Abstract
Aims
Studies suggest that metformin is associated with reduced COVID-19 severity in individuals with diabetes compared to other antihyperglycemics. We assessed if metformin is associated with reduced incidence of severe COVID-19 for patients with prediabetes or polycystic ovary syndrome (PCOS), common diseases that increase the risk of severe COVID-19.
Methods
This observational, retrospective study utilized EHR data from 52 hospitals for COVID-19 patients with PCOS or prediabetes treated with metformin or levothyroxine/ondansetron (controls). After balancing via inverse probability score weighting, associations with COVID-19 severity were assessed by logistic regression.
Results
In the prediabetes cohort, when compared to levothyroxine, metformin was associated with a significantly lower incidence of COVID-19 with “mild-ED” or worse (OR [95% CI]: 0.636, [0.455–0.888]) and “moderate” or worse severity (0.493 [0.339–0.718]). Compared to ondansetron, metformin was associated with lower incidence of “mild-ED” or worse severity (0.039 [0.026–0.057]), “moderate” or worse (0.045 [0.03–0.069]), “severe” or worse (0.183 [0.077–0.431]), and “mortality/hospice” (0.223 [0.071–0.694]). For PCOS, metformin showed no significant differences in severity compared to levothyroxine, but was associated with a significantly lower incidence of “mild-ED” or worse (0.101 [0.061–0.166]), and “moderate” or worse (0.094 [0.049–0.18]) COVID-19 outcome compared to ondansetron.
Conclusions
Metformin use is associated with less severe COVID-19 in patients with prediabetes or PCOS.
Keywords: Metformin, Polycystic ovary syndrome, Prediabetes, Glycemia, COVID-19
1. Introduction
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome (SARS)-associated coronavirus-2 (SARS-CoV-2), clinicians and researchers have sought new therapeutic options for COVID-19 patients. Current treatments, consisting of antiviral and immune-based interventions, have reduced but not eliminated COVID-19-related morbidity and mortality [1], [2]. One strategy for identifying new agents is drug repurposing: identifying previously approved medications that may be effective for treatment of COVID-19. This process reduces the time and cost required to identify new therapeutic options compared to novel drug discovery [3], [4].
Several studies have proposed that metformin, a routinely prescribed antihyperglycemic agent, reduces COVID-19 severity, the incidence of acute respiratory distress syndrome (ARDS), and intensive care admission in patients with diabetes taking the medication prior to onset of infection [5], [6], [7], [8], [9]. It has been suggested that metformin use may also reduce susceptibility to SARS-CoV-2 infection by increasing AMP-activated protein kinase (AMPK) cell signaling, inhibiting binding of the viral spike protein, and preventing viral entry into the cell [10], [11]. Metformin may attenuate immune inflammatory responses and release of proinflammatory cytokines [10]. As COVID-19 can cause cytokine storm, immune modulatory activity is desirable to avoid excessive inflammation and impaired organ function [12].
Metformin is primarily prescribed for individuals with Type 2 Diabetes Mellitus (T2DM), but it is also recommended for off-label use in other groups. Two sizable populations who may benefit from off-label metformin usage are individuals who have prediabetes and those with polycystic ovary syndrome (PCOS). Notably, these two populations overlap substantially [13].
Prediabetes is a state of intermediate hyperglycemia due to insulin resistance that poses a high risk for progression to diabetes. Individuals with prediabetes usually have a higher than normal variability of blood glucose concentration, but less than the threshold for diagnosing diabetes [14]. The American Diabetes Association (ADA) 2021 guidelines define prediabetes as the presence of any the following: a fasting plasma glucose of 100 mg/dl to 125 mg/dl (5.6–6.9 mmol/L), a 2-hour plasma glucose during a 75-gram oral glucose tolerance test between 140 mg/dl to 199 mg/dl (7.8–11 mmol/L), or a hemoglobin A1c of 5.7 % to 6.4 % (39–47 mmol/mol) [15]. Without proper management, between 25 % and 50 % of people with prediabetes progress to diabetes [16]. Management for prediabetes involves intensive lifestyle changes, including weight loss, to reduce the risk of progression to diabetes. In addition, metformin can reduce or delay the incidence of diabetes in individuals with prediabetes [17].
PCOS occurs in up to 12 % of reproductive-aged women worldwide and is a diagnosis of exclusion made by the presence of 2 of the 3 Rotterdam Criteria (oligomenorrhea/amenorrhea, clinical or biochemical evidence of hyperandrogenism, and polycystic morphology of ovaries on ultrasound [18], [19] in absence of thyroid disorders, states of prolactin excess, and congenital adrenal hyperplasia [20]. Although insulin resistance is not a defining feature of PCOS, it is present in approximately 75 % of patients with PCOS independent of their BMI [21], [22]. However, the presence of obesity has been shown to reduce insulin sensitivity twofold, which in turn is thought to worsen the hyperandrogenism characteristic of PCOS [22], [23], [24]. Despite extensive study, the mechanisms of impaired glucose utilization in PCOS remain unknown. There are no medications approved by the Food and Drug Administration (FDA) for treating PCOS; available therapies are largely used to manage symptoms [25]. One of these therapies is metformin, which is commonly used off-label in patients with PCOS to increase insulin sensitization, induce ovulation, regulate menstrual cycles, and aid weight loss [21].
For individuals at high risk for severe COVID-19, approaches to prevent poor COVID-19 outcomes are highly desirable. Given that many patients with prediabetes and/or PCOS are at high risk for severe COVID-19 and already take metformin, they are ideal populations for evaluating the impact of metformin usage prior to COVID-19 infection. We hypothesize that documented usage of metformin prior to COVID-19 infection will be associated with decreased severity of COVID-19 infection outcomes. Metformin use prior to infection may be a simple and affordable way to improve COVID-19 outcomes for patients with prediabetes or PCOS.
2. Methods
2.1. Data source
This retrospective observational study utilized clinical patient data aggregated in the National COVID Cohort Collaborative (N3C) (covid.cd2h.org). The N3C Data Enclave harmonizes electronic health record (EHR) data from over 13 million patients in the United States from a total of 74 institutions. This investigation leveraged data from 53 institutions that provided cases who met the inclusion criteria.
Source data within the N3C enclave is harmonized into Observational Medical Outcomes Partnership (OMOP) [26] version 5.3.1 format. The OMOP model includes standardized definitions of conditions, lab tests, procedures, and other relevant clinical data including positive COVID-19 laboratory tests [27], [28].
2.2. Study population and eligibility criteria
Our study included patients who were identified as being COVID-19 positive by positive SARS-CoV-2 laboratory test (polymerase chain reaction or antigen) after January 1, 2020. Positive patients were assigned to one or both of the prediabetes and PCOS cohorts as follows. Patients were identified as having prediabetes if they had either a documented history of the condition of prediabetes (included concepts in Table 1 ) or hemoglobin A1C (HbA1C) between 5.7 % and 6.4 % (ADA-recognized range for prediabetes) prior to COVID-19 diagnosis [29]. Patients were identified as having PCOS if they had a documented history of the condition (included concepts in Table 1).
Table 1.
Cohort definition concept sets for polycystic ovary syndrome (PCOS) and prediabetes.
| Cohort | OMOP Concept ID | Concept Name |
|---|---|---|
| PCOS | 3654296 | Polycystic ovary syndrome of bilateral ovaries |
| PCOS | 3654294 | Polycystic ovary syndrome of right ovary |
| PCOS | 40443308 | Polycystic ovary syndrome |
| PCOS | 3654295 | Polycystic ovary syndrome of left ovary |
| Prediabetes | 4029951 | Impaired glucose tolerance in MODY |
| Prediabetes | 4229140 | Impaired glucose tolerance associated with insulin receptor abnormality |
| Prediabetes | 40316773 | Prediabetes |
| Prediabetes | 4007106 | [D]Glucose tolerance test abnormal |
| Prediabetes | 4251180 | Impaired glucose tolerance associated with hormonal etiology |
| Prediabetes | 4311629 | Impaired glucose tolerance |
| Prediabetes | 37201113 | Prediabetes |
| Prediabetes | 3176858 | Prediabetes |
| Prediabetes | 4045297 | Impaired glucose tolerance in obese |
| Prediabetes | 4136531 | Impaired glucose tolerance in nonobese |
| Prediabetes | 4111906 | Impaired glucose tolerance associated with pancreatic disease |
| Prediabetes | 37018196 | Prediabetes |
| Prediabetes | 3107926 | Prediabetes |
| Prediabetes | 4027550 | Impaired glucose tolerance with hyperinsulinism |
| Prediabetes | 44808385 | Pre-diabetes |
| Prediabetes | 4047260 | Impaired glucose tolerance associated with genetic syndrome |
| Prediabetes | 4263688 | Impaired glucose tolerance associated with drugs |
| Prediabetes | 44826981 | Impaired fasting glucose |
| Prediabetes | 45597199 | Impaired fasting glucose |
We removed patients from both cohorts who had either 1) a documented history of the condition of diabetes mellitus type 1, diabetes mellitus type 2, gestational diabetes, or another diagnosed condition that is characterized by abnormal blood glucose (Supplemental File 1); or 2) a documented HbA1C ≥ 6.5 % (ADA-recognized range for diabetes) [29].
We defined metformin users as those patients who had recorded use [30] of metformin beginning on or before the start date of the visit during which the patient was diagnosed with COVID-19 and overlapping for at least one day with the COVID-19 visit.
As there were no other anti-hyperglycemic medications used frequently in patients without diabetes, we considered other medications unrelated to prediabetes or PCOS as controls. We chose levothyroxine and ondansetron within our study population as comparator drugs for the creation of our control sub-cohorts. Levothyroxine is a synthetic thyroid hormone medication that is used to treat hypothyroidism and other thyroid-related conditions [31]. Similar to metformin, levothyroxine is a prescription-only medication in the United States, is taken orally, and requires daily usage and adherence for maximal therapeutic benefit. Levothyroxine has substantial usage in our study population, and is not known to be a successful therapeutic intervention for COVID-19. Ondansetron is an antiemetic medication, often used in patients undergoing chemotherapy or radiotherapy [32]. Ondansetron is a prescription medication and can be administered orally or intravenously. Most of those prescribed ondansetron use it daily, and usage is adequate in our study population to use it as a comparator drug. Some recent studies found a decrease in all-cause mortality in COVID-19 patients when ondansetron is given after COVID onset [33], [34]. This suggests that ondansetron may be a conservative choice of comparator medication.
As with metformin, individuals were defined as using levothyroxine or ondansetron if they had a drug era for either medication beginning on or before the date of COVID-19 diagnosis and continuing for at least one day. Any patients using metformin in combination with levothyroxine and/or ondansetron were excluded from the study population (n < 20 for PCOS, n = 41 for prediabetes).
OMOP concept ID codes for all conditions and medications used in this analysis are listed in Supplemental File 1. Codesets containing the relevant concept IDs for each construct were formulated using ATLAS (https://atlas-covid19.ohdsi.org/), the graphical user interface for the OMOP common data model [35].
2.3. Study design
We explored the potential association between treatment with metformin and severity of COVID-19 within the prediabetes and PCOS cohorts. We assessed covariates for collinearity, imputed missing values, and conducted inverse probability weighting, followed by logistic regression analysis.
2.4. Missingness imputation
Missing values were imputed for BMI, race (white versus non-white), and ethnicity. We compared various imputation models including missRanger [36], [37] and different MICE imputers [38] on a (limited) complete dataset. All the models resulted in comparable estimates and we therefore chose the missForest implementation provided by missRanger (with a number of trees in the random forest equal to 101) because our experiments on a larger patient cohort showed its higher validity when compared to the MICE models [39].
2.5. Propensity score weighting
Inverse probability weighting was performed using the “Weightthem”method implemented in the R MatchThem package [40] using “propensity score” weights, the Average Treatment Effect on the Treated (ATT) estimand, and the “within” approach. Propensity score weighting was performed within each imputed dataset, all the weighted datasets were input to individual logistic regression models and the obtained estimates were then pooled via Rubin’s rule (Supplemental Figs. S1-S2) [41]. As such, for each imputed dataset, patients within a cohort (e.g. prediabetes, PCOS) with recorded metformin use were weighted to improve the overall matching with respect to another patient cohort with recorded levothyroxine or ondansetron use, but not metformin use. The propensity score formula included age, race, ethnicity, sex (only included for prediabetes), smoking status or nicotine dependence, Charlson Comorbidity Index, BMI, and a set of comorbidities, including PCOS (only for prediabetes patients) and prediabetes (only for PCOS patients), with prevalence higher than 2 % (Table 2A, Table 2B, Table 2C, Table 2D ).
Table 2A.
Demographics of the prediabetes cohort with levothyroxine comparator * Native Hawaiian or Other Pacific Islander.
| All patients | On Levothyroxine | On Metformin | p-value | |
|---|---|---|---|---|
| n | 3136 | 2736 | 400 | |
| DEMOGRAPHICS | ||||
| Age | 59.27 (15.03) | 61.18 (14.07) | 46.52 (14.99) | 0 |
| BMI | 33.74 (8.39) | 32.98 (7.97) | 38.20 (9.36) | 0 |
| Length of stay | 8.14 (39.64) | 7.88 (32.18) | 9.93 (72.44) | 0.336 |
| INPATIENT/OUTPATIENT | ||||
| Inpatient or ED | 1250 (39.9%) | 1161 (42.4%) | 89 (22.2%) | 0 |
| GENDER | 0.081 | |||
| Male | 766 (24.4%) | 651 (23.8%) | 115 (28.7%) | |
| Female | 2367 (75.5%) | 2082 (76.1%) | 285 (71.2%) | 0.041 |
| RACE | 0 | |||
| White | 2105 (79.3%) | 1887 (81.1%) | 218 (66.5%) | 0 |
| Black or African American | 418 (13.3%) | 325 (11.9%) | 93 (23.2%) | |
| Asian | 103 (3.3%) | >20 | <20 | |
| Other | 23 (0.7%) | <20 | <20 | |
| NHOPI* | <20 | <20 | <20 | |
| ETHNICITY | 0 | |||
| Hispanic or Latino | 669 (22.9%) | 555 (21.8%) | 114 (31.1%) | |
| Not Hispanic or Latino | 2247 (77.1%) | 1994 (78.2%) | 253 (68.9%) | |
| CCI | 1045 (33.3%) | 980 (35.8%) | 65 (16.2%) | 0 |
| Current or former smoker | 660 (21.0%) | 571 (20.9%) | 89 (22.2%) | 0.571 |
| HOSPITAL EVENTS | ||||
| Invasive ventilation | 105 (3.3%) | >20 | <20 | 0.019 |
| ECMO | <20 | <20 | <20 | 1 |
| COMORBIDITIES | ||||
| Hypertension | 1656 (52.8%) | 1489 (54.4%) | 167 (41.8%) | 0 |
| Neoplasm | 1206 (38.5%) | 1115 (40.8%) | 91 (22.8%) | 0 |
| Heart disease | 985 (31.4%) | 938 (34.3%) | 47 (11.8%) | 0 |
| Chronic respiratory disease | 817 (26.1%) | 732 (26.8%) | 85 (21.2%) | 0.023 |
| Liver disease | 351 (11.2%) | 322 (11.8%) | 29 (7.2%) | 0.01 |
| Kidney disease | 343 (10.9%) | >20 | <20 | 0 |
| Nicotine dependence | 269 (8.6%) | 245 (9.0%) | 24 (6.0%) | 0.061 |
| Cerebral infarction | 96 (3.1%) | >20 | <20 | 0.036 |
| Arthritis | 90 (2.9%) | >20 | <20 | 0.055 |
| Dementia | 88 (2.8%) | >20 | <20 | 0.005 |
| PCOS | 81 (2.6%) | <20 | >20 | 0 |
| Psoriasis | 63 (2.0%) | >20 | <20 | 0.177 |
Table 2B.
Demographics of the PCOS cohort with levothyroxine comparator * Native Hawaiian or Other Pacific Islander.
| All patients | On Levothyroxine | On Metformin | p-value | |
|---|---|---|---|---|
| n | 282 | 86 | 196 | |
| DEMOGRAPHICS | ||||
| Age | 33.18 (8.42) | 35.81 (8.06) | 32.03 (8.33) | 0 |
| BMI | 37.90 (8.94) | 36.73 (8.38) | 38.43 (9.15) | 0.173 |
| Length of stay | 7.04 (39.95) | 12.51 (64.96) | 4.63 (21.00) | 0.128 |
| INPATIENT/OUTPATIENT | ||||
| Inpatient or ED | 72 (25.5%) | 28 (32.6%) | 44 (22.4%) | 0.1 |
| RACE | 0.379 | |||
| White | 190 (67.4%) | 65 (75.6%) | 125 (63.8%) | 0.059 |
| Black or African American | 33 (11.7%) | < 20 | > 20 | |
| Asian | < 20 | < 20 | < 20 | |
| Other | < 20 | < 20 | < 20 | |
| NHOPI* | < 20 | < 20 | < 20 | |
| ETHNICITY | 0.85 | |||
| Hispanic or Latino | 63 (24.8%) | < 20 | >20 | |
| Not Hispanic or Latino | 191 (75.2%) | 59 (76.6%) | 132 (74.6%) | |
| CCI | 30 (10.6%) | < 20 | < 20 | 0.008 |
| Current or former smoker | 59 (20.9%) | < 20 | >20 | 0.267 |
| HOSPITAL EVENTS | ||||
| AKI in hospital | <20 | <20 | <20 | 0.671 |
| Invasive ventilation | <20 | <20 | <20 | 0.671 |
| ECMO | 0 | 0 | 0 | – |
| COMORBIDITIES | ||||
| Prediabetes | 84 (29.8%) | < 20 | > 20 | 0.044 |
| Neoplasm | 57 (20.2%) | 22 (25.6%) | 35 (17.9%) | 0.185 |
| Hypertension | 54 (19.1%) | < 20 | >20 | 0.992 |
| Chronic respiratory disease | 49 (17.4%) | < 20 | >20 | 1 |
| Heart disease | 21 (7.4%) | < 20 | < 20 | 0.302 |
| Liver disease | < 20 | < 20 | < 20 | 0.005 |
| Nicotine dependence | < 20 | < 20 | < 20 | 0.966 |
| Kidney disease | < 20 | < 20 | < 20 | 0.759 |
Table 2C.
Demographics of the prediabetes cohort with ondansetron comparator * Native Hawaiian or Other Pacific Islander.
| All patients | On Ondansentron | On Metformin | p-value | |
|---|---|---|---|---|
| n | 8015 | 7618 | 397 | |
| DEMOGRAPHICS | ||||
| age | 57.82 (16.14) | 58.41 (15.98) | 46.80 (15.09) | 0 |
| BMI | 33.22 (8.78) | 32.85 (8.62) | 37.88 (9.41) | 0 |
| length of stay | 10.66 (49.07) | 10.75 (47.57) | 8.91 (72.00) | 0.466 |
| INPATIENT/OUTPATIENT | ||||
| Inpatient or ED | 6614 (82.5%) | 6544 (85.9%) | 70 (17.6%) | 0 |
| GENDER | 0 | |||
| Male | 3346 (41.7%) | 3235 (42.5%) | 111 (28.0%) | |
| Female | 4661 (58.2%) | 4375 (57.4%) | 286 (72.0%) | |
| RACE | 0.07 | |||
| White | 4738 (59.1%) | 4515 (59.3%) | 223 (56.2%) | |
| Black or African American | 1873 (23.4%) | 1781 (23.4%) | 92 (23.2%) | |
| Asian | 228 (2.8%) | > 20 | < 20 | |
| Other | > 20 | > 20 | < 20 | |
| NHOPI* | 20 (0.2%) | < 20 | < 20 | |
| ETHNICITY | 0 | |||
| Hispanic or Latino | 1275 (17.3%) | 1163 (16.6%) | 112 (30.4%) | |
| Not Hispanic or Latino | 6088 (82.7%) | 5831 (83.4%) | 257 (69.6%) | |
| CCI > 1 | 2583 (32.2%) | 2520 (33.1%) | 63 (15.9%) | 0 |
| Current or former smoker | 1456 (18.2%) | 1368 (18.0%) | 88 (22.2%) | 0.04 |
| HOSPITAL EVENTS | ||||
| AKI in hospital | 973 (12.1%) | > 20 | < 20 | 0 |
| Invasive ventilation | 40 (0.5%) | > 20 | < 20 | 0.725 |
| ECMO | 552 (6.9%) | > 20 | < 20 | 0 |
| COMORBIDITIES | ||||
| hypertension | 3884 (48.5%) | 3720 (48.8%) | 164 (41.3%) | 0.004 |
| heart disease | 2659 (33.2%) | 2614 (34.3%) | 45 (11.3%) | 0 |
| neoplasm | 2492 (31.1%) | 2400 (31.5%) | 92 (23.2%) | 0.001 |
| chronic respiratory disease | 1787 (22.3%) | 1704 (22.4%) | 83 (20.9%) | 0.535 |
| kidney disease | 935 (11.7%) | > 20 | < 20 | 0 |
| liver disease | 849 (10.6%) | 822 (10.8%) | 27 (6.8%) | 0.015 |
| nicotine dependence | 759 (9.5%) | 734 (9.6%) | 25 (6.3%) | 0.033 |
| cerebral infarction | 327 (4.1%) | > 20 | < 20 | 0 |
| dementia | 213 (2.7%) | > 20 | < 20 | 0.01 |
| arthritis | 193 (2.4%) | > 20 | < 20 | 0.304 |
Table 2D.
Demographics of the PCOS cohort with ondansetron comparator * Native Hawaiian or Other Pacific Islander.
| All patients | On Ondansentron | On Metformin | p-value | |
|---|---|---|---|---|
| n | 501 | 309 | 192 | |
| DEMOGRAPHICS | ||||
| age | 32.54 (8.95) | 32.63 (9.21) | 32.39 (8.52) | 0.762 |
| BMI | 37.94 (9.39) | 37.87 (9.60) | 38.04 (9.13) | 0.862 |
| length of stay | 5.45 (53.44) | 6.94 (67.02) | 3.04 (15.00) | 0.427 |
| INPATIENT/OUTPATIENT | ||||
| Inpatient or ED | 229 (45.7%) | 195 (63.1%) | 34 (17.7%) | 0 |
| RACE | 0.561 | |||
| White | 331 (66.1%) | 210 (68.0%) | 121 (63.0%) | |
| Black or African American | 76 (15.2%) | 49 (15.9%) | 27 (14.1%) | |
| Asian | < 20 | < 20 | < 20 | |
| Other | < 20 | < 20 | < 20 | |
| NHOPI* | < 20 | < 20 | < 20 | |
| ETHNICITY | 0.224 | |||
| Hispanic or Latino | 100 (22.6%) | 55 (20.4%) | 45 (25.9%) | |
| Not Hispanic or Latino | 401 (87.4%) | 214 (79.6%) | 129 (74.1%) | |
| CCI > 1 | 75 (15.0%) | > 20 | < 20 | 0.001 |
| Current or former smoker | 96 (19.2%) | 50 (16.2%) | 46 (24.0%) | 0.042 |
| HOSPITAL EVENTS | ||||
| AKI in hospital | < 20 | < 20 | 0 | 0.286 |
| Invasive ventilation | 0 | 0 | 0 | – |
| ECMO | < 20 | < 20 | 0 | 0.698 |
| COMORBIDITIES | ||||
| prediabetes | 121 (24.2%) | 56 (18.1%) | 65 (33.9%) | 0 |
| neoplasm | 118 (23.6%) | 85 (27.5%) | 33 (17.2%) | 0.011 |
| hypertension | 117 (23.4%) | 85 (27.5%) | 32 (16.7%) | 0.007 |
| chronic respiratory disease | 101 (20.2%) | 71 (23.0%) | 30 (15.6%) | 0.06 |
| heart disease | 60 (12.0%) | > 20 | < 20 | 0 |
| liver disease | 45 (9.0%) | > 20 | < 20 | 0.001 |
| nicotine dependence | 41 (8.2%) | > 20 | < 20 | 0.037 |
2.6. Outcome definition
The outcome of interest for this investigation was COVID-19 clinical severity. Clinical severity was classified using the Clinical Progression Scale (CPS) established by the World Health Organization (WHO) for COVID-19 clinical research [42] modified into the five following categories: “mild” (outpatient, WHO severity 1–3); “mild ED” (outpatient with ED visit, WHO severity 3); “moderate” (hospitalized without invasive ventilation, WHO severity 4–6); “severe” (hospitalized with invasive ventilation or ECMO, WHO severity 7–9); and “mortality/hospice” (hospital mortality or discharge to hospice, WHO Severity 10) [28]. To develop ordinal classifications for patient severity, patients were assigned to severity groups according to the maximum clinical severity during their index encounter [28], which was defined as the medical encounter during which a positive COVID-19 test was documented for the first time. These classes were used to create four derived outcome variables based on COVID-19 severity: “mild ED” or worse, “moderate” or worse, “severe” or worse, and “mortality/hospice”.
The control-matched participants within the comparison were individuals with prediabetes or PCOS who used levothyroxine or ondansetron for conditions unrelated to prediabetes. The adjusted cohorts were then used to assess the association between metformin use and COVID-19 severity (“mild”, “mild ED”, “moderate”, “severe”, “mortality/hospice”) when compared to levothyroxine or ondansetron use.
2.7. Covariates
We evaluated COVID-19 severity and its association with metformin and other covariates: age, race, ethnicity, sex (only included for prediabetes), smoking status, Charlson Comorbidity Index, BMI, prediabetes (only included for PCOS), PCOS (only included for prediabetes) and other comorbidities (kidney disease, liver disease, hypertension, dementia, neoplasm, arthritis, heart disease, chronic respiratory disease, psoriasis, lupus, Alzheimer's disease, cerebral infarction, immunosuppression, PCOS, nicotine dependence) that occurred in>2 % of the cohort.
2.8. Statistical analysis
To investigate the association of metformin and other covariates with COVID-19 severity, we applied the glm function of the stats R package [43] to perform logistic regression on each imputed and matched dataset.
For each outcome, we independently performed a logistic regression (LR) to evaluate COVID-19 severity and its association with metformin and other covariates. Since in the PCOS cohort, the number of patients with COVID-19 severity of “severe” or “mortality/hospice” was very small, we omitted LR for these two outcome variables. For “on metformin” status, we pooled the LR estimates across all the imputed datasets by Rubin’s rule and we recorded the corresponding p-values, the pooled odds ratios and their 95 % confidence intervals. The p-values obtained for the “on metformin” status on the prediabetes and PCOS cohorts were adjusted by Bonferroni correction to account for family-wise False Discovery Rate (FDR).
Covariates were combined or removed when significant collinearity was detected to avoid biased regression coefficients. Collinearity between covariates was assessed by calculating the generalized variance inflation factor (GVIF) [44], [45]. For all cohorts, we observed high GVIF (>5) for race and Charlson Comorbidity Index (CCI) [46] (data not shown), likely due to low representation in a variety of race categories and CCI values. To alleviate that collinearity, while still including race and CCI within the regression, the race category was modified into a binary “white”/“non-white” variable, and the CCI variable was converted to a binary variable “CCI ≤ 1”/“CCI > 1”. No other included variables showed a GVIF>5.
We used the EValue R package (version 4.1.2) to determine the minimum strength of an unmeasured confounder in the logistic regression that would be required to change the conclusion that metformin was associated with reduced severity of COVID-19 [47]. We treated the outcome of decreased COVID-19 severity as a non-rare outcome (as it occurred more frequently than 15 %). For a more conservative estimate, we report the E-value estimate for the confidence interval of the odds ratio that is closest to the null, as recommended by VanderWeele et al. [47].
3. Results
In the current study, two cohorts of COVID-19 positive individuals were identified for assessing the association between metformin usage and COVID-19 severity outcomes. The two cohorts developed were prediabetes and PCOS (Fig. 1 ). Data for this project were derived from 53 total sites, among which only 26 provided PCOS case data. For this study, data was included up to May 12, 2022. The predictors with missing data were age, BMI, race, and ethnicity (see Supplemental Table 1), with an overall percentage of cases with any missing values equal to 30 % and 35 % in PCOS (for levothyroxine and ondansetron comparators respectively) and 38 % and 53 % in prediabetes patients (for levothyroxine and ondansetron comparators respectively,). Little’s test supported the assumption of Missing At Random over Missing Completely at Random data, p-value < 0.05 [48].
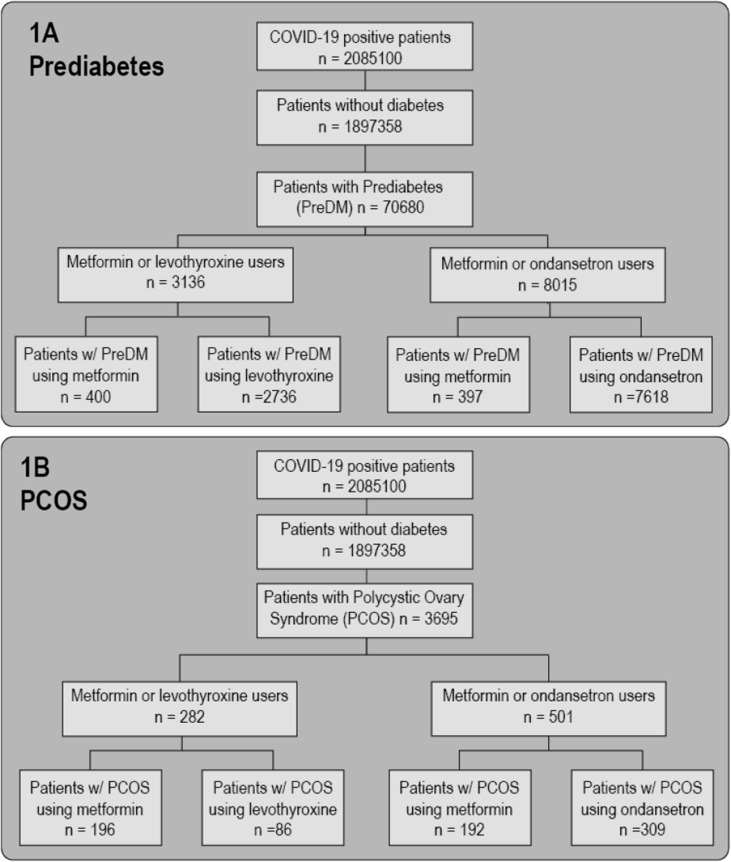
Fig. 1.
Definition of prediabetes and PCOS cohorts. COVID-19 positive patients were filtered to remove any individuals with diabetes and then separated into prediabetes (PreDM) (1A) and Polycystic Ovary Syndrome (PCOS) (1B) cohorts. For both cohorts, we selected patients that had a recorded usage of either metformin or levothyroxine (left) and metformin or ondansetron (right). Patients who used both metformin and the control medication (levothyroxine or ondansetron) were excluded from the analyses.
For the PCOS cohort, we evaluated 282 patients (metformin versus levothyroxine) and 501 patients (metformin versus ondansetron). For the prediabetes cohort, we evaluated 3136 patients (metformin versus levothyroxine) and 8015 patients (metformin versus ondansetron) (Fig. 1). Table 2A, Table 2B, Table 2C, Table 2D shows the demographics of both cohorts, including the CCI score. Distribution of reported COVID-19 severity scores are in Table 3 .
Table 3.
COVID-19 severity outcome of patients in prediabetes and PCOS cohorts for metformin or levothyroxine (3A) and metformin or ondansetron (3B). Cohen’s D is also reported as an estimate of the effect-size; large effect sizes (absolute value of Cohen’s D > 0.8) are indicated by an asterisk. Values<20 patients are obscured to reduce the risk of patient reidentification.
|
(A) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
PCOS |
Prediabetes |
|||||||
| All | Metformin | Levothyroxine | Cohen’s D | All | Metformin | Levothyroxine | Cohen’s D | |
| n | 282 | 196 | 86 | 3136 | 400 | 2736 | ||
| Mild | 210 (74.5%) | 152 (77.6%) | 58 (67.4%) | −0.233 | 1875 (59.8%) | 310 (77.5%) | 1565 (57.2%) | −0.418 |
| Mild ED | 34 (12.1%) | >20 | <20 | 0.084 | 191 (6.1%) | 31 (7.8%) | 160 (5.8%) | 0.080 |
| Moderate | 36 (12.8%) | >20 | <20 | 0.203 | 849 (27.1%) | 51 (12.8%) | 798 (29.2%) | 0.374 |
| Severe | < 20 | <20 | <20 | 0.079 | 57 (1.8%) | < 20 | > 20 | 0.070 |
| Mortality / hospice | < 20 | < 20 | <20 | 164 (5.2%) | < 20 | > 20 | ||
|
(B) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
PCOS |
Prediabetes |
|||||||
| All | Metformin | Ondansentron | Cohen’s D | All | Metformin | Ondansentron | Cohen’s D | |
| n | 501 | 192 | 309 | 8015 | 397 | 7618 | ||
| Mild | 270 (53.9%) | 158 (82.3%) | 112 (36.2%) | −1.040* | 1383 (17.3%) | 326 (82.1%) | 1057 (13.9%) | −1.963* |
| Mild ED | 93 (18.6%) | 21 (10.9%) | 72 (23.3%) | 0.324 | 889 (11.1%) | 30 (7.6%) | 859 (11.3%) | 0.119 |
| Moderate | 132 (26.3%) | < 20 | > 20 | 0.827* | 4711 (58.8%) | 34 (8.6%) | 4677 (61.4%) | 1.108* |
| Severe | < 20 | < 20 | < 20 | 0.131 | 328 (4.1%) | < 20 | > 20 | 0.179 |
| Mortality/ hospice |
< 20 | < 20 | < 20 | 704 (8.8%) | < 20 | > 20 | ||
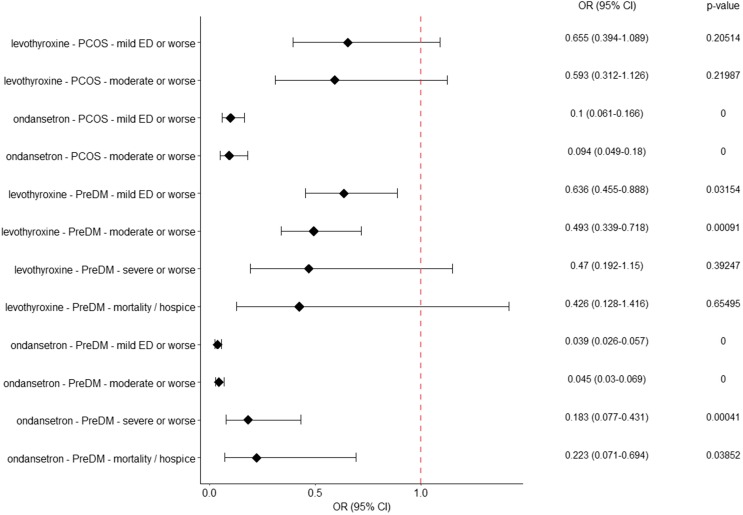
For the PCOS cohort, no significant association between metformin usage and decreased COVID-19 severity was observed as compared to the levothyroxine control group (Fig. 2 ). Within the metformin-levothyroxine assessment, white race was significantly associated with COVID-19 severity in both of the logistic regressions, but no other significant associations were observed (Supplementary Figs. S3a-b). However, in the metformin-ondansetron comparison for patients with PCOS (Fig. 2, Supplementary Figs. S3c-d), metformin use was significantly associated with a decreased incidence of COVID severity of “mild ED” or worse (OR: 0.101, 95 % CI 0.061–0.166, Bonferroni adj. p = 0), and “moderate” or worse (OR: 0.094, 95 % CI 0.049–0.18, adj. p = 0). In the “mild ED” or worse regression, the comorbidity of prediabetes indicated a potential increase in incidence of more severe COVID infection.
Fig. 2.
Association between metformin usage and COVID-19 severity in PCOS and prediabetes cohorts. Odds ratios (x-axis) and adjusted p-values are shown for the given combination of drug, cohort and outcome. For the PCOS cohort, there were insufficient patients with severe or worse disease or mortality/hospice for a logistic regression to be performed. All p-values are Bonferonni corrected. A comparison of the estimates obtained before and after cohort weighting provides an estimate of the effect size (Supplementary Fig. S5). Note that estimates are comparable, even for groups that show large effect sizes according to Cohen’s D (Table 2).
Assessment of the prediabetes cohort indicated a significant association of metformin usage with decreased incidence of COVID-19 severity of “mild ED” or worse (OR: 0.636, 95 % CI 0.455–0.888, adj. p = 0.0315) and “moderate” or worse (OR: 0.493, 95 % CI 0.339–0.718, adj. p = 0.0009) versus levothyroxine (Fig. 2). Additionally, lower incidence of “mild ED” or worse severity (OR: 0.039, 95 % CI 0.026–0.057, adj. p = 0), “moderate” or worse (OR: 0.045, 95 % CI 0.03–0.069, adj. p = 0), “severe” or worse (OR: 0.183, 95 % CI 0.077–0.431, adj. p = 0.0004), and “hospice or death from COVID” (OR: 0.223, 95 % CI 0.071–0.694, adj. p = 0.0385) were seen in metformin versus ondansetron analyses (Fig. 2).
In agreement with other prior investigations, we observed a significant association of COVID-19 severity “moderate” or worse with age for patients with prediabetes (Supplementary Figs. S4a-h) in several evaluations. Unexpectedly, for patients with prediabetes we saw significantly decreased incidence of more severe COVID-19 in patients with chronic respiratory disease (Supplementary Figs. S4a-h) or hypertension (Supplementary Figs. S4a-h and Supplementary Figs. S6), and for those with Hispanic or Latino ethnicity (Figs. S4a-h) in some evaluations, possibly due to the non-collapsibility of odds ratios [49], [50] and/or confounding factors such as behavior. A secondary analysis using logistic regression confirmed that metformin, as well as Hispanic or Latino ethnicity, are associated with decreased incidence of more severe COVID-19; on the other hand, chronic respiratory disease and hypertension do not show any significant association with COVID-19 severity (Supplementary Fig. S6). Heart disease (Figs. S4a-b) and history of neoplasm (Supplementary Fig. S3a) were variably associated with a higher incidence of severe disease outcomes.
To assess the sensitivity of our findings to uncorrected confounders, we calculated the E-value for the observed values of the odds ratio with significant p-values in Fig. 2 [47]. To explain the association of metformin use with decreased COVID-19 severity in the prediabetes cohort compared to levothyroxine, an unmeasured confounder would need to be associated with both the treatment and the outcome with an odds ratio of 0.79 (upper bound 0.93) (“mild ED” or worse) and 0.70 (upper bound 0.84) (“moderate” or worse) above and beyond the confounders included in the regression. To explain the association of metformin use with decreased COVID-19 severity in the PCOS cohort compared to ondansetron, an unmeasured confounder would need to be associated with both the treatment and the outcome with an odds ratio of 0.32 (upper bound 0.41) (“mild ED” or worse), 0.31 (upper bound 0.42) (“moderate” or worse), and in the prediabetes cohort, an odds ratio of 0.20 (upper bound 0.24) (“mild ED” or worse), 0.21 (upper bound 0.26) (“moderate” or worse), 0.43 (upper bound 0.66) (“severe” or worse), and 0.47 (upper bound 0.83) (“mortality/hospice”).
Since gastrointestinal symptoms, including nausea and vomiting, may be associated with more severe COVID-19 infection [51], [52], we also performed sensitivity analyses in which patients who initiated ondansetron (a medication frequently prescribed for nausea and vomiting) the day of their COVID-19 index diagnosis, possibly for nausea or vomiting related to COVID-19 infection, were excluded (Supplementary Fig. S7 and S8). Results of these sensitivity analyses were consistent with our main analyses. In individuals with prediabetes, protective effects of metformin were confirmed for the “mild ED” or worse, “moderate” or worse, and “severe” or worse analyses. Significance was not seen in the “mortality/hospice” analysis, potentially due to insufficient cohort size. The corresponding sensitivity analyses in the PCOS evaluation were largely unchanged compared with our main analyses.
4. Discussion
We observed a significant association between metformin use and less severe COVID-19 in the prediabetes cohort compared with levothyroxine or ondansetron use, and within the PCOS cohort compared to ondansetron use. While multiple studies have been conducted to assess metformin impact on COVID-19 in the type 2 diabetes population, this is the largest study to date investigating off-label usage including individuals with prediabetes or PCOS. These findings are consistent with prior investigations in type 2 diabetes patients, in which metformin users were reported to have lower COVID-19 severity [6], [10], [53].
The proposed mechanisms for metformin as a potential mediator of severe COVID-19 infection include its effect on blood glucose regulation via AMPK stimulation and glucose-6-phosphate (G6P) inhibition, which reduce hepatic gluconeogenesis [54], [55], as well as decreased reactive oxygen species production at the respiratory-chain complex 1 [56], [57]. Given the increased inflammation seen in both hyperglycemia and COVID-19, metformin’s capacity to attenuate immune inflammatory responses via reduced proinflammatory cytokines offers multiple potential benefits to individuals with altered glycemic control, including those with prediabetes or PCOS [10]. Reduced inflammation may be beneficial during the hyperinflammatory state of COVID-19 infection [2], and can also delay progression to diabetes or onset of cardiovascular disease in individuals with prediabetes [58].
Considering the impact of COVID-19 on populations investigated in this study, additional potential interventions to prevent or mitigate COVID-19 severity are greatly desired. While PCOS is a common endocrine disorder, its etiology and pathophysiology are not fully elucidated and clinical care for patients with PCOS can be inconsistent even during non-pandemic times [59]. Additionally, a wide swath of risk factors for severe COVID-19 are commonly observed in patients with PCOS, including obesity, hypertension, and metabolic syndrome [59]. As these cardio-metabolic risk factors are more frequently seen in patients with PCOS compared to the general population, patient risk for acquiring COVID-19 with severe presentation is increased [60]. One report indicated that individuals with PCOS have a 28 % increased risk of COVID-19 infection [61].
The current study’s association of metformin with reduced incidence of severe COVID-19 outcomes (compared to ondansetron) in the PCOS cohort supports a potential use case for metformin use in PCOS patients who are at elevated risk for severe COVID-19 infections. While no significant findings were observed in the metformin versus levothyroxine comparison for PCOS patients, it is possible that analyses were not sufficiently powered to detect the improvement mediated by metformin. Of some note, although not statistically significant, point estimates of the odds ratio (metformin versus levothyroxine) were directionally consistent in both populations. Given the potential impact an alternative COVID-19 therapy may pose for patients with PCOS, further investigation into this population with metformin and other drug repurposing candidates seems warranted.
Individuals with prediabetes also face increased COVID-19 risk compared to the general population. Although not meeting the threshold for type 2 diabetes diagnosis, individuals with prediabetes experience reduced insulin sensitivity and glycemic dysregulation [62]. The consistent moderate hyperglycemia experienced by individuals with prediabetes can be accompanied by chronic vascular complications including blood pressure changes and cognitive dysfunction [63]. Additionally, prediabetes may be an important risk factor for COVID-19 [62]. From our investigation, metformin usage in individuals with prediabetes was associated with a significant decrease in COVID severity when compared to levothyroxine or ondansetron users. The association of metformin use with reduced COVID severity may indicate metformin’s potential as a mitigator of severe COVID outcome.
Personalized clinical assessment should continue to be the standard of care for determining if metformin usage is appropriate. However, given these outcomes, clinicians may consider recommending metformin for high COVID-19 risk patients with prediabetes as it may support a reduced incidence of severe COVID. Future investigations using prospective trials and potentially randomized controlled trials are needed to further elucidate the relationship between metformin and COVID-19 outcome.
5. Limitations
This analysis is retrospective and is subject to confounding. Our study utilizes electronic health record data, which may contain some inaccuracies such as incomplete recording of patient data or incomplete/incorrect translation of source EHR data to the OMOP format used by N3C, despite efforts to harmonize and quality-check these data centrally. Further, while metformin and comparator drug usage were assumed to be consistent with prescriptions, medication compliance levels are not reported in our data set.
We were unable to identify a similar oral antihyperglycemic medication (e.g. sulfonylureas, thiazolidinediones) for comparison with metformin due to limited usage of those medications within our study cohorts in the populations of interest. We chose levothyroxine as an inactive comparator drug because of its similarity to metformin with respect to typical daily usage requirement, prescription requirement, and primarily oral administration modality [64], and because levothyroxine is not known to affect COVID-19 severity. However, it is possible that excessive or deficient levels of thyroid hormone in patients with thyroid disease may be associated with increased risk for poor COVID severity outcomes [65], [66], which may introduce a bias toward more severe COVID-19 outcome in patients using levothyroxine. This is contradicted in other investigations, particularly with thyroid diseases that are being actively managed [67], [68].
We chose a second comparator drug, ondansetron, to ensure the robustness of our finding using levothyroxine as a comparator. One of the indications for ondansetron is chemotherapy/radiotherapy induced nausea, which theoretically selects for a population with more cancer patients, who may be at risk for more severe COVID-19 outcome. However, we applied propensity weighting to balance our cohort with respect to neoplasm. Nonetheless, residual confounding by indication may still exist. Some reports have identified an association between ondansetron and a reduction in all cause mortality for patients who started taking ondansetron following admission to the hospital for COVID-19 [33], [34]. We believe ondansetron therefore represents a conservative choice of comparator drug, as our current knowledge of this drug indicates that it is likely neutral or associated with a slight benefit to COVID-19 outcome, and its use a comparator drug will therefore tend to underestimate any beneficial association of metformin with COVID-19 outcome. Sensitivity analysis performed by excluding patients prescribed ondansetron on the day of COVID-19 presentation confirmed the main results of our original analysis, indicating that ondansetron newly prescribed during COVID-19 presentation is not biasing our result.
We observed a limited number of patients (<20) in the severe and mortality/hospice categories in both the PCOS and prediabetes cohorts, which may have limited the statistical power to detect an association between metformin use and COVID-19 severity. This also made us unable to investigate the extent to which metformin lowers the risk of mechanical ventilation or death in patients with prediabetes. Future studies with greater cohort sizes may address this limitation.
6. Conclusion
In summary, this retrospective analysis of electronic health record data supports and extends the previously described association between lower COVID-19 disease severity and metformin usage. However, the inherent limitations of observational analyses leave opportunities for future work in this area. The generalizability of our findings should be evaluated and future research utilizing a larger sample and/or a prospective design, including relevant immunologic biomarkers, may help elucidate the mechanisms that underlie metformin's association with less severe COVID-19 outcome. Prediabetes and PCOS are common conditions and metformin is a safe, inexpensive and widely used drug, so our findings suggest its potential for improving COVID outcomes for many people at low cost.
Funding
This work was supported by NCATS U24 TR002306. Additionally, Justin T. Reese and Nomi L. Harris were supported by the Director, Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy Contract No. DE-AC02-05CH11231; Justin T. Reese, Melissa Haendel, Peter N. Robinson, and Nomi L. Harris were supported by National Human Genome Research Institute awards 7RM1HG010860-02 and 2R24-OD011883-10A1; Adnin Zaman was supported by NIH NIDDK grant F32 DK123878. Melissa Haendel was supported by the Marsico Family at the University of Colorado Anschutz Medical Campus; Elena Casiraghi and Giorgio Valentini were supported by Università degli Studi di Milano, Piano di sviluppo di ricerca, grant 2015-17 PSR2015-17.
Consortial authors
Christopher G Chute (Schools of Medicine, Public Health, and Nursing; Johns Hopkins University, Baltimore, MD, USA), John B Buse (Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA), G. Caleb Alexander, MD, MS, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA), Til Stürmer, MD, MPH, PhD (School of Public Health, UNC-Chapel Hill, Chapel Hill, NC, USA), and Jared Huling, PhD (University of Minnesota, Minneapolis, MN, USA).
We gratefully acknowledge the following core contributors to N3C:
Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O'Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O'Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang.
Details of contributions available at covid.cd2h.org/core-contributors. Authorship was determined using ICMJE recommendations.
The following institutions whose data is released or pending:
Available: Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Charleston Area Medical Center — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Indiana University School of Medicine — UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Loyola Medicine — Loyola University Medical Center • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours — U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University — U24TR002306 • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University — UL1TR003096: Center for Clinical and Translational Science • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington — UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI).
Submitted: Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute.
Pending: Arkansas Children’s Hospital — UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine — None (Voluntary) • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth — None (Voluntary) • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute — UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth — None (Voluntary) • Montana State University — U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research — None (Voluntary) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Florida — UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave (covid.cd2h.org/enclave) and supported by CD2H - The National COVID Cohort Collaborative (N3C) IDeA CTR Collaboration 3U24TR002306-04S2 NCATS U24 TR002306, DUR: RP-7BE1AC. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the on-going development of this community resource 27 The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the N3C program.
The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol #IRB00249128 or individual site agreements with NIH. The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2022.110157.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fischer W, Eron JJ, Holman W, et al. Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv [Internet] 2021; Available from: http://dx.doi.org/10.1101/2021.06.17.21258639.
- 2.Zhong J., Tang J., Ye C., Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2(7):e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacol Rep 2020;72(6):1479–508. [DOI] [PMC free article] [PubMed]
- 4.Dotolo S, Marabotti A, Facchiano A, Tagliaferri R. A review on drug repurposing applicable to COVID-19. Brief Bioinform 2021;22(2):726–41. [DOI] [PMC free article] [PubMed]
- 5.Ghany R., Palacio A., Dawkins E., Chen G., McCarter D., Forbes E., et al. Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA. Diabetes Metab Syndr. 2021;15(2):513–518. doi: 10.1016/j.dsx.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramante C.T., Buse J., Tamaritz L., Palacio A., Cohen K., Vojta D., et al. Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity. J Med Virol. 2021;93(7):4273–4279. doi: 10.1002/jmv.26873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey C.J., Gwilt M. Diabetes, metformin and the clinical course of covid-19: outcomes, mechanisms and suggestions on the therapeutic use of metformin. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.784459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Guo H., Qiu L., Zhang C., Deng Q., Leng Q. Immunomodulatory and antiviral activity of metformin and its potential implications in treating coronavirus disease 2019 and lung injury. Front Immunol. 2020;11:2056. doi: 10.3389/fimmu.2020.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramante C.T., Huling J.D., Tignanelli C.J., Buse J.B., Liebovitz D.M., Nicklas J.M., et al. Randomized trial of metformin, ivermectin, and fluvoxamine for covid-19. N Engl J Med. 2022;387(7):599–610. doi: 10.1056/NEJMoa2201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel S.M., Varghese E., Büsselberg D. Therapeutic potential of metformin in COVID-19: reasoning for its protective role. Trends Microbiol. 2021;29(10):894–907. doi: 10.1016/j.tim.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164:108183. doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibricevic D., Asimi Z. Frequency of prediabetes in women with polycystic ovary syndrome. Med Arh. 2013;67(4):282. doi: 10.5455/medarh.2013.67.282-285. [DOI] [PubMed] [Google Scholar]
- 14.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379(9833):2279–90. [DOI] [PMC free article] [PubMed]
- 15.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(Suppl 1):S15–33. [DOI] [PubMed]
- 16.Echouffo-Tcheugui J.B., Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42(1):59–77. doi: 10.1146/annurev-publhealth-090419-102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374(9702):1677–86. [DOI] [PMC free article] [PubMed]
- 18.Azziz R., Carmina E., Chen ZiJiang, Dunaif A., Laven J.S.E., Legro R.S., et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1) doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 19.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016;31(12):2841–55. [DOI] [PubMed]
- 20.Kyritsi E.M., Dimitriadis G.K., Kyrou I., Kaltsas G., Randeva H.S. PCOS remains a diagnosis of exclusion: a concise review of key endocrinopathies to exclude. Clin Endocrinol. 2017;86(1):1–6. doi: 10.1111/cen.13245. [DOI] [PubMed] [Google Scholar]
- 21.Vitek W, Alur S, Hoeger KM. Off-label drug use in the treatment of polycystic ovary syndrome. Fertil Steril 2015;103(3):605–11. [DOI] [PubMed]
- 22.Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine 2006;30(1):13–7. [DOI] [PubMed]
- 23.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38(9):1165–74. [DOI] [PubMed]
- 24.Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab 1996;81(8):2854–64. [DOI] [PubMed]
- 25.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98(12):4565–92. [DOI] [PMC free article] [PubMed]
- 26.Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015;22(3):553–64. [DOI] [PMC free article] [PubMed]
- 27.Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment. J Am Med Inform Assoc 2021;28(3):427–43. [DOI] [PMC free article] [PubMed]
- 28.Bennett TD, Moffitt RA, Hajagos JG, et al. The National COVID Cohort Collaborative: Clinical Characterization and Early Severity Prediction. medRxiv [Internet] 2021;Available from: http://dx.doi.org/10.1101/2021.01.12.21249511.
- 29.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hripcsak G, Duke JD, Shah NH, et al. Observational health data sciences and informatics (OHDSI): Opportunities for observational researchers. Stud Health Technol Inform 2015;216:574–8. [PMC free article] [PubMed]
- 31.Nagy E.V., Perros P., Papini E., Katko M., Hegedüs L. New formulations of levothyroxine in the treatment of hypothyroidism: trick or treat? Thyroid. 2021;31(2):193–201. doi: 10.1089/thy.2020.0515. [DOI] [PubMed] [Google Scholar]
- 32.Milne RJ, Heel RC. Ondansetron. Therapeutic use as an antiemetic. Drugs 1991;41(4):574–95. [DOI] [PubMed]
- 33.Bayat V, Ryono R, Phelps S, et al. Reduced mortality with ondansetron use in SARS-CoV-2-infected inpatients. Open Forum Infect Dis 2021;8(7):ofab336. [DOI] [PMC free article] [PubMed]
- 34.Miller G.M., Ellis J.A., Sarangarajan R., Parikh A., Rodrigues L.O., Bruce C., et al. Hypothesis-agnostic network-based analysis of real-world data suggests ondansetron is associated with lower COVID-19 any cause mortality. Drugs Real World Outcomes. 2022;9(3):359–375. doi: 10.1007/s40801-022-00303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier C., Kapsner L.A., Mate S., Prokosch H.-U., Kraus S. Patient cohort identification on time series data using the OMOP common data model. Appl Clin Inform. 2021;12(1):57–64. doi: 10.1055/s-0040-1721481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stekhoven DJ, Bühlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics 2012;28(1):112–8. [DOI] [PubMed]
- 37.Mayer M. Fast imputation of missing values [R package missRanger version 2.1.3]. 2021 [cited 2022 Jun 14];Available from: https://CRAN.R-project.org/package=missRanger.
- 38.van Buuren S. 2nd ed. CRC Press; 2018. Flexible imputation of missing data. [Google Scholar]
- 39.Casiraghi E, Wong R, Hall M, et al. A methodological framework for the comparative evaluation of multiple imputation methods: multiple imputation of race, ethnicity and body mass index in the U.S. National COVID Cohort Collaborative [Internet]. arXiv [cs.AI]. 2022;Available from: http://arxiv.org/abs/2206.06444.
- 40.Pishgar F., Greifer N., Leyrat C., Stuart E. MatchThem: Matching and weighting after multiple imputation. R J. 2021;13(2):228. [Google Scholar]
- 41.Rubin D.B. Wiley; New York: 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 42.WHO working group on the clinical characterisation and management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20(8):e192–7. [DOI] [PMC free article] [PubMed]
- 43.The R Stats Package [Internet]. [cited 2022 Jun 14];Available from: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/00Index.html.
- 44.Fox J. Applied regression analysis, linear models, and related methods. 1997;597. Available from: https://psycnet.apa.org/fulltext/1997-08857-000.pdf.
- 45.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc 1992;87(417):178–83.
- 46.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed]
- 47.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167(4):268–74. [DOI] [PubMed]
- 48.Li C. Little’s test of missing completely at random. Stata J. 2013;13(4):795–809. [Google Scholar]
- 49.Daniel R, Zhang J, Farewell D. Making apples from oranges: Comparing noncollapsible effect estimators and their standard errors after adjustment for different covariate sets. Biom J 2021;63(3):528–57. [DOI] [PMC free article] [PubMed]
- 50.Schuster N.A., Twisk J.W.R., Ter Riet G., Heymans M.W., Rijnhart J.J.M. Noncollapsibility and its role in quantifying confounding bias in logistic regression. BMC Med Res Methodol. 2021;21(1):136. doi: 10.1186/s12874-021-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng W., Qi K., Ye M., Zheng L.i., Liu X., Hu S., et al. Gastrointestinal symptoms are associated with severity of coronavirus disease 2019: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2022;34(2):168–176. doi: 10.1097/MEG.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 52.Chen R., Yu Y.-l., Li W., Liu Y.a., Lu J.-X., Chen F., et al. Gastrointestinal symptoms associated with unfavorable prognosis of COVID-19 patients: a retrospective study. Front Med. 2020;7 doi: 10.3389/fmed.2020.608259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020;19:100290. doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajjar J, Habra MA, Naing A. Metformin: an old drug with new potential. Expert Opin Investig Drugs 2013;22(12):1511–7. [DOI] [PubMed]
- 55.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batandier C., Guigas B., Detaille D., El-Mir M., Fontaine E., Rigoulet M., et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38(1):33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 58.Luc K., Schramm-Luc A., Guzik T.J., Mikolajczyk T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol [Internet] 2019;70(6) doi: 10.26402/jpp.2019.6.01. Available from: [DOI] [PubMed] [Google Scholar]
- 59.Kyrou I., Karteris E., Robbins T., Chatha K., Drenos F., Randeva H.S. Polycystic ovary syndrome (PCOS) and COVID-19: an overlooked female patient population at potentially higher risk during the COVID-19 pandemic. BMC Med. 2020;18(1):220. doi: 10.1186/s12916-020-01697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Medeiros SF, Yamamoto MMW, de Medeiros MAS, Yamamoto AKLW, Barbosa BB. Polycystic ovary syndrome and risks for COVID-19 infection: A comprehensive review. Rev Endocr Metab Disord 2022;23(2):251–64. [DOI] [PMC free article] [PubMed]
- 61.Subramanian A, Anand A, Adderley NJ, et al. Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study. Eur J Endocrinol 2021;184(5):637–45. [DOI] [PMC free article] [PubMed]
- 62.Sosibo AM, Khathi A. Pre-diabetes and COVID-19, could we be missing the silent killer? Exp Biol Med 2021;246(4):369–70. [DOI] [PMC free article] [PubMed]
- 63.Palladino R., Tabak A.G., Khunti K., Valabhji J., Majeed A., Millett C., et al. Association between pre-diabetes and microvascular and macrovascular disease in newly diagnosed type 2 diabetes. BMJ Open Diabetes Res Care [Internet] 2020;8(1):e001061. doi: 10.1136/bmjdrc-2019-001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eghtedari B., Correa R. StatPearls Publishing; 2021. Levothyroxine. [PubMed] [Google Scholar]
- 65.Hariyanto TI, Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr 2020;14(5):1429–30. [DOI] [PMC free article] [PubMed]
- 66.Permana H., Soeriadi E.A., Damara F.A., Mulyani Soetedjo N.N. The prognostic values of thyroid disorders in predicting COVID-19 composite poor outcomes: A systematic review and meta-analysis. Diabetes Metab Syndr. 2022;16(5):102464. doi: 10.1016/j.dsx.2022.102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boelaert K., Visser W.E., Taylor P.N., Moran C., Léger J., Persani L. ENDOCRINOLOGY IN THE TIME OF COVID-19: Management of hyperthyroidism and hypothyroidism. Eur J Endocrinol. 2020;183(1):G33–G39. doi: 10.1530/EJE-20-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S.-Y., Yoo D.-M., Min C.-Y., Choi H.-G. The effects of previous thyroid disease on the susceptibility to, morbidity of, and mortality due to COVID-19: A nationwide cohort study in South Korea. J Clin Med Res [Internet] 2021;10(16) doi: 10.3390/jcm10163522. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.