Abstract
The fbpABC locus of Neisseria gonorrhoeae has been proposed to encode a periplasmic protein-dependent iron transport system. Although the function of the gonococcal FbpA protein has been well characterized and its role as a periplasmic binding protein is well defined, little is known about the function of the FbpB and FbpC proteins. To define the function of the gonococcal FbpC protein, an N. gonorrhoeae F62 fbpC mutant was constructed by insertional inactivation with the kanamycin gene. The N. gonorrhoeae F62 fbpC mutant was observed to grow with heme, transferrin, or ferric nitrate as the sole exogenous iron source, indicating that the gonococcal FbpC protein is not absolutely required for growth with these iron sources. In previous studies we were unable to detect fbpB- or fbpC-specific transcripts by Northern analysis. Reverse transcription-PCR analysis with RNA obtained from N. gonorrhoeae F62 grown under iron-replete and -depleted conditions detected fbpA and fbpAB transcripts but failed to detect fbpC or fbpBC transcripts. These results indicate that FbpC does not play a pivotal role in iron transport in N. gonorrhoeae and suggest that additional ABC transport systems are functional in the gonococcus for the acquisition of iron.
Microorganisms have developed diverse and elaborate systems to obtain iron, which is present in limited quantities in the human host. Some pathogenic bacteria produce siderophores, which efficiently scavenge ferric iron from the environment and facilitate the growth of the organism (27). The pathogenic Neisseria species do not produce siderophores (17, 26) but instead produce an array of distinct iron-regulated outer membrane receptors which specifically interact with different iron binding proteins. These include the transferrin (TF) binding proteins, Tbp1 and Tbp2, the lactoferrin (LF) binding proteins, LbpA and LbpB (2, 5, 14, 24), and the hemoglobin receptors, HmbR (22, 23) and HpuB (6, 7, 15), which facilitate the uptake of heme iron from hemoglobin and hemoglobin-haptoglobin complexes, respectively. Although the binding of iron-containing ligands to these receptors is well characterized, the subsequent steps of iron removal and transport into the bacterial cytoplasm are not well defined. Previous studies have determined that iron is removed from TF and LF in an energy-dependent manner but that neither TF nor LF is internalized. Once the iron is removed from TF, it is bound by the ferric binding protein (FbpA). FbpA functions within the periplasm, shuttling iron from TF through the periplasm and to the cytoplasmic membrane. Our studies indicate that iron from heme interacts with FbpA (8); however, the specificity of this interaction and the fate of iron following its interaction with FbpA are not well defined. Neisseria meningitidis and N. gonorrhoeae fbpA mutants were recently demonstrated to be deficient in the ability to use iron from human TF and LF; however, the use of iron from heme and hemoglobin was unimpaired (12, 25). These results, together with our biochemical data obtained with N. gonorrhoeae, suggest that more than one periplasmic binding protein may be used for the transport of iron from hemin.
We have previously reported on the cloning and sequencing of the gonococcal fbpA gene (4). DNase footprinting studies indicate that E. coli Fur binds to a 42-bp site within the fbpA promoter (9). We have also established that the fbpA promoter is regulated by Fur and iron in E. coli and that the level of transcriptional regulation of fbpA in the gonococcus is directly related to the degree of iron restriction. Two open reading frames downstream of the gonococcal fbpA, designated fbpB and fbpC, have been identified (1). The proteins encoded by these loci are homologous to the products of portions of previously described operons expressed by Haemophilus influenzae (hitABC), Serratia marcescens (sfuABC), and Yersinia enterocolitica (yfuABC) (3, 19, 20). The fbpABC locus has been proposed to encode for a periplasmic protein-dependent transport system, with FbpA functioning as the periplasmic binding protein, FbpB (511 amino acids) functioning as the hydrophobic membrane protein, and FbpC (352 amino acids) functioning as the cytoplasmic membrane-associated nucleotide binding protein. However, attempts to detect proteins corresponding to FbpB and FbpC in iron-stressed gonococcal membranes or in Escherichia coli constructs expressing the fbpABC operon have failed. Likewise, in previous studies we were unable to detect fbpB- or fbpC-specific transcripts by Northern blot analysis (10), and thus the exact role of these proteins in iron transport remains to be defined. In this study, we have constructed and characterized a gonococcal fbpC mutant and have further defined the transcription of the N. gonorrhoeae fbpABC locus.
Bacterial strains and growth conditions.
N. gonorrhoeae F62 (obtained from R. P. Williams, Baylor College of Medicine, Houston, Tex.) was maintained on complex medium (gonococcal base; Difco Laboratories, Detroit, Mich.) agar containing 1% IsoVitaleX (BBL, Baltimore, Md.) and grown aerobically under 5% CO2 at 37°C. Broth cultures were grown in chemically defined medium (CDM) (16) supplemented with 4.2% NaHCO3. All glassware was washed with 10% nitric acid and thoroughly rinsed in deionized water to remove residual iron. Cold hemin (Na plus K salt) was purchased from Porphyrin Products Inc. (Salt Lake City, Utah), and apotransferrin and ferric nitrate were purchased from Sigma Chemical Co. (St. Louis, Mo.). Cold hemin was dissolved in distilled H2O and prepared fresh for each experiment. N. gonorrhoeae F62 was grown in CDM plus 25 μM Desferal (Ciba-Giegy) (CDM/25D) for 3 h aerobically at 37°C, washed, and resuspended in CDM/25D. This culture served as the inoculum into fresh CDM/25D with a starting absorbance at 660 nm (A660) of 0.06. To this was added either iron-loaded human TF (5 μM, 30% iron saturated), ferric nitrate (10 μM), or hemin (4 μM). Growth was monitored by measuring the A660 hourly for 5 h.
Construction and characterization of a gonococcal fbpC mutant.
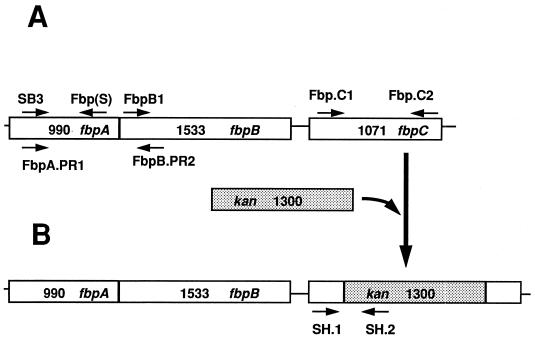
Plasmid pAFbpO, which contains a 3.6-kb DNA fragment corresponding to the fbpABC locus ligated into the EcoRV and BamHI sites of pBSKS (1) was used to construct an fbpC mutant. To disrupt the fbpC gene, a 600-bp BstEII-AccI internal fragment of the fbpC gene was deleted and the linearized plasmid was blunt ended with T4 DNA polymerase and subsequently dephosphorylated with calf intestinal alkaline phosphatase (Boehringer Mannheim) to reduce the religation background. A 1.3-kb EcoRI fragment encompassing the Tn903 kanamycin cassette was excised from pUC4K-KSAC (18) and blunt ended with T4 DNA polymerase. The kanamycin cassette was ligated with the linearized pAFbpOΔC vector in the presence of T4 DNA ligase overnight at 14°C, and the ligation mix was used to transform high-efficiency JM109 cells (Promega Corp., Madison, Wis.). Transformants were selected on Luria-Bertani agar plates containing kanamycin (50 μg/ml).
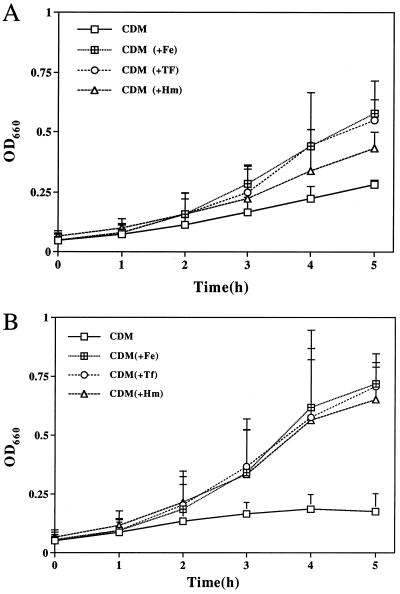
Three transformants were obtained, and one was used in further studies. Plasmid DNA was isolated from this transformant (designated pRSC.1), and the presence of the kan gene in the pRSC.1 construct was confirmed by the ability of PstI (unique restriction enzyme flanking the kan gene) to excise a 1.3-kb fragment corresponding to the kanamycin cassette. PstI digestion was also performed with pUC4K-KSAC (positive control) and pAFbpO (negative control) to confirm the presence of the kanamycin cassette in the plasmid construct pRSC.1 (data not shown). The presence of the kanamycin cassette in the fbpC gene was also confirmed by amplification and sequencing of the fbpC-kan junction (data not shown) with a fbpC gene-specific forward primer (SH.1) (Fig. 1B and Table 1) and a kan gene-specific reverse primer (SH.2) (Fig. 1B). After confirmation of the disruption of the fbpC gene in E. coli, pRSC.1 was used to transform piliated N. gonorrhoeae F62 (21) and transformants were selected on gonococcal base medium supplemented with kanamycin (50 μg/ml). Inactivation of the gonococcal chromosomal fbpC gene in the resulting transformants was confirmed by PCR with primers SH.1 and SH.2 (Fig. 1B). The expected DNA fragment of 281 bp was amplified from N. gonorrhoeae F62C.1 as well as from the plasmid construct pRSC.1 (positive control) (data not shown). We did not observe a PCR product with wild-type N. gonorrhoeae F62 chromosomal DNA as a template (data not shown). Sequencing of the 281-bp PCR product with primer SH.1 or SH.2 individually confirmed that proper allelic exchange had occurred (data not shown). To examine the ability of the fbpC mutant to grow with various iron sources, broth cultures of N. gonorrhoeae F62C.1 and wild-type N. gonorrhoeae F62 were grown in CDM/25D supplemented with hemin, TF, or ferric nitrate. As shown in Fig. 2B, N. gonorrhoeae F62C.1 was capable of growth with heme, TF, or ferric nitrate as the sole exogenous iron source. Although the gonococcal fbpC mutant exhibited slightly better growth with the different iron sources, these differences were not significant compared to the wild-type strain as determined by the Student t test with two-tailed analysis, (P > 0.1) (InStat 2.00; Graph Pad Software, Paul Stannard Soft Engine). These results indicate that gonococcal FbpC protein is not absolutely required for growth with these iron sources.
FIG. 1.
Schematic diagram of the Neisseria fbpABC operon. (A) Positions of the oligonucleotide primers used in RT-PCR. (B) Positions of the oligonucleotide primers used to confirm the presence of the kanamycin cassette (indicated by shading) in the recombinant plasmid construct and the fbpC mutant N. gonorrhoeae F62C.1.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence | Product |
|---|---|---|
| SH.1 | 5′ AAATACCAACCTTCCCGTCC 3′ | 281-bp fbpC-kan junction |
| SH.2 | 5′ CGATAGATTGTCGCACCTGA 3′ | |
| SB.3 | 5′ CAGACATTACCGTGTACAACGGCCAAC 3′ | 933-bp fbpA gene |
| FBP(S) | 5′ TTATTTCATACCGGCTTGCTCAAGC 3′ | |
| FbpA.PR.1 | 5′ AAAAGGATCCCGATATGAAAACATCTATCCGA 3′ | 1.1-kb fbpAB gene |
| FbpB.PR.2 | 5′ CGGGATCCGAGGCCGGTAAGCCAAATGGTAT 3′ | |
| FbpB1 | 5′ CGATGAAAAACACTATGTCTCCTAA 3′ | 2.5-kb fbpBC gene |
| Fbp.C2 | 5′ ATAAATATCCGCAGGCTTGTGGATG 3′ | |
| Fbp.C1 and Fbp.C2 | 5′ TTGAGGAAGCACCGCTATGACCGCC 3′ | 1.1-kb fbpC gene |
| RMP F1 | 5′ CATGTTTCTACAGCGGCCTG 3′ | 800-bp rmp gene |
| RMP R1 | 5′ CGGCAAGATATTACCTAGCCT 3′ |
FIG. 2.
Growth of N. gonorrhoeae F62 (A) and N. gonorrhoeae F62C.1 (B). N. gonorrhoeae was grown in CDM/25D for 3 h, washed, and resuspended in CDM/25D. This culture served as the inoculum into fresh CDM/25D with a starting A660 of 0.06. To this was added either iron-loaded human TF (5 μM, 30% iron saturated), ferric nitrate (Fe, 10 μM), or hemin (Hm, 4 μM). Cultures without added iron served as negative controls (-Fe). Growth was monitored hourly by A660 for 5 h. Results are from one experiment and are representative of three separate experiments. Mean values are plotted, and the standard deviations are included for each datum point. OD660, optical density at 660 nm (equivalent to A660).
Analysis of fbpABC transcription.
In previous studies we demonstrated by Northern blot analysis that the gonococcal fbpA gene is transcribed as a monocistronic mRNA independently of fbpB and fbpC (10). In this same study we could not detect fbpB- or fbpC-specific polycistronic or monocistronic transcripts by Northern blot analysis. To precisely define the mechanism of regulation of fbpABC, we examined transcription of the fbpABC locus by reverse transcription-PCR (RT-PCR) analysis. RT-PCR amplification was performed with the Titian one-tube RT-PCR system (Boehringer Mannheim) as specified by the manufacturer. To eliminate DNA contamination, RNA samples were treated with RNase-free DNase (Promega Corp., Madison, Wis.) and an RT-PCR negative control experiment with DNase-treated RNA samples was performed in parallel with the RT-PCR (Fig. 3, lane 9). RT-PCR analysis was performed with several sets of primers (Table 1 and Fig. 1) and with RNA isolated from N. gonorrhoeae F62 grown under iron-replete (CDM with 50 μM ferric nitrate) and iron-depleted (CDM with 20 μM Desferal) conditions. Constitutive expression of the gonococcal rmp gene (11) was confirmed by RT-PCR and used as a positive control (Fig. 3, lane 8).
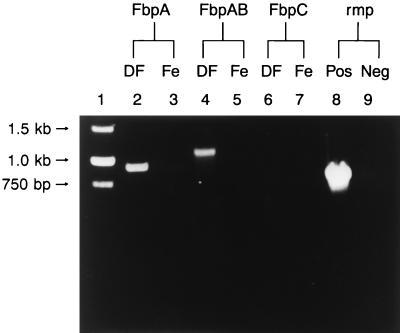
FIG. 3.
RT-PCR analysis for detection of fbpA, fbpAB, and fbpC transcripts in N. gonorrhoeae grown under iron-depleted and -replete conditions. Lanes: 1, DNA molecular size standards; 2, 4, 6, and 8, RNA obtained from N. gonorrhoeae under iron-depleted conditions; 3, 5, 7, and 9, RNA grown under iron-replete conditions; 2 and 3 RT-PCR with primer pairs SB3 and FBP(S) specific for fbpA; 4 and 5, RT-PCR with primer pairs FbpA.PR1 and FbpB.PR2 to amplify fbpA and the 5′ end of fbpB; 6 and 7, RT-PCR with primer pairs Fbp.C1 and Fbp.C2 to amplify fbpC; 8, RT-PCR with primer pairs RMP F1 and RMP R1 to amplify the rmp gene (positive control); 9, DNA control.
As predicted, a 900-bp fbpA-specific transcript was amplified with fbpA-specific oligonucleotides SB3 (forward primer) and FbpS (reverse primer). We also amplified a 1.1-kb fbpAB transcript, encompassing most of the fbpA gene and the 5′ region of the fbpB gene, with the fbpA-specific primer FbpA.PR1 (F.P) and the fbpB-specific primer FbpB.PR2 (R.P) (Table 1). Both the fbpA and fbpAB transcripts were detected only in RNA obtained from gonococci grown under iron-depleted conditions (Fig. 3). No fbpC- or fbpBC-specific transcript was detected in RNA samples from N. gonorrhoeae cultures grown under iron-replete or -depleted conditions (Fig. 3 and data not shown). The absence of fbpC and fbpBC transcripts under both iron-depleted and -replete conditions indicates that fbpC-specific RNA is not transcribed, or, alternatively, that if fbpC-specific RNA is transcribed, it may be highly unstable so that it is not detected by either Northern blot or RT-PCR analysis. From these results, we conclude that only the fbpA and fbpB genes are cotranscribed in N. gonorrhoeae.
Concluding remarks.
The gonococcal fbpC gene has been predicted to encode a cytoplasmic membrane-associated nucleotide binding protein which functions together with FbpA in the transport of iron into the cell (1). However, the exact role of the gonococcal FbpC in iron transport has not been previously defined. Characterization of the gonococcal fbpC mutant described here indicates that the gonococcal FbpC is not essential for iron transport from TF, heme, or inorganic iron (ferric nitrate). Our studies also indicate that the gonococcal fbpABC locus is not a functional equivalent of the ABC transport system described in H. influenzae (hitABC) (19). Previous studies with H. influenzae hitA and hitC mutants have demonstrated that the absence of either the hitA or hitC gene abolishes the function of the ABC transport system (13, 19). N. meningitidis fbpA and N. gonorrhoeae fbpA mutants were recently shown to be unable to use iron supplied from human TF, LF, or inorganic iron, supporting the pivotal role of FbpA in iron transport (12, 25). The observed phenotype of the meningococcal fbpA mutant could not be attributed to a specific gene of the operon, since a polar effect on downstream fbpB and fbpC genes was not ruled out by complementation analysis studies. However, characterization of a specific N. gonorrhoeae fbpC mutant, as described in the present study, indicates that FbpC is not essential for transport of iron in the gonococcus.
In this study, using RT-PCR of RNA obtained from N. gonorrhoeae F62 grown under iron-depleted conditions, we demonstrated that the fbpA and fbpB genes are cotranscribed. The inability to detect the fbpAB transcript in our previous studies (10) may be due to the instability of the fbpAB transcript such that the fbpAB transcript is rapidly degraded and not detected by Northern blot analysis. Sequencing data has confirmed the presence of a strong stem-loop structure which precedes the fbpA stop codon and may function to stabilize the fbpA transcript (1). Such a structure is absent in the fbpB gene. We were also unable to detect fbpC or fbpBC transcripts in RNA obtained from gonococcal cultures grown under iron-depleted or -replete conditions, indicating that these transcripts are either highly unstable or not transcribed at detectable levels.
Sequence analysis of the H. influenzae hitBC (19), S. marcescens sfuBC (3), and Y. enterocolitica yfuBC (20) genes suggests a potential for translational regulation of the C gene. In these systems, the translational start of the downstream C gene either overlaps or precedes the translational stop of the previous open reading frame (B gene), thus allowing an efficient translation of the downstream genes. In contrast, the translational start of the N. gonorrhoeae fbpC gene is 22 bp downstream of the translational stop of fbpB. These observations suggest that in the unlikely event that the fbpC transcript is produced, fbpC may not be efficiently translated. Previous attempts by other investigators to isolate FbpC proteins in iron-stressed gonococcal membrane or soluble extracts or in E. coli constructs expressing the fbpABC operon have been unsuccessful (1). Based on these observations, as well as on our characterization of the gonococcal fbpC mutant and our transcriptional analysis of the fbpABC locus, we propose that N. gonorrhoeae fbpC is a cryptic gene and that alternative ATPases may function together with the periplasmic binding protein FbpA in the transport of iron.
In summary, we have constructed a gonococcal fbpC mutant and have demonstrated that FbpC does not play a pivotal role in iron transport in N. gonorrhoeae. Our studies indicate that the gonococcal fbpABC locus is not a functional equivalent of the ABC transporter described in H. influenzae and suggest that other ABC systems may be functional in the gonococcus for the acquisition of iron.
Acknowledgments
We thank Pragnya Desai for helpful discussions.
This study was supported by Public Health Service grant AI30797 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Adhikari P, Berish S A, Nowalk A J, Veraldi K L, Morse S A, Mietzner T A. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J Bacteriol. 1996;178:2145–2149. doi: 10.1128/jb.178.7.2145-2149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J E, Sparling P F, Cornelissen C N. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angerer A, Gaisser S, Braun V. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J Bacteriol. 1990;172:572–578. doi: 10.1128/jb.172.2.572-578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berish S A, Meiztner T A, Mayer L W, Genco C A, Holoway B P, Morse S A. Molecular cloning and characterization of the structural gene for the major iron-regulated protein expressed by Neisseria gonorrhoeae. J Exp Med. 1990;171:1535–1546. doi: 10.1084/jem.171.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C J, Sparling P F, Lewis L A, Dyer D W, Elkins C. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun. 1996;64:5008–5014. doi: 10.1128/iai.64.12.5008-5014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C J, Elkins C, Sparling P F. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect Immun. 1998;66:987–993. doi: 10.1128/iai.66.3.987-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai P J, Nzeribe R, Genco C A. Binding and accumulation of hemin in Neisseria gonorrhoeae. Infect Immun. 1995;63:4634–4641. doi: 10.1128/iai.63.12.4634-4641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai P J, Angerer A, Genco C A. Analysis of Fur binding to operator sequences within the Neisseria gonorrhoeae fbpA promoter. J Bacteriol. 1996;178:5020–5023. doi: 10.1128/jb.178.16.5020-5023.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forng R Y, Ekechukwu C R, Subbarao S, Morse S A, Genco C A. Promoter mapping and transcriptional regulation of the iron-regulated Neisseria gonorrhoeae fbpA gene. J Bacteriol. 1997;179:3047–3052. doi: 10.1128/jb.179.9.3047-3052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotschlich E C, Seiff M, Blake M S. The DNA sequence of the structural gene of gonococcal protein III and the flanking region containing a repetitive sequence. Homology of protein III with enterobacterial OmpA proteins. J Exp Med. 1987;165:471–482. doi: 10.1084/jem.165.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heng H K, Kirby S D, Lee B C. A Neisseria meningitidis fbpABC mutant is incapable of using nonheme iron for growth. Infect Immun. 1998;66:2330–2336. doi: 10.1128/iai.66.5.2330-2336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirby S D, Gray-Owen S D, Schryvers A B. Characterization of a ferric binding-protein mutant in Haemophilus influenzae. Mol Microbiol. 1997;25:979–987. doi: 10.1111/j.1365-2958.1997.mmi535.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee B C, Schryvers A B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988;2:827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 15.Lewis L A, Dyer D W. Identification of an iron-regulated outer membrane protein of Neisseria meningitidis involved in the utilization of hemoglobin complexed to haptoglobin. J Bacteriol. 1995;177:1299–1306. doi: 10.1128/jb.177.5.1299-1306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse S A, Bartenstein L. Purine metabolism in Neisseria gonorrhoeae the requirement for hypoxanthine. Can J Microbiol. 1980;26:13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- 17.Norrod E P, Williams R P. Growth of Neisseria gonorrhoeae in medium deficient in iron without detection of siderophores. Curr Microbiol. 1978;1:281–284. [Google Scholar]
- 18.Oka A, Sugisaki H, Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 19.Sanders J D, Cope L D, Hansen E J. Identification of a locus involved in the utilization of iron by Haemophilus influenzae. Infect Immun. 1994;64:4515–4525. doi: 10.1128/iai.62.10.4515-4525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saken E M, Heesemann J. Molecular characterization of a novel iron transport system common in the genus Yersinia. GenBank accession no. Z47200. 1995. [Google Scholar]
- 21.Sparling P F. Genetic transforming of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966;92:1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stojiljkovic I, Larson J, Hwa V, Anjic S, So M. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J Bacteriol. 1996;178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stojiljkovic I, Hwa V, De Saint Martin L, O’Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsai J, Dyer D W, Sparling P F. Loss of transferrin receptor activity in Neisseria meningitidis correlates with inability to use transferrin as an iron source. Infect Immun. 1988;56:3132–3138. doi: 10.1128/iai.56.12.3132-3138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner P C, Thomas E, Elkins C, Clary S, Sparling P F. Neisseria gonorrhoeae heme biosynthetic mutants utilize heme and hemoglobin as a heme source but fail to grow within epithelial cells. Infect Immun. 1998;66:5215–5223. doi: 10.1128/iai.66.11.5215-5223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West S E H, Sparling P F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985;47:388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]