Abstract
Background
Approximately 5–10% of unselected breast cancer (BC) patients retain a hereditary predisposition related to a germline mutation in BRCA1/2 genes. The poly-ADP ribose polymerase (PARP)-inhibitors olaparib and talazoparib have been granted marketing authorization by both FDA and EMA for adults with BRCA1/2 germline mutations and HER2-negative (HER2-) advanced BC based on the results from the phase III OlympiAd and EMBRACA trials.
Methods
The panel of the Italian Association of Medical Oncology (AIOM) Clinical Practice Guidelines on Breast Cancer addressed two critical clinical questions, adopting the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach and the Evidence to Decision framework (EtD), to develop recommendations on the use of PARP-inhibitors, with respect to single-agent chemotherapy, in patients with BRCA-related triple-negative (clinical question 1) and hormone receptor-positive (HR+)/HER2- (clinical question 2) advanced BC.
Results
Two studies were eligible (OlympiAd and EMBRACA). For both clinical questions, the Panel judged the benefit/harm balance probably in favor of the intervention, given the favorable impact in terms of PFS, ORR, and QoL at an acceptable cost in terms of toxicity; the overall certainty of the evidence was low. The panel's final recommendations were conditional in favor of PARP-inhibitors over single-agent chemotherapy in both HR+/HER2-and triple-negative BC. Finally, the Panel identified and discussed areas of uncertainty calling for further exploration.
Conclusions
The Panel of AIOM BC Clinical Practice Guideline provided clinical recommendations on the use of PARP-inhibitors, with respect to single-agent chemotherapy, in patients with BRCA-related HER2-advanced BC by adopting the GRADE methodology.
Keywords: BRCA germline mutations, Breast cancer, HER2-negative, PARP-Inhibitors, GRADE methodology
Highlights
-
•
Use of PARP-inhibitors in BRCA-related HER2-advanced BC has been addressed by the Italian Association of Medical Oncology.
-
•
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach has been adopted.
-
•
HR+/HER2-and triple-negative BC subtypes have been addressed in two separate clinical questions.
-
•
Germline BRCA testing in HER2-advanced BC has a predictive value, being indicated independently from familial criteria.
1. Introduction
Approximately 5–10% of unselected breast cancer (BC) patients retain a hereditary predisposition [1], related to a germline mutation in high [2,3] or moderate [4] penetrance genes. Mutations in BRCA1 or BRCA2 genes are the major determinant of this BC genetic predisposition. In particular, BRCA1/2 mutations classified as pathogenic or likely pathogenic variants according to the ENIGMA classification [5] have been linked to specific patterns of hereditary BC (and ovarian cancer), and are typically characterized by high penetrance, with a probability of cancer development varying according the specific BRCA1/2 pathogenic/likely pathogenic variant, as well as additional genetic and environmental factors [6]. The mean cumulative risk of BC for women with BRCA1 or BRCA2 germline mutations by the age of 80 has been reported as ≈70% [7], with individuals with BRCA1 germline mutations more predisposed to triple-negative BC, while those with BRCA2 mutations are at higher risk of hormone-receptor (HR)-positive/HER2-negative BC [8,9].
BRCA-related BC predisposition builds on deficiency in the repair of DNA-double strand breaks through homologous recombination, thus making DNA single-strand break repair mechanisms, mainly regulated by the enzyme poly-ADP ribose polymerase (PARP), primarily responsible for the genomic integrity of BRCA-deficient cells. In BRCA-carriers, the inhibition of PARP is responsible for accumulation of irreparable DNA damage in cells presenting homozygous BRCA mutation, resulting in lethal consequences only in tumor cells (two hits), while sparing normal cells (heterozygous BRCA mutation = one hit), through the mechanism known as “synthetic lethality” [1,[10], [11], [12]]. Early-phase clinical trials reported promising antitumor activity of PARP-inhibitors in advanced BC patients with deleterious germline BRCA mutations [11,[13], [14], [15], [16]]. Subsequently, the OlympiAd and EMBRACA phase then III trials were conducted to assess the clinical efficacy of the PARP inhibitors olaparib and talazoparib, respectively, both meeting their primary endpoint by showing a Progression-free Survival (PFS) improvement favoring the PARP-inhibitor over the control arm (single agent chemotherapy). On the basis of these results, both FDA and EMA granted marketing authorization for olaparib and talazoparib for adults with BRCA1/2 germline mutations and HER2-negative locally-advanced or metastatic BC.
Current International guidelines regarding the use of PARP-inhibitors in BC patients with metastatic disease [[17], [18], [19]] are shown in Table 1.
Table 1.
Current International guidelines regarding the use of PARP-inhibitors in BC patients with metastatic disease.
| Society | Recommendation |
|---|---|
| ASCO[18] | Patients with TN metastatic BC with germline BRCA1 or 2 mutations who have previously been treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic disease setting may be offered an oral PARP inhibitor rather than chemotherapy (Type: evidence-based; benefits outweigh harms; Evidence quality: moderate; Strength of recommendation: strong). Patients with HR-positive/HER2-negative metastatic BC with germline BRCA1 or 2 mutations who are no longer benefiting from ET may be offered an oral PARP inhibitor in the first-through to third-line setting rather than chemotherapy (Type: evidence-based; benefits outweigh harms; Evidence quality: moderate; Strength of recommendation: strong) |
| NCCN[19] | The NCCN Panel recommends assessing for germline BRCA 1/2 mutations in all patients with recurrent or metastatic BC to identify candidates for PARP-inhibitor therapy. While olaparib and talazoparib are FDA indicated in HER2-negative disease, the NCCN Panel supports use in any BC subtype associated with germline BRCA 1/2 mutations. |
| ESMO[17] | Patients with HER2-negative metastatic BC and germline pathogenic or likely pathogenic variants in BRCA1 or BRCA2 should be offered treatment with a PARP inhibitor (olaparib or talazoparib), independent of HR status, as an alternative to chemotherapy [I, A; ESMO-MCBS v1.1 score: 4; ESCAT score: I-A]. Prior treatment with anthracyclines and taxanes should not be required before offering patients with metastatic BC and BRCA germline mutation treatment with a PARP inhibitor; nor should HR-positive patients be required to demonstrate complete endocrine resistance [I, D]. |
| ESO-ESMO [20] | For patients with a germline BRCA mutation, single agent PARP inhibitor (olaparib or talazoparib) is a preferred treatment option for those with triple-negative advanced BC. In ER-positive germline BRCA-associated advanced BC, the optimal sequence between a PARP inhibitor and ET with or without a CDK4/6 inhibitor is unknown. Given the OS benefit seen with CDK4/6 inhibitors, the panel recommends their use before a PARP inhibitor. |
Abbreviations: ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; ESMO, European Society of Medical Oncology; TN, triple-negative; BC, breast cancer; ET, endocrine therapy; MCBC, magnitude clinical benefit scale; ESCAT, ESMO Scale for Clinical Actionability of molecular Targets.
In Italy the use of olaparib was authorized by the Italian Authorization Drug Agency (AIFA) on 15th December 2020, for patients with HER2-negative locally advanced or metastatic BC harboring BRCA1/2 deleterious mutations, previously treated with anthracycline and taxane in the early (neo-adjuvant) or advanced setting (unless contraindicated). Patients with HR-positive/HER2-negative BC should have progressed during prior endocrine therapy (unless considered not eligible for such therapy). However, olaparib reimbursement by the Italian National Health System has only been granted for TN BC subtype, in patients treated with previous anthracycline, taxane and platinum salts in the (neo)-adjuvant or advanced settings (no evidence of disease progression to platinum in the advanced setting or disease-free interval ≥12 months since platinum in the early setting). The use of talazoparib was granted approval on 3rd July 2021 by AIFA for patients with HER2-negative locally advanced or metastatic BC harboring BRCA 1/2 deleterious mutations, previously treated with anthracycline and taxane in the early (neo-adjuvant) or advanced setting (unless contraindicated). Patients with HR-positive/HER2-negative BC should have progressed during a prior endocrine therapy (unless considered not eligible for such therapy). However, talazoparib reimbursement by the Italian National Health System was granted for TN BC subtype already treated also with platinum salts (no evidence of disease progression to platinum in the advanced setting or disease-free interval ≥6 months since platinum in the early setting) and for patients with HR-positive/HER2-negative BC previously given also CDK 4/6 inhibitor-based treatment.
The Panel of AIOM Clinical Practice Guidelines on Breast Cancer decided to systematically address a clinical question regarding the incorporation of PARP-inhibitors in the treatment armamentarium of HER2-negative BC patients with metastatic disease and BRCA germline mutation, considering HR-positive and TN subtypes separately, to provide clinical recommendations.
2. Methods
2.1. The AIOM breast cancer panel Clinical Practice Guidelines
The Panel of AIOM Breast Cancer Clinical Practice Guidelines encompasses academics and clinicians with expertise in clinical oncology, radiation therapy, surgery, radiology, pathology, oncogenetic and clinical research methodology in the field of breast cancer. Every year, an updated version of the AIOM Breast Cancer Clinical Practice Guidelines is uploaded to the AIOM official website, approved by a team of external reviewers nominated by AIOM itself and the following scientific organizations: Italian Society of Radiation Oncology (AIRO), Italian National Association of Breast Surgeons (ANISC), Regional Associations of Outpatient Cardiology (ARCA), Italian Society of Anatomic Pathology and Diagnostic Cytopathology (SIAPEC), Italian Society of Surgical Oncology (SICO), and Italian Society of Medical and Interventional Radiology (SIRM). In addition, in 2021, the AIOM Breast Cancer Clinical Practice Guidelines were considered suitable for publication in the National Guidelines System according to priority, no-redundancy, quality (of the reporting), methodological and clinical relevance criteria, and therefore recommended for clinical practice by the Italian National Health System.
2.2. Clinical question
The Panel of AIOM Guidelines addressed two clinical questions, adopting the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach, developed according to the PICO acronym (P = population; I = intervention, C = comparison, O = outcomes):
-
-
Population and Intervention: The Panel assessed the role of the single agent PARP-inhibitor in HER2-negative advanced BC patients harboring a deleterious BRCA1/2 germline mutation, who had already received both anthracycline and taxane in the early (neo-adjuvant) or advanced setting (unless contraindicated), as well as endocrine therapy in case of HR-positive status. The Panel addressed HR-positive/HER2-negative and TN BC populations separately, for the following reasons (discussed more fully below): advanced HR-positive/HER2-negative and TN BC constitute two very different settings, the latter representing a more challenging clinical scenario, given the poor survival rates and the paucity of effective targeted strategies in the setting of metastatic disease; in addition, in Italy the regulatory scenario of PARP inhibitors differs across the two subpopulations, imposing specific considerations, as outlined below.
-
-
Comparison: The Panel considered single-agent chemotherapy as the standard reference in this setting.
-
-Outcomes: The Panel identified the following outcomes:
-
oOutcomes of benefit: overall survival (OS), progression-free survival (PFS), objective response rate (ORR), quality of life (QoL). All these outcomes of benefit were deemed “critical” for decision-making.
-
oOutcomes of harm: any adverse event (AE) grade 3/4, anemia (grade 3/4), fatigue (grade 3/4), and discontinuation due to AEs were deemed “critical” for decision-making, while neutropenia (grade 3/4), and nausea (grade 3/4) were deemed “important”.
-
o
2.3. Search strategy selection and analysis of evidence
A systematic literature review was done, searching CENTRAL (Cochrane Controlled Register of Trials), PubMed/Medline, and Embase without language or date restriction up to 20th October 2022. The search was extended by cross-referencing all papers identified through this search strategy, as well as by searching ongoing clinical trials registered on ClinicalTrials.gov. Non-randomized clinical studies, narrative reviews and case reports were excluded.
Although the literature search was not restricted to trials selectively enrolling HER2-negative BC patients, only randomized controlled trials (RCTs) reporting efficacy data in this subgroup were included.
Information was collected on study designs, characteristics of the population enrolled, treatment received and results.
We entered data into the Cochrane Review Manager 5 software, and undertook analyses according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions [21]. Dichotomous outcomes were analyzed by calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed with a 95% confidence interval (CI). Continuous outcomes were analyzed using mean difference (MD) its 95%CI. For time-to-event outcomes, the HR was derived, as it is the most appropriate statistic. When possible, the HR and associated variances directly from the trial publication(s) were extracted. If it was not reported, we obtained it indirectly employing the methods described by Tierney and colleagues using other available summary statistics [22]. Data were synthetized with fixed or random effect depending on the I-squared statistic.
2.4. Certainty of evidence and evidence to decision framework (EtD)
The GRADE approach was adopted to evaluate the certainty of evidence for each selected outcome, considering five main domains: study limitations, imprecision, indirectness, inconsistency, publication bias.
The overall rating on the quality of evidence, on the basis on which the final recommendation is built, corresponds to one of the following: high, moderate, low, very low. A dedicated evidence profile table summarizes the quantitative synthesis of the effects and the certainty of evidence for each selected outcome.
A transparent, structured approach to move from evidence to decision -therefore supporting the decision-making process - is provided by the Evidence to Decision framework (EtD). It provides Panelists with a summary of the evidence in relation to: the priority of the problem, the desirable and undesirable effects, the certainty of evidence, the patients’ values and preferences, the balance of effects, the use of resources, equity, acceptability and feasibility.
The strength of the final recommendations is subsequently voted with the following options: strong in favor, conditional in favor, conditional against, strong against.
The AGREE-reporting checklist was followed to guide the reporting of the present recommendations [23].
3. Results
3.1. Search strategy results and summary of relevant trials
After removing duplicates, the search strategy identified 569 documents and two trials were ultimately included (EMBRACA, OlympiAd), with seven related publications [[24], [25], [26], [27], [28], [29]]. A PRISMA flowchart summarizing the selection process is reported in Fig. 1 and trials characteristics are comprehensively reported in Table 2.
Fig. 1.
PRISMA flowchart.
Table 2.
OlympiAd and EMBRACA trial characteristics.
| Study Name | Design (R ratio) | Population, N | Treatment armsa | Endpoints |
|---|---|---|---|---|
| OlympiAd | RCT (2:1) | HER2-negative BC, 302 - TN, 150 - HR+, 152 ≤2 prior CT lines for MBC (prior treatment with anthracycline and taxane for EBC or MBC; prior platinum salts permitted if DFI ≥12 months or no evidence of PDb) ≤1 prior ET lines for MBC in HR + BC |
Olaparib (tablets, 300 mg twice daily) Chemotherapy: - Capecitabine (tablets, 2500 mg/mq for 14 days on-7days off) - Eribulin-mesylate (1.4 mg/mq iv dd1-8, q3w) - Vinorelbine (30 mg/mq iv dd1-8, q3w) | Primary: PFS by blinded central review (according to RECIST 1.1) Secondary: OS; ORR; safety outcomes (adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events – CTCAE – v 4.0); QoL (30-item European Organization for Research and Treatment of Cancer Quality of Life Questionnaire -QLQ-C30). |
| EMBRACA | RCT (2:1) | HER2-negative BC, 431 - TN, 190 - HR+, 241 ≤3 prior CT lines for MBC (prior treatment with anthracycline and/or taxane for EBC or MBC; prior platinum salts permitted if DFI ≥6 months or no evidence of PDb) no limit on the number of prior ET lines in HR + BC | Talazoparib (tablets, 1 mg once daily) Chemotherapyc: - Capecitabine (oral, 1250 mg/m2, twice daily, for 14 days on-7days off) - Eribulin-mesylate (1.4 mg/mq iv dd1-8, q3w) - Vinorelbine (30 mg/m2, weekly iv dd1-8-15 q3w) - Gemcitabine (1250 mg/m2, ivdd1-8 q3w) | Primary: PFS by blinded central review (according to RECIST 1.1) Secondary: OS; ORR; CBR; safety (according to CTCA v 4.0), patient-reported outcomes (QLQ-C30 and breast cancer-specific QLQ-BR23) |
List of abbreviationsR, randomization; RCT, randomized clinical trial; BC, breast cancer; EBC, early breast cancer; MBC, metastatic breast cancer; TN, triple-negative; HR+, hormone receptor-positive; CT, chemotherapy; ET, endocrine therapy; PD, progressive disease; mq, square meters; iv, intravenous; dd, days; q3w, every 3 weeks; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; CBR, clinical benefit rate; QoL, quality of life
Cross-over was not permitted in neither OlympiAd nor Embraca.
In the OlympiAd trial, 29.3% of patients in the olaparib arm and 26.8% in the control arm had already received platinum-based therapy. In the EMBRACA trial, 16.0% of patients in the talazoparib arm and 20.8% in the control arms had already received platinum therapy.
Suggested dosing schedules were noted, but if institution dose and regimen guidelines differed, the site may utilize institution guidelines.
Clinical Question 1: In patients with advanced TN BC with deleterious BRCA germline mutation, who have already received previous chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, should single agent PARP-inhibitor be preferred over standard chemotherapy?
3.2. Outcomes of benefit
The evidence profile is reported in Supplementary Table 1.
3.3. PFS
For PFS analysis, a total of 340 TN BC patients were considered. There was a significant difference in PFS favoring the PARP-inhibitor group (relative risk: HR 0.51, 95% CI 0.39–0.67). The absolute risk of a PFS event was 24 per 100 lower (from 33 lower to 14 lower) in the PARP-inhibitor group compared to the single-agent chemotherapy group. The certainty of evidence for PFS was judged as “moderate” for imprecision of estimates since the optimal information size was not reached.
3.4. OS
This analysis included a total of 340 TN BC patients. No significant differences were detected (HR 0.91, 95% CI 0.70–1.19). The absolute risk of an OS event was 3 per 100 lower (from 13 lower to 6 higher) for the PARP-inhibitor group as compared to the control group. The certainty of evidence supporting the OS event was judged as “moderate” due to imprecision of the estimates since the optimal information size was not reached.
3.5. ORR in patients with measurable disease
A total of 269 TN BC patients with measurable disease were included in the analysis. There was a significant difference in ORR, favoring the PARP-inhibitor group compared to the control group, corresponding to a HR of 3.63 (95% CI 2.18–6.05). The absolute probability of ORR was 42 per 100 higher (from 19 higher to 81 higher). The certainty of evidence supporting the ORR outcome was judged as “moderate” due to imprecision of estimates, since the optimal information size was not reached.
3.6. QoL
The QoL outcome was assessed according to the following scales: GHS/QoL, QLQ-C30 functional scale: physical functioning, QLQ-C30: symptom scale: fatigue, QLQ-C30: symptom scale: pain, QLQ-C30: symptom scale: appetite loss. The scales included 576, 431, 642, 642, and 640 patients. The mean QoL was found to be significantly higher in patients receiving PARP-inhibitors as compared to the control group, in terms of GHS/QoL, QLQ-C30 functional scale, QLQ-C30 symptom scale: fatigue, QLQ-C30 symptom scale: pain. No significant differences were observed between the PARP-inhibitors and control groups in QLQ-C30 symptom scale: appetite loss. The certainty of evidence for QoL was judged overall as “low” for the following reasons: detection bias, attrition bias (large loss of patients who did not complete the questionnaires) and indirectness (QoL assessment also includes patients with HR + BC, corresponding to 33%).
Estimates of effect for each outcome of benefit related to Clinical Question 1 are summarized in Fig. 2a.
Fig. 2.
Estimates of effect for each outcome of benefit; a) Clinical Question 1, b) Clinical Question 2.
3.7. Outcomes of harm
OlympiAd and EMBRACA trials both reported safety data for the entire population, not specifically in TN vs HR + subgroups. However, it might be assumed that HR status should have no impact on safety. For each outcome of harm, 708 patients were considered.
3.8. Any AE grade 3/4
Treatment with PARP-inhibitor was not associated with a higher incidence of any AE grade 3/4 (RR 0.97, 95% CI 0.85–1.11). In terms of absolute effect, the risk of any AEs was 2 per 100 lower (from 6 lower to 6 higher). The certainty of evidence for the outcome any grade 3/4 AEs was judged as “low” for the following reasons: indirectness, detection bias, performance bias.
3.9. Anemia grade 3/4
The treatment with PARP-inhibitor resulted in a significant increase in the risk of anemia grade 3/4 (RR 6.53, 95% CI 3.52–12.15). In terms of absolute effect, the risk of anemia grade 3/4 was 25 per 100 higher (from 12 higher to 51 higher). The certainty of evidence for anemia grade 3/4 was judged as “low” for the following reasons: indirectness and performance bias.
3.10. Neutropenia grade 3/4
The treatment with PARP-inhibitor resulted with a significant decrease in the risk of neutropenia grade 3/4 (RR 0.44, 95% CI 0.30–0.64). In terms of absolute effect, the risk of neutropenia grade 3/4 was 15 per 100 lower (from 19 lower to 9 lower). The certainty of evidence for neutropenia grade 3/4 was judged as “low” for the following reasons: indirectness and performance bias.
3.11. Nausea grade 3/4
The treatment with PARP-inhibitor was found associated with a non-significant decrease in the risk of nausea grade 3/4 (RR 0.19, 95% CI 0.03–1.28). The absolute risk of nausea grade 3/4 was 1 per 100 lower (from 1 lower to 0 lower) in the PARP-inhibitor group than the chemotherapy group. The certainty of evidence for anemia grade 3/4 was judged as “low” for the following reasons: detection bias, and performance bias.
3.12. Fatigue grade 3/4
The treatment with PARP-inhibitors resulted in a non-significant increase in the risk of fatigue grade 3/4 (RR 1.24, 95% CI 0.45–3.39). In terms of absolute effect, the risk of fatigue grade 3/4 was 1 per 100 higher (from 1 lower to 6 higher) in the PARP-inhibitor group. The certainty of evidence for the outcome fatigue grade 3/4 was judged as “low” for the following reasons: indirectness, detection bias, performance bias.
3.13. Discontinuation due to AEs
No significant differences were detected between PARP-inhibitors and standard chemotherapy groups for the outcome discontinuation due to AEs (RR 0.74, 95% CI 0.43–1.28). The absolute risk of discontinuation due to AEs was 2 per 100 lower (from 5 lower to 2 higher) in the PARP-inhibitor group. The certainty of evidence for the outcome of discontinuation due to AEs was judged as “low” for the following reasons: indirectness, detection bias, performance bias.
Estimates of effect for each outcome of harm related to Clinical Question 1 are summarized in Fig. 3.
Fig. 3.
Estimates of effect for each outcome of harm related to both Clinical Question 1 and Clinical Question 2.
4. Benefit/harm balance
Overall, the Panel judged the benefit/harm balance probably in favor of the intervention, given the favorable impact of the intervention in terms of PFS, ORR, and QoL at an acceptable cost in terms of toxicity profile.
EdT (Evidence to decision) Framework
The Panel deemed the issue addressed by Clinical Question 1 a priority, given the fact that improving prognosis in BRCA-related TN metastatic BC is still an unmet need, as discussed below. The panel therefore judged the importance of desirable anticipated effects deriving from the use of PARP-inhibitor over single-agent chemotherapy as “high”. The Panel judged the substantiality of undesirable anticipated effects deriving from the use of PARP-inhibitor over single-agent chemotherapy as “low”. The complexity of the overall judgement in this regard was increased by the fact that the heterogeneity of chemotherapeutic agents adopted in the control arms may have an impact on the incidence of AEs.
The overall certainty of evidence was deemed “low” for the following reasons: imprecision of estimates, detection bias, performance bias, attrition bias and indirectness.
Although the Panel deemed unlikely the impact of the use of PARP-inhibitor on health equity, it still identified access to genetic testing for the detection of BRCA1/2 germline mutations as a possible issue in this regard. In particular, the Panel stressed the importance of offering BRCA genetic testing to all TN metastatic BC patients who may be considered for PARP-inhibitors, since the access to these agents is contingent on the ascertainment of BRCA germline mutations.
Final recommendation
The final recommendation was therefore as follows: “Patients with advanced TN BC with deleterious BRCA germline mutation, who have already received previous chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, may be offered single agent PARP-inhibitor.”
The Panel acknowledged that in patients with both germinal BRCA mutation and PD-L1 positive status, the OS benefit observed with immunotherapy + chemotherapy [30], and the consistent magnitude of benefit from immunotherapy irrespective of BRCA status [31], support prioritizing immunotherapy + chemotherapy as preferred first-line option in this setting reserving the single-agent PARP-inhibitor in a subsequent line.
Clinical question 2: In patients with advanced HR-positive/HER2-negative BC with deleterious BRCA germline mutation, who have already received previous endocrine therapy, chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, should single agent PARP-inhibitor be preferred over standard chemotherapy?
Outcomes of benefit
The evidence profile is reported in Supplementary Table 2.
PFS
For PFS analysis, a total of 393 HR-positive/HER2-negativeBC patients were considered. A significant difference in PFS favoring the PARP-inhibitor group was detected (relative risk: HR 0.61, 95% CI 0.46–0.82). The absolute risk of a PFS event was 15 per 100 lower (from 21 lower to 6 lower) in the PARP-inhibitor group than in the single-agent chemotherapy group. The certainty of evidence for PFS was judged as “moderate” because of imprecision of the estimates since the optimal information size was not reached.
OS
This analysis included a total of 393 HR-positive/HER2-negative BC patients. No significant differences were detected (HR 0.84, 95% CI 0.64–1.09). The absolute risk of an OS event was 6 per 100 lower (from 16 lower to 3 higher) for the PARP-inhibitor group compared to the control group. The certainty of evidence supporting the OS event was judged as “moderate” due to imprecision of the estimates since the optimal information size was not reached.
ORR in patients with measurable disease
This analysis included a total of 297 HR-positive/HER2-negative BC patients with measurable disease. A significant difference in terms of ORR was detected, favoring the PARP-inhibitor group as compared to the control group, corresponding to a HR of 1.71 (95% CI 1.30–2.26). The absolute probability of ORR was 27 per 100 higher (from 11 higher to 47 higher). The certainty of evidence supporting the ORR outcome was judged as “moderate” due to imprecision of the estimates, given the fact that the optimal information size was not reached.
QoL
The QoL outcome was assessed according to the following scales: GHS/QoL (follow-up interval: 24–30 months), QLQ-C30 functional scale: physical functioning (median follow up 11.2 months), QLQ-C30: symptom scale: fatigue, QLQ-C30: symptom scale: pain, QLQ-C30: symptom scale: appetite loss. For these scales 576, 431, 642, 642, and 640 patients were included. The QoL was assessed in the overall population of the OlympiAd and EMBRACA trials, with no separate evaluation for HR-positive/HER2-negative and TN subgroups. The QoL outcome is described under in the Clinical Question 1.
Estimates of effect for each outcome of benefit related to Clinical Question 2 are summarized in Fig. 2b.
Outcomes of harm
As already mentioned, safety data were reported for the overall population of OlympiAd and EMBRACA trials, with no separate evaluation for HR-positive/HER2-negative and TN subgroups. A detailed description of all outcomes of harm is given under in Clinical Question 1.
Estimates of effect for each outcome of harm related to Clinical Question 2 are summarized in Fig. 3.
Benefit/harm balance
Overall, the Panel judged the benefit/harm balance probably in favor of the intervention, given the favorable impact of the intervention in terms of PFS, ORR, and QoL at an acceptable cost in terms of toxicity. The overall certainty of the evidence was low.
EtD framework
The Panel deemed the issue addressed by the Clinical Question 2 a priority.
However, the panel judged the importance of desirable anticipated effects deriving from the use of PARP-inhibitors over single-agent chemotherapy in the HR-positive/HER2-negative BC subgroup as “moderate”, since more effective treatment options are currently available in this subgroup compared to TN disease in the advanced setting, as discussed below.
The Panel judged the importance of undesirable anticipated effects deriving from the use of PARP-inhibitors over single-agent chemotherapy as “low”. As already mentioned, the appraisal regarding the undesirable effects was complex given the heterogeneity of chemotherapeutic agents adopted in the control arms, which may have influenced the incidence of AEs.
The overall certainty of evidence resulted as “low” for the following reasons: imprecision of estimates, detection bias, and indirectness.
The final recommendation was therefore as follows: “Patients with advanced HR-positive/HER2-negative BC with deleterious BRCA germline mutation, who have received previous endocrine therapy, chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, may be offered single agent PARP-inhibitors.”
Table 3 shows the final recommendation.
Table 3.
Final recommendations and the summary of GRADE evaluations.
| Clinical Question 1 |
|---|
| In patients with advanced TN BC with deleterious BRCA germline mutation, who have already received previous chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, should single agent PARP-inhibitor be preferred over standard chemotherapy? |
| Recommendation: Patients with advanced TN BC with deleterious BRCA germline mutation, who have already received previous chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, may be offered single agent PARP-inhibitors. |
| Benefit/Harm ratio votes: probably in favor of the intervention (unanimity) |
| Strength of recommendation votes: conditional in favor (unanimity) |
| Certainty of Evidence: Low |
|
Clinical Question 2 |
| In patients with advanced HR-positive/HER2-negative BC with deleterious BRCA germline mutation, who have already received previous endocrine therapy, chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, should single agent PARP-inhibitor be preferred over standard chemotherapy? |
| Recommendation: Patients with advanced HR-positive/HER2-negative BC with deleterious BRCA germline mutation, who have already received previous endocrine therapy, chemotherapy with anthracycline and taxane, with no evidence of progressive disease during platinum salt-based treatment, may be offered single agent PARP-inhibitors. |
| Benefit/Harm ratio votes: probably in favor of the intervention (unanimity) |
| Strength of recommendation votes: conditional in favor (unanimity) |
| Certainty of Evidence: Low |
The Panel acknowledged in this regard that, despite the lack of a direct comparison between PARP-inhibitors and CDK 4/6 inhibitors, the impact on OS demonstrated with CDK 4/6 inhibitors in association with endocrine therapy in unselected HR-positive advanced BC both in endocrine-sensitive and endocrine-resistant settings [[32], [33], [34], [35]] (and the lack of OS impact with both olaparib and talazoparib) may be worth prioritizing, thus supporting the use of CDK 4/6 inhibitors before PARP-inhibitors in patients with HR-positive/HER2-negative metastatic BC harboring a BRCA germline mutation.
Discussion
The present work summarizes the rigorous methodology applied according to the GRADE system for the formulation of clinical recommendations regarding the use of PARP-inhibitors in HER2-negative BRCA-related advanced BC. The Panel addressed two separate clinical questions for TN and HR-positive advanced BC given the substantial diversity in clinical features and regulatory scenarios across these subtypes.
The AIOM Breast Cancer Clinical Practice Guidelines Panel provided a conditional recommendation in favor of PARP-inhibitors over single agent chemotherapy in patients with HER2-negative metastatic BC, both in TN and HR-positive/HER2-negative subtypes.
The benefit/harm balance of these recommendations is probably in favor of the intervention in both cases, given the substantial improvement in the outcomes of benefit PFS, ORR and QoL, at the cost of acceptable toxicity.
Analysis of the substantiality of anticipated desirable effects showed a positive impact in terms of PFS, ORR and QoL in both TN and HR-positive/HER2-negative subtypes, with no OS benefit in either group. However, when the Panel was called to judge the substantiality of anticipated desirable effects, the conclusion was different according to the BC subtype. While in TN BC the importance of desirable effects was judged “high”, it was lowered to “moderate” in the HR-positive subgroup. Compared to the HR-positive/HER2-negative subtype, TN BC poses a more challenging and hard-to-treat clinical scenario, where chemotherapy is still the mainstay in the advanced setting. Although recent breakthroughs have contributed to improving the outcome of (a subset of) TN BC patients [[36], [37], [38], [39]], the gap in terms of survival rates and treatment availability with the other BC subtypes is still substantial [[40], [41], [42]]. Even in the absence of significant prolongation of OS with the PARP-inhibitors compared to chemotherapy, the clinically meaningful improvement in terms of PFS and ORR with a chemotherapy-free strategy was considered remarkable in the TN subgroup. Conversely, the treatment armamentarium of HR-positive/HER2-negative metastatic BC is wider and encompasses several chemotherapy-free options with proven effectiveness [17], thus relatively scaling down the magnitude of PARP inhibitor-driven progress.
As far as PARP-inhibitor-related safety is concerned, the Panel judged the importance of anticipated undesirable effects as “small” in both clinical questions. The use of PARP-inhibitors was associated with a significant increase in the risk of anemia as compared to the chemotherapy control arm, but there was a significantly lower risk of neutropenia. Overall, no marked safety concerns emerged with the PARP-inhibitors compared to chemotherapy in either TN or HR-positive subpopulations. Although no subgroup safety analysis was available, the Panel found that HR status was unlikely to affect the incidence of AEs.
The certainty of evidence for both Clinical Questions was low, thus outlining the importance of engaging in a shared decision-making process.
The possible impact of PARP-inhibitors in terms of health equity calls for further discussion. As already mentioned, the Panel strongly recommends that all HER2-negative BCpatients with metastatic BC undergo genetic testing for the identification of BRCA germline mutations, given that AIFA has recently granted approval to olaparib in TN BC and talazoparib in HER2-negative BC, thus disentangling the value of BRCA testing from the traditional genetic counseling criteria. The BRCA germline mutation acquires a pure predictive role for therapy selection and therefore ascertaining BRCA status in HER2-negative MBC should be prioritized independently of age and personal/family history.
The Panel's position in this regard has been further strengthened by data suggesting that a not negligible proportion of HER2-negative BC patients with advanced disease not fitting the traditional criteria for genetic testing might actually harbor BRCA1/2 germline mutations [43,44]. These observations therefore outline the possibility of “under-identification” of patients with BRCA germline mutations, ultimately resulting in an unacceptable risk of missing a proportion of patients who may benefit from PARP-inhibitors. Of course, then inclusion of BRCA 1/2 germline mutations among biomarkers that guide treatment decisions, imposes a rethinking of the infrastructure system on which the genetic testing is currently based, granting broader access as well as optimizing turn-around times, while maintaining sustainability by the Italian Health System, which is based on the principle of equal access to health services.
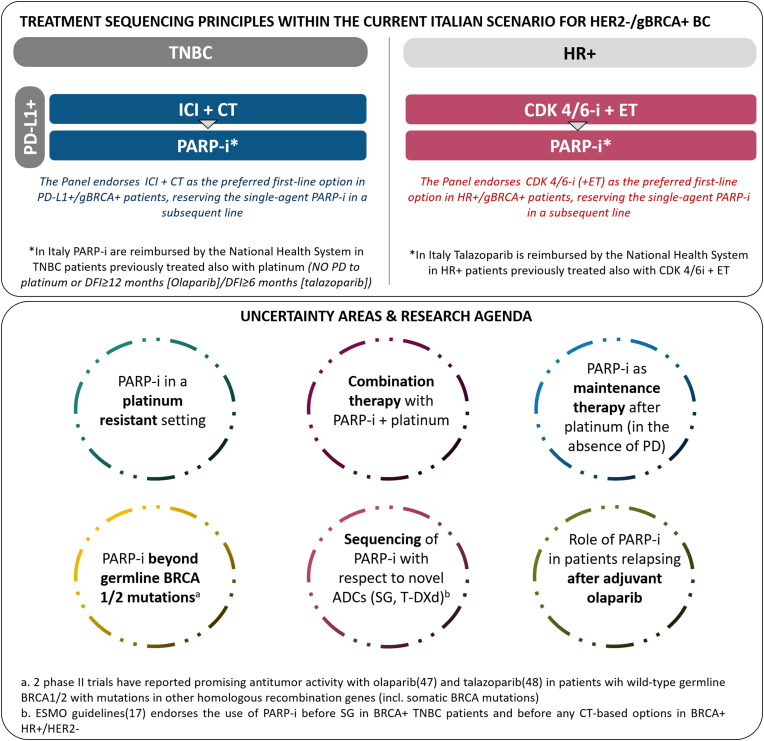
When formulating Clinical Questions 1 and 2, the Panel acknowledged the presence of areas of uncertainty calling for further exploration, summarized in Fig. 4.
Fig. 4.
Treatment sequencing principles within the current Italian scenario for HER2- gBRCA+ advanced breast cancer and areas of uncertainty acknowledged by the Panel when formulating Clinical Questions 1 and 2.
Abbreviations: gBRCA, germline BRCA; BC, breast cancer; TNBC, triple-negative breast cancer; ICI, immune checkpoint inhibitor; CT, chemotherapy; PARP-I, PARP-inhibitor; eBC, early breast cancer; mBC, metastatic breast cancer; PD, disease progression; DFI, disease-free interval; HR+, hormone receptor positive; CDK, 4/6-I CDK 4/6 inhibitor; ET, endocrine therapy; inc., including; SG, sacituzumab govitecan; T-DXd, trastuzumab deruxtecan.
No data are currently available on any formal head-to-head comparison of PARP-inhibitors and platinum salts. Indirect evidence comes from the phase II GeparOLA study, conducted in the neoadjuvant setting, where 106 HER2-negative BC patients with BRCA somatic/germline mutations and/or high homologous recombination deficiency (HRD) score were randomized to receive taxane-anthracycline-based neoadjuvant treatment in association with either carboplatin or the PARP-inhibitor olaparib. The trial, which failed to meet its primary endpoint, showed an absolute 6.5% pathological complete response (pCR) difference between olaparib- and carboplatin-containing arms (pCR rates 55.1%, [95%CI 44.5%–65.3%] vs 48.6% [95%CI 34.3%–63.2%], respectively). The non-comparative design of this study precludes any of formal comparison between arms [45]. For this reason, these findings must be considered merely exploratory.
In addition, it is currently not clear whether PARP-inhibitors should be used concomitantly or in sequence with platinum salts. Preliminary and indirect evidence comes from the BROCADE3 trial [46], where 509 HER2-negative BC patients harboring a germline mutation in BRCA1/2 genes were randomly assigned to chemotherapy with carboplatin and paclitaxel in association with either veliparib or placebo. The primary endpoint analysis indicated statistically significant improvement – although of doubtful clinical significance – in PFS (delta 1.9 months). However, there was a delayed separation of PFS curves of veliparib vs placebo-containing arms with a persistent tail in the PARP-inhibitor group; this warranted a post-hoc analysis, which revealed that the non-proportional hazard was probably sustained - at least in part - by the subgroup of patients receiving veliparib as monotherapy before progression. These data suggest that maintenance therapy after discontinuation of chemotherapy for reasons other than disease progression may potentially point to a strategical clinical positioning of PARP-inhibitors. However, these represent hypothesis-generating data, requiring confirmation in adequately designed and powered clinical trials.
Furthermore, it remains to be clarified whether PARP-inhibitors are effective in a platinum-resistant setting.
In the HR-positive subgroup - as already discussed - the proper placement of PARP-inhibitors in relation of CDK 4/6 inhibitors + endocrine therapy is currently not clear given the lack of direct comparison between these strategies. However, while an impact on OS has been demonstrated with CDK 4/6 inhibitors in association with endocrine therapy in unselected HR-positive advanced BC both in endocrine-sensitive and endocrine-resistant settings [[32], [33], [34], [35]] this was not the case with either olaparib or talazoparib. This might support the use of CDK 4/6 inhibitors before PARP-inhibitors in patients with HR-positive/HER2-negative metastatic BC harboring a BRCA germline mutation. This position is formally reflected in the Italian regulatory scenario regarding access to talazoparib, reimbursement of which is granted in HR-positive/HER2-negative metastatic BC patients already treated also with a CDK 4/6 inhibitor. However, a degree of uncertainty regarding the use of PARP-inhibitors in a post-CDK 4/6 inhibitor setting should be accounted, given the under-representation in both the OlympiAd and EMBRACA trials of patients previously treated with this endocrine-based strategy (less than 10% of the HR-positive/HER2-negative subpopulation in the EMBRACA trial, data not reported in the OlympiAd trial).
Another clinical scenario involves the subgroup of TN BC patients with both germline BRCA mutation and PD-L1 positive status, where the decision about sequencing PARP inhibitors with immunotherapy (/+ chemotherapy) may be controversial. A translational analysis from the Impassion130 trial showed that the magnitude of benefit from the incorporation of immunotherapy with chemotherapy was consistent irrespective of BRCA status [31], this being reassuring on the value of this strategy also in BRCA-related TN advanced BC. These data, with the current regulatory placement of immunotherapy as the standard first-line treatment in this setting, support the Panel of AIOM Clinical Practice Guidelines on Breast Cancer position for considering immunotherapy + chemotherapy the preferred first-line choice in patients with both germline BRCA mutation and PD-L1 positive status, possibly reserving the single-agent PARP-inhibitor in a subsequent line.
Beyond first-line treatment, the contemporary therapeutic landscape of pre-treated patients is constantly evolving, with the novel antibody-drug conjugates (ADCs) Sacituzumab Govitecan [39] and Trastuzumab Deruxtecan [38] recently receiving approval in TN and HER2-low BC patients, respectively. Within this framework, no head-to-head comparisons are currently available that could support the prioritization of one agent rather than the other (PARP-inhibitors versus ADCs) in the subgroup of patients harboring BRCA1/2 germline mutations. However, the most recent version of the ESMO guidelines [17], endorses the use of PARP-inhibitors before sacituzumab govitecan in BRCA-mutated TNBC patients and before any chemotherapy-based options in BRCA-mutated HR+/HER2-, thus indirectly supporting PARP-inhibitors as the preferred treatment option after CDK 4/6 inhibitors.
An important consideration also regards access to PARP inhibitors beyond germline BRCA 1/2 mutations. The “TBCRC 048” [47] and “Talazoparib Beyond BRCA” [48] single-arm phase II studies, which enrolled MBC patients harboring mutations in homologous recombination-related genes other than BRCA 1/2 (patients with somatic BRCA1/2 mutations were also included in the TBCRC-048 study), reported promising antitumor activity with olaparib and talazoparib, respectively, in patients with germline PALB2 mutations [47,48] and, the TBCRC-048 study, also in patient with somatic BRCA1/2 mutations [47]; this certainly points to a further research area worth delving into. Currently, the use of PARP inhibitors in this particular subset of patients is not allowed in Italy, but is enshrined by ESMO Clinical Practice Guidelines as a viable option [17].
A final aspect deserving to be pointed out is the evolving scenario of PARP-inhibitor landscape based on the OlympiA trial results [49,50], demonstrating the value in terms of invasive disease free survival, distant-disease free survival and overall survival benefit from the incorporation of adjuvant olaparib in HER2- BRCA mutated patients at high risk of relapse. In this context, unavoidably, we will be dealing in a near future with a subgroup of HER2-negative BRCA-mutated patients relapsing while on or after adjuvant olaparib. In both OlympiAd and EMBRACA trial patients previously treated with PARP-inhibitors were not eligible, and the OlympiA trial did not report post-recurrence treatments. It is however reasonable to hypothesize that in a similar scenario, the decision on whether to consider a re-challenge with PARP-inhibitor, will be driven, among other, by timing of recurrence with respect to adjuvant olaparib treatment end, treatments received in the (neo)-adjuvant setting and tumor phenotype.
Conclusions
To conclude, we have provided an overview of clinical recommendations on the use of PARP-inhibitors in HER2-negative MBC, applying the rigorous methodology of the GRADE system, outlining a conditional recommendation in favor of PARP-inhibitors over single-agent chemotherapy in both HR-positive/HER2-negative and TN BC subtypes.
Funding
AIOM (Italian Association of Medical Oncology).
Declaration of competing interest
FM personal fee from Roche, Novartis and Gilead outside the submitted work. MVD: personal fees from Eli Lilly, MSD, Exact Sciences, Novartis, Pfizer, Seagen, outside the submitted work; CC: consultan-cy/advisory role/speaker bureau: Pfizer, Novartis, Lilly, Roche, Gilead, MSD, Seagen, outside the submitted work; FM: Fees for advisory board participation; Novartis, Astra Zeneca, Daiichi Sankyo, SeaGen, MSD, Pfizer, Roche, PUMA, outside the submitted work; LDM: grants from Eli Lilly, personal fees from Eli Lilly, personal fees from Novartis, personal fees and non-financial support from Roche, personal fees from MSD, personal fees and non-financial support from Pfiz-er, personal fees from Genomic health, personal fees from Pierre Fabre, personal fees from Daiichi Sankyo, personal fees from Astrazeneca, personal fees from Seagen, personal fees and non-financial support from Eisai, personal fees from Ipsen, personal fees from Gilead, outside the submitted work; AZ: fees for advisory board; Lilly, Novartis, Astra Zeneca, Daiichi Sankyo, SeaGen, MSD, Pfizer, Roche, ExactSciences; LB: honoraria, consulting or advisory role Astra-Zeneca, Daiichi-Sankyo, Eisai, Exact Sciences, Gilead, Lilly, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, Seattle Genetics; research Funding Celgene, Genomic Health, Novartis, all outside the submitted work; CM: personal consultancy fees from Bayer, Roche, Astrazeneca, Daiichi Sankyo, outside the submitted work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.10.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cortesi L., Rugo H.S., Jackisch C. An overview of PARP inhibitors for the treatment of breast cancer. Targeted Oncol. 2021;16:255–282. doi: 10.1007/s11523-021-00796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miki Y., Swensen J., Shattuck-Eidens D., Futreal P.A., Harshman K., Tavtigian S., et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 3.Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 4.Couch F.J., Shimelis H., Hu C., Hart S.N., Polley E.C., Na J., et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190. doi: 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://enigmaconsortium.org/library/general-documents/enigma-classification-criteria/n.d.
- 6.2022. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) - genetic/familial high-risk assessment: breast Ovarian, and Pancreatic -Version 1. — September 7, 2022 n.d. [DOI] [PubMed] [Google Scholar]
- 7.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.-A., Mooij T.M., Roos-Blom M.-J., et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 8.Barnes D.R., Antoniou A.C. Unravelling modifiers of breast and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: update on genetic modifiers. J Intern Med. 2012;271:331–343. doi: 10.1111/j.1365-2796.2011.02502.x. [DOI] [PubMed] [Google Scholar]
- 9.Mavaddat N., Barrowdale D., Andrulis I.L., Domchek S.M., Eccles D., Nevanlinna H., et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the consortium of investigators of modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomarkers Prev. 2012;21:134–147. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javle M., Curtin N.J. The role of PARP in DNA repair and its therapeutic exploitation. Br J Cancer. 2011;105:1114–1122. doi: 10.1038/bjc.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lord C.J., Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bono J de, Ramanathan R.K., Mina L., Chugh R., Glaspy J., Rafii S., et al. Phase I, dose-escalation, two-Part Trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;7:620–629. doi: 10.1158/2159-8290.CD-16-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner N.C., Telli M.L., Rugo H.S., Mailliez A., Ettl J., Grischke E.-M., et al. A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations (ABRAZO) Clin Cancer Res. 2019;25:2717–2724. doi: 10.1158/1078-0432.CCR-18-1891. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman B., Shapira-Frommer R., Schmutzler R.K., Audeh M.W., Friedlander M., Balmaña J., et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tutt A., Robson M., Garber J.E., Domchek S.M., Audeh M.W., Weitzel J.N., et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 17.Gennari A., André F., Barrios C.H., Cortés J., Azambuja E de, DeMichele A., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Burstein H.J., Somerfield M.R., Barton D.L., Dorris A., Fallowfield L.J., Jain D., et al. Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39:3959–3977. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.2022. NCCN Clinical Practice Guidelines in Oncology - Breast Cancer - Version 4.2022 — June 21.https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419 n.d - available at: [Google Scholar]
- 20.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J.P.T., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for systematic reviews of interventions.training.cochrane.org/handbook/archive/v5.1/ updated March 2011. [Google Scholar]
- 22.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwers M.C., Kerkvliet K., Spithoff K. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016:i1152. doi: 10.1136/bmj.i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurvitz S.A., Gonçalves A., Rugo H.S., Lee K.-H., Fehrenbacher L., Mina L.A., et al. Talazoparib in patients with a germline BRCA -mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncol. 2020;25:e439–e450. doi: 10.1634/theoncologist.2019-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litton J.K., Hurvitz S.A., Mina L.A., Rugo H.S., Lee K.-H., Gonçalves A., et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526–1535. doi: 10.1016/j.annonc.2020.08.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robson M.E., Tung N., Conte P., Im S.-A., Senkus E., Xu B., et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robson M., Ruddy K.J., Im S.-A., Senkus E., Xu B., Domchek S.M., et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer. 2019;120:20–30. doi: 10.1016/j.ejca.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ettl J., Quek R.G.W., Lee K.-H., Rugo H.S., Hurvitz S., Gonçalves A., et al. Quality of life with talazoparib versus physician's choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol. 2018;29:1939–1947. doi: 10.1093/annonc/mdy257. [DOI] [PubMed] [Google Scholar]
- 30.Rugo H, Cortés J, Cescon D, Im S, Yusof MM, Gallardo C, et al. KEYNOTE-355: final results from a randomized, double-blind phase III study of first-line pembrolizumab + chemotherapy vs placebo + chemotherapy for metastatic TNBC, n.d.
- 31.Emens L.A., Molinero L., Loi S., Rugo H.S., Schneeweiss A., Diéras V., et al. Atezolizumab and nab -paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. JNCI J Natl Cancer Inst. 2021;113:1005–1016. doi: 10.1093/jnci/djab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slamon D.J., Neven P., Chia S., Fasching P.A., Laurentiis M.D., Im S.-A., et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 33.Im S.-A., Lu Y.-S., Bardia A., Harbeck N., Colleoni M., Franke F., et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 34.Sledge G.W., Toi M., Neven P., Sohn J., Inoue K., Pivot X., et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—monarch 2. JAMA Oncol. 2020;6:116. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.-S., Sonke G.S., Hart L., et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 36.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 37.Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.-A., Yusof M.M., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 38.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardia A., Hurvitz S.A., Tolaney S.M., Loirat D., Punie K., Oliveira M., et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 40.Brok WD den, Speers C.H., Gondara L., Baxter E., Tyldesley S.K., Lohrisch C.A. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat. 2017;161:549–556. doi: 10.1007/s10549-016-4080-9. [DOI] [PubMed] [Google Scholar]
- 41.Bonotto M., Gerratana L., Poletto E., Driol P., Giangreco M., Russo S., et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncol. 2014;19:608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianchini G., Angelis C.D., Licata L., Gianni L. Treatment landscape of triple-negative breast cancer — expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19:91–113. doi: 10.1038/s41571-021-00565-2. [DOI] [PubMed] [Google Scholar]
- 43.O'Shaughnessy J., Brezden-Masley C., Cazzaniga M., Dalvi T., Walker G., Bennett J., et al. Prevalence of germline BRCA mutations in HER2-negative metastatic breast cancer: global results from the real-world, observational BREAKOUT study. Breast Cancer Res. 2020;22:114. doi: 10.1186/s13058-020-01349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadav S., Hu C., Hart S.N., Boddicker N., Polley E.C., Na J., et al. Evaluation of germline genetic testing criteria in a hospital-based series of women with breast cancer. J Clin Oncol. 2020;38:1409–1418. doi: 10.1200/JCO.19.02190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fasching P.A., Link T., Hauke J., Seither F., Jackisch C., Klare P., et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study) Ann Oncol. 2021;32:49–57. doi: 10.1016/j.annonc.2020.10.471. [DOI] [PubMed] [Google Scholar]
- 46.Diéras V., Han H.S., Kaufman B., Wildiers H., Friedlander M., Ayoub J.-P., et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:1269–1282. doi: 10.1016/S1470-2045(20)30447-2. [DOI] [PubMed] [Google Scholar]
- 47.Tung N.M., Robson M.E., Ventz S., Santa-Maria C.A., Nanda R., Marcom P.K., et al. Tbcrc 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38:4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 48.Gruber J.J., Afghahi A., Timms K., DeWees A., Gross W., Aushev V.N., et al. A phase II study of talazoparib monotherapy in patients with wild-type BRCA1 and BRCA2 with a mutation in other homologous recombination genes. Nat Can (Que) 2022 doi: 10.1038/s43018-022-00439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geyer C.E., Garber J.E., Gelber R.D., Yothers G., Taboada M., Ross L., et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high risk, early breast cancer. Ann Oncol. 2022 doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., et al. Adjuvant olaparib for patients with BRCA1 - or BRCA2 -mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.