decisional needs due to the COVID‐19 pandemic. Med Decis Making. 2021;41:233‐239. doi: 10.1177/0272989X20979693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McAlpine K, Breau RH, Knee C, et al. Venous thromboembolism and transfusion after major abdominopelvic surgery. Surgery. 2019;166:1084‐1091. doi: 10.1016/j.surg.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 49. Beal EW, Tumin D, Chakedis J, et al. Identification of patients at high risk for post‐discharge venous thromboembolism after hepato‐pancreato‐biliary surgery: which patients benefit from extended thromboprophylaxis? HPB (Oxford). 2018;20:621‐630. doi: 10.1016/j.hpb.2018.01.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3


