Abstract
The multiple effects of Pseudomonas aeruginosa type III secretion have largely been attributed to variations in cytotoxin expression between strains. Here we show that the target cell type is also important. While lung epithelial cells showed significant changes in morphology but not viability when infected with P. aeruginosa, macrophages were efficiently killed by P. aeruginosa. Both responses were dependent on the type III secretion system.
Pseudomonas aeruginosa is an opportunistic gram-negative pathogen capable of causing both acute and chronic infections in susceptible individuals. Patients who suffer acute infections include individuals with severe burns, those with indwelling venous lines, and those requiring mechanical ventilation. Individuals with cystic fibrosis suffer chronic infections in which P. aeruginosa colonizes the airways and causes progressive damage. The synthesis and accumulation of bacterial products result in a nonspecific inflammatory response causing further damage to injured tissues. Unfortunately, eradication of P. aeruginosa from the hospital setting where the most susceptible individuals reside is considered impossible because of its intrinsic resistance to antibiotics, its ability to form biofilms, and its ubiquitous occurrence in the environment.
A novel virulence regulon in P. aeruginosa has recently been identified (5, 17). This regulon comprises what is now known as a type III secretion system. Similar complex systems for the secretion of proteins have been described for other gram-negative bacteria, including certain members of the Yersinia, Salmonella, and Shigella genera; pathogenic Escherichia coli; and several plant pathogens (7). Type III secretion systems encode on the order of 20 proteins that apparently assemble into a complex designed to deliver cytotoxins (or effectors) directly across both membranes of the bacterium and into the cytoplasmic compartment of eukaryotic cells. Certain in vitro culture conditions can at least in part mimic the signals required to trigger this so-called contact-dependent secretion mechanism.
Model systems in which to study the effects of the P. aeruginosa type III secretion on cultured mammalian cells have been established (3, 4, 6, 15, 20). In this study, we compared the responses of distinct types of cultured cells to a single P. aeruginosa strain, 388. This strain secretes the cytotoxins ExoS, ExoT, and ExoY, but not ExoU or exotoxin A (15, 19). ExoS and ExoT are closely related ADP-ribosyltransferases, ExoY is an adenylate cyclase, and ExoU is a cytotoxin of unknown mechanism. Exotoxin A is an ADP-ribosyltransferase that is secreted independently of the type III system. We were particularly interested in the interactions of P. aeruginosa with epithelial cells and macrophages, because of the apparent importance of epithelial barriers and phagocytic cells in guarding against P. aeruginosa infection.
For all experiments described here, established cell lines were cultured in the medium recommended by the American Type Culture Collection (supplemented with 100 U of penicillin per ml and 100 μg of streptomycin per ml) at 37°C under 5% CO2. Cells were dispensed into 24-well tissue culture plates to achieve a density of approximately 1 × 105 to 2 × 105 cells per well on the day of the experiment. Overnight cultures of bacteria in L broth were diluted into fresh broth and grown for 2 h at 30°C. The bacteria were pelleted by centrifugation, washed once in phosphate-buffered saline, and then resuspended and diluted in the appropriate cell culture medium without antibiotics. Bacteria were added to cell layers from which the culture medium had been siphoned; the plates were then centrifuged at 1,200 × g for 10 min and incubated at 37°C under 5% CO2. For each data point shown, a minimum of 500 cells were examined.
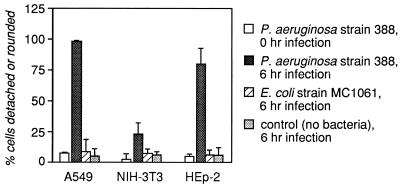
In an initial screen of cultured cell lines for P. aeruginosa-dependent cytotoxicity, the human epithelial cell lines A549 (lung carcinoma derived) and HEp-2 (laryngeal carcinoma derived) and the murine fibroblast line NIH 3T3 were tested in parallel. The eukaryotic cells were infected for various periods with either P. aeruginosa 388 or E. coli MC1061 (a nonpathogenic laboratory strain). In this initial experiment, multiplicities of infection (MOI; the number of bacteria per cultured cell) ranging from 0.1 to 1,000 were tested. Cytotoxicity was assessed visually by determining the percentage of cells that had rounded up and/or detached from the well. Figure 1 shows data for the 0- and 6-h points with an MOI of 10. By 22 h, virtually all of the epithelial cells infected with P. aeruginosa were either detached or rounded, while only approximately 38% of the Pseudomonas-infected NIH 3T3 cells appeared to be affected. At higher MOI, the time course of P. aeruginosa-specific response was not statistically different from the results shown in Fig. 1, with the exception that at the 22-h point all cell layers showed significant damage.
FIG. 1.
Human lung epithelial cells are susceptible to P. aeruginosa intoxication. The human epithelial cell lines A549 (lung carcinoma derived) and HEp-2 (laryngeal carcinoma derived) and the murine fibroblast line NIH 3T3 were infected for various times with E. coli MC1061 or with P. aeruginosa 388 at an MOI of 10 (bacterium/cell ratio = 10) or with the medium without bacteria. Detachment was scored with a hemocytometer, and rounding was scored visually at a magnification of ×100. Shown are the means plus standard deviations of four fields in two wells for each condition; data are representative of two independent experiments. Data are shown for only the 0- and 6-h points.
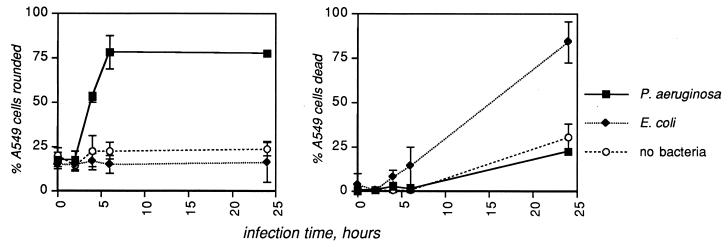
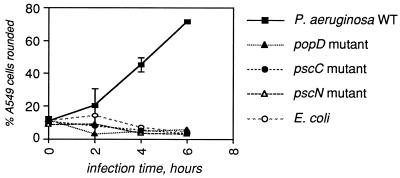
Based on the observation that the A549 cell line displayed the greatest specific sensitivity to P. aeruginosa infection, confluent layers (approximately 2 × 105 cells/well) of A549 cells were infected with either wild-type (WT) P. aeruginosa 388 or E. coli MC1061 or with medium alone at MOI of 0.1, 1.0, or 10 bacteria/cell. In these experiments, the percentage of cells that had rounded up (percent rounded) and the percentage that were no longer able to exclude trypan blue (percent dead) were quantitated. P. aeruginosa at an MOI of 10 caused significant rounding but not death of A549 cells when the two responses were quantitated separately (Fig. 2); similar trends were observed at lower MOI. To determine whether the type III secretion system was responsible for the rounding up of A549 cells, a panel of mutants in the P. aeruginosa type III secretory apparatus (15, 17) was analyzed (Fig. 3). The results demonstrate that the morphological changes in A549 cells after infection by P. aeruginosa are dependent on the type III secretion system.
FIG. 2.
P. aeruginosa causes rounding but not death of human lung epithelial cells. Layers of the human lung epithelial cell line A549 were infected with the medium control, WT P. aeruginosa 388, or E. coli MC1061, at an MOI of 10 bacteria/cell. Rounding was assessed visually at a magnification of ×100; death was assessed by trypan blue exclusion. Shown are the means plus standard deviations of four fields in each of three replicate wells. Data are representative of several independent experiments.
FIG. 3.
The type III secretion system is required for intoxication of A549 lung epithelial cells by P. aeruginosa. A panel of mutants in various components of the type III secretion apparatus (15, 17) was tested as described for Fig. 1 and 2. The results shown were obtained with an MOI of 10 for each bacterial strain; similar results were obtained at an MOI of 1. Shown are the means plus standard deviations of four fields in each of three replicate wells.
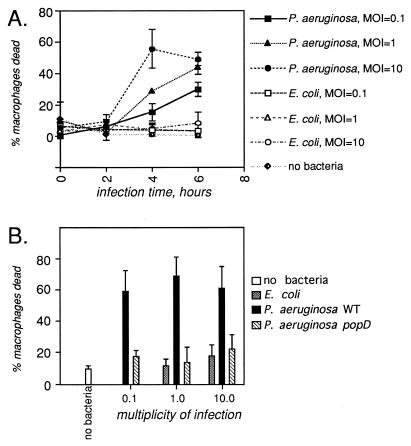
Primary cultures of bone marrow-derived macrophages from A/J mice (14) (kindly provided by the laboratory of Ralph Isberg) were infected for varying amounts of time with MOI ranging from 0.1 to 1,000 with WT P. aeruginosa 388 or E. coli MC1061. Gross changes in macrophage morphology and detachment from the wells were assessed visually; viability was assessed by trypan blue exclusion. Although the macrophages are inherently a more heterogeneous population than are epithelial cell lines, there were no significant differences in overall morphology between cells infected by WT P. aeruginosa and those infected by E. coli (data not shown). In contrast to the situation with A549 cells, however, the WT P. aeruginosa appeared to kill the macrophages very efficiently (Fig. 4A). Like the morphological changes observed in A549 cells, macrophage killing by P. aeruginosa is dependent on the type III secretion system, since a popD mutant did not cause any toxic effect on the macrophages (Fig. 4B).
FIG. 4.
Macrophages are killed by P. aeruginosa. Primary cultures of bone marrow-derived macrophages from strain A/J mice were infected as described for Fig. 1 and 2 with either P. aeruginosa or E. coli. Death was quantitated by trypan blue exclusion. Changes in gross morphology were assessed visually, but no significant differences between infection conditions were observed. (A) Time course of toxicity in response to different MOI of P. aeruginosa and E. coli; shown are the means plus standard deviations of four fields in three wells. (B) Toxicity is dependent on the type III secretion system. The data shown are the means plus standard deviations of six fields in two wells for the 6-h point only.
Exoenzyme S and a closely related gene product, ExoT, are two of the effectors of the P. aeruginosa type III secretion system. ExoS ADP-ribosylates several low-molecular-weight GTPases and the intermediate filament protein vimentin in vitro (1, 2). ExoS expression by P. aeruginosa causes a contact-dependent inhibition of DNA synthesis in mammalian cells in culture (10, 11), but it has never been shown that purified, enzymatically active ExoS is toxic to either cultured mammalian cells or animals. ExoT has approximately 0.2 to 1.0% of the in vitro ADP-ribosyltransferase activity of ExoS (9, 16). The amino-terminal halves of ExoS and ExoT have some homology to YopE, a cytotoxin of Yersinia species that disrupts the actin cytoskeleton (13, 18). The ADP-ribosyltransferase domain of ExoS is located in the C-terminal portion of the protein (8). This portion of the protein alone was toxic when expressed in CHO cells in culture (12) but is not shared with YopE. It therefore appears that ExoS and ExoT have two distinct domains, each having different potentially cytotoxic activities.
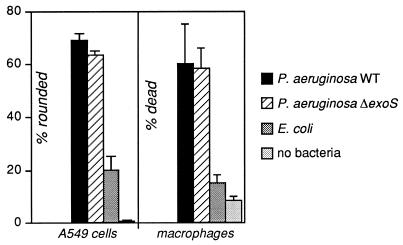
To determine whether ExoS and ExoT are involved in the cytotoxicity to A549 cells and macrophages, infections were performed with the WT P. aeruginosa 388, with isogenic derivatives in which the relevant genes had been replaced by a selectable marker (ΔexoS or ΔexoT, respectively [10, 11]), with E. coli MC1061, or with no bacteria. Cell rounding and death were then quantitated (Fig. 5 and data not shown). No significant differences were observed among the WT, ΔexoS, or ΔexoT strains of P. aeruginosa. These results show that, although the type III secretion system is necessary for intoxication of both cell types, neither ExoS nor ExoT alone can be implicated as the critical cytotoxin.
FIG. 5.
The A549 epithelial cell line and macrophages are intoxicated to the same extent by WT and exoS mutant strains of P. aeruginosa. Layers of each cell type were infected for 6 h at an MOI of 10 with the WT P. aeruginosa 388, with a derivative of that strain in which the gene encoding ExoS had been disrupted (11), with E. coli MC1061, or with no bacteria. Cell rounding was assessed visually at a magnification of ×100. Cell death was assessed by trypan blue exclusion. The A549 data represent the means plus standard deviations of four fields in three wells; the macrophage data are from six fields in two wells.
There are several possible explanations for these results. First, ExoS and ExoT may have similar activities inside cells. This may be due to more comparable ADP-ribosyltransferase activities in vivo than have been observed in vitro (9) or due to the as-yet-uncharacterized activity carried in the domains of ExoS and ExoT that are homologous to the Yersinia cytotoxin YopE (5). It is also possible that the rounding and death observed are independent of both ExoS and ExoT and instead are due to the activity of another cytotoxin secreted by P. aeruginosa. Two other effector proteins of the P. aeruginosa type III secretion system are ExoU and ExoY. ExoU is responsible for the majority of cytotoxic effects that have been reported for cultured mammalian cells (3, 4, 6) but does not appear to be encoded in the genome of strain 388 (3a). ExoY is a recently described adenylate cyclase toxin that causes rounding of CHO cells in culture (20). It is therefore possible that ExoS, ExoT, ExoY, an as-yet-unidentified cytotoxin, or a combination of toxins is responsible for the effects that we have observed. Based on in vitro studies, however, it appears unlikely that the ADP-ribosyltransferase activity of ExoS is the critical factor.
While by no means comprehensive, this survey of mammalian cell types has revealed two that are exquisitely sensitive to the P. aeruginosa type III secretion system, each in a unique way. Comparison of the human epithelial lines to the murine fibroblasts and macrophages suggests that the observations reported here are less likely to reflect species differences than cell-type-specific responses, given the functional conservation of cell types between species and the sensitivity of cells of both human and murine origin to the P. aeruginosa type III secretion system. Furthermore, the type III secretion systems of P. aeruginosa 388 (15) and PA103 (20) caused morphological changes in CHO cells, an epithelial line of hamster origin. Further analyses of additional cell lines will be required to support this hypothesis. The different in vitro responses to P. aeruginosa, namely, death without gross morphological change in murine macrophages in contrast to morphological changes without significant death in human epithelial cells, may have implications in the pathogenesis of P. aeruginosa infections. Disruption of lung epithelia may contribute to the ability of P. aeruginosa to penetrate into subsurface tissues. In addition, killing of macrophages may facilitate colonization of the airways and systemic dissemination during infection. The results presented here suggest specific avenues to be pursued in future experiments, especially in animal models of P. aeruginosa infection.
Acknowledgments
We appreciate the technical assistance of Joshua E. Eaton. We thank John M. Leong and Lisa Glickstein for critical review of the manuscript and members of the laboratory of Ralph Isberg for providing macrophages and instruction in the preparation and cultivation of these cells.
This work was supported by a Research Grant from the Cystic Fibrosis Foundation (J.C.), by PHS grants K04-AI01289 and R01-AI31665 (D.W.F.), and by the Center for Gastroenterology Research on Absorptive and Secretory Processes at New England Medical Center, PHS grant 1 P30DK39428, awarded by NIDDK.
REFERENCES
- 1.Coburn J, Dillon S T, Iglewski B H, Gill D M. Exoenzyme S of Pseudomonas aeruginosa ADP-ribosylates the intermediate filament protein vimentin. Infect Immun. 1989;57:996–998. doi: 10.1128/iai.57.3.996-998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coburn J, Wyatt R T, Iglewski B H, Gill D M. Several GTP-binding proteins, including p21c-H-ras, are preferred substrates of Pseudomonas aeruginosa exoenzyme S. J Biol Chem. 1989;264:9004–9008. [PubMed] [Google Scholar]
- 3.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 3a.Finck-Barbançon, V., and D. W. Frank. Unpublished hybridization data.
- 4.Fleiszig S M, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanada D, Sawa T, Yen T S, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank D W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 6.Hauser A R, Kang P J, Engel J N. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 7.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight D A, Finck-Barbançon V, Kulich S M, Barbieri J T. Functional domains of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1995;63:3182–3186. doi: 10.1128/iai.63.8.3182-3186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Yahr T L, Frank D W, Barbieri J T. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme S of Pseudomonas aeruginosa. J Bacteriol. 1997;179:1609–1613. doi: 10.1128/jb.179.5.1609-1613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuffie E M, Frank D W, Vincent T S, Olson J C. Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1998;66:2607–2613. doi: 10.1128/iai.66.6.2607-2613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson J C, McGuffie E M, Frank D W. Effects of differential expression of the 49-kilodalton exoenzyme S by Pseudomonas aeruginosa on cultured eukaryotic cells. Infect Immun. 1997;65:248–256. doi: 10.1128/iai.65.1.248-256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pederson K J, Barbieri J T. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas aeruginosa exoenzyme S is cytotoxic to eukaryotic cells. Mol Microbiol. 1998;30:751–759. doi: 10.1046/j.1365-2958.1998.01106.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallis A J, Yahr T L, Barbieri J T, Frank D W. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun. 1999;67:914–920. doi: 10.1128/iai.67.2.914-920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yahr T L, Barbieri J T, Frank D W. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J Bacteriol. 1996;178:1412–1419. doi: 10.1128/jb.178.5.1412-1419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yahr T L, Goranson J, Frank D W. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 18.Yahr T L, Hovey A K, Kulich S M, Frank D W. Transcriptional analysis of the Pseudomonas aeruginosa exoenzyme S structural gene. J Bacteriol. 1995;177:1169–1178. doi: 10.1128/jb.177.5.1169-1178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yahr T L, Mende-Mueller L M, Friese M B, Frank D W. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yahr T L, Vallis A J, Hancock M J, Barbieri J T, Frank D W. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]