Abstract
Background
With the introduction of investigational human epidermal growth factor receptor 2 (HER2) targeting treatments, thorough understanding of breast cancer with different HER2 expression levels is critical. The aim of this study was to compare clinicopathologic characteristics and survival of patients with metastatic breast cancer according to the level of HER2 expression.
Methods
Women with distant metastatic breast cancer during 2008–2016 were selected from PALGA, the Dutch Pathology Registry, and linked to the PHARMO Database Network. Breast cancer samples were categorised as HER2 immunohistochemistry score 0 (IHC0), HER2-low or HER2+.
Results
Among women with hormone receptor (HR) positive metastatic breast cancer (n = 989), 373 (38%) cancers were HER2 IHC0, 472 (48%) were HER2-low and 144 (15%) were HER2+. Among HR negative patients (n = 272), the proportion of HER2 IHC0, HER2-low and HER2+ was 110 (40%), 104 (38%) and 58 (21%) respectively.
Within the HR + cohort, patients with HER2 IHC0 or HER2-low cancer were significantly older compared to HER2+ patients. This age difference was not seen in the HR-cohort. The localisation of distant metastases differed significantly between HER2 IHC0 or HER2-low versus HER2+ cases. Survival rates did not differ markedly by subtypes.
Conclusion
Substantial proportion of patients had a HER2-low breast cancer. No clear differences in survival were found when comparing HER2 and HR status. Getting more granular insights in the level of HER2 expression and addressing HER2-low as a separate category could help to assess the impact of emerging treatment strategies. Therefore, more detailed information on HER2 expression should be routinely reported.
Keywords: HER2 status, HER2-low, Hormone receptor status, Metastatic breast cancer
Highlights
-
•
A substantial proportion of patients had HER2-low breast cancer.
-
•
HER2 expression levels differed between the primary tumour and the metastases.
-
•
It is important to assess HER2 status in both the primary tumour and metastases.
-
•
Similar survival in HER2-low and HER2-0 BC patients, stratified for HR.
1. Background
Breast cancer is the most frequently diagnosed solid cancer and is the leading cause of cancer related deaths amongst women worldwide [1]. In the year 2020, there were 2.3 million newly diagnosed women with breast cancer globally and around 685,000 deaths [1]. Although features of breast cancer are highly heterogenous, the increased understanding of its molecular biology over the past three decades has led to novel targeted therapies that have improved patients’ outcomes [2,3]. Nonetheless, more knowledge about the biological and clinical features would help to further support optimal use of available and emerging therapeutic approaches.
The human epidermal growth factor receptor 2 (HER2) is an important predictive and prognostic marker, commonly detected on the primary breast cancer and/or the distant metastases [4]. Immunohistochemistry (IHC) and in situ hybridization (ISH) are mainly used to determine HER2 status, which is used for treatment decisions [5]. In current clinical practice, HER2 status is classified dichotomously, as either positive (IHC3+ or IHC2+ ISH+) or negative (IHC0, IHC1+ or IHC2+ ISH-) [6]. However, as HER2 negative includes a wide spectrum of HER2 expression levels, emerging treatment options underline the need for a more granular stratification including a HER2-low expression segment in which some expression of HER2 is seen (IHC score 2+ and ISH negative or IHC 1+ and ISH negative or untested), thus leaving HER2 negative as true negative (HER2 IHC0) [2,7]. Hormone receptor (HR) status of the oestrogen receptor (ER) and progesterone receptor (PR) are, next to HER2, routinely assessed in breast carcinoma to further assess the prognosis of the patient and to choose optimal treatment, i.e. endocrine therapy for HR positive breast cancer.
HER2 is overexpressed in several cancer types, including breast and gastric tumours [8]. Previously, HER2+ breast cancer was associated with an aggressive biology, high recurrence and poor survival. However, due to agents targeting the HER2 pathway, prognosis has improved substantially [4,5,7]. Trastuzumab was the first agent developed to target the HER2 pathway. Due to the significant improvement in response rate, time to disease progression and survival, anti-HER2 targeted has become a standard therapeutic approach for HER2+ breast cancer [9]. However, most breast cancers are HER2 IHC0 or HER2-low (15–20% and 55–60%, respectively) while HER2+ is seen in about 15–20% of the newly diagnosed patients [7,[10], [11], [12]]. Even though HER2-low breast cancer has some HER2 expression, it is generally considered and treated as HER2 negative [7]. Currently, there are several investigational HER2 targeting treatments, including the antibody-drug conjugate (ADC) trastuzumab-deruxtecan, whom in addition to their proven/established efficacy and safety in HER2+ metastatic BC are being evaluated in HER2-low expressing mBC [13]. To illustrate, trastuzumab-deruxtecan has resulted in a significant longer progression-free and overall survival in patients with HER2-low metastatic breast cancer compared to the physician's choice of chemotherapy [13]. Therefore, a thorough understanding of the demographics and characteristics of patients with different levels of HER2 expression is critical to identify optimal patient populations per type of therapy.
The aim of this retrospective study was to describe patient demographics, disease characteristics and survival in a population-based cohort of metastatic breast cancer patients in the Netherlands, stratified by HER2 and HR status in real-world clinical practice. While most previous studies only distinguished between HER2+ and HER2 negative breast cancer, we included HER2-low as a subcategory within HER2 negative, to get more insight in the biology of HER2-low breast cancer [2,3,[10], [11], [12],14].
2. Methods
2.1. Data source
Data from women with distant metastatic breast cancer were obtained from the Dutch Pathology Registry (PALGA), the nationwide network and registry of histo- and cytopathology in the Netherlands [15,16]. Records from PALGA were linked to the PHARMO Database Network [17]. This population-based network of electronic healthcare databases combines data from different primary and secondary healthcare settings in the Netherlands [17]. These different data sources are linked on a patient level through validated algorithms [18]. A detailed description of the databases used for this study is included in previous published papers [16,17].
For the current study, permission has been obtained from PHARMO as well as the PALGA foundation to link the data with the PHARMO Database Network via a trusted third party.
2.2. Patient selection
All women diagnosed with distant metastatic breast cancer between January 1, 2008 until December 31, 2016 were selected from the linked Pathology Registry and Out-patient Pharmacy Database of the PHARMO Database Network. Distant metastatic breast cancer (at primary diagnosis or during follow-up) was defined as breast cancer that was spread to distant organs, distant lymph nodes and/or distant skin localisation, according to the TNM classification [19].
The index date was defined as the date of the first occurrence of either hospitalization for metastatic disease, the first pathology report for distant metastatic breast cancer, or the first dispensing of a drug used to treat metastatic breast cancer. Patients were excluded if the HER2 status was unknown, if data was not available in the Hospital Database of the PHARMO Database Network or of they could not be followed in the PHARMO Database Network around the index date. Women were followed until the end of data collection, death, or end of study period (December 31, 2017), whichever occurred first.
2.3. Baseline characteristics
Patient characteristics were determined at the index date and are presented for all women, overall and stratified by HR and HER2 status. Patient characteristics include age (categorised, mean ± standard deviation (SD)), year of index date (categorised), length of available follow-up after index date in the PHARMO Database Network (years) (categorised, mean ± SD, median [IQR]) and site of distant metastases (skin, pleural fluid, liver, bones, lymph nodes, brain, other). Differences between patient characteristics were made between HER2 categories per HR status.
Information on HER2 status was derived from the Dutch Pathology Registry, which included information on the results of the IHC and the ISH test to determine HER2 status, according to international guidelines [20,21]. If the result of the IHC test was equivocal, HER2 status was clarified with ISH. Breast cancers were categorised as HER2 IHC0, HER2-low or HER2+ according to Yao et al. (see Table 1) [5]. As HER2 status can differ between primary breast cancers and paired metastases, HER2 status of the metastases was used if available. If not available, information of the primary breast cancer was used. We also assessed differences in the level of HER2 expression between the primary tumour and the distant metastasis.
Table 1.
Definition of HR and HER2 status.
| HRa | ER | PR |
|---|---|---|
| Positive | + | + |
| + | – | |
| – | + | |
| Negative | – | – |
| HER2 | IHC | ISH |
| overexpression (HER2+) | 3+ | – |
| 2+ | + | |
| 1+ | + | |
| low expression (HER2-low) | 2+ | – |
| 1+ | – | |
| 1+ | Unknown | |
| negative (HER2 IHC0) | 0 | – |
Cut-off for HR positive versus negative was 10%. IHC, Immunohistochemistry; ISH, In Situ Hybridization; HR, hormone receptor; ER, Oestrogen Receptor; PR, Progesterone receptor [6].
Furthermore, women were categorised by HR status (HR positive or HR negative). According to the Dutch guideline for breast cancer treatment, ER and PR are considered positive in case more than 10% of tumour cells showed nuclear staining [22]. This information was also derived from the Pathology Registry (see Table 1).
2.4. Survival
Survival of distant metastatic breast cancer patients was determined after the index date and was presented per HER2 and HR status. Furthermore, comparisons in survival were made between the three HER2 categories (HER2 IHC0, HER2-low and HER2+) per HR status.
2.5. Statistical analysis
Categorical data are presented as counts (n) and proportions (%) and continuous data are presented as means with standard deviation (SD) and/or medians with interquartile range (IQR). Categorical characteristics were assessed with chi-squared tests, normally distributed continuous characteristics were compared using t-tests and skewed characteristics were analysed using Mann Whitney U tests. Comparison of survival was analysed with a Cox regression model with age and year of index date (to account for newer treatments coming available over the years) as covariates in the model. The results are presented in a Kaplan-Meier plot by HER2 and HR status. Statistical analysis was performed by SAS programs organised within SAS Enterprise Guide version 8.2 (SAS Institute Inc., Cary, NC, USA) and conducted under Windows using SAS version 9.4. A statistical test result was considered significant when p ≤ 0.05.
3. Results
3.1. Selection of the study population
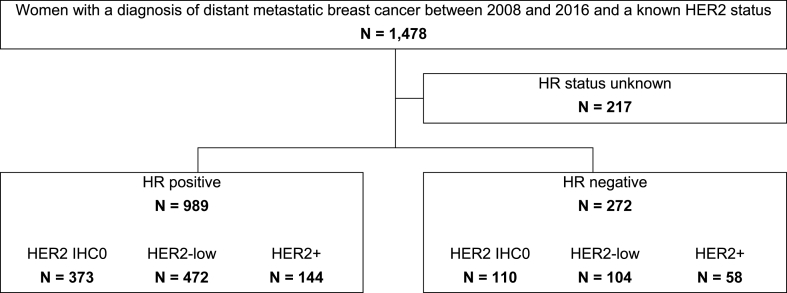
1478 women with a diagnosis of distant metastatic breast cancer and detailed HER2 information were identified from the linked Pathology Registry and Out-patient Pharmacy Database of the PHARMO Database Network cohort between 2008 and 2016. The HR status was unknown for 217 patients, resulting in 1261 women included in this study (Fig. 1).
Fig. 1.
Flow chart of patient selection. PHARMO, PHARMO Database Network; HR, hormone receptor.
3.2. HER2 and HR status distribution
Among women with HR + breast cancer (n = 989), 373 (38%) were classified as HER2 IHC0, 472 (48%) as HER2-low and 144 (15%) as HER2+. Furthermore, among women with HR-breast cancer (n = 272), the number of women with HER2 IHC0, HER2-low expression and HER2+ was correspondingly: 110 (40%), 104 (38%) and 58 (21%) (Fig. 1).
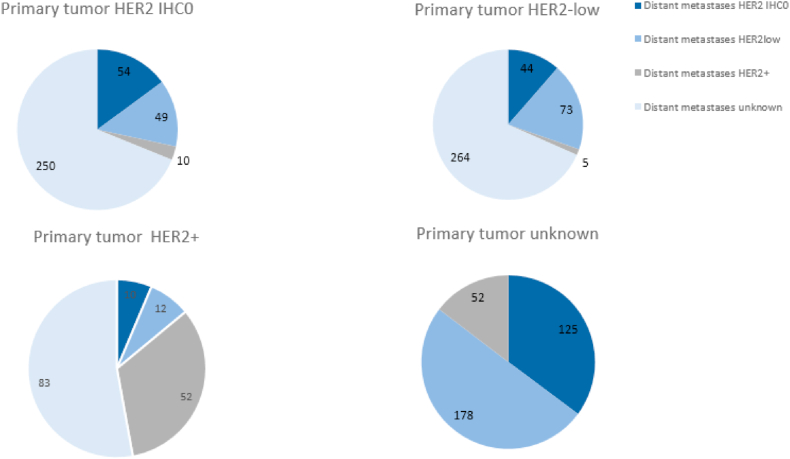
For 664 out of 1261 women (52%), HER2 status of the metastases was available (Fig. 2), We determined HER2 status of the primary tumour versus HER2 status of the distant metastases. For 309 women, detailed HER2 information (0, low, +) was available of both the primary tumour as well as the distant metastases (Fig. 2). In 179 out of 309 patients (58%), there was no difference in the level of HER2 expression. In the remaining patients (n = 130, 42%), there was a difference in the level of HER2 expression between the primary tumour and the distant metastases. Mostly, a HER2-low primary breast cancer versus HER2 IHC0 metastases was observed (n = 44/309, 14%), or vice versa (n = 49/309, 16%). A conversion from a HER2+ primary tumour to a HER2 IHC0 or HER2-low distant metastasis or the other way around occurred in 22 (out 309, 7%) and 15 (out of 309, 5%) of the patients, respectively.
Fig. 2.
HER2 status of the primary tumour versus HER2 status of the distant metastases.
3.3. Characteristics of the study population
Within the HR + breast cancer cohort, women with HER2 IHC0 or HER2-low cancer (mean age 61 and 62 years respectively) were older compared to women with HER2+ breast cancer (mean age 57 years) (p < 0.01). Among HR-breast cancer patients, mean age at index date was 58–59 years and did not differ significantly between the different HER2 categories (Table 2). Year of index date differed significantly between women with HER2 IHC0 or HER2-low and HER2+ cancer; a diagnosis of HER2+ breast cancer was more frequently seen in earlier years compared to a or HER2 IHC0 diagnosis in the HR + group. Concerning the HR-group, this difference was only observed when comparing HER2-low and HER2+. Mean length of available follow-up ranged from 2.4 to 2.9 years in women with HR-disease and 2.8–3.0 years in women with HR + breast cancer (Table 2).
Table 2.
Demographics and baseline characteristics of the study population.
| HR positive |
HR negative |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2 IHC0 |
HER2-low |
HER2+ |
-vs low |
-vs + |
low vs + |
HER2 IHC0 |
HER2-low |
HER2+ |
-vs low |
-vs + |
low vs + |
|
| N = 373 |
N = 472 |
N = 144 |
N = 110 |
N = 104 |
N = 58 |
|||||||
| n (%) | n (%) | n (%) | p-val | p-val | p-val | n (%) | n (%) | n (%) | p-val | p-val | p-val | |
| Age (years) | 0.96 | <.01 | <.01 | 0.58 | 0.78 | 0.94 | ||||||
| ≤35 | 5 (1) | 6 (1) | 6 (4) | 3 (3) | 4 (4) | 1 (2) | ||||||
| 36-49 | 67 (18) | 76 (16) | 42 (29) | 30 (27) | 19 (18) | 11 (19) | ||||||
| 50-59 | 100 (27) | 126 (27) | 27 (19) | 33 (30) | 36 (35) | 21 (36) | ||||||
| 60-69 | 102 (27) | 133 (28) | 41 (28) | 21 (19) | 24 (23) | 12 (21) | ||||||
| ≥70 | 99 (27) | 131 (28) | 28 (19) | 23 (21) | 21 (20) | 13 (22) | ||||||
| Mean ± SD | 61.0 ± 12.0 | 62.0 ± 12.9 | 57.2 ± 13.5 | 57.9 ± 13.8 | 59.0 ± 13.8 | 58.9 ± 11.7 | ||||||
| Year of index date | 0.55 | <.01 | <.01 | 0.56 | 0.05 | <.01 | ||||||
| <2008a | 13 (3) | 13 (3) | 6 (4) | 2 (2) | 0 (<0.5) | 6 (10) | ||||||
| 2008–2010 | 93 (25) | 124 (26) | 62 (43) | 35 (32) | 33 (32) | 13 (22) | ||||||
| 2011–2013 | 120 (32) | 168 (36) | 37 (26) | 36 (33) | 33 (32) | 23 (40) | ||||||
| 2014–2016 | 147 (39) | 167 (35) | 39 (27) | 37 (34) | 38 (37) | 16 (28) | ||||||
| Length of available follow-up (years) | 0.21 | 0.41 | 0.97 | 0.30 | 0.32 | 0.38 | ||||||
| <1 | 76 (20) | 110 (23) | 32 (22) | 27 (25) | 26 (25) | 14 (24) | ||||||
| 1-<2 | 93 (25) | 115 (24) | 33 (23) | 34 (31) | 29 (28) | 16 (28) | ||||||
| 2-<3 | 57 (15) | 78 (17) | 24 (17) | 20 (18) | 15 (14) | 5 (9) | ||||||
| 3-<4 | 64 (17) | 55 (12) | 16 (11) | 7 (6) | 16 (15) | 6 (10) | ||||||
| ≥4 | 83 (22) | 114 (24) | 39 (27) | 22 (20) | 18 (17) | 17 (29) | ||||||
| Mean ± SD | 2.8 ± 2.2 | 2.8 ± 2.4 | 3.0 ± 2.5 | 2.4 ± 2.2 | 2.5 ± 2.1 | 2.9 ± 2.5 | ||||||
| Site of distant metastasesb | ||||||||||||
| Skin | 34 (9) | 36 (8) | 10 (7) | 0.44 | 0.43 | 0.79 | 10 (9) | 7 (7) | 5 (9) | 0.52 | 0.92 | 0.66 |
| Pleural fluid | 79 (21) | 85 (18) | 16 (11) | 0.25 | <.01 | 0.05 | 27 (25) | 21 (20) | 10 (17) | 0.45 | 0.28 | 0.65 |
| Liver | 85 (23) | 105 (22) | 37 (26) | 0.85 | 0.49 | 0.39 | 24 (22) | 13 (13) | 13 (22) | 0.07 | 0.93 | 0.10 |
| Bones | 93 (25) | 116 (25) | 18 (13) | 0.91 | <.01 | <.01 | 9 (8) | 14 (13) | 11 (19) | 0.21 | 0.04 | 0.35 |
| Lymph nodes | 48 (13) | 35 (7) | 23 (16) | <.01 | 0.36 | <.01 | 14 (13) | 16 (15) | 4 (7) | 0.58 | 0.25 | 0.12 |
| Brain | 11 (3) | 21 (4) | 19 (13) | 0.26 | <.01 | <.01 | 9 (8) | 5 (5) | 10 (17) | 0.32 | 0.08 | <.01 |
| Otherc | 82 (22) | 127 (27) | 41 (28) | 0.10 | 0.12 | 0.71 | 35 (32) | 41 (39) | 12 (21) | 0.25 | 0.13 | <.01 |
As the date of biopsy of distant metastatic breast cancer may not represent the actual, earlier date of distant metastatic breast cancer diagnosis, the date of diagnosis was shifted when a hospitalization for distant metastatic disease or a dispensing of a drug to treat metastatic breast cancer was seen before the date of distant metastatic breast cancer biopsy, but after primary breast cancer diagnosis. As a result, for a small proportion of women the year of index date was before 2008.
Multiple sites of metastases per patient is possible.
Only sites of metastases occurring in more than 5% of the study population are presented and all others were placed in the ‘other’ category which included metastases to the gall bladder, bowel, adrenal gland, bladder, mamma (other site), endometrium, cervix, ovary, peritoneum, stomach, vertebra, axilla, lung, soft tissue, bronchus, and abdominal cavity. HR, Hormone Receptor; -: HER2 IHC0.
The site of metastases was assessed among all women and those cases with more than one metastatic location were included in more than one category. Among women with HR+ and HER2 IHC0 or HER2-low cancer, the bone was the most commonly diagnosed site of metastases, followed by the liver. Among women with HR- and HER2 IHC0 or HER2-low cancer, pleural fluid was the most commonly identified site of metastases. Among women with HR + or HR- and HER2+ cancer, the liver was the most commonly diagnosed site of metastases (Table 2). In the HR + group, women with HER2 IHC0 or HER2-low cancer more often developed pleural fluid and bone metastases and less often brain metastases compared to women with HER2+ disease. In the HR-group, women with HER2-low and HER2 IHC0 cancer were less often diagnosed with brain metastases than HER2+ patients (Table 2).
3.4. Survival
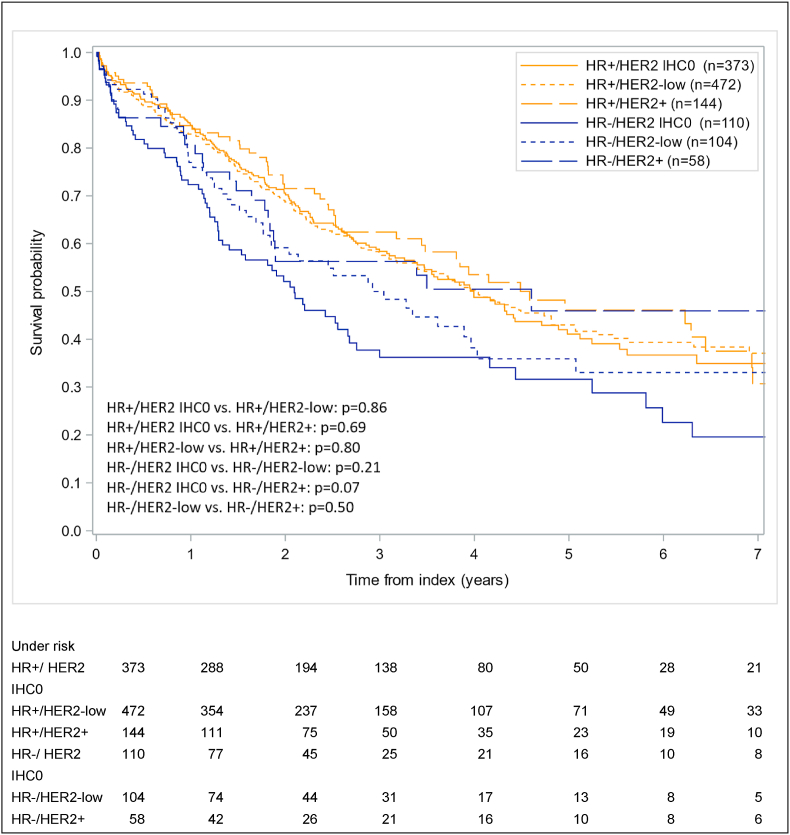
The results regarding survival after the index date are illustrated in Fig. 3. The median overall survival in this study ranged from 1.9 years for HR-/HER2 IHC0 to 4.6 years for HR+/HER2+ breast cancer patients. Five years after index date the survival was lowest amongst women with HR-/HER2 IHC0 breast cancer (28%) and highest among women with HR+/HER2+ breast cancer (48%). Furthermore, 5-year overall survival rates for HER2-low metastatic breast cancer patients were 45% and 32% for HR+ and HR-women respectively. No significant differences in survival among women with breast cancer were found when comparing the different HER2 classes, nor when comparing the HR+ and HR-groups.
Fig. 3.
Survival after index date among women with distant metastatic breast cancer, stratified by HR status and HER2. HR, Hormone Receptor Status.
4. Discussion
The aim of this retrospective study was to describe patient demographics, disease characteristics and survival in a population-based cohort of metastatic breast cancer patients in the Netherlands, stratified by HER2 and HR status in real-world clinical practice. Rather than stratifying by HER2+ versus HER2 negative, this study also included HER2-low as a distinct category within HER2 negative breast cancer.
For a substantial proportion of women included in our study (42%), a difference existed in the level of HER2 expression between the primary tumour and the distant metastases. Previous literature has also shown that discrepancies, previously defined as overexpression versus no overexpression, exist and can be explained by several reasons such as interpretation difficulties, heterogeneity of HER2 amplification and change in HER2 status over time [4,23,24]. It is therefore of high importance to assess HER2 status in both the primary tumour and the distant metastases, to rule out discrepancies and to guarantee optimal care [4]. Nonetheless, due to heterogeneity of the disease, there is often still some discrepancy which cannot be ruled out [25]. However, with the introduction of novel HER2 targeting treatment options, differences between HER2 IHC0 and HER2-low could become clinically relevant. Though, the lower threshold for HER expression in relation to therapy response is unknown, since the Destiny-Breast04 study did not include patients with IHC0 breast cancer. In the DAISY trial, a cohort of patients with IHC0 was included, according to the current version of the ASCO/CAP guidelines [26]. A substantial proportion of these patients (30%) responded to trastuzumab-deruxtecan [26]. This raises the question whether the difference between HER2 IHC0 and HER2-low remains clinically relevant.
The distribution of HER2 expression levels among women with HR + breast cancer was HER2 IHC0 (38%), HER2-low (48%) and HER2+ (15%). Among women with HR-disease, this distribution was 40%, 38% and 21% respectively. Therefore, the HER2-low category should be recognized as a considerable number of patients that could have an indication for novel anti-HER2 agents [7]. Prior available literature shows that around 10–12% of breast cancers are HER2+ in the Netherlands [27]. The slightly high rate of HER2+ cases in our study, which were mainly detected in the earlier years of the study period, might be explained by false-positivity in the early years of HER2 testing [28]. In our study, 78% of patients had a HR + breast tumour, which is in line with previous reports [27,29].
In our study, 20% of the patients were still alive after 10 years. Based on data from IKNL, 10-year survival data among women with stage IV breast cancer at primary diagnosis was 12% [27]. However, it is difficult to compare these results, because the 10-year survival based on IKNL data only included women with metastatic disease at primary diagnosis, while we also included women with non-metastatic disease at primary diagnosis who developed metastases during follow-up. The median overall survival in our study ranged from 1.9 years for HR-/HER2 IHC0 breast cancer to 4.6 years for HR+/HER2+ breast cancer. A recently published review indicated that the median overall survival for metastatic HR-/HER2 IHC0 breast cancer is approximately 1 year versus approximately 5 years for the other subtypes [30]. A potential explanation for this relatively favourable survival in our study is that we selected patients with confirmed distant metastases. Those patients that are fit for treatment might have been more likely to undergo a biopsy of the metastases compared to patients that are unfit for treatment since this would not have therapeutic consequences. Some recent studies also included HER2-low breast cancer as a distinct category, with conflicting results. In the study of Agostinetto et al. overall survival of patients with HER2-low breast cancer did not significantly differ compared to patients with HER2+ or HER2 IHC0 breast cancer [3]. Likewise, the studies of Horisawa et al. and Schettini et al. compared the prognosis of HER2-low patients with that of HER2 IHC0 patients and did not observe a difference in prognosis, regardless of HR status [12,14]. These findings are very much in line with our results (see Appendix, Table S1). On the other side, Carsten et al. demonstrated that HER2-low breast cancer patients had a significantly better prognosis compared to patients with HER2 IHC0 breast cancer, particularly in patients with HR-disease [31].
There are several limitations in this study that need to be addressed. Firstly, detailed information about IHC and ISH is required to distinguish between the three different HER2 categories. However, the reporting of HER2 status was often not specific enough to distinguish between HER2 IHC0 and HER2-low, which resulted in excluding many patients. Secondly, HER2 status of the metastatic tumour was not known for all women and in these cases, HER2 status of the primary tumour was used. This could have affected survival outcomes. Thirdly, the relatively short follow-up duration is another limitation in this study, especially in the HR + group as these patients often have late recurrences. Ideally, survival is stratified by treatment options and treatment lines. However, due to the sample size and data availability between the databases this was not possible. Fourthly, the lack of adjustment and multivariate analysis to balance age and other characteristics could have contributed to the results of the survival data. Finally, HER2 expression could be heterogeneous between different metastatic sites. Also, a strength of this study needs to be addressed. Namely, the ability to differentiate between HER2+, HER2-low and HER2 IHC0 due to structured data capture in the pathology registry is a clear strength of our study and is unique compared to other studies assessing the breast cancer landscape. The Pathology Registry collects very specific information on – among other things – breast cancer biopsies, making it a unique source to study the level of HER2 expression.
5. Conclusion
In this study, using a trichotomous HER2 classification, a substantial proportion of patients (48% of the HR + cohort and 38% of the HR-cohort) had HER2-low breast cancer. No differences in survival probability were observed among the patients using this real-world data. Nonetheless, addressing HER2-low as a separate category could have a major clinical impact as these patients could benefit from HER2-targeting therapy. To achieve this, detailed information on the level of HER2 expression, rather than a dichotomous reporting, on both primary tumours and distant metastases should be routinely reported. Further research with larger populations with detailed information regarding the level of HER2 expression could contribute to the optimal use of HER2-targeting agents.
Funding
This study was funded by Daiichi Sankyo Europe GmbH.
Conflict of interest/Competing interests
EH, JO and JK were employees of the PHARMO Institute for Drug Outcomes Research during the conduct of the study. This independent research institute performs financially supported studies for government and related health care authorities and several pharmaceutical companies.
GV was employed by Daiichi Sankyo Europe GmbH during the conduct of the study. Daiichi Sankyo Europe GmbH funded the study.
VD received traveling funding by Daiichi Sankyo Europe GmbH during the conduct of the study and was involved in an advisory board of Astra Zeneca/Daiichi Sankyo and Novartis.
MR was employed by Daiichi Sankyo Europe GmbH during the conduct of the study. Daiichi Sankyo Europe GmbH funded the study.
CD was involved in an advisory board of Astra Zeneca/Daiichi Sankyo and received research funding from Roche and AstraZeneca.
Authors contributions
JO and JK contributed to the study conception and design. Material preparation and analysis were performed by EH, JO and JK. The first draft of the manuscript was written by GV and EH. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This observational study analysed de-identified data from the PHARMO Database Network; therefore, the study was exempt from ethical review and informed consent was not required.
Consent for publication.
Not applicable.
Consent to participate.
Not applicable.
Availability of data and material.
Not applicable.
Acknowledgements
The authors would like to thank all the healthcare providers contributing information to the PHARMO Database Network.
List of abbreviations
- ADC
Antigen-drug conjugate
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor 2
- HR
Hormone receptor
- IHC
Immunohistochemistry
- ISH
In Situ Hybridization
- IQR
Interquartile range
- PR
Progesterone receptor
- SD
Standard deviation
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.11.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.(WHO) WHO https://www.who.int/news-room/fact-sheets/detail/breast-cancer Breast Cancer 2021 [Available from:
- 2.Miglietta F., Griguolo G., Bottosso M., Giarratano T., Lo Mele M., Fassan M., et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021;7(1):137. doi: 10.1038/s41523-021-00343-4. http://europepmc.org/abstract/MED/34642348 https://europepmc.org/articles/PMC8511010 https://europepmc.org/articles/PMC8511010?pdf=render [Internet]. 2021. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostinetto E., Rediti M., Fimereli D., Debien V., Piccart M., Aftimos P., et al. HER2-Low breast cancer: molecular characteristics and prognosis. Cancers. 2021;13(11):2824. doi: 10.3390/cancers13112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapia C., Savic S., Wagner U., Schönegg R., Novotny H., Grilli B., et al. HER2gene status in primary breast cancers and matched distant metastases. Breast Cancer Res. 2007;9(3):R31. doi: 10.1186/bcr1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao M., Fu P. Advances in anti-HER2 therapy in metastatic breast cancer. Chin Clin Oncol. 2018;7(3):27. doi: 10.21037/cco.2018.05.04. [DOI] [PubMed] [Google Scholar]
- 6.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 7.Eiger D., Agostinetto E., Saúde-Conde R., de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers. 2021;13(5) doi: 10.3390/cancers13051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi T., Shitara K., Naito Y., Shimomura A., Fujiwara Y., Yonemori K., et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512–1522. doi: 10.1016/S1470-2045(17)30604-6. [DOI] [PubMed] [Google Scholar]
- 9.Sendur M.A., Aksoy S., Altundag K. Cardiotoxicity of novel HER2-targeted therapies. Curr Med Res Opin. 2013;29(8):1015–1024. doi: 10.1185/03007995.2013.807232. [DOI] [PubMed] [Google Scholar]
- 10.Marchiò C., Annaratone L., Marques A., Casorzo L., Berrino E., Sapino A. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol. 2021;72:123–135. doi: 10.1016/j.semcancer.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Mutai R., Barkan T., Moore A., Sarfaty M., Shochat T., Yerushalmi R., et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–69. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horisawa N., Adachi Y., Takatsuka D., Nozawa K., Endo Y., Ozaki Y., et al. Breast Cancer; 2021. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. [DOI] [PubMed] [Google Scholar]
- 13.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schettini F., Chic N., Brasó-Maristany F., Paré L., Pascual T., Conte B., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casparie M., Tiebosch A.T.M.G., Burger G., Blauwgeers H., van de Pol A., van Krieken J.H.J.M., et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Anal Cell Pathol. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foundation P. www.palga.nl [Available from:
- 17.Kuiper J.G., Bakker M., Penning-van Beest F.J.A., Herings R.M.C. Existing data sources for clinical epidemiology: the PHARMO database network. Clin Epidemiol. 2020;12:415–422. doi: 10.2147/CLEP.S247575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Herk-Sukel M.P., van de Poll-Franse L.V., Lemmens V.E., Vreugdenhil G., Pruijt J.F., Coebergh J.W., et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer. 2010;46(2):395–404. doi: 10.1016/j.ejca.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Classification UT.
- 20.Wolff A.C., Hammond M.E., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 21.Wolff A.C., Hammond M.E., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 22.Oncologie L.W.D. integraal kankercentrum Nederland; 2021. Borstkanker landelijke richtlijn, versie: 3.0. [Google Scholar]
- 23.Regitnig P., Schippinger W., Lindbauer M., Samonigg H., Lax S.F. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J Pathol. 2004;203(4):918–926. doi: 10.1002/path.1592. [DOI] [PubMed] [Google Scholar]
- 24.Edgerton S.M., Moore D. II, merkel D, thor AD. erbB-2 (HER-2) and breast cancer progression. Appl Immunohistochem Mol Morphol. 2003;11(3):214–221. doi: 10.1097/00129039-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Pasha N., Turner N.C. Understanding and overcoming tumor heterogeneity in metastatic breast cancer treatment. Nat Can (Que) 2021;2(7):680–692. doi: 10.1038/s43018-021-00229-1. [DOI] [PubMed] [Google Scholar]
- 26.Mosele Al M.F., Dieras V., Deluche E., Ducoulombier A., Pistilli B., Bachelot T., Viret F., Levy C., Signolle N., Tran D., Garberis I.J., Le-Bescond L., Tran Dien A., Droin N., Kobayashi M., Kakegawa T., Jimenez M., Lacroix-Triki M., André F. LBA1 Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): biomarker analyses from patients from DAISY trial. Ann Oncol. 2022:123. [Google Scholar]
- 27.IKNL . 2020. Borstkanker in Nederland: trends 1989-2019 gebasseerd op cijfers uit de Nederlandse Kankerregistratie. [Google Scholar]
- 28.Moelans RadW C.B., Hoefnagel L.D.C., E. van der Wall en P.J. van Diest Nieuwe ontwikkelingen in HER2-detectie bij het mammacarcinoom. Nederlands Tijdschrift voor Oncologie. 2010:194. [Google Scholar]
- 29.kankernl. HER2-positieve borstkanker.
- 30.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 31.Denkert C., Seither F., Schneeweiss A., Link T., Ju Blohmer, Just M., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.