Abstract
Oxidative stress by reactive oxygen species (ROS) has been hypothesized to be the major mediator of SARS-CoV-2-induced pathogenesis. During infection, the redox homeostasis of cells is altered as a consequence of virus-induced cellular stress and inflammation. In such scenario, high levels of ROS bring about the production of pro-inflammatory molecules like IL-6, IL-1β, etc. that are believed to be the mediators of severe COVID-19 pathology. Based on the known antioxidant, anti-inflammatory, mucolytic and antiviral properties of NAC, it has been hypothesized that NAC will have beneficial effects in COVID-19 patients. In the current study efforts have been made to evaluate the protective effect of NAC in combination with remdesivir against SARS-CoV-2 induced lung damage in the hamster model. The SARS-CoV-2 infected animals were administered with high (500 mg/kg/day) and low (150 mg/kg/day) doses of NAC intraperitoneally with and without remdesivir. Lung viral load, pathology score and expression of inflammatory molecules were checked by using standard techniques. The findings of this study show that high doses of NAC alone can significantly suppress the SARS-CoV-2 mediated severe lung damage (2 fold), but on the contrary, it fails to restrict viral load. Moreover, high doses of NAC with and without remdesivir significantly suppressed the expression of pro-inflammatory genes including IL-6 (4.16 fold), IL-1β (1.96 fold), and TNF-α (5.55 fold) in lung tissues. Together, results of this study may guide future preclinical and clinical attempts to evaluate the efficacy of different doses and routes of NAC administration with or without other drugs against SARS-CoV-2 infection.
Keywords: COVID‐19, Remdesivir, Lung pathology, Macrophage, Bronchoalveolar lavage fluid (BALF), D-Dimer (D2D)
Graphical abstract
Abbreviations
- ABSL-3
Animal Biosafety Level 3
- ANOVA
Analysis of variance
- ARDS
Acute respiratory distress syndrome
- BALF
Bronchoalveolar lavage fluid
- D2D
D-Dimer
- IFNγ
Interferon gamma
- IL1β
Interleukin 1 beta
- IL-6
Interleukin 6
- IL-6R
Interleukin 6 receptor
- I.P.
Intraperitoneal
- NAC
N-acetyl cysteine
- ROS
Reactive oxygen species
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- TCID50
Median Tissue Culture Infectious Dose
- TNF-α
Tumor necrosis factor alpha
1. Introduction
In COVID-19 patients the dominant pathophysiology includes acute pneumonia with profuse radiological opacity, diffuse alveolar damage, extensive inflammatory cells infiltration and microvascular thrombosis in lungs (Carsana et al., 2020). In severe cases, it can lead to acute respiratory distress syndrome (ARDS) and life-threatening pneumonia. Although the exact mechanisms of pathogenesis are yet to be elucidated, the hyper-inflammatory state in severe COVID-19 patients is believed to promote cellular damage in multiple organs including lungs (Chen et al., 2020). Though in clinical cases, remdesivir has been useful to shorten the hospitalization period, it is not routinely recommended for patients under mechanical ventilation and until now, no drugs are effective in reducing the SARS-CoV-2-associated mortality (Beigel et al., 2020; Horby et al., 2021). Hence, continuous efforts have been made to identify effective antiviral and/or therapeutics to mitigate the SARS-CoV-2-associated pathologies.
Several therapeutic interventions have been proposed to counter the inflammation-induced organ damage in COVID-19. Although glucocorticoid therapy has been widely adapted to control inflammatory organ injuries in COVID-19, its clinical usefulness has been widely debated (Shang et al., 2020). The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial showed that dexamethasone reduces 28-day mortality in COVID-19 patients who are receiving respiratory support (Horby et al., 2021). At the same time, it was also noticed that dexamethasone treatment was prominent when the patients were treated more than 7 days after onset of symptoms. This suggests that dexamethasone is more useful when the extent of inflammatory lung damage is more (Horby et al., 2021). Efficacy of corticosteroids as an adjunct therapy in combination with other drugs in managing severe COVID-19 conditions indicates the involvement of the host immune system in the manifestation of the disease severity.
Interleukin-6 (IL-6), proinflammatory cytokine, is expressed by various cell types like immune cells, endothelial cells, fibroblasts, epithelial cells and pneumocytes. The level of IL-6 is reported to be high in COVID-19 patients. However, its level is comparatively lower than other ARDS patients, and thus targeting IL-6 for COVID-19 therapy is a matter of debate (Jones and Hunter, 2021; Sinha et al., 2020). Dysregulation of IL-6 signaling pathway has been linked to multiple inflammatory diseases including cytokine release syndrome linked with chimeric antigen T-cell therapy. Moreover, tocilizumab and sarilumab, the monoclonal antibodies against IL-6R are approved for the treatment of these conditions. The upregulation of IL-6 in severe COVID-19 patients led to the hypothesis that IL-6R blocking might be beneficial. However, despite multiple studies so far no confirmatory conclusion has been reached yet, and variability in different trials has been assumed as a major hindrance in this effort (Angriman et al., 2021).
During infection, viruses often promote a pro-oxidant microenvironment to replicate and escape the cellular defense. Recent study shows that SARS-CoV-2 infection impairs the metabolism and redox function of cellular glutathione (Bartolini et al., 2021). Virus replication leads to the secretion of pro-inflammatory molecules which attract neutrophils and macrophages to the site of infection. These infiltrations further release the pro-inflammatory signals leading to a hyper-inflammatory state to restrict the infection and thus can induce lung damage through pneumonia or ARDS, which is the major cause of SARS-CoV-2-associated mortality. Simultaneously, these pro-inflammatory molecules also lead to the release of ROS, which can restrict the viral dissemination. However, the localized release of these free radicals also damages the uninfected cells (Parasher, 2021). Therefore, ROS production is a necessary evil. To maintain the homeostasis, redox balance is essential (Chernyak et al., 2020), and is controlled through the release of antioxidants mainly glutathione. In the diseased state, the release of ROS surpasses the secretion of antioxidants, leading to oxidative stress and cellular destruction. Under these conditions, the supplementary intake of antioxidants has proven to be effective, especially in the infectious disease models.
N-acetyl cysteine (NAC), a precursor to glutathione, is a plant antioxidant naturally found in onion and garlic (Salamon et al., 2019). NAC has a protective effect in the mouse model of lethal influenza infection. A combination of an antiviral drug oseltamivir and NAC had a better outcome in this model (Garozzo et al., 2007). Infusion of NAC attenuated the lung damage, decreased the neutrophil influx and lung leak in rats intratracheally administered with IL-1 (Leff et al., 1993). Different independent clinical studies have reported the protective effects of NAC in suppressing lung injury (Bernard et al., 1997; Suter et al., 1994). Although multiple literatures have strongly suggested the potential use of NAC in COVID-19, direct evidence for the effect of NAC in SARS-CoV-2 is still lacking.
Based on potent antioxidant and mucolytic properties of NAC, it has been proposed as an adjunct therapy for SARS-CoV-2 treatment (Andreou et al., 2020; de Alencar et al., 2021; De Flora et al., 2020; Dominari et al., 2021; Ibrahim et al., 2020; Liu et al., 2020; Poe and Corn, 2020; Shi and Puyo, 2020; Wong et al., 2021; Zhou et al., 2021). NAC showed efficacy in preventing the SARS-CoV-2-mediated dysregulation of cellular glutathione (Bartolini et al., 2021). An observational study suggests that NAC in combination with antiviral drugs, can act as a promising medication for COVID-19 patients (Bhattacharya et al., 2020). Recently, it was also deciphered that NAC can bind to RBD of spike protein and can inhibit the SARS-CoV-2 entry (Fu et al., 2021). However, NAC as an antiviral drug is quite controversial. In a double-blinded, randomized, placebo-controlled trial, NAC did not show any better clinical outcome in severe COVID-19 cases (de Alencar et al., 2021).
Drugs that are believed to be effective on immune cells and other cell types are often difficult to evaluate in cell culture systems and their human trials have several limitations. Thus, preclinical animal models are resourceful for both the drug efficacy and its associated outcome. Among different models, the hamster model have been successfully addressed to evaluate the drug and vaccine candidates for SARS-CoV-2 (Chen et al., 2021; de Melo et al., 2021; Kaptein et al., 2020; Wang et al., 2021).
Recently, we have established the SARS-CoV-2-Syrian golden hamster model (Suresh et al., 2021). Like earlier reports, our model also manifested a self-limiting form of the disease in which the lung pathology on 4-dpi was severe clearly mimicking several clinical features of COVID-19. The quantitative proteomics also revealed a differential expression of host proteins involved in cellular redox homeostasis. In this study we investigated the effect of parenteral dose of NAC (high- 500 mg/kg/day; low- 150 mg/kg/day) against SARS-CoV-2-mediated infection and lung damage in Syrian golden hamster model.
2. Materials and methods
2.1. Chemicals, cell and virus
N-acetyl cysteine (NAC) was procured from Sigma and reconstituted to a stock concentration of 200 mg/ml in sterile water as per manufacturer's instruction. The original NAC solution had acidic pH, hence the pH was adjusted to 7–7.4 with sodium hydroxide prior to administration. From the stock concentrations, NAC was administrated according to the body weight. Remdesivir (CIPREMI RTU) injection was purchased from Cipla India Ltd. Vero-E6 cells (ATCC, CRL-1586) were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco) and antibiotics (100 U/ml penicillin and 100 μg/mL streptomycin) in 5% CO2 environment at 37 °C. The SARS-CoV-2 strain, IND- ILS January 2020 (Genbank accession ID- MW559533.2) isolated from clinical sample in Odisha, India during the COVID-19 pandemic was used in the study (Suresh et al., 2021). The virus was propagated in Vero-E6 cells in DMEM containing 2% FBS supplemented with antibiotics and the end-point infectivity titre (TCID50) was determined as described (Mendoza et al., 2020).
2.2. Syrian golden hamster study design
All experiments were carried out in the containment facility (ABSL-3) of Institute of Life Science (ILS), Bhubaneswar as per the Institutional Biosafety Committee (IBSC) and Institutional Animal Ethical Committee (IAEC) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India guidelines (Project No: ILS/IAEC-235-AH/JUN-21 and ILS/IAEC-236-AH/JUN-21). Forty eight Syrian golden hamsters (male and female) aged 3–4 months procured from Animal House, ILS and were acclimatized in the ABSL-3 facility with food and water ad libitum for 4–6 days prior to the experiment. Anaesthetized (ketamine 200 mg/kg and xylazine 10 mg/kg) animals were infected with 100 μl inoculum containing 105 TCID50 of SARS-CoV-2 in PBS through intranasal route (50 μl/nare) as described earlier (Suresh et al., 2021). The infected hamsters were categorized into six groups (each containing eight animals); two NAC (alone) groups (high dose – 500 mg/kg, low dose - 150 mg/kg body weight), two NAC-in combination with remdesivir (15 mg/kg body weight), and two control groups (vehicle and only remdesivir group). Because of the restrictions in use of lung tissue for histopathological analysis, only three hamsters from each group were considered for albumin analysis from the bronchoalveolar lavage fluid (BALF) and these animals' lung tissues were not included for any further analysis.
2.3. Clinical scoring and sample collection and processing
All animals were monitored daily till 4-day post infection (dpi) for survivability and body weight measurement. On 4-dpi the animals were sacrificed, blood, bronchoalveolar lavage fluid (BALF) and lungs were collected for further analysis. The whole right-lobe of the lung was collected in RNAlater for RNA extraction and the left-lobes were collected in 10% buffered-formalin for histopathology. For BALF, 2.5 ml of PBS was flushed twice into the lungs through trachea using catheter-tubing connected to a 26 G needle.
2.4. Hematoxylin and eosin (H&E) staining and lung pathology scoring
The lung tissues collected in buffered-formalin were processed for H&E staining as described earlier (Suresh et al., 2021). Briefly, tissues were paraffin embedded and sectioned into 5 μm thick sections and stained with H&E solution (Sigma). For precise/unbiased pathological scoring between the groups, sections were randomly assigned and were independently evaluated by two experts. The lung pathology was scored as per the reported parameters (Li et al., 2021; Suresh et al., 2021). Vasculopathy was scored considering the endothelial hypertrophy, endothelialitis and vasculitis as discussed before (Becker et al., 2021). The highest score for each pathological lesion was set as four. The highest composite score was 12.
2.5. RNA extraction, cDNA synthesis and qPCR
RNA extraction was carried out as per the RNeasy Kit (Qiagen). Briefly, the whole right lung was homogenized with the tissue lysis buffer (supplemented with 2-Mercaptoethanol) and on-column treated with RNase-Free DNase (Qiagen). Two microgram of RNA were reverse transcribed with random hexamer primer using High-Capacity cDNA Reverse Transcription system (Applied Biosystem) and SYBR Green qPCR was carried out. For viral copy number calculation, NP gene standard curve of SARS-CoV-2 was generated (data not shown). Fold-change in host genes (cytokine/chemokine) was carried out using 1 μl of cDNA as a template. The Ct values of samples were normalized with β-Actin and fold-change in transcript levels were estimated by ΔΔCt method. Primers used in the study are listed in (Supplementary Table 1). All reactions were carried out in triplicates and melt curve analysis was performed at the end of the PCR step.
2.6. Immunofluorescence and viral antigen analysis in the lungs
The paraffin-embedded sections were processed for antigen retrieval as mentioned earlier (Suresh et al., 2020, 2021). Sections were deparaffinized in xylene (Sigma) and rehydrated in graded-ethanol (100%–70%) and rinsed in distilled water as reported earlier (Suresh et al., 2020). The sections were blocked with horse serum for 30 min at room temperature and treated with SARS-CoV-2 NP antibody (#11–2003; Abgenex, 1:200) overnight at 4 °C in a humidified chamber. Following this, sections were washed thrice with PBST and treated with 1:500 dilution of anti-Rabbit Alexa Fluor-594 secondary antibody (#A-11037; Life technologies), and incubated in dark for 45 min. After three washings, sections were mounted with ProLong Gold Antifade reagent containing DAPI (#P36935; Invitrogen) and images were captured in Leica TCS SP8STED confocal microscope.
2.7. Immunohistochemistry for macrophage estimation in lung tissue
The blocks were deparaffinized, processed for antigen retrieval as performed earlier and treated with 3% hydrogen peroxide (prepared in methanol) for 20 min at room temperature to quench the endogenous peroxidase activity. Following this, sections were blocked with horse serum and incubated overnight at 4 °C with anti-Iba1 antibody (#019–19741, Wako) diluted 1:1000 in PBS. After three washings, biotinylated IgG secondary antibody (Vector Laboratories) was added and after 45 min incubation at room temperature, these were further treated with ABC reagent (Vector Laboratories) for 30 min. For staining, diaminobenzidine (Vector Laboratories) was used as a substrate and hematoxylin was used as a counterstain. Sections were visualized under the microscope (Leica DM500) and images were captured at different magnifications.
2.8. D-Dimer protein quantification in serum samples
Three serum samples from each group were tested in duplicate for D-Dimer protein quantification using Hamster D-Dimer (D2D) ELISA Kit (#MBS744338, MyBioSource) as per the manufacturer's instructions. Briefly, 100 μl of serum were added to each well of the plate and incubated with the conjugate buffer for 1 h at 37 °C. After three washings, substrate (A and B) solutions were added and the plate was incubated in dark for 20 min. Following the incubation, stop solution was added and optical density (OD) was measured at 450 nm using microplate reader (Thermo Scientific). Blank and D2D standard solutions were also included in the test and calculation was performed as described in the kit procedure.
2.9. PAGE, coomassie staining of BALF for albumin quantification
The BALF collected from hamsters (three animals per group) were processed for albumin quantification by SDS PAGE. The fluids were centrifuged at 8000 rpm for 20 min at 4 °C to remove cellular debris and 25 μl of clear supernatant was treated with 4X Laemmli buffer (to a final concentration of 1X). Samples were incubated in a boiling water bath for 10 min and run on 10% SDS PAGE (gel thickness 1.5 mm) in vertical mini-slab gel (Mini-Protean III; BioRad). After electrophoresis, gel was stained with standard procedure of Coomassie Brilliant Blue solution and destained with methanol and glacial acetic acid solution as described elsewhere. Densitometry quantification of the albumin bands were done by using the Image J program (NIH). The treated groups' albumin band intensity was normalized to vehicle group.
2.10. CT scanning of animals
Micro-CT scanning of only infected and treated hamsters' lungs were carried out using Quantum GX-2 micro-CT imaging system (PerkinElmer) in ABSL-3 facility. Hamsters were anaesthetized, using ketamine/xylazine cocktail, each animal was kept in a supine position in the sample bed. A scout view was obtained to position the animal. Animals' correct position was checked at 90° and 180° and then scan was completed using QuantumGX2 software. For hamster lungs scanning the following parameters were used, X-ray filter: Cu + Al (0.5 mm); field of views (FOVs): 72 mm; Scan mode: High resolution; pixel size: 144 μm, X-ray source voltage: 90 kVp, current: 88 μA and projection radiographs were taken throughout the 360° gantry rotation for a total scan time of 4 min.
2.11. Analysis and quantification
The data obtained from CT scan were analyzed and quantified using the Analyze 14.0 Direct Software from PerkinElmer. The subregioned files were processed using the process tool, for the median correction with kernel size X: Y: Z; 3:3:3. Then the processed file was subjected to segmentation in the segment tool. The segmentation was performed in semi-automatic mode using the object extractor tool. The segmented file was further processed using the measure tool, where the volume of the aerated and consolidated lungs was obtained.
2.12. Statistical analysis
The GraphPad Prism 6.0 software was used for the statistical analysis (GraphPad Software, Inc., San Diego, CA). Statistical analysis performed using one-way ANOVA with Tukey's Multiple Comparisons Test and p<0.05 was considered statistically significant. The data were represented as the mean value ± SEM.
3. Results
3.1. High dose of NAC reduced the clinical features and lung pathology in SARS-CoV-2 infected hamsters
NAC can be administered in humans and animals in multiple routes. As lung inflammation and damage occurs very early (within 3–4 dpi) in hamsters infected with SARS-CoV-2, we assumed that administration of NAC through a parenteral route will be more effective. Multiple doses of drug administration in this model by intravenous route in ABSL-3 facility are technically very challenging. Hence, the intraperitoneal route was opted to achieve the systemic effect of NAC. For the study, administration of NAC was performed with high dose (500 mg/kg/day) and low dose (150 mg/kg/day) for three consecutive days (Fig. 1 A). Loss of body weight during the early phase of SARS-CoV-2 infection in hamsters is a distinct feature of the model and our study also corroborated to this finding. Analysis of daily body weight in all the animals indicated an overall, significant loss up to 15% by 4 dpi (Fig. 1B). Though the weight loss within the groups from 1 dpi to 4 dpi was not statistically significant, from 2 dpi onwards we observed an increased weight loss in high dose NAC group followed by the low dose NAC and the remdesivir group in comparison to the vehicle group.
Fig. 1.
Administration of NAC reduces the clinical and lung pathology of hamsters infected with SARS-CoV-2.
(A) Study design, administration of NAC and Remdesivir in SARS-CoV-2 infected hamsters. (B) Change in daily body weight of hamster after SARS-CoV-2 infection and treatment (n = 5). (C) Representative digital images showing gross morphology and pathological lesions in lungs harvested on 4dpi. H&E stained tissue sections revealing the lung pathology and vasculopathy (scale bar = 200 μm). * Indicates the blood vessel. (D & E) Vasculopathy and lung pathology score of only infected or treated hamsters.
During necropsy, except for the high dose NAC groups, congestion and hemorrhages were clearly observed on the surface of the lung for all other groups (Fig. 1C). In the vehicle, remdesivir and low dose NAC group, these pathological signs were more severe with large, widely dispersed hemorrhagic lesions covering nearly 50% of the total lung area. In the high dose NAC-remdesivir group, lungs were apparently clear, with mild-to-minimal focal hemorrhages, whereas no such gross visible lesion was noticed in high dose NAC (only) group. These findings also correlated to the histopathological observations, with noticeably lower multifocal necrosis, infiltration of inflammatory cells and vasculatures-associated lesions such as endotheliitis, perivascular cuffing, etc. in high dose NAC group (with and without remdesivir). Scoring for vasculopathy including endothelial hypertrophy, endothelialitis and vasculitis in H&E stained sections showed a significant increase in vascular deformities in vehicle, remdesivir and low dose NAC group compared to the high dose NAC group with and without remdesivir (Fig. 1D).
Gross evaluation by two independent experts for lung pathology (alveolitis, bronchiolitis, and endotheliitis) in different H&E stained sections revealed significant lung damage in vehicle, remdesivir and low dose NAC treated animals (Fig. 1E). Administration of high dose of NAC suppressed the SARS-CoV-2 associated lung inflammation and damage compared to the vehicle and remdesivir treated group.
Further, the effect of different doses of NAC and remdesivir on lung damage and pathology was assessed by micro-CT on 4 dpi just prior to euthanasia (n = 3). In the vehicle and remdesivir treated groups, the consolidated area in lungs (hypoaerated region) was nearly around 30%, whereas in the NAC high dose group the consolidation was significantly lower than the vehicle (Fig. 2 A and B). Similarly in the high dose NAC with remdesivir group the consolidation was low (∼10%) which corroborates with the histopathological scoring for lungs damage.
Fig. 2.
Micro-CT evaluation of hamster lungs on 4 dpi.
(A) Axial, coronal and sagittal lungs micro-CT images of vehicle, NAC high dose, remdesivir and NAC high dose with remdesivir group. The arrows indicate the consolidated/hypo-aerated regions. (B) Micro-CT derived aerated and consolidated/hypo-aerated lung volume relative to respective total lung volume (n = 3).
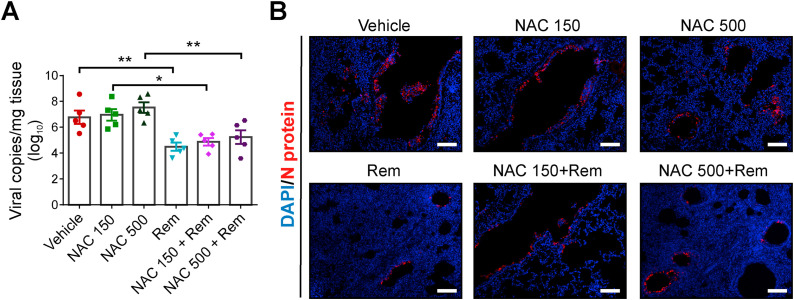
3.2. Administration of NAC alone did not suppressed the lung viral load in SARS-CoV-2 infected hamsters
To evaluate the effect of NAC and to rule out the difference in viral RNA copies due to variation in the sampling region(s) in the lungs, the whole right lobe was processed for RNA extraction. We estimated similar RNA copies in the vehicle group and low dose NAC treated group however, a marginal increase in viral copies were observed in high dose NAC group (Fig. 3 A). In concurrent to previous findings, remdesivir treatment has significant effect on viral load and approximately two-log10 reduction was measured compared to the vehicle groups.
Fig. 3.
Viral load in lung tissue of only infected or treated hamsters
(A) Viral RNA copy number expressed as log10 copies/mg of lung tissue (n = 5). (B) Representative immunofluorescence images indicating the presence of viral Nucleocapsid (N) protein in only infected or treated lungs (scale bar = 100 μm).
In parallel, immunofluorescence study was performed using Nucleoprotein antibody in lung tissue. Distinct punta pertaining to viral protein was observed in the bronchial epithelial cells and alveolar regions of the lungs (Fig. 3B). Treatment with NAC exhibited similar viral load within the low and high dose of NAC. The effect of remdesivir in restricting virus replication was prominent, however, in presence of NAC it was suppressed.
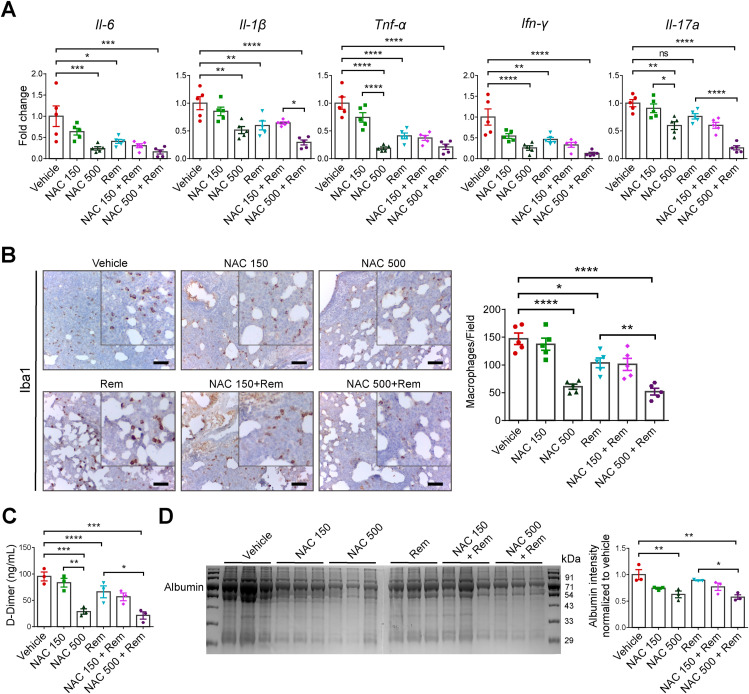
3.3. Administration of NAC inhibited the pro-inflammatory cytokine response and ameliorated the cytokine-associated lung damage in SARS-CoV-2 infected hamsters
During virus infection, dendritic cells and macrophages mediate cytokine production through cell-mediated immune response to contain the intracellular pathogen. Reports suggest that, irrespective of the route of inoculation, cytokines genes are upregulated during SARS-CoV-2 infection in animals (Francis et al., 2021; Lee et al., 2020), and with remdesivir treatment in the early time points, their expression levels are comparatively low (He et al., 2022; Ye et al., 2021). Our data revealed similar findings in which the pro-inflammatory cytokines, IL-6, IL-1β, TNF-α, and IFN-γ are significantly low in remdesivir group compared to the vehicle group (Fig. 4 A). Although the statistics associated with low dose NAC group are not significant, the mean fold-change indicated a decreasing trend compared to the vehicle group. Additionally, we observed a significant reduction in these cytokines in the high dose NAC indicating their lower expression level. However, within the NAC groups, a dose dependent effect was observed for the pro-inflammatory cytokines (with and without remdesivir treatment).
Fig. 4.
NAC administration and host cellular response of SARS-CoV-2 infected hamster.
(A) Expressions profile of pro-inflammatory cytokines in lung tissues (n = 5). (B) Representative Immunohistochemistry images indicating the presence of Iba1 positive macrophages in lungs (scale bar = 50 μm). Graph showing the quantification of the macrophages/field of stained sections. (C) Serum D-Dimer quantification in only infected or treated hamsters (n = 3). (D) Gel image showing albumin in the BALF (n = 3). The bar diagram estimates the relative albumin level in only infected or treated groups.
It is reported elsewhere that an increase in cytokines level (IL-6, TNF-α, etc) is attributed to macrophage infiltration to the site of infection. Thus, we performed immunohistochemistry using the anti-Iba1 antibody to monitor the macrophage infiltration into the lungs. Data analysis using ImageJ software revealed a higher macrophage population in the vehicle group compared to the remdesivir and NAC treated group (Fig. 4B). These cells were significantly lower in the remdesivir group due to the reduction in viral load and the level of associated cytokines. The high dose of NAC revealed a significant decrease in lung macrophages (both with and without remdesivir), however in the low dose, the effect was visible only in presence of remdesivir.
During the COVID pandemic, several clinical reports have highlighted the implication of D-Dimer protein as a biomarker for SARS-CoV-2-associated disease severity. To check its level in different groups of animals, we quantified the D2D protein in serum by competitive ELISA. Compared to the vehicle group, the quantum of D2D protein in the remdesivir group was significantly low (Fig. 4C). We observed a significant reduction in the D2D with high dose of NAC, however with low dose, though the differences were inconsequential, yet a dose dependent trend was observed suggesting that administration of NAC can significantly alleviate SARS-CoV-2-associated hematological complications.
Several reports have indicated albumin as a marker of tissue damage and vascular leakage. To correlate this, we checked its level in BALF by SDS-PAGE and performed densitometric analysis of the bands. The data revealed that in the vehicle group, higher albumin levels were noted compared to the high dose NAC group (Fig. 4D). In the low dose NAC group and remdesivir group the albumin quantification was almost similar. We observed a significant reduction in albumin level either because of the solitary effect of NAC or combinatorial effects of NAC with remdesivir. These results further corroborated to the total protein levels in BALF as estimated by BCA method (Fig. S1).
4. Discussion
In this study, we examined the effects of NAC (low and high dose) with and without remdesivir in the hamster model of SARS-CoV-2 infection. Analysis of treated and control hamsters lung tissues through immunofluorescence staining against viral protein and q-PCR based viral copy number estimation clearly showed no beneficial effect of NAC in reducing the viral load in the treated animals. In fact, some animals showed a minor increase in viral load, although statistically not significant. In the hamster model, dexamethasone boosts virus replication and this has been attributed to its anti-inflammatory or immunosuppressive effects (Wyler et al., 2022). Although a high dose of NAC showed anti-inflammatory effects, but it did not enhance the lung viral load to a greater extent indicates that the NAC-induced immunosuppression might not be that severe as dexamethasone. This assumption needs further validation in different models. Inability of NAC to neutralize SARS-CoV-2 virus in vivo contradicts earlier prediction (Fu et al., 2021). The antiviral property of NAC has been proposed based on its property to disrupt interaction between SARS-CoV-2 RBD and human ACE2 (Fu et al., 2021). However, multiple studies including ours have reported no or minimal expression of ACE2 proteins in hamster lung tissues (Lean et al., 2022; Suresh et al., 2020). Moreover, in these animals involvement of other cell entry mechanisms but not through ACE2 reporter has been proposed. In future, the antiviral effect of NAC in animal models where ACE2 plays a significant role in SARS-CoV-2 pathogenesis might help in addressing these discrepancies.
We noticed that treatment of SARS-CoV-2-infected hamsters with NAC significantly reduced lung damage observed both in micro-CT and histopathological evaluation, immune cell infiltration and expression of inflammatory molecules in lungs. Interestingly, high dose of NAC significantly suppressed the D-Dimer protein level in SARS-CoV-2 infected animals, which corroborates similar findings with COVID-19 patients (Assimakopoulos et al., 2021). Together, these findings are in line with the known anti-inflammatory properties of NAC in other conditions.
The clinical outcome of COVID-19 is often complicated due to manifestation of vascular abnormalities like endothelial dysfunction, vasculopathy and thrombosis. The major mechanisms behind pathogenesis of COVID-19 associated pulmonary vasculopathy are believed due to over production of cytokines, chemokines and reactive oxygen species (Nicosia et al., 2021). These mediators induced by SARS-CoV-2 indirectly cause vascular damage. Hence, any intervention that could suppress these inflammatory mediators is anticipated to improve the clinical course of COVID-19. Various studies with hamster model of SARS-CoV-2 infection including ours have clearly demonstrated that this model exhibits pulmonary vascular lesions as seen in COVID-19 patients (Allnoch et al., 2021; Becker et al., 2021; Suresh et al., 2021). In the current study, high dose of NAC significantly suppressed the SARS-CoV-2 infection-induced pulmonary vascular damage and leakiness. This beneficial effect of NAC might be due to its anti-inflammatory properties. At the same time, NAC is known to protect endothelial cells from LPS-induced apoptosis (Xiong et al., 2019). Hence, in the current study the direct effect of NAC on endothelial cells can't be ruled out and needs further investigation. Together, the reduced vascular damage conferred by NAC may be helpful to improve gas exchange in NAC-treated COVID-19 patients.
In literature NAC has been used in different doses and routes in different animal species (Supplementary Table 2). Heterogeneity observed for the NAC efficacy in different clinical trials is believed to be due to differences in dose, route and timing of NAC administration. In a dose escalation study, intravenous and intra-arterial administration of 450 mg/kg NAC has been reported as the maximum tolerable dose (MTD) in adults with impaired kidney function undergoing digital subtraction angiography (DSA) with or without intervention (Dosa et al., 2017). In different animal studies, through i.p. route NAC has been given at different doses and a high dose of 500 mg/kg have also been used in certain studies (Breitbart et al., 2011; Kurutas et al., 2005; Shih et al., 2018; Somdas et al., 2020). Hence, to evaluate the effect of NAC on SARS-CoV-2 induced lung damage in hamsters, we decided to go for two different doses of NAC through the i.p. route in a therapeutic mode.
Although NAC is considered as a safe drug, the findings from different studies are controversial or need additional information. Gastrointestinal discomfort is a known side effect of NAC in humans. An earlier study also reported significant gas production and bloating in rats receiving 1200 mg/kg NAC orally for thirty days (Arfsten et al., 2004). Mild adverse reactions like bloating and intestinal gas formation has also been reported in some human patients treated with NAC (Hirai et al., 2017). In our study, although we didn't observe any significant gross gastrointestinal complications in NAC treated animals, but the high dose NAC associated toxicities cannot be completely ruled out.
The results of our findings suggest that parenteral administration of high dose NAC has a protective effect on SARS-CoV-2 induced lung damage. Effect of high doses of NAC in reducing vasculopathy in infected hamster lungs mimics a similar effect of NAC against LPS induced vascular leakage (Schmidt et al., 1998). Further studies are needed to evaluate suitable dose, route and time of administration to confirm NAC efficacy as an adjuvant treatment for severe form of SARS-CoV-2 infection. This approach is particularly appealing to individuals with elevated risk of respiratory complications or patients who are receiving respiratory support. Like dexamethasone, use of NAC along with antiviral drugs might have better clinical outcomes and needs further investigation. We believe that the potential role of high-dose of N-acetyl cysteine in the treatment of future waves of emerging SARS-CoV-2 variants might warrant for further investigations.
CRediT authorship contribution statement
Voddu Suresh: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, The final manuscript was approved by all authors, The final manuscript was approved by all authors. Padmanava Behera: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, The final manuscript was approved by all authors. Deepti Parida: Data curation, Formal analysis, Methodology, Writing – review & editing, The final manuscript was approved by all authors. Amlan Priyadarshee Mohapatra: Methodology, Writing – review & editing, The final manuscript was approved by all authors. Suraja Kumar Das: Methodology, Writing – review & editing, The final manuscript was approved by all authors. Sneha Kumari: Methodology, Writing – review & editing, The final manuscript was approved by all authors. Kiran Avula: Methodology, Writing – review & editing, The final manuscript was approved by all authors. Amruta Mohapatra: Methodology, Writing – review & editing, The final manuscript was approved by all authors. Gulam Hussain Syed: Resources, Writing – review & editing, The final manuscript was approved by all authors. Shantibhusan Senapati: Conceptualization, Formal analysis, Funding acquisition, Investigation, Resources, Project administration, Supervision, Validation, Writing – original draft, The final manuscript was approved by all authors.
Declaration of competing interest
The funding agency has no role in design and execution of the project. The authors declare no conflict of interest.
Acknowledgements
We thank the Director ILS for providing the research infrastructures. VS and DP duly acknowledge the Council of Scientific and Industrial Research (CSIR), and APM acknowledges Indian Council of Medical Research (ICMR), Government of India for research fellowship. SS is financially supported by DBT-ILS and DBT-BIRAC grant (BT/CS0004/CS/02/20). We acknowledge Bhabani S. Sahoo, Madan Mohan Mallick, Sushanta Kumar Swain and Jajati Keshari Ray for their assistance. We duly acknowledge the SS Lab members for constant support during the study. We sincerely acknowledge Dr. Shahzada Asad, Senior Product Specialist (Small Animal In vivo Imaging, Discovery & Analytical Solutions), PerkinElmer for his constant support and help in analyzing the micro-CT data. The graphical abstract has been created with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejphar.2022.175392.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Allnoch L., Beythien G., Leitzen E., Becker K., Kaup F.J., Stanelle-Bertram S., Schaumburg B., Mounogou Kouassi N., Beck S., Zickler M., Herder V., Gabriel G., Baumgärtner W. Vascular inflammation is associated with loss of aquaporin 1 expression on endothelial cells and increased fluid leakage in SARS-CoV-2 infected golden Syrian hamsters. Viruses. 2021;13:639. doi: 10.3390/v13040639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou A., Trantza S., Filippou D., Sipsas N., Tsiodras S. COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. In vivo (Athens, Greece) 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angriman F., Ferreyro B.L., Burry L., Fan E., Ferguson N.D., Husain S., Keshavjee S.H., Lupia E., Munshi L., Renzi S., Ubaldo O.G.V., Rochwerg B., Del Sorbo L. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir. Med. 2021;9:655–664. doi: 10.1016/S2213-2600(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfsten D., Johnson E., Thitoff A., Jung A., Wilfong E., Lohrke S., Bausman T., Eggers J., Bobb A. Impact of 30-day oral dosing with N-acetyl-L-cysteine on Sprague-Dawley rat physiology. Int. J. Toxicol. 2004;23:239–247. doi: 10.1080/10915810490502041. [DOI] [PubMed] [Google Scholar]
- Assimakopoulos S.F., Aretha D., Komninos D., Dimitropoulou D., Lagadinou M., Leonidou L., Oikonomou I., Mouzaki A., Marangos M. N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study. Infectious Diseases (London, England) 2021;53:847–854. doi: 10.1080/23744235.2021.1945675. [DOI] [PubMed] [Google Scholar]
- Bartolini D., Stabile A.M., Bastianelli S., Giustarini D., Pierucci S., Busti C., Vacca C., Gidari A., Francisci D., Castronari R., Mencacci A., Di Cristina M., Focaia R., Sabbatini S., Rende M., Gioiello A., Cruciani G., Rossi R., Galli F. SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., Beythien G., de Buhr N., Stanelle-Bertram S., Tuku B., Kouassi N.M., Beck S., Zickler M., Allnoch L., Gabriel G., von Köckritz-Blickwede M., Baumgärtner W. Vasculitis and neutrophil extracellular traps in lungs of golden Syrian hamsters with SARS-CoV-2. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.640842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard G.R., Wheeler A.P., Arons M.M., Morris P.E., Paz H.L., Russell J.A., Wright P.E. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R., Ghosh R., Kulshrestha M., Chowdhury S., Mukherjee R., Ray I. 2020. Observational Study on Clinical Features, Treatment and Outcome of COVID 19 in a Tertiary Care Centre in India- a Retrospective Case Series. [Google Scholar]
- Breitbart R., Abu-Kishk I., Kozer E., Ben-Assa E., Goldstein L.H., Youngster I., Berkovitch M. Intraperitoneal N-acetylcysteine for acute iron intoxication in rats. Drug Chem. Toxicol. 2011;34:429–432. doi: 10.3109/01480545.2011.564176. [DOI] [PubMed] [Google Scholar]
- Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., Galli M., Catena E., Tosoni A., Gianatti A., Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. The Lancet. Infectious diseases. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.E., Winkler E.S., Case J.B., Aziati I.D., Bricker T.L., Joshi A., Darling T.L., Ying B., Errico J.M., Shrihari S., VanBlargan L.A., Xie X., Gilchuk P., Zost S.J., Droit L., Liu Z., Stumpf S., Wang D., Handley S.A., Stine W.B., Jr., Shi P.Y., Davis-Gardner M.E., Suthar M.S., Knight M.G., Andino R., Chiu C.Y., Ellebedy A.H., Fremont D.H., Whelan S.P.J., Crowe J.E., Jr., Purcell L., Corti D., Boon A.C.M., Diamond M.S. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature. 2021;596:103–108. doi: 10.1038/s41586-021-03720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak B.V., Popova E.N., Prikhodko A.S., Grebenchikov O.A., Zinovkina L.A., Zinovkin R.A. COVID-19 and oxidative stress. Biochemistry. Biokhimiia. 2020;85:1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alencar J.C.G., Moreira C.L., Müller A.D., Chaves C.E., Fukuhara M.A., da Silva E.A., Miyamoto M.F.S., Pinto V.B., Bueno C.G., Lazar Neto F., Gomez Gomez L.M., Menezes M.C.S., Marchini J.F.M., Marino L.O., Brandão Neto R.A., Souza H.P. vol. 72. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2021. pp. e736–e741. (Double-blind, Randomized, Placebo-Controlled Trial with N-Acetylcysteine for Treatment of Severe Acute Respiratory Syndrome Caused by Coronavirus Disease 2019 (COVID-19)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S., Balansky R., La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. Faseb. J. : official Pub. Federation Am. Soc. Experimental Biol. 2020;34:13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo G.D., Lazarini F., Larrous F., Feige L., Kornobis E., Levallois S., Marchio A., Kergoat L., Hardy D., Cokelaer T., Pineau P., Lecuit M., Lledo P.M., Changeux J.P., Bourhy H. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominari A., Hathaway D., Iii, Kapasi A., Paul T., Makkar S.S., Castaneda V., Gara S., Singh B.M., Agadi K., Butt M., Retnakumar V., Chittajallu S., Taugir R., Sana M.K., Kc M., Razzack S., Moallem N., Alvarez A., Talalaev M. Bottom-up analysis of emergent properties of N-acetylcysteine as an adjuvant therapy for COVID-19. World J. Virol. 2021;10:34–52. doi: 10.5501/wjv.v10.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosa E., Heltai K., Radovits T., Molnar G., Kapocsi J., Merkely B., Fu R., Doolittle N.D., Toth G.B., Urdang Z., Neuwelt E.A. Dose escalation study of intravenous and intra-arterial N-acetylcysteine for the prevention of oto- and nephrotoxicity of cisplatin with a contrast-induced nephropathy model in patients with renal insufficiency. Fluids Barriers CNS. 2017;14:26. doi: 10.1186/s12987-017-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M.E., Goncin U., Kroeker A., Swan C., Ralph R., Lu Y., Etzioni A.L., Falzarano D., Gerdts V., Machtaler S., Kindrachuk J., Kelvin A.A. SARS-CoV-2 infection in the Syrian hamster model causes inflammation as well as type I interferon dysregulation in both respiratory and non-respiratory tissues including the heart and kidney. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Chen Y., Wang K., Hettinghouse A., Hu W., Wang J.Q., Lei Z.N., Chen Z.S., Stapleford K.A., Liu C.J. Repurposing FDA-approved drugs for SARS-CoV-2 through an ELISA-based screening for the inhibition of RBD/ACE2 interaction. Protein & cell. 2021;12:586–591. doi: 10.1007/s13238-020-00803-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garozzo A., Tempera G., Ungheri D., Timpanaro R., Castro A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int. J. Immunopathol. Pharmacol. 2007;20:349–354. doi: 10.1177/039463200702000215. [DOI] [PubMed] [Google Scholar]
- He Z., Ye F., Zhang C., Fan J., Du Z., Zhao W., Yuan Q., Niu W., Gao F., He B., Cao P., Zhao L., Gao X., Gao X., Sun B., Dong Y., Zhao J., Qi J., Liang X.J., Jiang H., Gong Y., Tan W., Gao X. A comparison of Remdesivir versus gold cluster in COVID-19 animal model: a better therapeutic outcome of gold cluster. Nano Today. 2022;44 doi: 10.1016/j.nantod.2022.101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai D.M., Jones J.H., Zelt J.T., da Silva M.L., Bentley R.F., Edgett B.A., Gurd B.J., Tschakovsky M.E., O'Donnell D.E., Neder J.A. Oral N-acetylcysteine and exercise tolerance in mild chronic obstructive pulmonary disease. J. Appl. Physiol. 2017;122:1351–1361. doi: 10.1152/japplphysiol.00990.2016. [DOI] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim H., Perl A., Smith D., Lewis T., Kon Z., Goldenberg R., Yarta K., Staniloae C., Williams M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clinical immunology (Orlando, Fla. 2020;219 doi: 10.1016/j.clim.2020.108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., Hunter C.A. Is IL-6 a key cytokine target for therapy in COVID-19? Nat. Rev. Immunol. 2021;21:337–339. doi: 10.1038/s41577-021-00553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein S.J.F., Jacobs S., Langendries L., Seldeslachts L., Ter Horst S., Liesenborghs L., Hens B., Vergote V., Heylen E., Barthelemy K., Maas E., De Keyzer C., Bervoets L., Rymenants J., Van Buyten T., Zhang X., Abdelnabi R., Pang J., Williams R., Thibaut H.J., Dallmeier K., Boudewijns R., Wouters J., Augustijns P., Verougstraete N., Cawthorne C., Breuer J., Solas C., Weynand B., Annaert P., Spriet I., Vande Velde G., Neyts J., Rocha-Pereira J., Delang L. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc. Natl. Acad. Sci. U.S.A. 2020;117:26955–26965. doi: 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurutas E.B., Cetinkaya A., Bulbuloglu E., Kantarceken B. Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediat. Inflamm. 2005:390–394. doi: 10.1155/MI.2005.390. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean F.Z.X., Núñez A., Spiro S., Priestnall S.L., Vreman S., Bailey D., James J., Wrigglesworth E., Suarez-Bonnet A., Conceicao C., Thakur N., Byrne A.M.P., Ackroyd S., Delahay R.J., van der Poel W.H.M., Brown I.H., Fooks A.R., Brookes S.M. Differential susceptibility of SARS-CoV-2 in animals: evidence of ACE2 host receptor distribution in companion animals, livestock and wildlife by immunohistochemical characterisation. Transboundary and Emerging Diseases. 2022;69:2275–2286. doi: 10.1111/tbed.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.C., Zhang A.J., Chan J.F., Li C., Fan Z., Liu F., Chen Y., Liang R., Sridhar S., Cai J.P., Poon V.K., Chan C.C., To K.K., Yuan S., Zhou J., Chu H., Yuen K.Y. Oral SARS-CoV-2 inoculation establishes subclinical respiratory infection with virus shedding in golden Syrian hamsters. Cell reports. Medicine. 2020;1 doi: 10.1016/j.xcrm.2020.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff J.A., Wilke C.P., Hybertson B.M., Shanley P.F., Beehler C.J., Repine J.E. Postinsult treatment with N-acetyl-L-cysteine decreases IL-1-induced neutrophil influx and lung leak in rats. Am. J. Physiol. 1993;265:L501–L506. doi: 10.1152/ajplung.1993.265.5.L501. [DOI] [PubMed] [Google Scholar]
- Li C., Chen Y.X., Liu F.F., Lee A.C., Zhao Y., Ye Z.H., Cai J.P., Chu H., Zhang R.Q., Chan K.H., Chiu K.H., Lung D.C., Sridhar S., Hung I.F., To K.K., Zhang A.J., Chan J.F., Yuen K.Y. vol. 73. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2021. pp. e719–e734. (Absence of Vaccine-Enhanced Disease with Unexpected Positive Protection against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by Inactivated Vaccine Given within 3 Days of Virus Challenge in Syrian Hamster Model). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang M., Luo G., Qian X., Wu C., Zhang Y., Chen B., Leung E.L., Tang Y. Experience of N-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19: a case report. Medicine. 2020;99 doi: 10.1097/MD.0000000000022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza E.J., Manguiat K., Wood H., Drebot M. Two detailed plaque assay protocols for the quantification of infectious SARS-CoV-2. Current Protocols Microbiol. 2020;57 doi: 10.1002/cpmc.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia R.F., Ligresti G., Caporarello N., Akilesh S., Ribatti D. COVID-19 vasculopathy: mounting evidence for an indirect mechanism of endothelial injury. Am. J. Pathol. 2021;191:1374–1384. doi: 10.1016/j.ajpath.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. 2021;97:312–320. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe F.L., Corn J. N-Acetylcysteine: a potential therapeutic agent for SARS-CoV-2. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon S., Kramar B., Marolt T.P., Poljsak B., Milisav I. Medical and dietary uses of N-acetylcysteine. Antioxidants. 2019;8 doi: 10.3390/antiox8050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Walther A., Gebhard M.M., Martin E., Schmidt H. Influence of N-acetylcysteine treatment on endotoxin-induced microcirculatory disturbances. Intensive Care Med. 1998;24:967–972. doi: 10.1007/s001340050697. [DOI] [PubMed] [Google Scholar]
- Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Puyo C.A. N-acetylcysteine to combat COVID-19: an evidence review. Therapeut. Clin. Risk Manag. 2020;16:1047–1055. doi: 10.2147/TCRM.S273700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih W.L., Chang C.D., Chen H.T., Fan K.K. Antioxidant activity and leukemia initiation prevention in vitro and in vivo by N-acetyl-L-cysteine. Oncol. Lett. 2018;16:2046–2052. doi: 10.3892/ol.2018.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P., Matthay M.A., Calfee C.S. Is a "cytokine storm" relevant to COVID-19? JAMA Intern. Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- Somdas M.A., Gunturk I., Balcioglu E., Avci D., Yazici C., Ozdamar S. Protective effect of N-acetylcysteine against cisplatin ototoxicity in rats: a study with hearing tests and scanning electron microscopy. Braz. J Otorhinolaryngology. 2020;86:30–37. doi: 10.1016/j.bjorl.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh V., Mohanty V., Avula K., Ghosh A., Singh B., Reddy R.K., Parida D., Suryawanshi A.R., Raghav S.K., Chattopadhyay S., Prasad P., Swain R.K., Dash R., Parida A., Syed G.H., Senapati S. Quantitative proteomics of hamster lung tissues infected with SARS-CoV-2 reveal host factors having implication in the disease pathogenesis and severity. Faseb. J. : official Pub. Federation Am. Soc. Experimental Biol. 2021;35 doi: 10.1096/fj.202100431R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh V., Parida D., Minz A.P., Sethi M., Sahoo B.S., Senapati S. Tissue distribution of ACE2 protein in Syrian golden hamster (Mesocricetus auratus) and its possible implications in SARS-CoV-2 related studies. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.579330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter P.M., Domenighetti G., Schaller M.D., Laverriere M.C., Ritz R., Perret C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest. 1994;105:190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang C., Song Y., Coleman J.R., Stawowczyk M., Tafrova J., Tasker S., Boltz D., Baker R., Garcia L., Seale O., Kushnir A., Wimmer E., Mueller S. vol. 118. Proceedings of the National Academy of Sciences of the United States of America; 2021. (Scalable Live-Attenuated SARS-CoV-2 Vaccine Candidate Demonstrates Preclinical Safety and Efficacy). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.K., Lee S.W.H., Kua K.P. N-acetylcysteine as adjuvant therapy for COVID-19 - a perspective on the current state of the evidence. J. Inflamm. Res. 2021;14:2993–3013. doi: 10.2147/JIR.S306849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler E., Adler J.M., Eschke K., Teixeira Alves G., Peidli S., Pott F., Kazmierski J., Michalick L., Kershaw O., Bushe J., Andreotti S., Pennitz P., Abdelgawad A., Postmus D., Goffinet C., Kreye J., Reincke S.M., Prüss H., Blüthgen N., Gruber A.D., Kuebler W.M., Witzenrath M., Landthaler M., Nouailles G., Trimpert J. Key benefits of dexamethasone and antibody treatment in COVID-19 hamster models revealed by single-cell transcriptomics. Mol. Ther. : the journal of the American Society of Gene Therapy. 2022;30:1952–1965. doi: 10.1016/j.ymthe.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong T., Zhang Z., Zheng R., Huang J., Guo L. N-acetyl cysteine inhibits lipopolysaccharide-induced apoptosis of human umbilical vein endothelial cells via the p38MAPK signaling pathway. Mol. Med. Rep. 2019;20:2945–2953. doi: 10.3892/mmr.2019.10526. [DOI] [PubMed] [Google Scholar]
- Ye Z.W., Yuan S., Chan J.F., Zhang A.J., Yu C.Y., Ong C.P., Yang D., Chan C.C., Tang K., Cao J., Poon V.K., Chan C.C., Cai J.P., Chu H., Yuen K.Y., Jin D.Y. Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection. Emerg. Microb. Infect. 2021;10:291–304. doi: 10.1080/22221751.2021.1885998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Yang X., Huang A., Chen Z. The potential mechanism of N-acetylcysteine in treating COVID-19. Curr. Pharmaceut. Biotechnol. 2021;22:1584–1590. doi: 10.2174/1389201021999201228212043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.