Abstract
Objective
The aim of this systematic review (SR) was to gather all available epidemiological evidence on former participation in any type of sport, at a professional and varsity level, as a potential risk factor for neurodegenerative diseases (NDs) and neurocognitive disorders (NCDs).
Design
Systematic searches were performed on PubMed, the Cochrane databases, and the ISI Web of Knowledge databases. Included studies were assessed using the NOS checklist.
Eligibility criteria for selecting studies
All epidemiological studies reporting data on the possible association between a clinical diagnosis of amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND), dementia or mild cognitive impairment (MCI), Parkinson’s disease (PD), chronic traumatic encephalopathy (CTE) at any stage and with any clinical pattern and the former participation in any types of sport at a varsity and professional level were included.
Results
Data from the 17 included studies showed a higher frequency of NDs and NCDs in former soccer and American football players. Updating the previous SR confirmed a higher frequency of ALS/MND in former soccer players. Data reported a significantly higher risk of dementia/AD in former soccer players, and of MCI in former American football players. Results also showed a significantly higher risk of PD in former soccer and American football players, and a significantly higher risk of CTE in former boxers and American football players.
Summary/conclusions
This SR confirmed a higher risk of NDs and NCDs in former professional/varsity athletes. However, the pathological mechanisms underlying this association remain unclear, and further high-quality studies should be performed to clarify whether the association could be sport specific.
Keywords: Systematic review, Neurodegenerative diseases, Risk factors, Athletes, Clinical epidemiology
Background
Previous epidemiological studies reported an association between professional sport and neurodegenerative diseases (NDs) and neurocognitive disorders (NCDs). These include a range of conditions primarily affecting brain and central nervous system (CNS) functioning [1, 2]. NDs are multifactorial, still incurable, and debilitating conditions resulting in the progressive degeneration and/or death of nerve cells [1]. The pathophysiological mechanisms underlying these conditions can differ, causing deficits ranging from memory loss and cognitive impairment to movement and gait deficits and impairments in language and breathing abilities [3]. Amyotrophic lateral sclerosis (ALS) represents the most common motor neuron disease (MND) and described as a rare progressive neuromuscular disease, typically causes the degeneration of both the upper and lower motor neurons but may also include cognitive and behavioral symptoms [4–6]. Parkinson’s disease (PD) is primarily characterized by a specific set of motor symptoms, including tremor, rigidity, and bradykinesia, and by an impairment of balance and postural reflexes. It can also cause several non-motor symptoms, such as gastrointestinal and urogenital disorders, and psychiatric symptoms, including depression [7]. NCDs is a broad term for defining several different chronic and progressive conditions affecting the ability to think, remember and make decisions. Symptoms may vary according to the type and severity of disease, and include memory loss, attention and communication deficits, and impairments in the ability to reason and solve problems [8, 9]. According to the DSM-5 dementia and mild NCD (MCI) are the main categories of NCDs. As for dementia, some subtypes are included among NDs, as they are caused by neurodegenerative mechanisms, while some types of dementia and other NCDs can be due to other causes, including secondary causes as intoxication or trauma [2]. The most common cause of ND dementia is Alzheimer's disease (AD), which is characterized by memory loss and specific deficits in language, sensory, motor and/or executive functions [8, 10]. Among the risk factors for dementia, the most known are genetic factors and aging. However, gender, educational level, and some health conditions are also included among risk factors [11].
Some studies investigated previous participation in some contact sports as a potential risk factor for neurodegenerative diseases (ND) and neurocognitive disorders (NCD) [12–14] focusing on pathological changes in former players [15, 16] in particular, soccer [14, 17–21] and American football players [12, 13]. Some small studies and reports have suggested that sports involving repeated traumatic brain injuries (TBI) may cause a long-term risk of NDs [22]. When considering specific NDs or NCDs, a relatively recent systematic review reported a significantly higher frequency of ALS in former professional and varsity soccer, and American football players [23]. The most frequently investigated potential sport-related risk factors for ALS and for all other NDs and NCDs, include multiple trauma and concussion [24, 25] along with oxidative stress [26] and exposure to toxic substances/chemicals (e.g., pesticides) [27, 28] or drugs/dietary supplements [28]. However, as for ALS, a more recent hypothesis also suggested that former athletes might share a phenotype causing simultaneously a higher athletic predisposition, with lower body mass index (BMI) and cardiovascular risk and higher physical fitness, and a higher risk of ALS [28, 29]. Another relatively recent systematic review, specifically focusing on long-term consequences of repeated concussions, reported that former participants in contact, collision, and combat sports seem to be at an increased risk of cognitive impairment and other mental health problems [30]. The review reported a higher frequency of cognitive decline and some NCDs and NDs in former professional athletes, considering NDs/NCDs along with diseases of the nervous system and sense organs, and other mental health conditions, including suicide and cognitive decline, hypothesizing multiple trauma and head injuries as potential causes of such condition. Multiple concussion and trauma and their potential role in the long-term onset of NDs or NCDs have been widely investigated, and are still a matter of debate, particularly in relation to popular sports such as soccer and rugby [31]. However, even though repeated head trauma has been linked to irreversible cognitive deficits [32–34] the pathophysiological mechanisms underlying this potential association are still to be clarified.
Traditionally, multiple concussion and head trauma had been known to cause the so-called ‘‘punch-drunk syndrome’’ firstly descripted in 1928 in retired boxers [35] which was associated to a basic lesion due to traumatic multiple hemorrhages, and replaced few years later by the wording ‘‘Dementia pugilistica’’ term coined in 1937 and which took into consideration in its definition the hypothesis of a type of dementia induced by brain trauma [36]. In the following decades, the scientific community focused the attention on the possible association between this type of sport and NDs, suggesting an etiological link between exposure to repeated head trauma and concussion and dementia and/or PD, even hypothesizing synergistic effects between head trauma and apolipoprotein-epsilon four in patients with Alzheimer's disease [37, 38]. In recent times, although the debate is still open, a study investigated the association between the former participation in contact and collision sports such as American football, boxing, soccer, rugby, and hockey, and a higher risk of chronic traumatic encephalopathy (CTE), highlighting a strong dose–response relationship between the duration of American football played and this pathology [39].
Based on all these hypotheses, evidence seems to suggest an association between former participation in professional sports, and mainly in contact and collision sports, with a higher risk of NDs or NCDs. Therefore, the aim of this systematic review (SR) was to gather all available epidemiological evidence on former participation in any type of sport, at a professional and varsity level, as a potential risk factor for NDs or NCDs.
Materials and methods
This SR was performed following the methodology of the Cochrane handbook for SRs [40] and reported based on the PRISMA statement for reporting SRs and meta-analyses [41]. Systematic searches for all available literature up to February 1, 2022, were performed on PubMed, the Cochrane Databases (that gives access to: Cochrane Database of Systematic Reviews, Embase, PubMed, CT.gov, ICTRP and CINAHL), and ISI Web of Knowlegde (that gives access to: Science Citation Index, Social Sciences Citation Index, Arts and Humanities Citation Index, Emerging Sources Citation Index, Conference Proceedings Citation Index–Science, Conference Proceedings Citation Index-Social Sciences and Humanities, Book Citation Index–Science, Book Citation Index–Social Sciences and Humanities, Current Chemical Reactions, and Index Chemicus). The following terms were used: ((neurodegenerative* OR neuro-degenerative* OR “amyotrophic lateral sclerosis” OR ALS OR “motor neuron disease” OR “motor neuron diseases” OR “motor-neuron disease” OR “motor-neuron diseases” OR parkinson* OR parkinsonism* OR Alzheimer* OR dementia* OR encephalopath* OR ((cognitive OR neurocognitive OR neuro-cognitive) AND (impairment* OR degenerati* OR disord*))) AND (((professional* OR competitive* OR competition* OR elite* OR varsity* OR college* OR “high school” OR “high-school”) AND (sport* OR player*)) OR athlete* OR rider* OR fighter* OR boxeur* OR boxer* OR runner* OR skier* OR rower* OR “martial art” OR “martial arts” OR “martial artist” OR “martial artists” OR “combat sport” OR “combat sports” OR “players”)). Further relevant articles were also identified by reviewing the references of considered studies. No limitations were applied for date of publication nor study design; only studies published in English were considered. Retrieved literature was screened by two independent reviewers (GB, PP), and studies were selected based on their pertinence and relevance to the topic of the review. The full texts of selected studies were assessed for inclusion by applying the following predefined eligibility criteria. Potential disagreements were resolved by discussion or, where needed, involvement of a third reviewer (EL). All case–control and cohort studies addressing the possible association between any types of sport practiced as a varsity and/or a professional athlete and the risk of NDs and/or NCDs, were included. Since studies on this topic are performed using any type of available data, including data from structured information sources, ranging from death certificates to biological tissue banks, we included all studies reporting a diagnosis of any of the considered condition based on any type of criteria, including clinical criteria and neurobiological criteria. Therefore, all studies reporting by any means (e.g., death certificates, clinical records) a clinical, neurobiological or neuropathological diagnosis of ALS or motor neuron disease (MND), dementia of any type or mild cognitive impairment (MCI), PD, CTE at any state of disease and with any clinical pattern were also included considering the wider diagnostic category. Case reports, case series, non-systematic or narrative reviews, letters, commentaries, and editorials were excluded. Only epidemiological studies (i.e., cohort and case–control) reporting data on the frequency of any of the considered NDs and/or NCDs were included.
For studies analyzing the association between professional and/or varsity sports and ALS, we updated a previously published systematic review [23] therefore only studies published from January 2016 to February 2022 were included.
Studies meeting the eligibility criteria were qualitatively assessed using the Newcastle–Ottawa Scale (NOS) for quality assessment of non-randomized studies [42]. The NOS scale is structured in 8 items assessing the adequacy of 3 major domains: selection of the study group, comparability of the groups, and ascertainment of the exposure of interest for case–control studies or of the outcome of interest for cohort studies. A maximum of 9 stars can be assigned to each assessed study, and specifically a maximum of 4 stars for the Selection domain, 2 stars for the Comparability domain, and 3 stars for either the Outcome or the Exposure domain. The potential presence of other biases and/or methodological flaws was also considered in assessing the included studies. Data were extracted using structured tables. Specific information was gathered on the type of sport considered by each study, along with how the enrolled population of athletes was defined, and on the diagnostic criteria and procedure used to define the identified cases. Moreover, for cohort studies, specific information was also extracted on the source of the reference population. Gathered information also included study design, year of publication, characteristics of the included population, definition of the exposure(s), diagnostic criteria, length of the follow-up, and results for each analysis.
Results
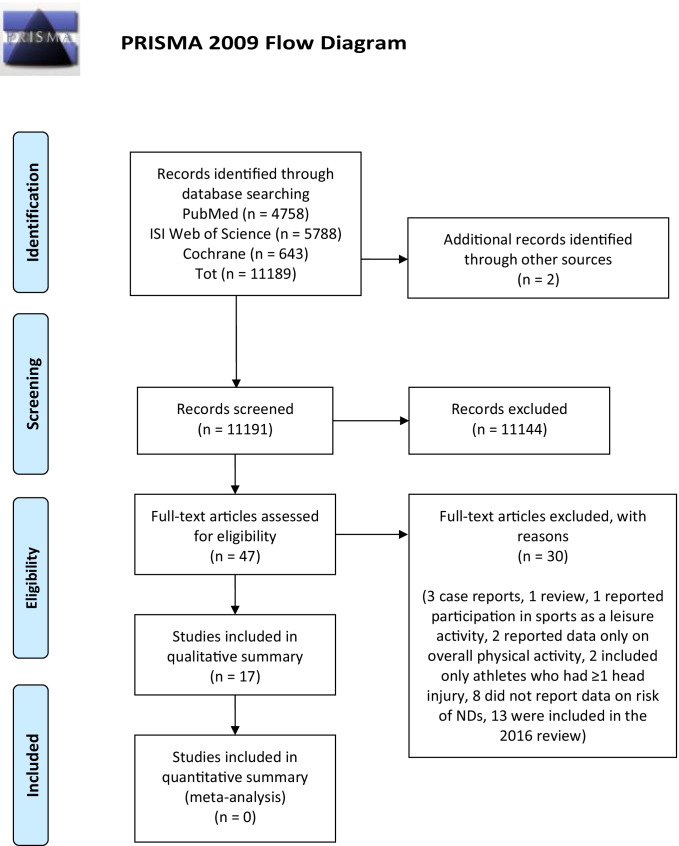
Structured search on bibliographic databases yielded 11,189 articles. Two further studies were identified by reviewing the bibliographic references of included studies [43, 44]. After selection based on the pertinence and relevance to the topic of the review, 11,191 records were excluded, leaving 47 articles meeting the selection criteria. The full texts of the selected articles were assessed for inclusion, and 30 further articles were excluded as 3 were case reports [17, 19, 45], 1 review [46], 1 reported participation in sports as a leisure activity [47] 2 reported data only on an overall measure of physical activity [48, 49] 2 included only athletes who had ≥ 1 head/brain injury [50, 51] 8 did not report any measure of risk of NDs and/or NCDs [20, 52–58] and 13 were already included in the 2016 review [59–71].
The flow diagram of the literature is reported in Fig. 1.
Fig. 1.
Flow diagram of literature
A total of 17 articles were included in the qualitative summary [43, 44, 72–86].
Overall, five cohort studies reported data on the frequency of all NDs/NCDs included in this review in former professional or varsity athletes [74, 77, 79, 82, 84]. Considering each single pathology, 10 cohort studies [82, 84] and 1 case–control study [44] reported data on the frequency of dementia/MCI in former athletes, 8 cohort studies [43, 73–75, 77, 79, 82, 84], and 1 case–control study [44] reported data on the frequency of PD or Parkinsonism, 1 cohort study [80] reported data on CTE, and 7 new cohort studies [77, 82–86] and 1 new case–control study [81] reported data on the frequency of ALS or MND. The summary of the main characteristics, quality assessment and results from the included studies were reported according to each considered outcome in Tables 1, 2, 3, 4, and 5.
Table 1.
Summary of results from studies reporting data on the frequency of any ND and/or NCD
| Characteristics | Quality assessment | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. of subjects | Results | |||||||||
| Fist author, year | Sport | Source of cases | Reference population | Exposure | Selection | Comparability | Outcome/exposure | n. cases among the exposed/total exposed | n. cases among the reference group/ total reference |
Data ORs/HRs (95%CIs) or SIRs/PRs/SMRs/SPMRs |
| Cohort studies | ||||||||||
| Russell 2021 [84] | Soccer | Hospital records, prescriptions and death certificates with ICD-9 and ICD-10 codes | Random sample of matched individuals (3:1 ratio) from the community health index database | Having been a soccer player in the Scottish League as documented in the archives of the Scottish Football Museum and individual league clubs | *** | ** | ** | 386/7676 players | 366/23028 non players |
Frequency of all NDs/NCDs diagnosis HR 3.66 (95% CI 2.88–4.65) p < 0.001 |
| Mackay 2020 [82] | Soccer | Death certificates with ICD-9 and ICD-10 codes (primary and contributory cause), and prescriptions for dementia from electronic health records | Controls from general population matched to the players according to sex, age, and degree of social deprivation | Former professional soccer player born before January 1, 1977 | ** | ** | ** | 222/7676 players |
238/23028 non players |
*HR for Death (95% CI) HR-U: 4.10 (2.88–5.83) < 0.001 HR-U&C: 3.53 (2.72–4.57) p: < 0.001 |
| Nguyen 2019 [79] |
American football baseball |
Death certificates from 1979 to 2013 classified with ICD underlying (U) and underlying or contributing (U&C) | MLB cohort: from LBD of all players that appeared in ≥ 1 game at a professional level in 1871–2006 | NFL cohort: from NIOSH consisting in players who played ≥ 1 season in 1959–1988 | ** | ** | ** |
NFL U: 22/3419 U&C: 39/3419 |
MLB U: 13/2708 U&C: 16/2708 |
HR (95%CI) U: 2.07 (1.01–4.23) U&C: 2.99 (1.64–5.45) |
| Janssen 2017 [77] |
American football basketball swimming wrestling |
3-step procedure: screening of medical index through REP tool, review of diagnoses, diagnosis confirmed by senior neurologist | Varsity athletes (football vs non-football) enrolled in the Mayo and Rochester High Schools in 1956 to 1970 | Varsity activity in football, basketball, swimming, or wrestling documented in the yearbooks | ** | * | ** | 10/296 football players | 8/190 non-football athletes |
frequency all NDs/NCDs football: 10/296 (3%) non-football: 8/190 (4%) |
| Lehman, 2012 [74] | American football | Death certificates with ICD code for ALS in effect at death | US male mortality rates (1960–2007) for 119 cause of death categories | Having played in the National Football League for at least 5 seasons between 1959 and 1988 | **** | ** | * |
U: 10/3439 players U&C: 17/3439 players |
– |
All NDs/NCDs (standardized mortality rates) SMR U: 2.83 (1.36–5.21) SMR U&C: 3.26 (1.90–5.22) SMR-non-speed: 1.58 (0.33–4.61), 3 cases SMR-speed: 4.74 (2.59–7.95), 14 cases |
MLB US Major League Baseball, NFL National Football League, NIOSH National Institute for Occupational Safety and Health, LBD Lahman Baseball Database, HR Hazard ratio, SMR standardized mortality ratio, CI confidence interval, U underlying, U&C underlying and contributing
Table 2.
Summary of results from studies reporting data on the frequency of ALS or MND
| Characteristics | Quality assessment | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. of subjects | Results | |||||||||
| Fist author, year | Sport | Source of cases | Reference population | Exposure | Selection | Comparability | Outcome/Exposure | n. cases among the exposed/total exposed |
n. cases among the reference group/ total reference |
Data ORs/HRs (95%CIs) or SIRs/PRs/SMRs/SPMRs |
| Cohort studies | ||||||||||
| Daneshvar 2021 [86] | American football | Diagnoses and death records from news reports and/or obituaries matched to NFL records and National Death Index |
Centers for Disease Control and Prevention National Institute for Occupational Safety and Health Life Table Analysis System |
Having played at least 1 regular season as a professional in the NFL from 1970 to 2019 | *** | * | *** | 55/19423 players | – |
SIR 3.59 (95% CI 2.58–4.93) SMR 3.94 (95% CI 2.62–5.69) |
| Russell 2021 [84] | Soccer | Hospital records, prescriptions and death certificates with ICD-9 and ICD-10 codes | Random sample of matched individuals (3:1 ratio) from the community health index database | Having been a soccer player in the Scottish League as documented in the archives of the Scottish Football Museum and individual league clubs | *** | ** | ** | 24/7676 players | 23/23028 non-players |
frequency of MND diagnosis HR 3.52 (95% CI 1.81–6.88) p < 0.001 |
| Gamez 2021 [85] | Soccer | Personal archives from a leading ALS Unit, PubMed, specialized soccer websites, self-reports of patients in the media | Male population of Catalonia | Having played in the first, second and third tiers of the Spanish league between 1968 and 2013, and who developed ALS between 2000 and 2020 | ** | - | * | 7/10780 players | – | SIR 2.11 (95% CI 0.85–4.37) |
| Mackay 2020 [82] | Soccer | Death certificates with ICD-9 and ICD-10 codes (primary and contributory cause) for MND from electronic health records | controls from the general population who were matched to the players based on sex, age, and degree of social deprivation | Former professional soccer players born before January 1, 1977 | ** | ** | ** | 22/7676 players | 17/23028 non-players |
MND HR for death (95% CI) 4.33 (2.05–9.15) p: < 0.001 |
| Pupillo 2020 [83] | Soccer | cases identified using different sources: Google, 2 published books, a recent scientific report | ISTAT mortality tables (since 1975) | Professional soccer players practicing in the period 1959–2000 (Panini almanacs) | ** | * | * | 34/23586 players | - |
ALS overall SIR: 1.91 (95% CI 1.32–2.67) Subjects aged < 45 years SIR: 4.66 (95% CI 2.66–7.57) League A SIR: 5.69 (95%CI 2.73–10.47) |
| Nguyen 2019 [79] |
American football baseball |
Death certificates from 1979 to 2013 classified with ICD underlying (U) and underlying or contributing (U&C) | MLB cohort: from LBD of all players that appeared in ≥ 1 game at a professional level in 1871–2006 | NFL cohort: from NIOSH consisting in players who played ≥ 1 season in 1959–1988 | ** | ** | ** |
NFL U: 9/3419 U&C: 10/3419 |
MLB U: 3/2708 U&C: 3/2708 |
ALS HR (95%CI) U: 2.81 (0.75–10.51) U&C: 3.10 (0.84–11.38) |
| Janssen 2017 [77] |
American football basketball swimming wrestling |
3-step procedure: screening of medical index through REP tool, review of diagnoses, diagnosis confirmed by senior neurologist | Varsity athletes (football vs non-football) enrolled in the Mayo and Rochester High Schools in 1956 to 1970 | Varsity activity in football, basketball, swimming or wrestling documented in the yearbooks | ** | * | ** | 0/296 football players | 0/190 non-football athletes |
ALS IRR football: 0.00 (0.00–1.92) non-football: NA p NA |
| Case–control studies | ||||||||||
| Filippini 2020 [81] |
Any sport Soccer Volleyball Cycling Swimming |
Recruited either at the Neurology Units of the study area or by contacting them by phone and/or mail Cases from 2 registries (ERRALS and PARALS) diagnosed with ‘definite’ and ‘probable’ ALS based on El Escorial revised criteria |
National Health Service directory of the residents in the study provinces Random controls, matched by sex, age (± 5) and province of residence, from 4 populations |
Having played a sport at a competitive level | ** | ** | - |
Cases 95/230 Controls 135/230 |
- |
Any sport Cases 10 vs controls 22 adj OR 0.50 (0.22–1.17) Soccer Cases 6 vs controls 6 adj OR 1.19 (0.35–4.02) Volleyball Cases 1 vs controls 4 adj OR 0.28 (0.03–2.64) Cycling Cases 1 vs controls 5 adj OR 0.31 (0.03–2.94) Swimming Cases 1 vs controls 1 adj OR 1.61 (0.09–27.33) |
MLB US Major League Baseball, NFL National Football League, SIR standardized incidence ratio, SMR standardized mortality ratio, HR hazard ratio, IRR incidence rate ratio, OR odds ratio, NA not applicable, CI confidence interval, U underlying, U&C underlying and contributing
Table 3.
Summary of results from studies reporting data on the frequency of MCI and dementias
| Characteristics | Quality assessment | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. of subjects | Results | |||||||||
| Fist author, year | Sport | Source of cases | Reference population | Exposure | Selection | Comparability | Outcome/exposure | n. cases among the exposed/total exposed | n. cases among the reference group/total reference | Data ORs/HRs (95%CIs) or SIRs/PRs/SMRs/SPMRs |
| Cohort studies | ||||||||||
| Russell 2021 [84] | Soccer | Hospital records, prescriptions and death certificates with ICD-9 and ICD-10 codes | Random sample of matched individuals (3:1 ratio) from the community health index database | Having been a soccer player in the Scottish League as documented in the archives of the Scottish Football Museum and individual league clubs | *** | ** | ** | 338/7676 players | 312/23028 non-players |
frequency of dementia (not otherwise specified) diagnosis HR 3.59 (95% CI 2.93–4.39) p < 0.001 |
| Mackay 2020 [82] | Soccer |
Electronic health records to obtain data on death certification and medications typically prescribed for the treatment of dementia ICD-9 and ICD-10 codes that were used to identify outcomes as death with neurodegenerative disease listed as the primary or a contributory cause |
Controls from general population matched to players based on sex, age, and degree of social deprivation | Former professional soccer player born before January 1, 1977 | ** | ** | ** |
Dementia 180/7676 players 178 non-players AD 64/7676 players non-AD dementia 121/7676 players |
Dementia 178/23,028 non-players AD 47/23,018 non-players Non-AD dementia 133/23,018 non-players |
dementia HR for Death (95% CI): 3.87 (2.86–5.24) p: < 0.001 AD HR for Death (95% CI): 5.07 (2.92–8.82) p: < 0.001 non-AD dementia HR for Death (95% CI): 3.48 (2.42–5.00) p: < 0.001 |
| Nguyen 2019 [79] |
American football baseball |
Death certificates from 1979 to 2013 classified with ICD underlying (U) and underlying or contributing (U&C) | MLB cohort: from LBD of all players that appeared in ≥ 1 game at a professional level in 1871–2006 | NFL cohort: from NIOSH consisting in players who played ≥ 1 season in 1959–1988 | ** | ** | ** |
NFL U: 6/3419 players U&C: 16/3419 players |
MLB U 7/2708 players U&C 10/2708 players |
Dementia/AD HR (95%CI) U: 1.28 (0.41–4.07) U&C: 2.26 (0.99–5.17) |
| Willer 2018 [78] |
American football hockey |
Comprehensive neuro-cognitive assessment | Age-matched noncontact sport athlete controls | Retired professional hockey and football athletes (average age 56 years) | ** | * | * | 8/21 contact athletes | 3/21 non-contact athletes |
MCI frequency contact sport vs noncontact sport 8/21 vs 3/21 p = 0.08 |
| Janssen 2017 [77] |
American football basketball swimming wrestling |
3-step procedure: screening of medical index through REP tool, review of diagnoses, diagnosis confirmed by senior neurologist | Varsity athletes (football vs non-football) enrolled in the Mayo and Rochester High Schools in 1956 to 1970 | Varsity activity in football, basketball, swimming, or wrestling documented in the yearbooks | ** | * | ** | 7/296 football players | 5/190 non-football athletes |
> 1 year of playing football: 4.99 (1.62–11.65) non-football: 3.24 (0.88–8.28) IRR: 1.54 (0.50–3.60), p = 0.26 |
| Vann Jones 2013 [76] | Soccer | Test Your Memory, self-administered questionnaire | Frequency also compared to data from a large UK-based MCI prevalence study | Having been a soccer player from Former Player Associations (FPAs) of 4 professional football clubs in the UK | ** | * | * |
MCI—possible dementia 10/92 soccer players |
– |
Overall MCI/dementia 10/92 MCI: 8/92 dementia: 2/92 No significant difference in cognitive impairment prevalence between ex-professional footballers and a large sample of men in Wales |
| Lehman 2012 [74] | American football | Death certificates with ICD code for AD in effect at death | US male mortality rates (1960–2007) for 119 cause of death categories | Having played in the National Football League for at least 5 seasons between 1959 and 1988 | **** | ** | * |
U: 2/3439 players C: 7/3439 |
– |
AD SMR-U: 1.80 (0.22–6.50) SMR-U&C: 3.86 (1.55–7.95) |
| Savica 2012 [75] | American football | Medical records confirmed by a neurologist |
Having been a band, glee club, or choir member as for the yearbooks of the only 2 high schools in Rochester in 1946–1956 Expected incidence calculated based on previously published rates for that area |
Having been a football player as for the yearbooks of the only 2 high schools in Rochester in 1946–1956 | *** | ** | ** | 13/438players | 2/140 non-players |
Dementia HR (95%CI) 1.58 (0.36–7.01) (p = 0.55) SIR (95%CI) observed vs expected players: 0.72 (0.38–1.23) non-players: 0.47 (0.05–1.68) |
| Guskiewicz 2005 [72] | American football |
AD diagnosed by clinicians MCI diagnosed by clinician, self-reported or reported by informant |
General US Population (AD) | Members of the National Football League Retired Player’s Association | ** | * | * |
AD 33/2252 MCI diagnosed 22/758 MCI reported by informant 77/758 |
– |
AD 33/2252 overall age-adjusted prevalence ratio 1.37 (95% CI 0.98–1.56) higher in (≤ 70 years) 22 diagnosed MCI and 77 memory impairment |
| Schulte 1996 [43] | Athletes (any) | Death certificates with ICD-9 code for NDs | Deaths for all causes 1982–1991, and proportion of NDs deaths in all occupations | occupation and industry coded as “writers, artists, entertainers, and athletes” according to the 1980 Bureau of the Census classification | **** | ** | * | 265 deaths (white female writers, artists, entertainers, or athletes)/130420 total deaths | – |
AD PMR: 135 (95% CI 111–162) |
| CASE–CONTROL STUDIES | ||||||||||
| Park 2005 [44] |
Athletes (any) dancers |
Death certificates with ICD-9 code for NDs | Deaths for all other causes (excluding CNS neurologic, degenerative, and neoplasms of brain, lymphatic, or hematopoietic tissue) | Certificates, coded according to the 1980 Bureau of Census (BOC) system | *** | ** | ** |
Pre-senile dementia 27,374/2,614,346 AD 47,783/2,614,346 (total n° of deaths 2,614,346) |
MOR (95%CI) presenile dementia: 0.93 (0.46–1.66) AD: 1.46 (0.96–2.10) |
|
MLB US Major League Baseball, LBD Lahman Baseball Database, HR hazard ratio, IRR incidence rate ratio, SMR standardized mortality ratio, SIR standardized incidence ratio, PMR proportionate mortality ratio, MOR mortality odds ratio, CI confidence interval, U underlying, U&C underlying and contributing
Table 4.
Summary of results from studies reporting data on the frequency of PD
| Characteristics | Quality assessment | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. of subjects | Results | |||||||||
| Fist author, year | Sport | Source of cases | Reference population | Exposure | Selection | Comparability | Outcome/exposure | n. cases among the exposed/total exposed | n. cases among the reference group/total reference | Data ORs/HRs (95%CIs) or SIRs/PRs/SMRs/SPMRs |
| Cohort studies | ||||||||||
| Russell 2021 [84] | Soccer | Hospital records, prescriptions and death certificates with ICD-9 and ICD-10 codes | Random sample of matched individuals (3:1 ratio) from the community health index database | Having been a soccer player in the Scottish League as documented in the archives of the Scottish Football Museum and individual league clubs | *** | ** | ** | 28/7676 players | 45/23028 non players |
frequency of PD diagnosis HR 2.09 (95% CI 1.20–3.61) p = 0.009 |
| Mackay 2020 [82] | Soccer | Death certificates with ICD-9 and ICD-10 codes (primary and contributory cause) for PD from electronic health records | Controls from general population matched to the players according to sex, age, and degree of social deprivation | Former professional soccer players born before January 1, 1977 | ** | ** | ** | 28/7676 players |
44/23028 non players |
PD HR for death (95% CI): 2.15 (1.17–3.96) p = 0.01 |
| Nguyen 2019 [79] |
American football baseball |
Death certificates from 1979 to 2013 classified with ICD underlying (U) and underlying or contributing (U&C) | MLB cohort: from LBD of all players that appeared in ≥ 1 game at a professional level in 1871–2006 | NFL cohort: from NIOSH consisting in players who played ≥ 1 season in 1959–1988 | ** | ** | ** |
NFL U: 3/2708 U&C: 14/3419 |
MLB U: 3/2708 U&C: 5/2708 |
HR (95%CI) U: 3.08 (0.76–12.42) U&C: 3.45 (1.21–9.83) |
| Janssen 2017 [77] |
American football basketball swimming wrestling |
3-step procedure: screening of medical index through REP tool, review of diagnoses, Diagnosis confirmed by senior neurologist | Varsity athletes (football vs non-football) enrolled in the Mayo and Rochester High Schools in 1956 to 1970 | Varsity activity in football, basketball, swimming or wrestling documented in the yearbooks | ** | * | ** | 3/296 football players | 3/190 non-football athletes |
Incidence rate ratio (IRR) parkinsonism (95% CI) football: 2.08 (0.57–5.32) Non-football:0.86 (0.23–2.19) p > 0.99 |
| Lehman 2012 [74] | American football | Death certificates with ICD code for PD in effect at death | US male mortality rates (1960–2007) for 119 cause of death categories | Having played in the National Football League for at least 5 seasons between 1959 and 1988 | **** | ** | * |
U: 2/3439 players C: 3/3439 players |
– |
PD SMR-U: 2.14 (0.26–7.75) SMR-U&C: 4.31 (1.73–8.87) |
| Savica 2012 [75] | American football | Medical records confirmed by a neurologist |
Having been a band, glee club or choir member as for the yearbooks of the only 2 high schools in Rochester in 1946–1956 Expected incidence calculated based on previously published rates for that area |
Having been a football player as for the yearbooks of the only 2 high schools in Rochester in 1946–1956 | *** | ** | ** | 10/438 players | 5/140 non-players |
HR (95%CI) 0.48 (0.17–1.42) (p = 0.19) SIR (95%CI) observed vs expected players: 2.36 (1.13–4.34) non-players: 4.94 (1.61–11.51) |
| Lolekha, 2010 [73] | Boxing |
2-phase procedure: screening with validated Tanner’s questionnaire and Sevillano weighted score and diagnosis with UKPDSBB clinical diagnostic criteria |
Several populations | Registered retired boxers from the Sports Authority of Thailand, the Boxer’s Club of Thailand and the old Thai Boxing Foundation | *** | * | – | 8/704 boxers | – |
8/704 Parkinsonism (5 PD, 1 PSP, 2 vascular parkinsonism) crude prevalence: 0.71% (95% CI 0.09–1.33) comparable to general populations |
| Schulte 1996 [43] | Athletes (any) | Death certificates with ICD-9 code for NDs | Deaths for all causes 1982–1991, and proportion of NDs deaths in all occupations | Occupation and industry coded as “writers, artists, entertainers, and athletes” according to the 1980 Bureau of the Census classification | **** | ** | * | 128 deaths (white female writers, artists, entertainer, or athletes)/130420 total deaths | PMR: 128 (95% CI 107–152) | |
| Case–control studies | ||||||||||
| Park, 2005 [44] |
Athletes (any) dancers |
Death certificates with ICD-9 code for NDs | Deaths for all other causes (excluding CNS neurologic, degenerative, and neoplasms of brain, lymphatic or hematopoietic tissue) | Certificates, coded according to the 1980 Bureau of Census (BOC) system | *** | ** | ** |
33,678 PD total deaths: 2,614,346 |
– | MOR 1.26 (95% CI 0.76–1.97) |
MLB US Major League Baseball, LBD Lahman Baseball Database, HR hazard ratio, IRR incidence rate ratio, SMR standardized mortality ratio, SIR standardized incidence ratio, PMR proportionate mortality ratio, MOR mortality odds ratio, CI confidence interval, U underlying, U&C underlying and contributing, PSP progressive supranuclear palsy
Table 5.
Summary of results from studies reporting data on the frequency of CTE
| Characteristics | Quality assessment | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. of subjects | Results | |||||||||
| Fist author, year | Sport | Source of cases | Reference population | Exposure | Selection | Comparability | Outcome/exposure | n. cases among the exposed/total exposed | n. cases among the reference group/total reference | Data ORs/HRs (95%CIs) or SIRs/PRs/SMRs/SPMRs |
| Cohort studies | ||||||||||
| Bieniek 2019 [80] |
Baseball Basketball Boxing American football Hockey Soccer Wrestling |
Mayo Clinic Tissue Registry | consecutive autopsies from the Mayo Clinic Tissue Registry with available information on sport participation | information on sport participation and participation age/level/duration taken from high school yearbooks or obituaries | *** | – | *** |
300 athletes CTE positive:r 15 athletes CTE features: 12 |
450 non-athletes CTE positive: 6 CTE features: 9 |
OR* for combined CTE pathology (males) adj for age at death, baseball, boxing, and American football American football beyond high school OR = 13.23 (4.23, 41.36) p < 0.001 OR for combined CTE positive (CTE +) (males) adj for boxing and American football boxing OR = 10.29 (1.79, 59.28) p = 0.009 American football OR = 2.87 (1.17, 7.01) p = 0.021 American football beyond high school OR = 6.84 (1.76, 26.66) p = 0.0056 |
OR odds ratio
*Only adjusted data reporting statistically significant differences have been reported
Methodological quality
The overall methodological quality of included studies was moderate. The highest score achieved was 7, while the lowest score was 3. Five cohort studies achieved a score of 7 [43, 74, 75, 84, 86] while 4 studies, 3 cohort [79–81] and 1 case–control [44] were assigned 6 stars, 2 cohort studies were assigned 5 stars [73, 77], 5 studies, 4 cohort [72, 76, 78, 83] and 1 case–control [81] were assigned 4 stars, and 1 cohort study [85] was assigned 3 stars.
Selection
Only two cohort studies reached 4 stars, which is the highest possible score, in this domain, while most studies were scored 2 stars. All included cohort studies enrolled professional or varsity athletes from secure records (e.g., sports leagues, yearbooks), thus the enrolled exposed cohort is often adequately defined and representative, and data on exposure are drawn from secure sources. However, the unexposed cohort is often enrolled from different sources from the exposed cohort, and most of the studies did not or could not (e.g., studies using census data) provide information on the absence of the outcome at the beginning of the study.
As for the two case–control studies, cases were enrolled through either registries or death certificates. Therefore, samples were representative, but the definition of cases did not have an independent validation. In both studies, controls were enrolled from the community, as they were either randomly selected from general population or selected among death certificates due to other non-degenerative causes. However, both studies could not and did not ascertain the absence of the outcome at the start of the study.
Comparability
Most studies reached 2 stars (n = 8), which is the highest possible score, in this domain, while 6 studies were assigned 1 star, and 2 studies had no stars [73, 80]. Most studies were either matched or had the analysis adjusted for age [72, 76–78, 83] and/or for other variables. In some studies, matched controls were selected from general population, while in some other studies controls were either other athletes [77–79] or students from the same schools [75]. In case–control studies, controls were either matched for sex, age, and province of residence [81] or selected based on the availability of enough information for adjusting all analyses [44].
Exposure/outcome
Only 2 cohort studies reached 3 stars in this domain, which is the highest possible score [80, 86] while 6 studies had 2 stars, 6 studies had 1 star, and 1 case–control study and 1 cohort study had no stars [81, 85].
Most of the cohort studies used structured information from death certificates, clinical records, registries, or record-linkage. Of the five remaining studies, 2 studies obtained data on the outcome by clinicians [72, 78] 2 from questionnaires [73, 77] and 2 only from databases or search engines (i.e., PubMed, Google) or books and magazines [83, 85]. Using secure sources such as hospital records and certificates or using an independent validation of the diagnosis to confirm the outcome can minimize the potential for inaccurate information. However, even if death certificates are an extremely valuable source of data, their accuracy in reporting deaths due to NDs/NCDs can be inaccurate. In fact, deaths due to the considered NDs/NCDs were shown to be underreported as primary cause of death [87–94] and studies on mortality are often of poor methodological quality [95].
Moreover, most of the included studies used, for all the considered outcomes, widely heterogeneous diagnostic criteria. Using a heterogeneous set of criteria prevent the direct comparison of data, as different criteria can lead to different prevalence and incidence rates [96–98].
Another issue was the length of the follow-up, as at least part of the included sample in most studies using administrative data had a follow-up length insufficient to observe the considered outcome.
As for case–control studies, one [44] had a secure source for ascertaining the exposure, while the other [81] used an unstructured questionnaire and did not use the same method for cases and controls. Moreover, neither reported the response rate for both cases and controls. Moreover, most of the studies did not report the number of participants lost to follow-up.
Qualitative analysis
Results from the included studies were reported according to outcome. A summary of the characteristics and results from each study are reported in Tables 1, 2, 3, 4, and 5.
All NDs/NCDs
Only 5 cohort studies reported data on the overall risk of any type of NDs and/or NCDs in former professional/varsity athletes. Specifically, 3 of the 5 cohort studies reported the risk of NDs/NCDs in either former professional American football players [74, 79] or former varsity American football players [77] while the remaining 2 studies reported the risk of NDs/NCDs in former professional soccer players [82, 84]. Both studies on professional American football players enrolled former players from the National Football League (NFL) and used death certificates to calculate their risk of death due to NDs/NCDs as underlying (U) and/or contributing (C) cause of death. The studies reported an overall higher standardized mortality ratio (SMR) due to NDs/NCDs in former NFL players (SMR-U 2.83, 95%CI 1.36–5.21; SMR-U&C 3.26, 95%CI 1.90–5.22) [74] with a specifically higher risk of NDs/NCDs in former NFL players compared to baseball players (HR-U 2.07, 95%CI 1.01–4.23; HR-U&C 2.99, 95%CI 1.64–5.45) [79]. A significantly higher risk of death due to NDs/NCDs was also observed in the 2 studies enrolling former professional soccer players, with HRs ranging from 3.53 to 4.10 (HR-U 4.10, 95%CI 2.88–5.83; HR-U&C 3.53, 95%CI 2.72–4.57; HR 3.66, 95%CI 2.88–4.65) [82, 84]. No risk measure was calculated for former varsity athletes, but the study observed that 3% of the enrolled football athletes reported a diagnosis of NDs/NCDs compared to 4% of the athletes enrolled in the basketball, swimming, or wrestling teams [77].
ALS or MND
The previous review [23] on the risk of ALS and MND in former professional and varsity athletes reported a significantly higher frequency of ALS and MND in professional soccer and American football players, and a slightly increased risk of ALS in varsity athletes. The update of the SR allowed to identify 6 new cohort studies [77, 79, 82–85] and 1 new case–control study [81]. When considering professional athletes, 5 large cohort studies reported a significantly higher risk of ALS and MND in former soccer and American football players (MND: HR 3.52, p < 0.001; HR 4.33, p < 0.001; ALS: SIR 1.91; ALS: SIR 2.11; ALS: SIR 3.59) [82–86] compared to either general population [79–82] or matched controls [78]. Two studies specifically reported a significantly higher risk in subjects aged < 45 (SIR: 4.66, 95% CI 2.66–7.57) years and in athletes playing in the top league (SIR 5.69, 95%CI 2.73–10.47) [83] and in athletes having longer careers (OR 1.2, 95% CI 1.1–1.3) [86]. One study also reported a higher risk of death due to ALS compared to general population (SMR 3.94) [86]. Another cohort study used death certificates and did not report a higher risk of death due to ALS in former NFL players compared to baseball players [79]. The last cohort study enrolled varsity athletes from yearbooks and compared the frequency of ALS (i.e., healthcare information system) between football and non-football player, reporting no cases of ALS during its follow-up period [77]. The only, relatively small, case–control study enrolled cases from 2 registries and matched controls from general population and did not observe a significantly higher risk of ALS in athletes (i.e., any type of sport) compared to non-athletes [81].
Dementia and/or MCI
Ten cohort studies [43, 72, 74–79, 82, 84] and 1 case–control study [44] reported data on the risk of dementia in former professional and/or varsity athletes. Seven cohort studies [72, 74–76, 78, 79, 82, 83] reported information on the frequency of dementias or MCI in former professional athletes. When considering former soccer players, 2 large cohort studies enrolled participants from museum and league club archives and structured healthcare information systems, reporting a higher frequency of dementia (HR 3.59, p < 0.001; HR 3.87, p: < 0.001) and AD (HR 5.07, p: < 0.001) in former soccer players compared to general population [82, 84]. A small study enrolled former soccer players from 4 soccer clubs and did not report an increased risk NCDs as tested with a self-administered questionnaire in former players, irrespective of role and length of career, compared to general population [76]. As for American football, 3 cohort studies reported a slightly higher risk of AD in former NFL players [72, 74, 79]. The first two studies enrolled former NFL player from retired players associations and reported respectively a higher risk of AD compared to general US population (prevalence ratio 1.37) [72], and of death due to AD compared to US male mortality rates (SMR-U 1.80, SMR-U&C 3.86) [74], with the second reporting a significantly higher risk in athletes who played in speed positions (SMR 6.02) [74]. The third cohort study used data from the National Institute of Safety and Health (NIOSH) and reported a slightly, non-significantly, higher risk of death due to dementia/AD (i.e., death certificates) in former NFL players compared to baseball players (HR-U 1.28, HR-U&C 2.26) [79]. When considering varsity athletes, 2 cohort study enrolled former athletes from yearbooks [75, 77]. The first reported no significant differences in the frequency of dementia and MCI in former football players compared to non-football players [77], while the second reported no significant increase in the risk of dementia in former football players compared to participants in non-sport-related clubs [75]. Two further studies, 1 cohort, and 1 case–control study, investigated the association between occupational exposure to professional sports and the risk of death due to NCDs using census data and death certificates [43, 44]. The cohort study reported a significantly higher risk of AD in subjects whose occupation was coded as “writers, artists, entertainers, and athletes” (PMR: 135 (95% CI 111–162) [43], while the case–control study reported no significant increase on risk of AD or pre-senile dementia in subjects whose occupation was coded as “athlete or dancer” [44].
When considering MCI, a small cohort study enrolled participants from alumni associations and reported a slightly, non-significantly, higher risk of MCI (i.e., comprehensive neurocognitive assessment) in former NFL and hokey players compared to former non-contact sport athletes [78]. A small cohort study reported no increased risk of MCI based on a self-administered test in former soccer players from former player associations compared to general population (i.e., data from a large population study on MCI) [76]. However, one study on former NFL players from retired NFL players associations reported a correlation between MCI or memory impairment (MI) (i.e., diagnosed, self-reported, reported by informant) and self-reported recurrent concussion (MCI p = 0.02; self-reported MI p = 0.001; spouse/relative-reported MI p = 0.04) [72].
Parkinson's disease (PD)
Eight cohort studies [43, 73–77, 79, 82, 84] and 1 case–control study [44] reported data on the risk of PD in professional and varsity athletes. As for professional athletes, 2 cohort studies used data from archives of museums or clubs and hospital records, prescriptions, and death certificates, and reported a significantly higher frequency of PD in former soccer players (HR 2.09, p = 0.009; HR 2.15, p = 0.01) [82, 84]. Two further cohort studies used data from information systems (e.g., NIOSH database), and reported a higher risk of death due to PD (i.e., death certificates) in former American football players compared to general population (SMR-U&C 4.31) [74] or to former baseball players (HR-U&C 3.45) [79], with no significant difference between athletes playing in speed vs non-speed position [74]. One cohort study in a relatively limited sample of former Thai boxers enrolled from registers of former boxers (i.e., sports authority, boxers’ clubs, and Thai boxing foundation) reported a prevalence of PD and Parkinsonism, as assessed through a validated scales and questionnaires, comparable to the prevalence observed in several Asian general populations [73]. Two further studies, 1 cohort and 1 case–control, investigated the association between occupational exposure to professional sports and the risk of death due to PD using census data and death certificates [43, 44]. Of these, 1 reported a higher risk of death due to PD in the category of subjects whose occupation was categorized as “Writers, artists, entertainers, and athletes” (PMR: 128) [43], while the other reported no significantly increased risk of PD in subjects whose previous occupation was classified as “athlete” [44]. When considering varsity athletes, 2 studies enrolled former athletes from yearbooks and did not report a higher risk of PD or Parkinsonism in former American football players compared to either members of other non-sport-related clubs [75] or to non-football athletes [77].
Chronic traumatic encephalopathy (CTE)
Only one study reported data on the risk of CTE in former competitive athletes. The study enrolled participants from a brain tissue bank, ascertaining exposure to previous competitive sport participation (e.g., varsity, professional) through obituaries or school yearbooks. In this study, participants were classified as CTE positive (CTE +) in case of pathology fitting with all aspects of the consensus criteria for CTE neuropathology, and as “features of CTE” in case of multiple lesions suggestive of CTE pathology without being consistent with all parts of the criteria verbatim. CTE + and features of CTE were classified together as “combined CTE patology”. CTE + was also evaluated separately as secondary outcome. The study reported a significantly higher risk of CTE in participants who played American football both at during (CTE + OR = 2.87, p = 0.021) and after high school (CTE pathology OR = 13.23, p < 0.001, CTE + OR = 6.84, p = 0.0056), and in former boxers (CTE + OR = 10.29, p = 0.009) [80].
Quantitative analysis
No meta-analyses were carried out due to the clinical, methodological, and statistical heterogeneity of the included studies. The main reasons that prevented the cumulative analysis of data were due to the study designs being different, and their methodological quality not being homogeneous. As for clinical heterogeneity, the definition and source of both the reference population and the cases were widely variable across studies. Moreover, the investigated cohorts of athletes were mostly the same cohorts observed in different time-periods, as in the case of the two large cohort studies on mortality due to NDs among Scottish professional soccer players.
Discussion
Overall, the present systematic review reported a higher frequency of NDs/NCDs in former professional athletes. Specifically, published studies showed an increased frequency of any type of NDs/NCDs in former soccer players [82, 84] and American football players [74] even when compared to other non-contact sport athletes [79]. When considering specific NDs and NCDs, data from the update of the previous SRs confirmed a higher frequency of ALS or MND in former soccer players [82–85]. Gathered data also reported a significantly higher risk of dementia and AD in former soccer players [82, 84] and a higher risk of MCI in former American football players, mainly associated to a higher risk of concussion [72]. Moreover, data also showed a significantly higher risk of PD in former soccer and American football players [74, 75, 79, 82, 84] and a significantly higher risk of CTE in former boxers and in former American football players [80].
Some studies reported either non-significant results or no increase in risk in some categories of athletes. However, most of these studies were of low or relatively low methodological quality and included very small or relatively small samples defining cases based on self-reports or non-standardized/validated tools. Moreover, some studies only compared different types of sports (i.e., contact vs non-contact, football vs non-football). The only large and high-quality study [44] reporting no difference in the mortality rate for any of the considered NDs and NCDs considered as exposed participants classified as “athlete/dancer” based on the occupational census, which is a wider definition that could lead to potentially underestimating results. Most of the included studies attempted to take into consideration potential confounding factors by either matching non exposed subjects or controls by at least the main characteristics (e.g., gender, age, ethnicity), with some of them also stratifying for potential interacting factors such as length of career and type of sport or position/role played. However, the factors considered for adjustment where heterogeneous across some of the included studies, and most studies only include male professional players due to the availability of data. This further affected the direct comparison of data. Previous reviews suggested that the higher risk of NDs observed in some specific categories of former athletes could be sport specific. However, no studies on sports other than soccer, American football and marginally boxing, were available. Therefore, conclusion can only be based on available studies, as no data are currently available on different sports. Further large studies should be performed on other sports, mainly in other contact sports (e.g., boxing, martial arts) and sports requiring running/speed roles (e.g., rugby, hokey, etc.), should be carried out, as these types of sports resulted to be associated with the highest risk of NDs and NCDs.
As for ALS and MND, our results confirmed the higher risk observed in formed soccer and American football player reported in the previously published SR [23]. Included studies reported an increased risk of ALS in soccer and American football players also confirmed an earlier onset of ALS in soccer players, and a higher frequency of ALS in players with a longer career, in specific positions or roles, and playing in main leagues [82–84, 86]. Therefore, based on results from the previous SR and the present update, soccer and American football players seem to be at risk of a specific, more severe, type of ALS or MND, with earlier onset (< 45 years) and shorter survival.
Our data also showed an increased risk of dementia, AD and MCI in former soccer and American football players. This increase in the frequency of these conditions was linked, in some studies, to an increased exposure to brain injuries and concussions, specifically in former American football players. In this last category of athletes, a higher frequency of CTE was also observed. As expected, the risk of CTE resulted the highest in former boxers, who were among the first categories of athletes in whom the formerly called “punch drunk syndrome” and “dementia pugilistica” were investigated [99, 100].
Moreover, a higher risk of PD and Parkinsonism was observed in former soccer and American football players, which was not linked to specific roles or positions. This increased frequency of PD, as for dementia and cognitive decline, was also generally linked to an increased exposure to brain injury and trauma.
On this basis, our results seem to suggest that competitive athletes, and specifically those participating in contact sports, are exposed to a higher risk of trauma, concussion, and subsequent brain injuries, thus leading to a higher risk of developing cognitive outcomes or PD/Parkinsonism later in life. However, professional athletes appear to also be at a higher risk of what seems to be a specific form of ALS/MND. Based on our data and data from our previous SR, in fact, the type of ALS/MND observed in former soccer players seem to have a much earlier, bulbar onset, and a much shorter survival. This increased risk has been linked to several possible genetic and environmental risk factors. Several gene variants are associated with a susceptibility to ALS/MND, and when considering environmental risk factors, the exposure to heavy metals, trace elements, solvents, and other volatile organic compounds (e.g., pesticides and radiation) or toxins (e.g., cyanobacteria), has been linked to a higher risk of developing this condition. As for some life-style factors, the widespread use or abuse of branched-chain amino acids in professional athletes has been hypothesized to be potentially linked to a higher risk of ALS/MND [28]. This led to the specific hypothesis that professional athletes might share a specific phenotype linked to both a better predisposition to high-level sport and a risk of developing ALS/MND. However, all these genetic, environmental, and lifestyle risk factor are shared risk factors for all the considered NDs and NCDs. The exposure to some pesticides, including organophosphorus compounds and carbamates, in fact, has been reported to be associated not only to an increased risk of ALS, but also to a higher risk of AD and PD [101]. Several other compounds, such as solvents and neurotoxic agents, have also been proven to be shared risk factors for ALS and other NDs and NCDs, in particular PD [102]. Moreover, several polymorphisms and gene variants have been reported to be associated to a higher frequency of specific NDs, and some have also been investigated as involved in the epigenetic mechanisms leading to the onset of the disease [103–106]. The exposure to several lifestyle factors, including smoking, physical activity, and dietary habits along with the use/abuse of some dietary supplements, have also been proven to have a role in the etiological mechanisms of some NDs and NCDs. The evident interlinking and interaction among these factors, and their being shared risk factors for several NDs and NCDs underline the need to start considering neurodegeneration as a process starting with an early, subclinical, phase, which might be common to all NDs. The neurodegenerative process in NDs, in fact, has been proven to start several decades before the onset of symptoms [107]. This long latent period, along with the shared association between several risk factors and some specific NDs suggests that this early process might be common to different NDs and only in a subsequent phase cause specific symptoms according to a subjective susceptibility and specific interactions between risk factors [3, 108]. Thus, based on this hypothesis, research should focus on investigating the interacting role of genetic, environmental, and lifestyle risk factors in this early phase of neurodegeneration to explore the possibility of defining potential preventive strategies [106]. Moreover, former professional athletes should be further investigated, as these risk factors and their interactions, as well as the risk of some specific NDs, appear to be significantly higher in this population. Studies on this population should also aim at clarifying whether the risk might be specific to some type of sports or roles, and whether the clinical characteristics of the NDs are typical of this population.
Our SR has some limitations. The main limitation is the relatively small number of included studies. Studies on this topic are relatively few and are limited to a very small number of sports, mainly soccer and American football. This limits the generalizability of results and the reliability of results. The small number of published studies might be due to the difficulty of investigating a relatively rare exposure as having been a professional athlete, and the subsequent difficulty of enrolling large samples of professional athletes. Moreover, when considering ALS, the difficulty of carrying out large studies might also be due to the rarity of the disease, which would require very large samples. Furthermore, NDs and NCDs are diseases whose typical onset is later in life, thus longitudinal studies require covering very long observation periods. Therefore, prospective studies are almost unfeasible, and retrospective studies might be very difficult to carry out in absence of effective and efficient informative systems and databases. However, the use of data from information systems, such as death certificates and hospital records, largely underestimates the frequency of NDs and NCDs, thus limiting the quality of studies, as does the use, inevitable in these systems, of different diagnostic criteria. A further limitation, due to the above-mentioned difficulties, is that the considered cohorts of athletes should be intended as mostly the same cohorts observed in different time periods. This prevents the comparison and the cumulative analysis of data, allowing only to observe a possible trend in the risk of NDs and NCDs over time within the same cohort. Further high-quality studies including athletes participating in different sports would therefore be extremely useful to assess whether the observed higher risk might be sport-specific or shared with other types of sport, and to further investigate possible characteristic or risk factors suggesting specific pathophysiological mechanisms explaining the observed increase in the risk of NDs and NCDs.
Funding
None.
Data availability
The present SR was not registered. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Key points
- An increased frequency of any type of NDs or NCDs has been identified in former soccer players and American football players.
- An increased risk of dementia and mild cognitive impairment, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and chronic traumatic encephalopathy has been identified among former professional and varsity athletes.
- NDs could share common etiopathogenic process, and only in a subsequent phase specific symptoms can occur, according to a subjective susceptibility and specific interactions between risk factors. This can be explained by the long latent period, along with the shared association between several risk factors and specific NDs.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.JPND. Available at: https://www.neurodegenerationresearch.eu/what/ (Last visited: 10/11/2021)
- 2.American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed.)
- 3.Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech. 2017;10(5):499–502. doi: 10.1242/dmm.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddique N, Siddique T. Amyotrophic Lateral Sclerosis Overview. 2001 Mar 23 [updated 2021 Sep 30]. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2022
- 5.Beeldman E, Raaphorst J, Klein Twennaar M, de Visser M, Schmand BA, de Haan RJ. The cognitive profile of ALS: a systematic review and meta-analysis update. J Neurol Neurosurg Psychiatry. 2016;87(6):611–619. doi: 10.1136/jnnp-2015-310734. [DOI] [PubMed] [Google Scholar]
- 6.Pinto WBVR, Debona R, Nunes PP, Assis ACD, Lopes CG, Bortholin T, Dias RB, Naylor FGM, Chieia MAT, Souza PVS, Oliveira ASB. Atypical Motor Neuron Disease variants: Still a diagnostic challenge in Neurology. Rev Neurol (Paris) 2019;175(4):221–232. doi: 10.1016/j.neurol.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Lang AE, Lozano AM (1998) Parkinson's disease. First of two parts. N Engl J Med 339(15):1044–53. 10.1056/NEJM199810083391506 [DOI] [PubMed]
- 8.Sacuiu SF. Dementias. Handb Clin Neurol. 2016;138:123–151. doi: 10.1016/B978-0-12-802973-2.00008-2. [DOI] [PubMed] [Google Scholar]
- 9.CDC 2019. Available at: https://www.cdc.gov/aging/dementia/index.html (Last visited: 10/11/2021)
- 10.Kovacs GG. Concepts and classification of neurodegenerative diseases. Handb Clin Neurol. 2017;145:301–307. doi: 10.1016/B978-0-12-802395-2.00021-3. [DOI] [PubMed] [Google Scholar]
- 11.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248):413–446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed]
- 12.Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 13.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol. 2014;127:29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay J, Johnson VE, Smith DH, Stewart W. Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Annu Rev Pathol. 2016;11:21–45. doi: 10.1146/annurev-pathol-012615-044116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart W, McNamara PH, Lawlor B, et al. Chronic traumatic encephalopathy: a potential late and under recognized consequence of rugby union? QJM. 2016;109:11–15. doi: 10.1093/qjmed/hcv070. [DOI] [PubMed] [Google Scholar]
- 17.Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307–2309. doi: 10.1212/WNL.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130:877–889. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinberg LT, Anghinah R, Nascimento CF, et al. Chronic Traumatic Encephalopathy Presenting as Alzheimer's Disease in a Retired Soccer Player. J Alzheimers Dis. 2016;54(1):169–174. doi: 10.3233/JAD-160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling H, Morris HR, Neal JW, et al. Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol. 2017;133(3):337–352. doi: 10.1007/s00401-017-1680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EB, Kinch K, Johnson VE, et al. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol. 2019;138:389–399. doi: 10.1007/s00401-019-02030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce N, Gallo V, McElvenny D. Head trauma in sport and neurodegenerative disease: an issue whose time has come? Neurobiol Aging. 2015;36:1383–1389. doi: 10.1016/j.neurobiolaging.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Lacorte E, Ferrigno L, Leoncini E, et al. Physical activity, and physical activity related to sports, leisure and occupational activity as risk factors for ALS: A systematic review. Neurosci Biobehav Rev. 2016;66:61–79. doi: 10.1016/j.neubiorev.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Piazza O, Sirén AL, Ehrenreich H. Soccer, neurotrauma and amyotrophic lateral sclerosis: is there a connection? Curr Med Res Opin. 2004;20(4):505–508. doi: 10.1185/030079904125003296. [DOI] [PubMed] [Google Scholar]
- 25.Patricios JS, Kemp S. Chronic traumatic encephalopathy: rugby's call for clarity, data and leadership in the concussion debate. Br J Sports Med. 2014;48(2):76–79. doi: 10.1136/bjsports-2013-093031. [DOI] [PubMed] [Google Scholar]
- 26.Harwood CA, McDermott CJ, Shaw PJ. Physical activity as an exogenous risk factor in motor neuron disease (MND): a review of the evidence. Amyotroph Lateral Scler. 2009;10(4):191–204. doi: 10.1080/17482960802549739. [DOI] [PubMed] [Google Scholar]
- 27.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- 28.Beghi E. Are professional soccer players at higher risk for ALS? Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(7–8):501–506. doi: 10.3109/21678421.2013.809764. [DOI] [PubMed] [Google Scholar]
- 29.Chiò A, Mora G. Physical fitness and amyotrophic lateral sclerosis: dangerous liaisons or common genetic pathways? J Neurol Neurosurg Psychiatry. 2012;83(4):389. doi: 10.1136/jnnp-2012-302351. [DOI] [PubMed] [Google Scholar]
- 30.Manley G, Gardner AJ, Schneider KJ, et al. A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med. 2017;51(12):969–977. doi: 10.1136/bjsports-2017-097791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutherford A, Stewart W, Bruno D. Heading for trouble: is dementia a game changer for football? Br J Sports Med. 2019;53(6):321–322. doi: 10.1136/bjsports-2017-097627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer’s disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC, Stevens JC, Ganguli M et al. (2001) Practice parameter: Early detection of dementia–-MCI (an evidencebased review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56:1133–1142 [DOI] [PubMed]
- 35.Martland HS. Punch drunk. JAMA. 1928;91(15):1103–1107. [Google Scholar]
- 36.Millspaugh JA. Dementia pugilistica. U S Nav Med Bull. 1937;35:297–303. [Google Scholar]
- 37.Roberts AH. Brain damage in boxers. A study of prevalence of traumatic encephalopathy among ex-professional boxers. London: Pitman Medical & Scientific Publication Company 1969:1–132
- 38.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 39.Mez J, Daneshvar DH, Abdolmohammadi B, et al. Duration of American Football Play and Chronic Traumatic Encephalopathy. Ann Neurol. 2020;87(1):116–131. doi: 10.1002/ana.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JPT, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5 1.0. The Cochrane Collaboration. Available: www.cochrane-handbook.org (accessed 12.14.2021).
- 41.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(21):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available: www.ohri.ca/programs/clinical epidemiology/ oxford.asp (accessed 12.14.2021)
- 43.Schulte PA, Burnett CA, Boeniger MF, Johnson J. Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through1991. Am J Public Health. 1996;86(9):1281–1288. doi: 10.2105/ajph.86.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park RM, Schulte PA, Bowman JD, et al. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48(1):63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- 45.Vanacore N, Binazzi A, Bottazzi M, Belli S. Amyotrophic lateral sclerosis in an Italian professional soccer player. Parkinsonism Relat Disord. 2006;12(5):327–329. doi: 10.1016/j.parkreldis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Dhote VV, Raja MKMM, Samundre P, Sharma S, Anwikar S, Upaganlawar AB. Sports-Related Brain Injury and Neurodegeneration in Athletes. Curr Mol Pharmacol. 2022;15(1):51–76. doi: 10.2174/1874467214666210910114324. [DOI] [PubMed] [Google Scholar]
- 47.Adani G, Filippini T, Garuti C, et al. Environmental Risk Factors for Early-Onset Alzheimer's Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy. Int J Environ Res Public Health. 2020;17(21):7941. doi: 10.3390/ijerph17217941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feddermann-Demont N, Junge A, Weber KP, et al. Prevalence of potential sports-associated risk factors in Swiss amyotrophic lateral sclerosis patients. Brain Behav. 2017;7(4):e00630. doi: 10.1002/brb3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho J, Lee I, Park S, et al. Physical activity and all-cause mortality in Korean older adults. Ann Hum Biol. 2018;45(4):337–345. doi: 10.1080/03014460.2018.1478448. [DOI] [PubMed] [Google Scholar]
- 50.Schaffert J, Didehbani N, LoBue C, et al. (2021) Frequency and predictors of traumatic encephalopathy syndrome in a prospective cohort of retired professional athletes. Front Neurol 12:617526. Published 2021 Feb 23. 10.3389/fneur.2021.617526 [DOI] [PMC free article] [PubMed]
- 51.Schwab N, Wennberg R, Grenier K, et al. Association of position played and career duration and chronic traumatic encephalopathy at autopsy in elite football and hockey players. Neurology. 2021;96(14):e1835–e1843. doi: 10.1212/WNL.0000000000011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins MW, Lovell MR, Iverson GL et al. (2002) Cumulative effects of concussion in high school athletes. Neurosurgery 51(5):1175–9;discussion1180–1 [DOI] [PubMed]
- 53.Adams JW, Alvarez VE, Mez J, et al. Lewy body pathology and chronic traumatic encephalopathy associated with contact sports. J Neuropathol Exp Neurol. 2018;77(9):757–768. doi: 10.1093/jnen/nly065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanacore N, Barbariol P, Caffari B, et al. Amyotrophic Lateral Sclerosis and soccer: an internet survey of 29 Italian players. Ann Ist Super Sanita. 2018;54(4):364–369. doi: 10.4415/ANN_18_04_14. [DOI] [PubMed] [Google Scholar]
- 55.Rose SC, Yeates KO, Nguyen JT, et al. Exposure to head impacts and cognitive and behavioral outcomes in youth tackle football players across 4 seasons. JAMA Netw Open. 2021;4(12):e2140359. doi: 10.1001/jamanetworkopen.2021.40359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Char Bray MJ, Tsai J, Bryant B et al. (2022) Effect of professional fighters' weight class on regional brain volume, cognition, and other neuropsychiatric outcomes. Neurology 98(1 Supplement 1):S10. 10.1212/01.wnl.0000801824.22603.9a [DOI] [PMC free article] [PubMed]
- 57.Walia B, Kmush BL, Ehrlich J, Mackowski M, Sanders S. Age at league entry and early all-cause mortality among national football league players. Int J Environ Res Public Health. 2021;18(24):13356. doi: 10.3390/ijerph182413356.PMID:34948966;PMCID:PMC8706978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tenforde AS, Cortez B, Coughlan-Gifford E, et al. Individual and cumulative health afflictions are associated with greater impairment in physical and mental function in former professional American style football players. PM R. 2022;14(1):30–39. doi: 10.1002/pmrj.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longstreth WT, McGuire V, Koepsell TD, et al. Risk of amyotrophic lateral sclerosis and history of physical activity: a population-based case-control study. Arch Neurol. 1998;55:201–206. doi: 10.1001/archneur.55.2.201. [DOI] [PubMed] [Google Scholar]
- 60.Scarmeas N, Shih T, Stern Y, et al. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2002;59:773–775. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- 61.Belli S, Vanacore N. Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005;20(3):237–242. doi: 10.1007/s10654-004-6879-7. [DOI] [PubMed] [Google Scholar]
- 62.Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(Pt 3):472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 63.Valenti M, Pontieri FE, Conti F, Altobelli E, Manzoni T, Frati L. Amyotrophic lateral sclerosis and sports: a case-control study. Eur J Neurol. 2005;12(3):223–225. doi: 10.1111/j.1468-1331.2004.00978.x. [DOI] [PubMed] [Google Scholar]
- 64.Abel EL. Football increases the risk for Lou Gehrig’s disease, amyotrophic lateral sclerosis. Percept Mot Skills. 2007;104(3):1251–1254. doi: 10.2466/pms.104.4.1251-1254. [DOI] [PubMed] [Google Scholar]
- 65.Taioli E. All causes of mortality in male professional soccer players. Eur J Public Health. 2007;17(6):600–604. doi: 10.1093/eurpub/ckm035. [DOI] [PubMed] [Google Scholar]
- 66.Chiò A, Calvo A, Dossena M, et al. LS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph Lateral Scler. 2009;4:205–209. doi: 10.1080/17482960902721634. [DOI] [PubMed] [Google Scholar]
- 67.Beghi E, Logroscino G, Chiò A, et al. Amyotrophic lateral sclerosis, physical exercise, trauma and sports: results of a population-based pilot case-control study. Amyotroph Lateral Scler. 2010;11(3):289–292. doi: 10.3109/17482960903384283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanacore N, Cocco P, Fadda D, Dosemeci M. Job strain, hypoxia and riskof amyotrophic lateral sclerosis: results from a death certificate study. Amyotroph Lateral Scler. 2010;11(5):430–434. doi: 10.3109/17482961003605796. [DOI] [PubMed] [Google Scholar]
- 69.Trojsi F, Sagnelli A, Vanacore N, et al. Clinical features and lifestyle of patients with amyotrophic lateral sclerosis in Campania: brief overview of an Italian database. Ann Ist Super Sanita. 2012;48(3):287–291. doi: 10.4415/ANN_12_03_09. [DOI] [PubMed] [Google Scholar]
- 70.Gotkine M1, Friedlander Y, Hochner H (2014) Triathletes are over-represented in a population of patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener 15(7–8):534–536 [DOI] [PubMed]
- 71.Fang F, Hållmarker U, James S, et al. Amyotrophic lateral sclerosis among cross-country skiers in Sweden. Eur J Epidemiol. 2016;31(3):247–253. doi: 10.1007/s10654-015-0077-7. [DOI] [PubMed] [Google Scholar]
- 72.Guskiewicz KM, Marshall SW, Bailes J et al. (2005) Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57(4):719–26;discussion719–26 [DOI] [PubMed]
- 73.Lolekha P, Phanthumchinda K, Bhidayasiri R. Prevalence and risk factors of Parkinson's disease in retired Thai traditional boxers. Mov Disord. 2010;25(12):1895–1901. doi: 10.1002/mds.23210. [DOI] [PubMed] [Google Scholar]
- 74.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savica R, Parisi JE, Wold LE, et al. High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc. 2012;87(4):335–340. doi: 10.1016/j.mayocp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vann Jones SA, Breakey RW, Evans PJ. Heading in football, long-term cognitive decline and dementia: evidence from screening retired professional footballers. Br J Sports Med. 2014;48(2):159–161. doi: 10.1136/bjsports-2013-092758. [DOI] [PubMed] [Google Scholar]
- 77.Janssen PH, Mandrekar J, Mielke MM, et al. High school football and late-life risk of neurodegenerative syndromes, 1956–1970. Mayo Clin Proc. 2017;92(1):66–71. doi: 10.1016/j.mayocp.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willer BS, Zivadinov R, Haider MN, et al. A preliminary study of early-onset dementia of former professional football and hockey players. J Head Trauma Rehabil. 2018;33(5):E1–E8. doi: 10.1097/HTR.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen VT, Zafonte RD, Chen JT, et al. Mortality Among Professional American-Style Football Players and Professional American Baseball Players. JAMA Netw Open. 2019;2(5):e194223. doi: 10.1001/jamanetworkopen.2019.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bieniek KF, Blessing MM, Heckman MG, et al. Association between contact sports participation and chronic traumatic encephalopathy: a retrospective cohort study. Brain Pathol. 2020;30(1):63–74. doi: 10.1111/bpa.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filippini T, Fiore M, Tesauro M et al. (2020) Clinical and Lifestyle Factors and Risk of Amyotrophic Lateral Sclerosis: A Population-Based Case-Control Study. Int J Environ Res Public Health 30;17(3):857 [DOI] [PMC free article] [PubMed]
- 82.Mackay DF, Russell ER, Stewart K, et al. Neurodegenerative Disease Mortality among Former Professional Soccer Players. N Engl J Med. 2019;381(19):1801–1808. doi: 10.1056/NEJMoa1908483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pupillo E, Bianchi E, Vanacore N, et al. Increased risk and early onset of ALS in professional players from Italian Soccer Teams. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(5–6):403–409. doi: 10.1080/21678421.2020.1752250. [DOI] [PubMed] [Google Scholar]
- 84.Russell ER, Mackay DF, Stewart K, et al. Association of field position and career length with risk of neurodegenerative disease in male former professional soccer players. JAMA Neurol. 2021;78(9):1057–1063. doi: 10.1001/jamaneurol.2021.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gamez J, Carmona F. Confirmation of early non-bulbar onset of amyotrophic lateral sclerosis in Spanish league soccer players. J Neurol Sci. 2021;428:117586. doi: 10.1016/j.jns.2021.117586. [DOI] [PubMed] [Google Scholar]
- 86.Daneshvar DH, Mez J, Alosco ML, et al. Incidence of and mortality from amyotrophic lateral sclerosis in national football league athletes. JAMA Netw Open. 2021;4(12):e2138801. doi: 10.1001/jamanetworkopen.2021.38801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiò A, Magnani C, Oddenino E, et al. Accuracy of death certificate diagnosis of amyotrophic lateral sclerosis. J Epidemiol Community Health. 1992;46(5):517–518. doi: 10.1136/jech.46.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ragonese P, Filippini G, Salemi G, et al. Accuracy of death certificates for amyotrophic lateral sclerosis varies significantly from north to south of Italy: implications for mortality studies. Neuroepidemiology. 2004;23(1–2):73–77. doi: 10.1159/000073978. [DOI] [PubMed] [Google Scholar]
- 89.Yeo L, Lynch C, Hardiman O. Validating population-based registers for ALS: how accurate is death certification? J Neurol. 2010;257(8):1235–1239. doi: 10.1007/s00415-010-5494-7. [DOI] [PubMed] [Google Scholar]
- 90.Stickler DE, Royer JA, Hardin JW. Accuracy and usefulness of ICD-10 death certificate coding for the identification of patients with ALS: results from the South Carolina ALS Surveillance Pilot Project. Amyotroph Lateral Scler. 2012;13(1):69–73. doi: 10.3109/17482968.2011.614253. [DOI] [PubMed] [Google Scholar]
- 91.Perera G, Stewart R, Higginson IJ, Sleeman KE. Reporting of clinically diagnosed dementia on death certificates: retrospective cohort study. Age Ageing. 2016;45(5):668–673. doi: 10.1093/ageing/afw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao L, Calloway R, Zhao E, et al. Accuracy of death certification of dementia in population-based samples of older people: analysis over time. Age Ageing. 2018;47(4):589–594. doi: 10.1093/ageing/afy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hobson P, Meara J. Mortality and quality of death certification in a cohort of patients with Parkinson's disease and matched controls in North Wales, UK at 18 years: a community-based cohort study. BMJ Open. 2018;8(2):e018969. doi: 10.1136/bmjopen-2017-018969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi H, Counsell C. Accuracy of death certificates for recording parkinsonian syndromes and associated dementia. J Neurol. 2021;268(1):140–146. doi: 10.1007/s00415-020-10113-0. [DOI] [PubMed] [Google Scholar]
- 95.Marin B, Couratier P, Preux PM, Logroscino G. Can mortality data be used to estimate amyotrophic lateral sclerosis incidence? Neuroepidemiology. 2011;36(1):29–38. doi: 10.1159/000321930. [DOI] [PubMed] [Google Scholar]
- 96.Erkinjuntti T, Ostbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med. 1997;337(23):1667–1674. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- 97.Wancata J, Börjesson-Hanson A, Ostling S, et al. Diagnostic criteria influence dementia prevalence. Am J Geriatr Psychiatry. 2007;15(12):1034–1045. doi: 10.1097/JGP.0b013e31813c6b6c. [DOI] [PubMed] [Google Scholar]
- 98.Naik M, Nygaard HA. Diagnosing dementia – ICD-10 not so bad after all: a comparison between dementia criteria according to DSM-IV and ICD-10. Int J Geriatr Psychiatry. 2008;23(3):279–282. doi: 10.1002/gps.1874. [DOI] [PubMed] [Google Scholar]
- 99.Fesharaki-Zadeh A. Chronic traumatic encephalopathy: a brief overview. Front Neurol. 2019;10:713. doi: 10.3389/fneur.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inserra CJ, DeVrieze BW. Chronic traumatic encephalopathy. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from:https://www.ncbi.nlm.nih.gov/books/NBK470535/. Accessed 10/11/2021 [PubMed]
- 101.Baltazar MT, Dinis-Oliveira RJ, de Lourdes BM, et al. Pesticides exposure as etiological factors of Parkinson's disease and other neurodegenerative diseases–a mechanistic approach. Toxicol Lett. 2014;230(2):85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 102.Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology. 2013;80(22):2035–2041. doi: 10.1212/WNL.0b013e318294b3c8. [DOI] [PubMed] [Google Scholar]
- 103.Bradley WG, Andrew AS, Traynor BJ, et al. Gene-Environment-Time Interactions in Neurodegenerative Diseases: Hypotheses and Research Approaches. Ann Neurosci. 2018;25:261–267. doi: 10.1159/000495321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fleming SM. Mechanisms of Gene-Environment Interactions in Parkinson's Disease. Curr Environ Health Rep. 2017;4(2):192–199. doi: 10.1007/s40572-017-0143-2. [DOI] [PubMed] [Google Scholar]
- 105.Berson A, Nativio R, Berger SL, Bonini NM. Epigenetic regulation in neurodegenerative diseases. Trends Neurosci. 2018;41(9):587–598. doi: 10.1016/j.tins.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nativio R, Donahue G, Berson A, et al. Dysregulation of the epigenetic landscape of normal aging in Alzheimer's disease. Nat Neurosci. 2018;21(4):497–505. doi: 10.1038/s41593-018-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Calne DB, Eisen A, McGeer E, Spencer P. Alzheimer's disease, Parkinson's disease, and motoneurone disease: abiotrophic interaction between ageing and environment? Lancet. 1986;2(8515):1067–1070. doi: 10.1016/s0140-6736(86)90469-1. [DOI] [PubMed] [Google Scholar]
- 108.Jellinger KA. Recent advances in our understanding of neurodegeneration. J Neural Transm (Vienna) 2009;116(9):1111–1162. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The present SR was not registered. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.