Abstract
Background
Recurrence risk of systemic lupus erythematosus (SLE)‐associated venous thromboembolism (VTE) is unclear.
Aim
To determine the recurrence risk of SLE‐associated VTE overall and by presence of provoking factors and SLE flares.
Methods
A multicenter, retrospective cohort study was conducted among patients with first SLE‐associated VTE who discontinued anticoagulation. SLE flares were defined as Systemic Lupus Erythematosus Disease Activity Index 2000 greater than 4. The primary outcome was recurrent VTE. Incidence rates and cumulative incidences were calculated by presence of provoking factors and antiphospholipid syndrome (APS) at index VTE. The hazard ratio (HR) for recurrence after SLE flare–associated index VTE was estimated with Cox regression, adjusted for provoking factor presence and APS.
Results
Eighty patients were included with 21 recurrent VTEs in median 8 years. For provoked index VTE, the recurrence rate in patients without APS was 1.1 per 100 person‐years (PY; 95% confidence interval [CI], 0.1–3.1) and in the presence of APS 3.5 per 100 PY (95% CI, 0.9–8.9), yielding cumulative incidences of 7.5% (95% CI, 1.2%–21.7%) and 31.4% (95% CI, 6.3%–61.6%) respectively. For unprovoked index VTE, these analogous rates were 3.8 per 100 PY (95% CI, 1.2–9.0) and 16.7 per 100 PY (95% CI, 4.5–42.7), with cumulative incidences of 33.7% (95% CI, 10.7%–58.9%) and 54.2% (95% CI, 10.7%–84.5%), respectively. Forty‐six index VTEs were flare associated, and the adjusted HR for recurrence was 0.4 (95% CI, 0.1–1.8) compared to those without flares at their index VTE.
Conclusion
Antiphospholipid syndrome is the main determinant for recurrence risk of SLE‐associated VTE irrespective of presence of a provoking factor. Future research should attempt to confirm that flare‐associated VTE has a lower recurrence risk.
Keywords: antiphospholipid syndrome, inflammation, systemic lupus erythematosus, thrombosis, venous thromboembolism
Essentials.
Recurrence risk of systemic lupus erythematosus (SLE)‐associated venous thrombosis is not known.
We determined the recurrence risk stratified by provoking factor and antiphospholipid syndrome (APS).
APS seems to mainly determine recurrence risk, besides provoking factor.
1. INTRODUCTION
Systemic lupus erythematosus (SLE) is associated with a thrombotic tendency due to chronic inflammation and the potential presence of antiphospholipid antibodies. 1
In general, after the initial phase of anticoagulant therapy for venous thromboembolism (VTE), a decision is made for either discontinuation or indefinite continuation of anticoagulant therapy depending on the estimated risk of recurrent VTE. 2 Only patients in whom the recurrence risk exceeds the risk of potential anticoagulant‐associated bleeding are deemed to benefit from anticoagulant therapy. Although we know that the risk of incident VTE is higher in patients with SLE, a systematic review by Borjas Howard et al. 3 showed that a gap in knowledge about the recurrence risk is present.
Several aspects of SLE might contribute to a higher or lower recurrence risk and, consequently, are an argument to continue or discontinue anticoagulant therapy, respectively. As a chronic disorder, SLE could be considered as a persistent risk factor for recurrent VTE, especially in the presence of secondary APS. 4 However, data on the recurrence risk in relation to other transient provoking factors of VTE (e.g., estrogen‐containing oral contraceptives) are lacking.
Although SLE is a persistent risk factor, the relapsing–remitting disease course may indicate a more fluctuating character. In inflammatory bowel disease, the risk of VTE is higher during flares compared to periods of remission. 5 Furthermore, Zöller et al. 6 demonstrated that the risk of incident of pulmonary embolism (PE) was highest in the first year after SLE diagnosis and declined steeply thereafter. This suggests that when inflammatory activity subsides by adequate treatment, the trigger for a hypercoagulable state wanes. This concept for a lower recurrence risk after inflammation‐associated VTE was recently demonstrated for transient mild ambulatory infections. 7
Altogether, we aimed to described the natural history of recurrent VTE in patients with SLE and explore the effect of SLE disease activity at time of the VTE on this risk.
2. METHODS
2.1. Study design
Between 2018 and 2021, we conducted a retrospective cohort study in 12 academic medical centers and teaching hospitals in the Netherlands (Supplementary Material). Data were anonymously collected from medical records. Permission for the use of personal and medical data was obtained following local practices in each hospital (i.e., informed consent or an opt‐out procedure) in accordance with the Dutch Medical Treatment Contracts Act and the European General Data Protection Regulation. A waiver for the Medical Research Involving Human Subjects Act was granted by the Medical Ethical Committee of the University Medical Centre Groningen (UMCG) (METc 2019/139), and the study was locally registered (UMCG research register 201900135).
2.2. Participants
Patients with a record of a confirmed SLE diagnosis, in the period of 1980 to 2019, and a VTE were identified by screening medical reports by the treating physician and/or researcher. VTE was defined as a proximal deep vein thrombosis (DVT) of the lower extremities and/or a PE treated with at least 3 months of anticoagulant therapy. Patients with a first VTE (the index VTE) that was SLE associated and who stopped anticoagulation therapy were eligible for inclusion. A VTE was considered SLE associated, if the VTE was diagnosed within a year before formal diagnosis of SLE or thereafter, as stated in the medical records.
A distinction was made between objective and nonobjective index VTE. DVT and/or PE confirmed by imaging results was considered as an objective index VTE. Nonobjective index VTEs were VTE stated in medical records with confirmed anticoagulation treatment for at least 3 months.
Patients younger than 18 years of age at the time of the index VTE and those treated with indefinite anticoagulation therapy or with a history of VTE before a SLE‐associated VTE were excluded.
2.3. Follow‐up
Follow‐up started at the date of discontinuation of anticoagulation therapy for the index VTE and ended at date of last known contact at the hospital, date of death, or start of anticoagulation therapy for a reason other than recurrent VTE. A visual representation of the study timeline is provided in Figure 1.
FIGURE 1.
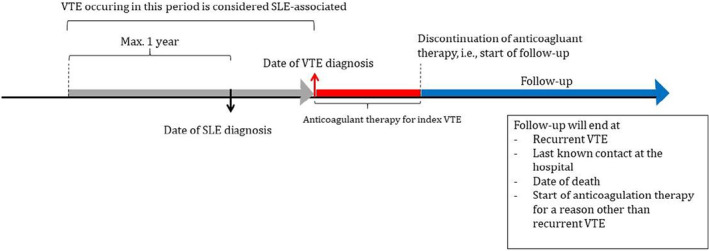
Study timeline. SLE, systemic lupus erythematosus; VTE, venous thromboembolism.
2.4. Outcome
The primary outcome is recurrent VTE, defined as thrombosis in any deep venous system, including the calf veins, and pulmonary embolism. We made a distinction between objectively confirmed (i.e., by medical imaging) and nonobjectively confirmed (i.e., only a mention of VTE and 3 months of anticoagulation therapy) recurrent VTE. In case of recurrent DVT after an index VTE, a recurrence was adjudicated if recurrent DVT occurred in the contralateral leg. If a recurrence occurred in the ipsilateral leg, an independent reviewer was consulted (JB or KM). In this case, a recurrence was adjudicated a new venous segment was affected compared to baseline ultrasound after index DVT or, when a baseline ultrasound was not available, if a new venous segment was affected compared to the index DVT. Deaths were independently adjudicated by two reviewers (SB and JB) according to the cause‐of‐death classification in VTE studies of the Standardization and Scientific Committee of the ISTH. 10
2.5. Covariates
Presence of provoking factor, antiphospholipid syndrome (APS), and SLE activity, expressed as the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI‐2K), 9 at index VTE were collected as covariates from medical records.
Presence of a provoking factor and APS were used as dichotomous covariates. Provoking factors were surgery, use of estrogen‐containing contraception, pregnancy and/or puerperium, being bedridden for more than 3 days, (cast) immobilization of a lower extremity, hospital admission, and presence of a central venous catheter. APS was defined according to the revised Saporro classification 2006 and was considered present if diagnosed before or in the course of the index VTE. 12
Systemic lupus erythematosus activity was expressed as the SLEDAI‐2K, a clinical score quantifying disease activity over different organ systems. The SLEDAI‐2K was treated as a continuous and dichotomous covariate with a cutoff of greater than 4, which is considered as active SLE (i.e., a flare). 10
We collected the SLEDAI‐2K from patient visits at the rheumatologist or clinical immunologists related to the index VTE or a visit closest to the index VTE with a maximum of a month. If the SLEDAI‐2K was not reported, the score was manually calculated by clinical information available for this visit. Here, we assumed that a certain sign or symptom from the SLEDAI‐2K was not present if not reported. If there were no visits around the index VTE, the SLEDAI‐2K was considered missing.
Ethnicity, sex, age at SLE, and VTE diagnosis were collected as descriptive variables.
2.6. Statistical analysis
We estimated the incidence of recurrent VTE by dividing number of events by total follow‐up time in years and stratified by presence of provoking factor and APS at index VTE.
Cumulative incidence was estimated, with death as competing risk. Follow‐up was censored at last known hospital visit.
The influence of SLE activity at index VTE on the risk of recurrent VTE was estimated in a cause‐specific Cox regression adjusted for presence of provoking factor and APS at index VTE.
Primarily, complete cases were analyzed. Due to missing data in the presence of a provoking factor and SLE activity at index VTE, a post hoc best‐ versus worst‐case analysis was performed, to explore the range of uncertainty in the found estimates. For the incidence calculation, the best case entailed that all missing values in the presence of a provoking factor at index VTE were imputed to unprovoked and worst case the opposite (i.e., all missing values imputed to provoked). Similarly, we created four extreme scenarios for the influence of SLE activity on the recurrence risk. Missing values in provoking factor and dichotomous SLEDAI‐2K at index VTE were imputed according to following combinations: (1) unprovoked and SLEDAI‐2K of 4 or less, (2) unprovoked and SLEDAI‐2K index greater than 4, (3) provoked and SLEDAI‐2K of 4 or less, and (4) provoked and SLEDAI‐2K greater than 4. Finally, we performed a sensitivity analysis including only patients with an objectively confirmed index VTE.
Analyses were performed in R 3.6.2. (R Core Team) with packages “survival,” “cmprsk,” and “ggplot2.”
3. RESULTS
3.1. Baseline characteristics
We identified 152 patients with SLE and a history of VTE. After exclusion of ineligible patients, 80 patients with a SLE‐associated index VTE were included (Figure 2). Baseline characteristics are provided in Table 1.
FIGURE 2.
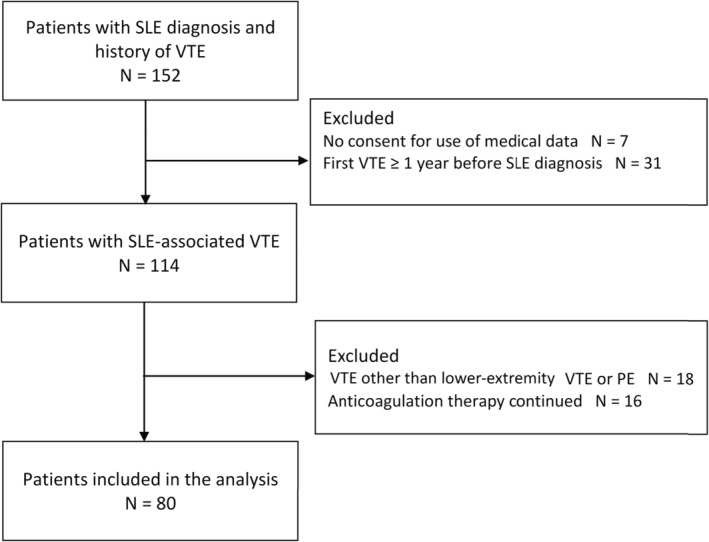
Patient flow.
TABLE 1.
Baseline characteristics
| Total = 80 | |
|---|---|
| Age at SLE diagnosis (years), median (IQR) | 29 (21–40) |
| Age at first VTE (years), median (IQR) | 31 (26–51) |
| Sex (female), n (%) | 66 (83) |
| Ethnicity, n (%) | |
| Caucasian | 51 (64) |
| African/Caribbean | 8 (10) |
| Asian | 3 (4) |
| Other | 2 (3) |
| Missing | 16 (20) |
| Type index‐VTE, n (%) | |
| PE with or without DVT | 45 (56) |
| Proximal DVT | 35 (44) |
| Objectively confirmed index VTE, n (%) | 58 (73) |
| SLEDAI‐2K at index‐VTE, median (IQR) | 8 (5–12) |
| Missing, n (%) | 22 (28) |
| Months since SLE diagnosis, median (IQR) | 27 (0–128) |
| Provoked, n (%) | |
| Yes | 40 (50) |
| No | 29 (36) |
| Missing, n (%) | 11 (14) |
| APS, n (%) | |
| Yes | 21 (26) |
| LAC only | 5 |
| AC or B2GP only | 9 |
| LAC + AC or B2GP | 2 |
| Triple | 2 |
| Unknown | 3 |
| No | 55 (69) |
| Missing | 4 (5) |
| Hydroxochloroquine, n (%) | |
| Yes | 20 (25) |
| No | 34 (43) |
| Unknown | 26 (33) |
| Mortality, n (%) | 5 (6) |
| FU time (years), median (IQR) | 8 (3–16) |
Abbreviations: AC, anticardiolipin antibiodies; APS, antiphospholipid syndrome; B2GP, anti‐b2‐glycoprotein antibodies; DVT, deep vein thrombosis; FU, follow‐up; IQR, interquartile range; LAC, lupus anticoagulant; SD, standard deviation; SLE, systemic lupus erythematosus; PE, pulmonary embolism; VTE, venous thromboembolism.
Half of the index VTEs were provoked by the use of estrogen‐containing contraceptives (n = 22 [28%]), hospitalization (n = 14 [18%]), pregnancy/puerperium (n = 10 [13%]), and surgery/(trauma) immobilization (n = 10 [12%]). Fifty‐eight of 80 index VTEs were objectively confirmed (i.e., confirmed by medical imaging).
Median follow‐up time was 8 (interquartile range [IQR], 3–16) years. Follow‐up differed between patients with an objectively confirmed and nonobjectively confirmed VTE (8 [IQR, 3–9] vs. 13 [IQR, 9–12] years). Four patients were lost to follow‐up, and five patients died during follow‐up. Cause of death was other than PE in four patients and could not be determined in one patient. Three patients were censored because of start of anticoagulation therapy for reasons other than recurrent VTE.
3.2. Risk of recurrence after SLE‐associated VTE
During 674 person‐years (PY) of follow‐up (median, 8 years), 21 patients suffered from recurrent VTE, of which 16 were objectively confirmed. Seven recurrences concerned PE, 11 proximal DVT, and three were other VTE. Four recurrences were provoked (Table S1).
This accumulated in an incidence rate of 3.1 (95% CI, 1.9–4.8) per 100 PY. Incidence rates of recurrent VTE stratified by the presence of provoking factor and secondary APS at index VTE are displayed in Table 2. This analysis suggests that there is an incremental association between the existence of these factors on the risk of recurrent VTE, with secondary APS having the strongest influence. A cumulative incidence plot is shown in Figure 3, again stratified by provoking factor and secondary APS at index VTE. A table with the cumulative incidences at 1, 5, and 10 years is available in Table S2.
TABLE 2.
Incidence rate stratified by presence of provoking factor at index VTE and APS (complete cases: 67)
| Provoked | APS | Events (N/total) | Follow‐up (years) | Incidence rate recurrence per 100 PY (95% CI) |
|---|---|---|---|---|
| Yes | No | 3/29 | 283 | 1.1 (0.2–3.1) |
| Yes | Yes | 4/10 | 115 | 3.5 (0.9–8.9) |
| No | No | 5/20 | 130 | 3.8 (1.2–9.0) |
| No | Yes | 4/8 | 24 | 16.7 (4.5–42.7) |
Abbreviations: APS, antiphospholipid syndrome; CI, confidence interval; PY, person‐years.
FIGURE 3.
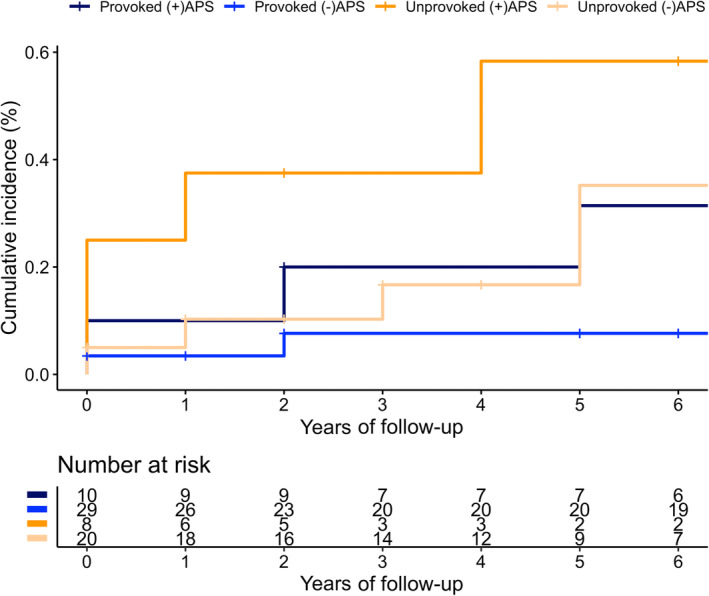
Cumulative incidence of recurrent venous thromboembolism (VTE) stratified by presence of provoking factor and antiphospholipid syndrome at index VTE with death as competing risk.
3.3. Influence of SLE activity at index VTE on risk of recurrent VTE
Table 3 shows the results of the cause‐specific Cox regression analysis estimating the influence of SLE activity at index VTE on the risk of recurrent VTE. When modeled as a continuous variable, the risk of recurrent VTE is decreased by 10% per SLEDAI‐2K index point, although not statistically significant. A consistent result was seen when the SLEDAI‐2K was modeled as a categorical variable with a cutoff of 4. A disease flare (i.e., SLEDAI‐2K greater than 4) at time of index VTE was associated with a lower risk of recurrent VTE (adjusted hazard ratio [HR], 0.4 [95% CI, 0.1–1.8]). However, this was again not statistically significant.
TABLE 3.
Influence of disease activity at time of index VTE at risk of recurrent VTE
| SLEDAI‐2K | Events N/total | Follow‐up (years) | Incidence rate recurrence per 100 PY (95% CI) | Unadjusted HR (95% CI) | Adjusted a HR (95% CI) |
|---|---|---|---|---|---|
| Continuous | — | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) | ||
| ≤4 | 5/12 | 58 | 8.6 (2.8–20.1) | Ref | |
| >4 | 9/46 | 387 | 2.3 (1.1–4.4) | 0.3 (0.1–1.0) | 0.4 (0.1–1.8) |
Abbreviations: CI, confidence interval; HR, hazard ratio; PY, person‐years; SLE, systemic lupus erythematosus; SLEDAI‐2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
Adjusted for presence of provoking factor and secondary APS at index VTE. SLEDAI‐2K score >4 is associated with active SLE (i.e., a flare).
3.4. Sensitivity analysis
Results of the best–worst‐case analysis to explore the range of uncertainty introduced by missing data in the presence of a provoking factor and SLE activity at index VTE are provided in the Supporting Information.
The point estimates for the incidences of recurrent VTE after provoked and unprovoked index VTE without APS in the best and worst case were quite similar (Tables S3–S5). The point estimates recurrences after provoked and unprovoked index VTE with APS were farther apart (Tables S3–S5). Overall, in both scenarios, trends were unchanged from the main analysis.
Regarding the influence of SLE activity (dichotomous) at index VTE on the risk of recurrent VTE, all four explored scenarios showed similar associations, with point estimates of nonsignificant adjusted HRs ranging from 0.5 to 0.8 (Table S6).
When restricting the analysis to only objectively confirmed index VTE, all results were in line with the main analysis (Table S7).
4. DISCUSSION
In this retrospective cohort study with 80 patients with SLE, one quarter of whom had a secondary APS, we have provided incidence estimates of recurrence after a first VTE. As expected, we found an incremental relationship between the existence of an unprovoked index VTE and secondary APS. The point estimate for recurrence risk was lowest in patients with a provoked index VTE without a secondary APS (i.e., 1.1 per 100 PY). The point estimate of provoked index VTE with a secondary APS was three times higher and similar to the point estimate of an unprovoked index VTE without secondary APS (i.e., 3.5 and 3.8 per 100 PY, respectively). Patients with an unprovoked index VTE with a secondary APS had the highest risk of recurrence, which was fourfold the risk of unprovoked index VTE without a secondary APS (i.e., 16.7/100 PY). Of note, CIs were imprecise and overlapping, except for unprovoked VTE with secondary APS and provoked VTE without secondary APS. However, not unexpectedly, this suggests that the recurrence risk is mainly determined by the presence of APS at index VTE. Furthermore, we explored the effect of SLE activity at index VTE on the risk of recurrent VTE. This analysis suggested that active disease (i.e., a flare) may act as a transient provoking factor and might be associated with a lower risk of recurrence. However, these results should be interpreted with caution because of the small sample size of this study.
Not many studies have looked into the risk of recurrent VTE in autoimmune diseases. We are not aware of any that have studied SLE specifically. The largest study included 1305 patients from the RIETE registry with different autoimmune disorders, including 97 patients with SLE. 11 The overall incidence of recurrent VTE in patients with the autoimmune disorders was higher than in our study (6.5 vs. 3.1/100 PY). This could likely be attributed to the fact that our population suffered from more provoked indexVTEs (50% vs. 43%), which were mainly estrogen‐containing oral contraceptives related, as could be expected from a population predominantly consisting of women in the reproductive age.
As mentioned before, the strength of our study is the use of real‐life clinical data. Patients with SLE are closely monitored at the hospital, allowing us to retrospectively collect data over a considerable length of follow‐up. Some concerns may arise regarding the quality of data in patients with the longest follow‐up, as older medical documentation may have been scantier. We considered this in the sensitivity analysis including only objectively confirmed index VTEs. Patients who contributed the longest follow‐up were the ones with nonobjectively confirmed index VTE. Excluding these patients did not change the results in comparison with the main analysis.
This study was limited in some aspects, among which was the small sample size, resulting in wide CIs. However, one should realize that this issue is hard to overcome. SLE is a rare disease with an incidence ranging from 0.9 to 5.5 per 100,000 PY. 13 The aforementioned study with data from the RIETE registry illustrated why it is hardly feasible to design a larger study. Of the eligible 51,913 patients with a first VTE, only 97 comprised patients with SLE.
To overcome the issue of a small sample size, we have included patients over a considerable period, namely, 1980 to 2019. During this period, the prescription of hydroxochloroquine has increased to improve the recurrence rate. Patients with a more recent first VTE are possibly more often treated with hydroxochloroquine. Hydroxochloroquine decreases the risk of thrombosis in patients with SLE, but its effect on risk of recurrent thrombosis is not known. We could not analyze the difference in recurrent VTE in patients who were treated with hydroxochloroquine at index VTE and those who did not. Also, we could not analyze the modifiable effect of hydroxochloroquine, initated after the index VTE.
Despite the small sample size, we have stratified the analyses by the presence of provoking factor and secondary APS at index VTE, as we considered these clinically relevant determinants of recurrent VTE. This has led to small subgroups and point estimates with rather wide CIs. These results should therefore be interpreted with caution. Ideally, we would have stratified by sex, but the proportion of men in this study was too small to do this reliably.
The retrospective nature of this study also limited the assessment of SLE activity at time of index VTE. Our method might have underestimated SLE activity and could therefore have led to an overestimation of the inverse association with recurrence risk. However, the SLEDAI‐2K is most suitable for explorative analysis in a retrospective study. 14 , 15 Data on changes in treatment regimen to define flare were not reliably available and therefore not collected.
There might have been an underestimation of the recurrence risk in this study. As depicted in the flowchart, 16 patients were not included in the analysis because anticoagulant therapy was continued indefinitely at the discretion of their treating physician. In a few patients, this was because of the presence of APS. In the remaining patients, the reason for continuation of anticoagulant therapy was not readily available in the medical charts. However, it is conceivable that this was because of an anticipated high recurrence risk.
Finally, there were some missing data in the presence of provoking factor and SLE disease activity at index VTE. Presumably, this missingness was not at random. Best‐ versus worst‐case analysis was performed as an attempt to account for this and did not lead to different results. Furthermore, we did not collect data on arterial thrombotic events and a population who continued anticoagulation.
The explorative inverse association between high SLE activity and recurrence risk should be interpreted with caution due to missing data but may introduce a new interesting hypothesis. The majority of index VTE occurred during active SLE in patients with available data on SLEDAI‐2K, which confirms the notion that a flare is a risk factor for VTE. A flare is temporary once adequately treated and may act as a transient provoking factor, similar to other known transient provoking factors (e.g., estrogen use and surgery). If a VTE occurs during SLE in remission, this could indicate that the nonmodifiable constitutional VTE risk of a person is higher, especially in the absence of other provoking factors, leading to a higher recurrence risk.
We acknowledge that we cannot provide strict recommendations for the duration of anticoagulant therapy for SLE‐associated VTE, as we did not investigate the bleeding risk of extended therapeutic anticoagulation therapy in this specific population. Data on this topic are scarce. However, we propose some clinical implications based on our data reflected against current guidelines. 15
First, our data are too preliminary to suggest that SLE disease activity should be taken into account in the decision for the duration of anticoagulant therapy. If an inverse association between SLE activity at index VTE with recurrence risk is confirmed in future research, it could be an argument against indefinite anticoagulant therapy in the presence of a high bleeding risk.
Overall, the evidence supports continuing anticoagulant treatment in patients with APS, as already recommended in international guidelines, 16 and supports discontinuation in patients with a provoked VTE without APS. Recurrence risk of unprovoked VTE without APS seems to be similar to the risk in the general population. This suggest that continuation of anticoagulant therapy should be considered.
5. CONCLUSION
This study indicated that APS is the main determinant for recurrence risk of a first SLE‐associated VTE. Our findings suggest that indefinite anticoagulant therapy may be warranted in patients with SLE with secondary APS. In the absence of APS treatment, decisions for SLE‐associated VTE may be approached similar to the general population. However, studies on the bleeding risk of anticoagulant therapy are necessary to make an adequate benefit–risk assessment. Furthermore, active SLE disease at the time of a VTE may act as a transient provoking factor, but this finding needs to be confirmed in future research.
AUTHOR CONTRIBUTIONS
Soerajja Bhoelan collected and cleaned the data, performed the analysis, interpreted the data, and drafted the manuscript. Jaime Borjas Howard designed the study, interpreted the data, and revised the manuscript critically for important intellectual content. Vladimir Tichelaar designed the study, interpreted the data, and revised the manuscript critically for important intellectual content. Paule van Daele provided the data and revised the manuscript critically for important intellectual content. Liesbeth Hak provided the data and revised the manuscript critically for important intellectual content. Alexandre Voskuyl provided the data and revised the manuscript critically for important intellectual content. Maarten Limper provided the data and revised the manuscript critically for important intellectual content. Robbert Goekoop provided the data and revised the manuscript critically for important intellectual content. Onno Teng provided the data. Jelle Vosters provided the data and revised the manuscript critically for important intellectual content. Marc Bijl provided the data and revised the manuscript critically for important intellectual content. Els Zirkzee provided the data and revised the manuscript critically for important intellectual content. Annemarie Schilder provided the data and revised the manuscript critically for important intellectual content. Hein Bernelot Moens provided the data and revised the manuscript critically for important intellectual content. Karina de Leeuw designed the study, interpreted the data, and revised the manuscript critically for important intellectual content. Karina Meijer designed the study, interpreted the data, and revised the manuscript critically for important intellectual content.
RELATIONSHIP DISCLOSURE
SB, JBH, VT, KdL, AV, PvD, JV, RG, OT, MB, EZ, HBM report no conflicts of interest. LH reports consulting fees form SOBI; payments were made to her institution. LH acts as secretary at the NVvAKI Dutch Society of Allergy and Clinical Immunology. This is an unpaid position. AS reports consulting fees from AbbVie and AstraZeneca, and lecture fees from Novartis and Eli LKM reports consulting fees from Uniqure, for participation in writing committee for gene therapy paper, speaker fees from Alexion, Bayer, and CLS Behring; participates in the Data Safety Monitoring Board of PCC trial by Octapharma; participates in a steering committee for a factor VIII study by Bayer; and is chair of the Dutch Society for Hematology; all payments were made to her institution. ML reports an unrestricted research grant from GSK, and fees for participation in advisory boards from GSK, Roche, and AstraZeneca; all payments were made to his institution. ML is chairman of the Dutch SLE/APS working group (ARCH) and a member of the scientific advisory board for the SLE patient organization NVLE. This is unpaid.
Supporting information
Tables S1‐S7
Bhoelan S, Borjas Howard J, Tichelaar V, et al. Recurrence risk of venous thromboembolism associated with systemic lupus erythematosus: A retrospective cohort study. Res Pract Thromb Haemost. 2022;6:e12839. doi: 10.1002/rth2.12839
Handling Editor: Dr Lana Antoinette Castellucci
Contributor Information
Soerajja Bhoelan, Email: b.s.bhoelan@umcg.nl, @soerajja94.
Jaime Borjas Howard, @JaimeBorjas11.
REFERENCES
- 1. Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi‐ethnic cohort. Ann Rheum Dis. 2009;68(2):238‐241. doi: 10.1136/ard.2008.093013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baglin T, Bauer K, Douketis J, Buller H, Srivastava A, Johnson G. Duration of anticoagulant therapy after a first episode of an unprovoked pulmonary embolus or deep vein thrombosis: guidance from the SSC of the ISTH. J Thromb Haemost. 2012;10(4):698‐702. doi: 10.1111/j.1538-7836.2012.04662.x [DOI] [PubMed] [Google Scholar]
- 3. Borjas‐Howard J, Leeuw K, Rutgers A, Meijer K, Tichelaar V. Risk of recurrent venous thromboembolism in autoimmune diseases: a systematic review of the literature. Semin Thromb Hemost. 2019;45(2):141‐149. doi: 10.1055/s-0038-1661387 [DOI] [PubMed] [Google Scholar]
- 4. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480‐1483. doi: 10.1111/jth.13336 [DOI] [PubMed] [Google Scholar]
- 5. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375(9715):657‐663. doi: 10.1016/S0140-6736(09)61963-2 [DOI] [PubMed] [Google Scholar]
- 6. Zöller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow‐up study from Sweden. Lancet. 2012;379(9812):244‐249. doi: 10.1016/S0140-6736(11)61306-8 [DOI] [PubMed] [Google Scholar]
- 7. Bhoelan BS, Borjas Howard JF, Tichelaar YIGV, Meijer K. Risk of recurrence after transient inflammation‐associated venous thromboembolism: similar to provoked, unprovoked or in‐between? Br J Haematol. 2020;190(6):e343‐e346. doi: 10.1111/bjh.16909 [DOI] [PubMed] [Google Scholar]
- 8. Tritschler T, Kraaijpoel N, Girard P, et al. Definition of pulmonary embolism‐related death and classification of the cause of death in venous thromboembolism studies: communication from the SSC of the ISTH. J Thromb Haemost. 2020;18(6):1495‐1500. doi: 10.1111/jth.14769 [DOI] [PubMed] [Google Scholar]
- 9. Yee C‐S, Farewell VT, Isenberg DA, et al. The use of systemic lupus erythematosus disease activity Index‐2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology. 2011;50(5):982‐988. doi: 10.1093/rheumatology/keq376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295‐306. doi: 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 11. Borjas Howard J, Ruiz‐Sada P, de Leeuw K, et al. Risk of recurrent venous thromboembolism in patients with autoimmune diseases: data from the Registro Informatizado de Enfermedad TromboEmbólica (RIETE) registry. Br J Haematol. 2021;194(1):195‐199. doi: 10.1111/bjh.17549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnaud L, Fagot J‐P, Mathian A, Paita M, Fagot‐Campagna A, Amoura Z. Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation‐wide population‐based study. Autoimmun Rev. 2014;13(11):1082‐1089. doi: 10.1016/j.autrev.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 13. Parodis I, Emamikia S, Gomez A, Gunnarsson I, van Vollenhoven RF, Chatzidionysiou K. Clinical SLEDAI‐2K zero may be a pragmatic outcome measure in SLE studies. Expert Opin Biol Ther. 2019;19(2):157‐168. doi: 10.1080/14712598.2019.1561856 [DOI] [PubMed] [Google Scholar]
- 14. Fitzgerald JD, Grossman JM. Validity and reliability of retrospective assessment of disease activity and flare in observational cohorts of lupus patients. Lupus. 1999;8(8):638‐644. doi: 10.1191/096120399680411443 [DOI] [PubMed] [Google Scholar]
- 15. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123(12):1794‐1801. doi: 10.1182/blood-2013-12-512681 [DOI] [PubMed] [Google Scholar]
- 16. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296‐1304. doi: 10.1136/annrheumdis-2019-215213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S7


