Abstract
Purpose
This study aimed to investigate the effect of continuous positive airway pressure (CPAP) on blood glucose fluctuation in patients with type 2 diabetes mellitus (T2DM) and obstructive sleep apnea (OSA).
Methods
Patients with T2DM and OSA were divided into an intervention group and a control group. All patients were treatment naïve. The intervention group was given CPAP therapy. The subjects were monitored using a continuous glucose monitoring system (CGMS) for 2 weeks.
Results
Of 60 patients, 30 were selected to receive CPAP intervention while 30 without CPAP served as controls. The CPAP tolerance of the intervention group was good, with average time on CPAP therapy of 55.2 ± 4.3 days, and average daily time on CPAP therapy of 8.3 ± 2.8 h. The postprandial blood glucose (PBG), fasting blood glucose (FBG), and HbA1c levels in the intervention group decreased significantly (P < 0.05). Significant variations in 24-h mean blood glucose and night-time mean blood glucose were significantly lower with CPAP therapy than without therapy (P < 0.05, respectively). The mean of daily differences and mean ambulatory glucose excursions were both considerably lower with treatment than without (P < 0.05, respectively). There was also a significant difference in time in range and time above range (P < 0.05, respectively).
Conclusion
CPAP treatment may significantly improve the blood glucose level and blood glucose stability in patients with T2DM and OSA. CPAP is an effective treatment method beyond lifestyle intervention and drug therapy.
Keywords: Obstructive sleep apnea, Type 2 diabetes mellitus, Continuous glucose monitoring system, Continuous positive airway pressure
Introduction
Because type 2 diabetes mellitus (T2DM) and obstructive sleep apnea (OSA) are both highly prevalent diseases, they frequently occur together. Furthermore, patients with T2DM have a considerably greater prevalence of OSA than the overall population. The prevalence of OSA in patients with T2DM is about 55% (24–86%), and the risk is 50% higher than that of patients without T2DM [1–3]. OSA affects more than 60% of hospitalized patients with T2DM. Studies have recently shown that patients with T2DM and OSA have a significantly higher risk of macrovascular and microvascular complications [4, 5]. Intermittent hypoxia produced by nocturnal apneas or hypoventilation during sleep may cause blood glucose fluctuations and impair insulin sensitivity.
CPAP is the treatment of first choice for patients with OSA [6]. As CPAP improves nocturnal hypoxia in patients with OSA, it may also improve the metabolic disorders in those patients.
With the advancement of continuous glucose monitoring systems (CGMS) in recent years, researchers have reported that blood glucose fluctuations, rather than glycosylated hemoglobin (HbA1c), play a significant role in the complications of diabetes. Blood glucose fluctuations have been linked to complications such as coronary microangiopathy, diabetic retinopathy, and diabetic nephropathy [7, 8].
Therefore, the goal of this study was to assess how blood glucose levels and fluctuations in glucose change in patients with T2DM and OSA before and during CPAP treatment.
Methods
Research subjects
Patients with T2DM patients and snoring symptoms in the endocrinology department of the International Hospital of Peking University were evaluated by polysomnography (PSG) and a sample of patients with OSA were enrolled from December 2019 to June 2021. All patients were naive to CPAP and had no prior surgery for OSA. Diet, lifestyle, and hypoglycemic drugs did not change before and during the study. The 1999 WHO diagnostic criteria were used to confirm diagnosis of T2DM: (1) fasting plasma glucose (FPG) ≥ 7.0 mmol/L. (2) Postprandial blood glucose was ≥ 11.1 mmol/l at 2 h or ≥ 11.1 mmol/l randomly. In the absence of clear hyperglycemia syndrome, the standard was confirmed by repeated testing. Criteria for exclusion were: (1) sinusitis, nasal polyps, deviation of the nasal septum, tongue hypertrophy, tonsil hypertrophy, hyperplasia of lymph tissue in the root of the tongue, and other anatomical stenosis of the respiratory tract; (2) endocrine diseases such as hypothyroidism, acromegaly, and adrenal hyperplasia; (3) patients who took insulin-sensitive drugs. The study was approved by the Peking University International Hospital’s Bioethics Committee. Participants gave written informed consent. The registry’s URL is www.chictr.org.cn and the trial’s registration number is ChiCTR1900023467.
General conditions
Medical records were obtained for all subjects in this study. Medical history and important clinical markers were documented including gender, age, weight, height, hip, waist, diabetes duration, diastolic blood pressure (DBP), and systolic blood pressure (SBP). Waist-to-hip ratio (WHR) was calculated by the formula waist/hip, and the formula weight/height2 (kg/m2) was used to compute the body mass index (BMI).
Polysomnography monitoring
Subjects were monitored in the sleep monitoring center in Peking University International Hospital for at least 7 h at night. The instrument was an Alice A6 (Philips, USA) multi-channel sleep monitor, which recorded (1) mouth and nose airflow; (2) snoring; (3) mean SaO2 and the lowest SaO2; (4) chest and abdominal breathing movement. The apnea hypopnea index (AHI) was established by international standards of the American Academy of Sleep Medicine (AASM) Sleep Apnea Definitions Task Force [9] as the average number of apneas and hypopneas during at least 7 h of sleep. Patients with an AHI ≥ 5 and mostly obstructive events were diagnosed with OSA. Patients were classified into two groups based on the results of the PSG: severe (AHI ≥ 30 times/h) and mild to moderate (AHI 5 to < 30 times/h). On the day of the monitoring, patients were advised not to take sedatives, strong tea, wine, or caffeine.
Continuous glucose-monitoring system
The subjects were monitored for 2 weeks before and after treatment with CGMS (Abbott, USA). The fluctuation index and its calculation formula were as follows:
Time in range (TIR1 and TIR2)
The percentage of time that blood glucose was in the range of 3.9–10.0 mmol/l during 24 h was TIR1, and the percentage of time between 0:00 and 6:00 am during the night that blood glucose was within the range of 3.9–10.0 mmol/l was TIR2.
Time below range (TBR1 and TBR2)
The percentage of time with blood glucose below 3.9 mmol/l in 24 h was TBR1, and the percentage of time with blood glucose below 3.9 mmol/l between 0:00 and 6:00 am during the night was TBR2.
Time above range (TAR1 and TAR2)
The percentage of time with blood glucose above 10 mmol/l in the 24 h was TAR1 and the percentage of time between 0:00 and 6:00 am during the night that blood glucose was higher than that of 10 mmol/l was TAR2.
Mean blood glucose (MBG1) and standard deviation (SD1)
The average of blood glucose during 24 h and its SD.
Mean ambulatory glucose excursions (MAGE1)
The average of all glucose excursions.
Absolute mean of daily differences (MODD)
The mean absolute deviation of matched values between two consecutive 24 h.
Mean blood glucose level (MBG2) and its standard deviation (SD2)
The average of blood glucose from 0 to 6 am during the night and its SD.
Mean ambulatory glucose excursions at night (MAGE2)
The average of all glucose excursions from 0 to 6 am during the night.
Laboratory biochemical examination
The biochemical blood tests included postprandial blood glucose (PBG), fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), fasting insulin (FINS), uric acid (UA), serum creatinine (sCr), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C). All blood tests were performed at Peking University International Hospital. HbA1c was quantified using high-performance liquid chromatography. PBG, UA, sCr, FBG, TC, TG, HDL-C, and LDL-C were measured using enzyme-based techniques (HPLC). Urinary microalbuminuria, urinary creatinine, and UACR values were calculated using the immunoturbidimetric method from our laboratory. The formula FINS (mIU/L) FBG (mmol/L)/22.5 was used to calculate the homeostasis model assessment index of insulin resistance (HOMA-IR).
CPAP therapy
After CGMS monitoring, 30 subjects were assigned to the intervention group. The intervention group was treated with a fully automatic single level continuous positive pressure S9 ventilator (RESMED, USA). We adjusted the pressure level of CPAP according to the AHI of the patients, so that the patients could receive CPAP treatment without discomfort. The subjects were treated for at least 6 weeks and at least 4 h a night. All subjects were given lifestyle guidance, and the glucose-lowering guidance before and after treatment was not changed.
Statistical analysis
Statistical analysis was carried out using the SPSS Version 21.0 software (IBM, Chicago, IL, USA) and used one-way analysis of variance (ANOVA) to compare the intervention group and control group. The χ2 test count data was used to compare count data among the groups and paired t-test was used to compare the data before and after treatment. The value of P less than 0.05 is considered to be statistically significant.
Results
CPAP therapy results
There were 30 patients (16 men) in the intervention group, with 17 having mild to moderate OSA and 13 having severe OSA, with an average age of 52.0 ± 11.2 years. In the control group of 30 patients (19 men), the average age was 54. 8 ± 9.0 years. CPAP treatment for the intervention group was well tolerated with average duration on therapy 55.2 ± 4.3 days and average daily wearing time at 8. 3 ± 2.8 h.
Comparison of laboratory indexes between intervention group and control group before and after treatment
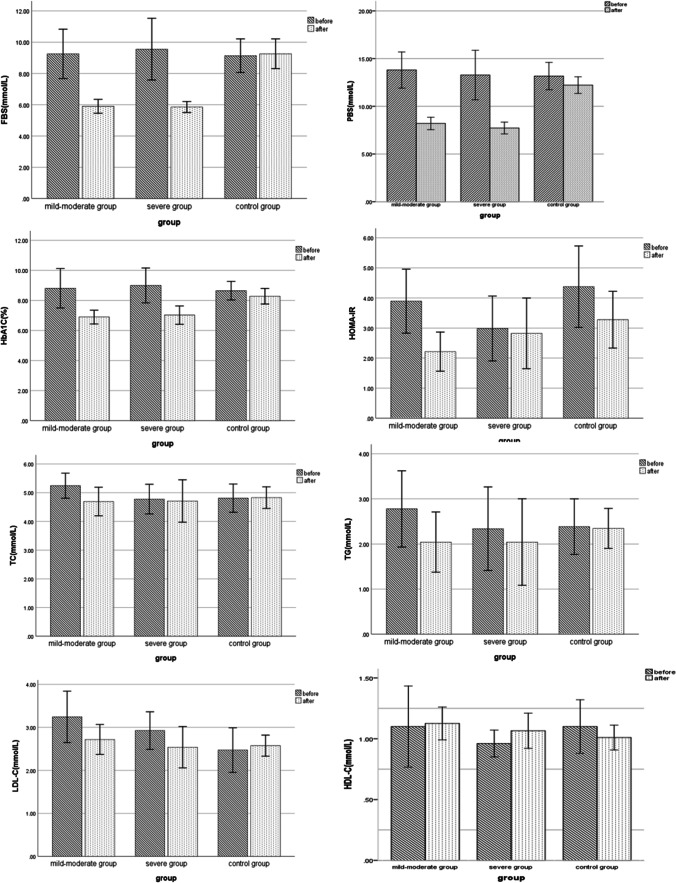
For the following attributes, there were no significant differences between the groups: gender, age, diabetic duration, WHR, FBG, PBG, BMI, HbA1c, TC, LDL-C, TG, UA, sCr, HOMA-IR, UACR, and HDL-C. After CPAP treatment, there were no significant differences in LDL-C, TG, HDL-C, TC, UA, UACR, sCr, and HOMA-IR between the groups. The level of HbA1c, PBG, and FBG decreased significantly compared with before CPAP in the intervention group (P < 0.05). In the control group, however, there were no significant differences in HbA1c, FBG, or PBG when compared to baseline measurements (P > 0.05) (shown in Table 1 and Fig. 1).
Table 1.
Comparison of laboratory indexes between intervention group and control group before and after treatment
| Index | Control group (n = 30) |
Mild-moderate group (n = 17) |
Severe group (n = 13) |
F(X2) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | |||
| Age (years) | 54.8 ± 9.0 | - | - | 52.8 ± 10.3 | - | - | 51.1 ± 14.1 | - | - | - | - |
| Sex (male%) | 19(63%) | - | - | 6(35%) | - | - | 10(77%) | - | - | - | - |
| BMI (kg/m2) | 30.9 ± 4.5 | 30.2 ± 4.4 | 0.06 | 30.2 ± 3.3 | 30.2 ± 3.34 | 0.57 | 30.3 ± 4.2 | 30.2 ± 4.3 | 0.23 | 1.78 | 0.18 |
| WHR | 0.99 ± 0.07 | 0.97 ± 0.32 | 0.34 | 0.99 ± 0.06 | 1.00 ± 0.21 | 0.35 | 0.98 ± 0.09 | 0.97 ± 0.01 | 0.46 | 0.31 | 0.78 |
| Duration (years) | 6.7 ± 6.8 | - | 8.6 ± 6.5 | - | 4.0 ± 5.1 | - | - | - | |||
| AHI | 34.5 ± 20.4 | - | 16.0 ± 7.3 | 3.5 ± 2.2 | < 0.05 | 44.4 ± 14.2 | 4.5 ± 2.6 | < 0.05 | 1.25 | 0.27 | |
| SBP (mmHg) | 136.6 ± 7.6 | 130.3 ± 8.2 | 0.31 | 129.2 ± 3.9 | 130.3 ± 4.1 | 0.23 | 129.7 ± 3.5 | 127.3 ± 7.3 | 0.56 | 1.02 | 0.42 |
| DBP (mmHg) | 82.7 ± 13.9 | 83.0 ± 20.3 | 0.67 | 89.7 ± 7.4 | 86.4 ± 11.0 | 0.24 | 87.3 ± 6.5 | 80.4 ± 8.6 | 0.43 | 2.09 | 0.29 |
| FBG (mmol/L) | 9.14 ± 2.87 | 9.26 ± 2.54 | 0.55 | 9.25 ± 3.07 | 5.90 ± 0.86 | < 0.05 | 9.55 ± 3.26 | 5.85 ± 0.58 | < 0.05 | 23.98 | < 0.05 |
| PBG (mmol/L) | 13.20 ± 3.83 | 12.22 ± 2.32 | 0.18 | 13.81 ± 3.68 | 8.21 ± 1.26 | < 0.05 | 13.28 ± 4.29 | 7.72 ± 1.03 | < 0.05 | 39.29 | < 0.05 |
| HbA1c (%) | 8.65 ± 1.66 | 8.28 ± 1.39 | 0.09 | 8.81 ± 2.55 | 6.89 ± 0.89 | < 0.05 | 9.00 ± 1.92 | 7.02 ± 1.01 | < 0.05 | 9.41 | < 0.05 |
| TC (mmol/L) | 4.77 ± 1.32 | 4.83 ± 0.99 | 0.79 | 5.24 ± 0.85 | 4.69 ± 0.97 | 0.10 | 4.78 ± 0.85 | 4.71 ± 1.22 | 0.86 | 0.11 | 0.89 |
| TG (mmol/L) | 2.39 ± 1.68 | 2.34 ± 1.16 | 0.91 | 2.78 ± 1.64 | 2.04 ± 1.30 | 0.21 | 2.34 ± 1.53 | 2.04 ± 1.59 | 0.67 | 0.40 | 0.67 |
| LDL-C (mmol/L) | 2.51 ± 1.39 | 2.58 ± 0.64 | 0.81 | 3.24 ± 1.16 | 2.72 ± 0.68 | 0.15 | 2.93 ± 0.72 | 2.54 ± 0.79 | 0.14 | 0.32 | 0.73 |
| HDL-C (mmol/L) | 1.08 ± 0.45 | 0.99 ± 0.21 | 0.32 | 1.10 ± 0.65 | 1.13 ± 0.26 | 0.90 | 0.96 ± 0.18 | 1.07 ± 0.24 | 0.17 | 0.98 | 0.38 |
| UA (umol/L) | 399.9 ± 87.3 | 350.6 ± 81.3 | 0.06 | 361.0 ± 131.5 | 315.6 ± 81.6 | 0.34 | 334.4 ± 84.0 | 320.2 ± 85.1 | 0.65 | 1.29 | 0.28 |
| sCr (mmol/L) | 80.6 ± 30.5 | 69.4 ± 17.2 | 0.10 | 66.2 ± 16.2 | 68.9 ± 16.3 | 0.62 | 69.9 ± 18.0 | 72.7 ± 24.5 | 0.77 | 0.39 | 0.68 |
| UACR | 31.4 ± 32.1 | 8.9 ± 5.7 | < 0.05 | 32.3 ± 67.9 | 9.9 ± 5.8 | 0.24 | 34.0 ± 69.0 | 24.7 ± 55.4 | 0.06 | 0.56 | 0.58 |
| HOMA-IR | 4.28 ± 3.70 | 3.28 ± 2.53 | 0.22 | 3.89 ± 2.07 | 2.21 ± 1.27 | < 0.05 | 2.98 ± 1.78 | 2.82 ± 1.94 | 0.78 | 1.37 | 0.26 |
BMI body mass index; WHR waist-to-hip ratio; SBP systolic blood pressure; DBP diastolic blood pressure; FBG fasting blood glucose; HbA1c glycosylated hemoglobin; sCr serum creatinine; UA uric acid; TC total cholesterol; TG triglycerides; LDL-C low-density lipoprotein cholesterol; HDL-C high-density lipoprotein cholesterol; PBG postprandial blood glucose; UACR urinary microalbumin/creatinine ratio; HOMA-IR homeostasis model assessment index of insulin resistance
Fig. 1.
Comparison of laboratory indexes between intervention group and control group before and after treatment. There are no significant differences in LDL-C, TG, HDL-C, and TC, between the three groups. The level of HbA1c, PBG, and FBG reduced significantly compared with before in the intervention group (P < 0.05). In the control group, however, there were no significant differences in HbA1c, FBG, or PBG when compared to baseline (P > 0.05)
Comparison of blood glucose fluctuation indexes between intervention group and control group before and after treatment
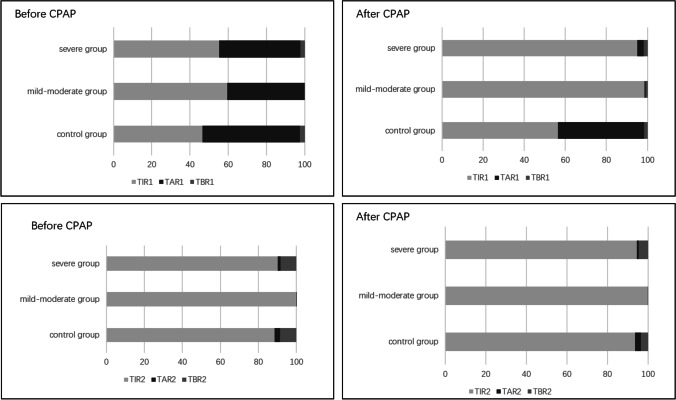
The levels of MBG1, MAGE1, MODD, SD1, MBG2, SD2, MAGE2, TIR1, TAR1, TBR1, TIR2, TAR2, and TBR2 did not differ significantly between the control group and the intervention group before treatment (P > 0.05). MBG1, MAGE1, MODD, and SD1 in the intervention group were considerably lower after CPAP treatment than before treatment (P < 0.05), and TIR1 and TAR1 were lower than those before treatment (P < 0.05). Furthermore, the findings revealed substantial alterations in MBG2, SD2, and MAGE2 before and after therapy (P > 0.05). However, the levels of MAGE1, MODD, SD1, MBG2, SD2, and MAGE2 did not change significantly in the control group (shown in Table 2 and Fig. 2).
Table 2.
Comparison of blood glucose fluctuation indexes between intervention group and control group before and after treatment
| Index | Control group (n = 30) |
Mild-moderate group (n = 17) |
Severe group (n = 13) |
F(X2) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Before | After | p | |||
| MBG1 (mmol/L) | 8.50 ± 2.13 | 7.90 ± 1.23 | 0.12 | 8.02 ± 1.58 | 6.66 ± 0.93 | < 0.05 | 8.06 ± 2.27 | 6.17 ± 1.41 | < 0.05 | 11.68 | < 0.05 |
| SD1 (mmol/L) | 2.22 ± 1.02 | 2.29 ± 1.04 | 0.44 | 1.75 ± 0.88 | 1.08 ± 0.31 | < 0.05 | 1.82 ± 0.86 | 0.78 ± 0.43 | < 0.05 | 22.25 | < 0.05 |
| MAGE1 (mmol/L) | 4.71 ± 2.09 | 4.29 ± 1.03 | 0.28 | 3.75 ± 1.36 | 2.44 ± 0.80 | < 0.05 | 3.82 ± 1.43 | 2.08 ± 1.40 | < 0.05 | 25.74 | < 0.05 |
| MODD (mmol/L) | 2.16 ± 0.97 | 2.09 ± 0.85 | 0.58 | 1.60 ± 0.94 | 1.14 ± 0.40 | < 0.05 | 1.97 ± 1.22 | 0.95 ± 0.59 | < 0.05 | 15.92 | < 0.05 |
| MBG2 (mmol/L) | 6.69 ± 1.91 | 6.08 ± 1.19 | 0.12 | 6.65 ± 1.15 | 5.94 ± 0.96 | < 0.05 | 6.72 ± 2.31 | 5.87 ± 1.41 | 0.12 | 0.01 | 0.99 |
| SD2 (mmol/L) | 0.78 ± 0.50 | 0.76 ± 0.43 | 0.78 | 0.55 ± 0.36 | 0.35 ± 0.16 | < 0.05 | 0.89 ± 0.66 | 0.48 ± 0.40 | < 0.05 | 1.49 | 0.24 |
| MAGE2 (mmol/L) | 1.90 ± 1.30 | 1.65 ± 1.34 | 0.17 | 1.52 ± 1.29 | 0.91 ± 0.73 | < 0.05 | 2.19 ± 1.51 | 1.22 ± 1.43 | < 0.05 | 2.09 | 0.13 |
| TIR1 (%) | 46.64 ± 28.72 | 56.35 ± 29.04 | 0.10 | 59.47 ± 25.62 | 98.46 ± 3.02 | < 0.05 | 55.22 ± 30.92 | 94.96 ± 12.16 | < 0.05 | 27.55 | < 0.05 |
| TAR1 (%) | 50.92 ± 30.06 | 41.92 ± 30.06 | 0.09 | 40.47 ± 25.70 | 0.42 ± 1.29 | < 0.05 | 42.56 ± 31.22 | 3.17 ± 11.13 | < 0.05 | 30.08 | < 0.05 |
| TBR1 (%) | 2.43 ± 6.29 | 1.73 ± 5.97 | 0.68 | 0.06 ± 0.24 | 1.12 ± 2.91 | 0.16 | 2.22 ± 5.35 | 1.87 ± 5.77 | 0.89 | 0.18 | 0.83 |
| TIR2 (%) | 88.48 ± 23.90 | 93.72 ± 21.63 | 0.31 | 99.95 ± 0.19 | 99.86 ± 0.59 | 0.33 | 90.19 ± 32.46 | 94.42 ± 14.19 | 0.31 | 2.00 | 0.15 |
| TAR2 (%) | 3.00 ± 13.32 | 2.86 ± 12.05 | 0.65 | 0.05 ± 0.61 | 0 | 0.33 | 1.54 ± 5.54 | 1.08 ± 3.88 | 0.34 | 0.62 | 0.54 |
| TBR2 (%) | 8.52 ± 21.14 | 3.42 ± 11.92 | 0.28 | 0 | 0.14 ± 0.59 | 0.23 | 8.27 ± 20.55 | 4.62 ± 14.11 | 0.63 | 0.82 | 0.45 |
MBG1 average blood glucose level; SD1 standard deviation of average blood glucose level; MAGE1 the average fluctuation of blood glucose in the day; MODD mean absolute difference of daytime blood glucose; MBG2 mean blood glucose level at night; SD2 standard deviation of average blood glucose level at night; MAGE2 the mean fluctuation of blood glucose at night; TIR1 and TIR2 the time percentage of blood glucose in the range of 3.9–10.0 mmol/l during 24 h was TIR1, and the time percentage between 0:00 and 6:00 in the night in the range of 3.9–10.0 mmol/l during night was TIR2; TBR1 and TBR2 the time percentage of blood glucose below 3.9 mmol/l in 24 h was TBR1, and the time percentage between 0:00 and 6:00 in the night in the range of 3.9–10.0 mmol/l was TBR2; TAR1 and TAR2:the time percentage of blood glucose in the range above 10 mmol/l was TAR1, and the time percentage between 0:00 and 6:00 in the night in the range of above 10 mmol/l was TAR2
Fig. 2.
Comparison of blood glucose fluctuation indexes between intervention group and control group before and after treatment. The levels of TIR1, TAR1, TBR1, TIR2, TAR2, and TBR2 did not differ significantly between the control group and the intervention group before treatment (P > 0.05). The level of TIR1 and TAR1 were lower than those before treatment in the intervention group (P < 0.05)
Discussion
In our study, after CPAP treatment, the glucose metabolism in the intervention group including FBG, PBG, and HbA1c improved significantly compared to the control group. At the same time, the BMI of the intervention group did not change significantly with CPAP treatment (P > 0.05). CPAP improved blood glucose levels in patients with T2DM regardless of body weight, and blood glucose levels in the intervention group were considerably lower than those in the control group. This study found that insulin resistance in patients with mild to moderate OSA decreased significantly, which is similar to most current research results.
It has previously been controversial whether or not CPAP treatment can effectively improve insulin resistance, decrease HbA1c, FBG, and PBG, and improve blood glucose metabolism in patients with OSA and T2DM [10, 11]. Two meta-analyses have shown that CPAP may not improve glycemic control [12, 13]. Also, there is limited evidence that CPAP therapy can increase a patient’s glucose time in range.
The mechanism of impaired glucose stability induced by OSA
At present, the mechanisms of impaired glucose stability induced by OSA in diabetes include the following:
Activation of the sympathetic nervous system
OSA causes sleep fragmentation and sympathetic adrenal axis excitability, which reduces tissue sensitivity to insulin, insulin secretion, and hepatic glucose breakdown, aggravating insulin resistance [14].
Direct effects of hypoxia
OSA-induced intermittent hypoxia and reoxygenation can result in intermittent tissue hypoxia and reoxygenation, which differs from chronic hypoxia. The continuous fluctuations in oxygen saturation contribute to the formation of reactive nitrogen and oxygen, increase oxidative stress, and activate the redox-sensitive cell signal transduction pathway in inflammation [15, 16]. Hypoxia can cause stress, mitochondrial damage, inflammation, and β cell apoptosis [17].
Sleep fragmentation
Patients with OSA have sleep fragmentation and frequent awakening, and sudden sleep fragmentation can reduce insulin sensitivity. A recent animal study [18] found that during natural sleep, insulin sensitivity of visceral adipocytes exposed to sleep interruption was reduced.
Systemic inflammatory response
Sleep fluctuation can cause systemic inflammation. Tumor necrosis factor (TNF-α) and the level of interleukin-6 (IL-6) is increased, and the phosphorylation of insulin receptor and insulin receptor substrate is inhibited. These inflammatory factors can affect glucose metabolism by increasing the hormone level against insulin and inhibiting the intake of glucose by muscle tissue and fat [19]. The level of inflammatory factors in the body is related to the fluctuation of blood glucose. In diabetes, good blood glucose control and reasonably constant blood glucose levels are advantageous in reducing the inflammatory response in vivo.
The damage of tissue and organs caused by glucose metabolism disorder is not only determined by the degree of blood glucose increase but also closely related to the fluctuation range of blood glucose. In diabetes patients with similar blood glucose or HbA1c, the magnitude of blood glucose fluctuation is quite variable. Similarly, blood glucose fluctuations have been shown to be a significant component in the pathogenesis of diabetes macrovascular and microvascular problems. Compared with chronic persistent hyperglycemia, blood glucose fluctuation has a more serious effect on the occurrence and development of diabetes complications [20]. The fluctuation of intraday blood glucose monitored by CGMS is closely related to vascular endothelial dysfunction, vascular stress, diabetes microangiopathy [21–24], and severity of T2DM. The current study not only investigated how blood glucose levels improved in patients with T2DM and OSA but also evaluated how blood glucose levels fluctuated after CPAP treatment. The results showed that, after treatment, the blood glucose fluctuation indexes of the intervention group, including MAGE, SD, and MODD, were much lower, and the differences were statistically significant compared to the control group. The results showed that the levels of MAGE and SD decreased significantly in the night compared with the control group. Similarly, prior studies [25, 26] have demonstrated that CPAP treatment may significantly improve the blood glucose fluctuation and blood glucose level of nocturnal interstitial fluid in patients with T2DM and OSA.
TIR is currently being utilized as a novel blood glucose evaluation index for assessing the variability of blood glucose in patients with diabetes. It has been shown that the shorter the TIR, the higher the incidence of microalbuminuria and retinopathy in diabetes [22]. In a retrospective study of 9028 critically ill patients with or without diabetes, the lower the TIR, the greater the chance of death [27]. The results in our study showed that the TIR and TAR levels of the intervention group were dramatically improved with CPAP treatment.
This study has limitations. First, the average age of patients in our study was too elderly to reflect all patients with T2DM. Second, the follow-up period was limited to 3 months due to time constraints, and it was not known if the improvements in blood glucose levels and their fluctuations would be durable. Third, the study sample size of 60 participants is relatively small, limiting the generalizability of the findings.
Conclusions
CPAP improves blood glucose levels and reduces fluctuation of blood glucose levels in patients with T2DM and OSA. It is an effective way to improve abnormal glucose metabolism in patients with T2DM and OSA, beyond lifestyle intervention and drug therapy.
Author contribution
XZ and XMZ conceived and designed research; XZ, WZ, and SXX collected data and conducted research; XZ and XFY analyzed and interpreted data; XZ wrote the initial paper; XMZ revised the paper; XZ and XMZ had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This study was supported by the Project of Peking University International Hospital Fund (YN2019XQ02).
Data availability
The data used to support the findings of this study are available from the corresponding author and first author upon request.
Declarations
Ethics approval
The study was approved by the Peking University International Hospital’s Bioethics Committee. All procedures performed in studies involving human participants were in accordance with the ethics standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethics standards.
Consent to participate
Written informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Subramanian A, Adderley NJ, Tracy A, Taverner T, Hanif W, Toulis KA, Thomas GN, Tahrani AA, Nirantharakumar K. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care. 2019;42:954–963. doi: 10.2337/dc18-2004. [DOI] [PubMed] [Google Scholar]
- 2.Tahrani AA. Obstructive sleep apnoea in diabetes: does it matter? Diab Vasc Dis Res. 2017;14:454–462. doi: 10.1177/1479164117714397. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Guo X, Guo L, Lu J, Zhou X, Ji L. Prevalence and associated factors of obstructive sleep apnea in hospitalized patients with type 2 diabetes in Beijing, China 2. J Diabetes. 2015;7:16–23. doi: 10.1111/1753-0407.12180. [DOI] [PubMed] [Google Scholar]
- 4.Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Yu X, Xin S, Zhang W, Zhang X, Ji L. Correlation between OSAHS and early peripheral atherosclerosis indices in patients with type 2 diabetes mellitus in China: a cross-sectional inpatient study. J Diabetes Res. 2021;2021:6630020. doi: 10.1155/2021/6630020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med. 2007;356:1751–1758. doi: 10.1056/NEJMct066953. [DOI] [PubMed] [Google Scholar]
- 7.Xia J, Xu J, Li B, Liu Z, Hao H, Yin C, Xu D. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin Chim Acta. 2017;466:162–166. doi: 10.1016/j.cca.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Cesana F, Giannattasio C, Nava S, et al. Impact of blood glucose variability on carotid artery intima media thickness and distensibility in type 1 diabetes mellitus. Blood Press. 2013;22:355–361. doi: 10.3109/08037051.2013.791413. [DOI] [PubMed] [Google Scholar]
- 9.Iber C, Ancoli-Israel S, Chesson A. The AASM manual for snoring of sleep and associated events: rules, terminology and technical specifications. 1. Weschester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 10.Henley DE, Buchanan F, Gibson R, Douthwaite JA, Wood SA, Woltersdorf WW, Catterall JR, Lightman SL. Plasma apelin levels in obstructive sleep apnea and the effect of continuous positive airway pressure therapy. J Endocrinol. 2009;203:181–188. doi: 10.1677/JOE-09-0245. [DOI] [PubMed] [Google Scholar]
- 11.Kurosawa H, Saisho Y, Fukunaga K, Haraguchi M, Yamasawa W, Kurihara I, Betsuyaku T, Itoh H. Association between severity of obstructive sleep apnea and glycated hemoglobin level in Japanese individuals with and without diabetes. Endocr J. 2018;65:121–127. doi: 10.1507/endocrj.EJ17-0356. [DOI] [PubMed] [Google Scholar]
- 12.Labarca G, Reyes T, Jorquera J, Dreyse J, Drake L. CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: systematic review and meta-analysis. Clin Respir J. 2018;12:2361–2368. doi: 10.1111/crj.12915. [DOI] [PubMed] [Google Scholar]
- 13.Loffler KA, Heeley E, Freed R, et al. Continuous positive airway pressure treatment, glycemia, and diabetes risk in obstructive sleep apnea and comorbid cardiovascular disease. Diabetes Care. 2020;43:1859–1867. doi: 10.2337/dc19-2006. [DOI] [PubMed] [Google Scholar]
- 14.Muraki I, Wada H, Tanigawa T. Sleep apnea and type 2 diabetes. J Diabetes Investig. 2018;9:991–997. doi: 10.1111/jdi.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drager LF, Yao Q, Hernandez KL, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SG, Hoffman GR, Poulogiannis G, et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol Cell. 2013;49:172–185. doi: 10.1016/j.molcel.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnaud C, Poulain L, Lévy P, Dematteis M. Inflammation contributes to the atherogenic role of intermittent hypoxia in apolipoprotein-E knock out mice. Atherosclerosis. 2011;219:425–431. doi: 10.1016/j.atherosclerosis.2011.07.122. [DOI] [PubMed] [Google Scholar]
- 18.Khalyfa A, Wang Y, Zhang SX, Qiao Z, Abdelkarim A, Gozal D. Sleep fragmentation in mice induces nicotinamide adenine dinucleotide phosphate oxidase 2-dependent mobilization, proliferation, and differentiation of adipocyte progenitors in visceral white adipose tissue. Sleep. 2014;37:999–1009. doi: 10.5665/sleep.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 20.Pickham D, Flowers E, Drew BJ. Hyperglycemia is associated with corrected QT prolongation and mortality in acutely ill patients. J Cardiovasc Nurs. 2014;29:264–270. doi: 10.1097/JCN.0b013e31827f174c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gimeno-Orna JA, Castro-Alonso FJ, Boned-Juliani B, Lou-Arnal LM. Fasting plasma glucose variability as a risk factor of retinopathy in type 2 diabetic patients. J Diabetes Complications. 2003;17:78–81. doi: 10.1016/S1056-8727(02)00197-6. [DOI] [PubMed] [Google Scholar]
- 22.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, Close KL. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22:72–78. doi: 10.1089/dia.2019.0251. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41:2370–2376. doi: 10.2337/dc18-1131. [DOI] [PubMed] [Google Scholar]
- 25.Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, Kripke DF, Kline LE. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–542. doi: 10.5664/jcsm.27347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallayova M, Donic V, Tomori Z. Beneficial effects of severe sleep apnea therapy on nocturnal glucose control in persons with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;81:e8–11. doi: 10.1016/j.diabres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Lanspa MJ, Krinsley JS, Hersh AM, Wilson EL, Holmen JR, Orme JF, Morris AH, Hirshberg EL. Percentage of time in range 70 to 139 mg/dL is associated with reduced mortality among critically ill patients receiving IV insulin infusion. Chest. 2019;156:878–886. doi: 10.1016/j.chest.2019.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author and first author upon request.