Abstract
Introduction
Subjective cognitive decline (SCD) may be an early symptom of Alzheimer's disease. We aimed to estimate the prevalence of SCD in Brazil and its association with dementia modifiable risk factors.
Methods
We used data of 8138 participants from the Brazilian Longitudinal Study of Aging (ELSI‐Brazil), a population‐based study that included clinical and demographic variables of individuals across the country. We calculated the prevalence of SCD and its association with dementia modifiable risk factors.
Results
We found that the prevalence of SCD in Brazil was 29.21% (28.22%–30.21%), varying according to region, sex, and age. SCD was strongly associated with hearing loss, low education, psychological distress, Brown/Pardo and Black races.
Discussion
The prevalence of SCD in Brazil is higher than in high‐income countries. Brown/Black races and dementia modifiable risk factors were associated with SCD. Public strategies that target SCD may help mitigate the incidence of dementia.
Keywords: Brazil, dementia, population‐based, prevalence, risk factors, subjective cognitive decline
1. BACKGROUND
Because life expectancy is increasing worldwide, a growing number of older individuals presenting cognitive decline is expected in the coming decades. By 2050, the global estimate of dementia is ∼150 million cases. 1 Among the strategies to mitigate the social and economic burden associated with dementia, the identification of the earliest manifestations of cognitive syndromes is essential. 2 Modifiable risk factors of dementia should be prioritized in individuals with the earliest signs of cognitive decline, such as hearing loss and physical inactivity. 3
In this context, the construct of subjective cognitive decline (SCD) has been shown to be useful in identifying individuals at greater risk of cognitive decline, especially in the context of the Alzheimer's disease continuum. 4 , 5 SCD is usually defined as a self‐reported cognitive complaint in persons with normal cognition, and it has been suggested as a predictor of dementia. 6 In addition, SCD appears to be associated with a broader risk of dementia. 7 Thus identifying individuals with SCD may help target preventable risk factors for dementia and guide the decisions of policymakers in public health. 8 A recent meta‐analysis indicates that the prevalence of SCD varies from 5% to 58% according to age, sociocultural background, and the criteria used. 9 Most of these estimations come from studies conducted in high‐income countries—especially Europe and North America—whereas, paradoxically, two‐thirds of all individuals with dementia live in low‐ and middle‐income countries (LMICs). 10 Although many of the modifiable risk factors for dementia are frequent in LMIC populations, such as low education and higher cardiovascular burden, 11 the frequency of these risk factors in SCD is still poorly understood. One study identified that older age, thyroid disease, and minimal anxiety symptoms among others are risk factors of SCD, but the prevalence of well‐known risk factors of dementia in SCD remains unclear. 12
Determining the profile and risk factors for SCD in the Brazilian population is both crucial and difficult for several reasons. First, Brazil is the largest Latin American country with more than 30 million older adults. 13 Second, it is a multicultural nation, composed of a myriad of racial groups. Third, Brazil has high rates of low education and unequal access to medical care. 14 Finally, Brazil is globally known for its unified health care system (Sistema Único de Saúde [SUS]), which provides an opportunity to study and organize public health strategies.
Herein, we aimed at investigating the prevalence of SCD individuals in Brazil and its associated risk factors. To this end, we obtained data from the Brazilian Longitudinal Study of Aging (ELSI‐Brazil), 15 a nationwide epidemiological taskforce.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources, meetings, and presentations. We did not find any study that evaluated the prevalence of subjective cognitive decline (SCD) and its associated risk factors in Brazil or even in Latin America.
Interpretation: Our findings are higher than the prevalence found in studies from high‐income countries. Hearing loss, psychological distress, and Brown/Pardo and Black races are strongly associated with SCD.
Future directions: The identification of individuals with SCD and knowing of its risk factors can lead to the elaboration of public health strategies aimed at reducing the impact of modifiable risk factors for dementia. Our findings may also help to understand the social and racial inequalities regarding the early diagnosis of dementia.
2. METHODS
2.1. Study design and sampling
The ELSI‐Brazil is a population‐based cohort designed to study the social and biological determinants of aging in Brazil. The baseline evaluation was performed in 2015 to 2016 and included individuals 50 years of age or older (range 50 to 105). A stratification sampling was used according to the size of municipalities. It comprised small, medium, and large cities, covering urban and rural areas, representing the whole country. Sample weights were derived to account for differential probability of selection and differential non‐response. All participants signed the informed consent form before enrollment. The study procedure was conducted by Oswaldo Cruz Foundation (FIOCRUZ), Minas Gerais, Brazil, in accordance with the principles of the Declaration of Helsinki. Interviewers were trained and certified by FIOCRUZ and the Federal University of Minas Gerais, in face‐to‐face evaluations using a tablet. ELSI‐Brazil is a public database and further details can be found elsewhere, 15 and all instruments used are detailed in the supplementary material of the corresponding study.
The ELSI‐Brazil adopted a methodology sto that of other longitudinal aging studies around the world (i.e., the Health and Retirement family of studies), which offers an opportunity for cross‐national comparison. To ensure that the sample represents the urban and rural areas of the small, medium, and large municipalities, the ELSI‐Brazil sampling adopts a design with selection stages, combining stratification of primary sampling units (municipalities), census tracts, and households. 16 Design data from the 2010 National Brazilian Census carried out by the Brazilian Institute of Geography and Statistics (IBGE) was used as a basis to conduct the ELSI‐Brazil as a representative cohort. The estimated sample size was 10,000 individuals, from 70 municipalities across the country. The effective sample design was 1.5, allowing an estimated prevalence of 1% with a sample error of 0.25%.
2.2. Workflow to define SCD
From the total sample (n = 9412 individuals), a subset with complete responses (n = 8138) was evaluated. Participants were classified as SCD according to international recommendations provided by the Subjective Cognitive Decline – Initiative Working Group. 17 , 18 Thus they were classified as SCD when they matched all the following criteria (see Table S1):
A self‐experienced persistent decline in cognitive capacity, compared with a previous normal cognitive status, unrelated to an acute event. The participant must have responded “Bad” or “Regular” to the question “Currently, how do you classify your memory?” AND responded “Similar” or “Worse” to the question “Comparing your memory to how it was 2 years ago, you think that your current memory is …”. This warrants that the complaint matched the criterion of persistence over time.
Negative for the brief cognitive screening: Raw scores above threshold in the Semantic Fluency test, as determined by normative data for the Brazilian population adjusted for age and education. 19 Semantic fluency has proved an adequate screening tool for dementia in previous studies. 20 , 21
Negative for depression symptomatology: Have answered no to the questions: “Has your doctor ever said you have Depression?” AND score below 3 on the Center for Epidemiological Studies Depression Scale (CES‐D8).
Individuals with criteria “b” and “c” but negative for the criterion “a” were classified as Cognitively Unimpaired (CU). A summary of the inclusion process is presented in Figure 1. We have tested two different methods of inclusion using one or two questions in the (a) criterion. Both criteria selected similar individuals with SCD (Table S2).
FIGURE 1.
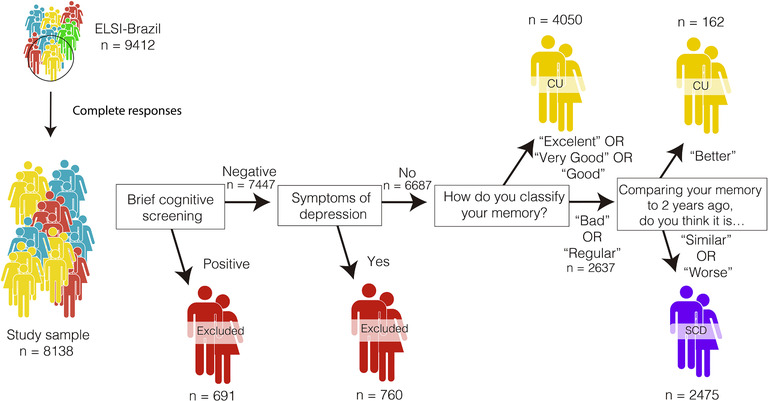
Detailed steps of inclusion criteria for this study. CU, cognitively unimpaired group; SCD, subjective cognitive decline
2.3. Variables collected
Several variables were collected in this study, including sociodemographic factors (i.e., age, sex, education, literacy, race, and income) and variables related to mental health (sleep quality, symptoms of depression, and feeling of loneliness), and modifiable risk factors for dementia were also analyzed (i.e., less than 5 years of education, hearing impairment, hypertension, excessive alcohol consumption, obesity, smoking, depression, social isolation, physical inactivity, and diabetes). 11
The definition of race used in this study was the same as in the Brazilian Census. 22 Race was self‐reported and it included White, Black, Brown/Pardo, Indigenous, and Asian options (Panel 1). Brown/Pardo individuals are defined as any continuum between White and Black individuals, which can include terms, such as “Mulato,” “Cafuzo,” and “Mixed,” as defined by the social construct that based the Brazilian Census. 22
A full description of the questions used for characterizing each variable are depicted in Table S3. Data on traumatic brain injury and air pollution were not collected in the original data set.
2.4. Statistical analysis
Summary demographic statistics were calculated accounting for complex survey analysis. Prevalence was estimated considering the sample weight due to the complex sample, for both whole sample and regionally, with 95% confidence intervals. Categorical variables were compared using chi‐square test and continuous variables were compared using independent t‐tests. We performed Bonferroni's correction for post hoc multiple comparisons, describing continuous variables as mean ± SD. Thus, p‐values were considered statistically significant when <0.002 after Bonferroni's correction (considering the adjustment for 20 variables).
Multicollinearity between variables within the regression model was evaluated with variance inflation factors, using a threshold of 5 for acceptance of the model (Table S4). Two multivariate logistic regression models were calculated with SCD as the primary outcome. Model 1 was hypothesis‐driven using 10 described risk factors for dementia. 3 Model 2 was data‐driven, in which variables were included in the model when presenting p < 0.05 in univariate analysis. Odds and prevalence ratios were also calculated for all variables that presented significance in the logistic regression model. A sensitivity analysis was performed to analyze the impact of missing data in the final sample. All analyses were performed using R (V4.1.0) using built‐in functions, the package survey, and epiR.
3. RESULTS
We included 8138 Individuals from the ELSI‐Brazil cohort in the final analysis with a mean age of 62.6 (± 9.4) years, 56% female, and mean education of 5.6 (± 4.3) years. The subsample excluded by the incomplete responses (n = 1274) had demographic and clinical characteristics that were similar to those of the whole sample (Table S5). In addition, the sensitive analysis showed that this subsample was similarly associated with the independent variables (Table S6).
Overall prevalence of SCD was 29.21% (95% confidence interval [CI] 28.22% to 30.21%), and this prevalence varied according to age, sex, and region (Figure 2 and Table S7). From all individuals that met the study criteria, 2475 were classified as SCD (Table 1), and those without memory complaint (4212) were classified as CU. Compared with CU individuals, those with SCD presented a higher frequency of female individuals, lower family income, and increased frequency of Black individuals.
FIGURE 2.
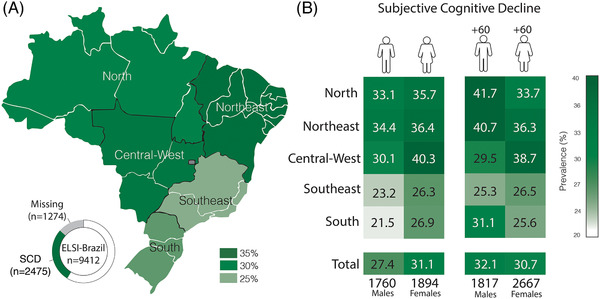
(A) Prevalence of subjective cognitive decline (SCD) according to each Brazilian region. (B) Prevalence of subjective cognitive decline (SCD) according to age and sex in each Brazilian region
TABLE 1.
Demographic characteristics of the sample, divided between cognitively unimpaired (CU) and subjective cognitive decline (SCD) subjects
| CU (n = 4212) | SCD (n = 2475) | |
|---|---|---|
| Age (years) | 62.4 (± 9.4) | 62.7 (± 9.3) |
| Sex (F) | 2102 (49.9%) | 1408 (56.9%) |
| Education (years) | 6.3 (± 4.5) | 4.8 (± 3.9) |
| Illiterate | 914 (21.7%) | 753 (30.5%) |
| Family income | 5.1 (± 3.7) | 4.2 (± 2.7) |
| Race/color skin | ||
| White | 1739 (42.7%) | 824 (34.3%) |
| Black | 375 (9.2%) | 256 (10.7%) |
| Brown/Pardo | 1821 (44.8%) | 1247 (52.0%) |
| Asian | 40 (1.0%) | 18 (0.8%) |
| Indigenous | 94 (2.3%) | 54 (2.3%) |
| Urban zone | 3627 (86.1%) | 2027 (81.9%) |
The frequency of well‐known risk factors for dementia was different between groups (Table 2). Compared with the CU, the SCD group showed increased rates of low education, with a higher percentage of illiterates and higher frequency of physically inactive individuals (p < 0.001 for both). Frequency of hearing loss was substantially higher in SCD (p < 0.0001), but the frequency of hearing aids use was similar to among CU individuals (p > 0.05). The SCD group also presented increased psychological distress, specifically higher symptoms of depression and frequent feeling of loneliness (p < 0.0001). Sleep quality was also worse in SCD when compared with CU (p < 0.001). Regarding sex‐specific differences, men with SCD showed increased frequency of hearing loss, heavy drinking, active smoking, but women with SCD had a higher frequency of hypertension, obesity, depression, and physical inactivity (Table S8).
TABLE 2.
Modifiable risk factors for dementia, and clinical and mental health conditions of the sample
|
Cognitively unimpaired (n = 4212) |
Subjective cognitive decline (n = 2475) |
Uncorrected p‐value |
|
|---|---|---|---|
| Modifiable risk factors for dementia | |||
| Low education | 2311 (54.9%) | 1749 (70.7%) | <0.0001 |
| Hearing loss | 811 (19.3%) | 1017 (41.1%) | <0.0001 |
| Hypertension | 2066 (49.1%) | 1297 (52.6%) | 0.007 |
| Heavy drinking | 217 (5.2%) | 98 (4.0%) | 0.027 |
| Obesity | 1183 (28.8%) | 644 (26.6%) | 0.064 |
| Active smoking | 749 (17.8%) | 386 (15.6%) | 0.022 |
| Depression | 274 (6.5%) | 195 (7.9%) | 0.037 |
| Social isolation | 801 (19.0%) | 505 (20.4%) | 0.17 |
| Physically inactive | 1807 (43.4%) | 1195 (48.9%) | <0.0001 |
| Diabetes | 607 (14.5%) | 392 (15.9%) | 0.12 |
| Mental health conditions | |||
| Poor quality of sleep | 1457 (34.6%) | 1307 (52.8%) | <0.0001 |
| Feeling of loneliness | 1590 (38.4%) | 1212 (43.2%) | <0.0001 |
| Total CES‐D8 scores | 1.9 (± 2.0) | 2.9 (± 2.3) | <0.0001 |
p‐Values in bold are statistically significant after correcting for multiple comparisons. CES‐D8, Center for Epidemiological Scale‐Depression 8.
A multivariate logistic regression analysis was conducted to identify the association between well‐known modifiable risk factors for dementia and SCD (Figure 3). Low education (odds ratio [OR] 1.9, 95% CI 1.7 to 2.1), hearing loss (OR 2.9, 95% CI 2.6 to 3.2), depression (OR 1.31, 95% CI 1.1 to 1.6), and physical inactivity (OR 1.2, 95% CI 1 to 1.3) were significant predictors of SCD. A second regression model (Model 2) accounted for variables that presented statistically significant in univariate analysis (Table 2). This model revealed that hearing loss was the strongest predictor of SCD, while low education, female, poor sleep quality, and frequent feeling of loneliness were also predictors (Figure 3). Black and Brown/Pardo races presented increased odds (OR 1.4, 95% CI 1.1 to 1.7 and OR 1.4, 95% CI 1.2 to 1.6, respectively) of SCD compared to White individuals (OR 1, reference).
FIGURE 3.
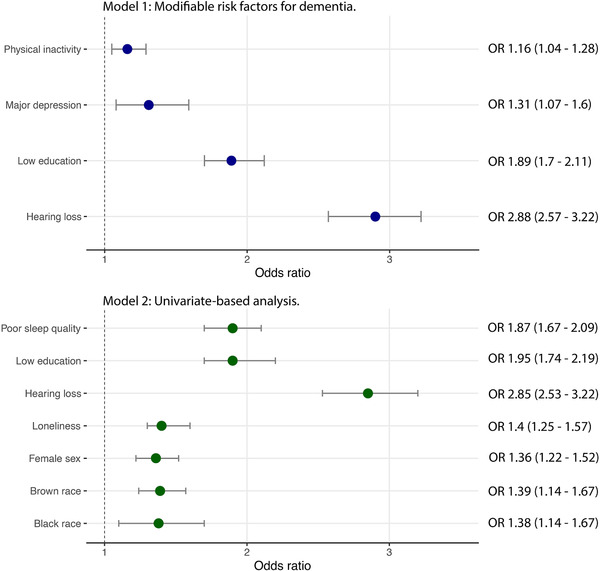
Odds ratio of identified risk factors associated with subjective cognitive decline in this sample. Model 1: established modifiable risk factors for dementia. Model 2: univariate‐based analysis of variables included in the study
4. DISCUSSION
This study provides epidemiological estimates of SCD in a continental‐sized country. As a large LMIC with very low education attainment, Brazil's population shares many similarities with most older adults living with cognitive decline around the globe. 11 These findings may support strategies applicable in public health systems in LMICs worldwide, with focus on modifiable risk factors for dementia in individuals with SCD. Our findings might guide multidomain lifestyle‐intervention studies to control risk factors for dementia, such as clinical trials focused on dementia prevention. 23
Herein we used the most recent definition of SCD, 6 which is characterized by consistent cognitive complaints in cognitively normal individuals. We found an estimated prevalence of SCD of 29.21% among Brazilian older adults, with variations related to sex, age, and region of the country. Comparatively, our estimate of SCD is higher than the prevalence calculated for the United States 24 of 11.1%, and in a Chinese cohort, ranging from 14.4% to 18.8%. 25 Other studies performing harmonization of 15 different cohorts found a prevalence of SCD ranging from 5% to 58%, 9 , 26 which may be explained by the use of different diagnostic criteria and cultural perception of memory complaints. Some studies also in high‐income countries have found higher estimates of cognitive complaints in the elderly, ranging from 50% to 80%. 27 , 28
The prevalence of SCD varied across ages, genders, and regions. We demonstrated that women present increased frequency of SCD. In general, women presented higher rates of SCD than men in both adults and older adults and in all regions. Previous studies have demonstrated that Alzheimer's disease is more present in women than men 29 , 30 and that female sex is also a risk factor for SCD. 12 In addition, depression and anxiety are more prevalent in women worldwide 31 and in Brazil, 32 which could account for a higher rate of SCD. The strength of the association is higher in women for some risk factors, such as hypertension 33 and diabetes, 34 which indicates that there are differences between the sexes in the impact of some cardiovascular risk factores on cognitive complaints. 35 We also found that older adults (60 years of age or older) presented a higher frequency of SCD than adults (between 50 and 60 years old) in this cohort, which is expected as cognitive complaints increase with age, together with the incidence of Alzheimer's disease. 10
Modifiable risk factors for dementia were also identified as predicting likelihood of SCD. The evaluation of these risk factors in individuals with SCD may represent a unique opportunity for preventive interventions and with guiding policy‐making decisions. 7 We observed a particular increased frequency of low educational attainment, hearing loss, and physical inactivity in this study, as related to SCD. These findings were similar to those found by a Chinese SCD cohort. 25 In fact, educational level was inversely related with the prevalence of SCD in our study, which corroborates previous findings. 24 Although ideally all individuals should be targeted to control risk factors for dementia, individuals with SCD may be prioritized for aforementioned risk factor control due to an optimal momentum of intervention, that is, patients that looked for medical assistance with cognitive complaints.
Our findings suggest that hearing loss was the most robust predictor of SCD in the ELSI‐Brazil sample. The association of hearing loss with SCD has been poorly explored in previous studies, but it was observed in a large US SCD cohort. 36 , 37 This finding has not yet been described in LMIC populations, where most individuals with hearing loss reside. 38 Age‐related hearing loss poses a complex interaction with depression and cognitive decline 39 and potential mechanisms explaining this link include social deprivation as a result of reduced interaction with others and increased cognitive load to compensate for diminished hearing (such as lip‐reading). 38 Moreover, male individuals presenting SCD had a higher frequency of hearing loss along with other factors than female individuals, which corroborates previous findings. 36 , 40 Early identification and treatment of hearing loss complaints are cornerstones in avoiding complications associated with this condition. In this context, public health strategies focused on primary care are likely to be more appropriate for the early identification of this condition.
Individuals characterized as SCD presented worse depressive symptoms and feelings of loneliness. Increased psychological distress symptoms may reflect the mental health status of this population. Even though we excluded individuals with a previous diagnosis of depression, the association of the depressive score with SCD could mean undiagnosed depression. We also found an association of SCD with feelings of loneliness and poor sleep quality. Feeling of loneliness 41 and self‐reported poor sleep quality have been increasingly associated with cognitive decline 42 and sleep dysregulation has been repeatedly pointed to as a risk factor for dementia. 43 Further studies may clarify whether these factors are predictors of future cognitive decline.
Along with the established risk factors for dementia, SCD was robustly associated with self‐identified race. It is notable that Brown/Pardo and Black races were associated with increased frequency of SCD in our study. Not only poor cardiovascular health, but also racial inequalities and social background disparities may favor the increase in risk of SCD among Brown/Pardo and Black individuals, which corroborated previous data associating ethnicity disparities with dementia risk. 44 These populations are particularly vulnerable to worse health outcomes, 45 such as increased mortality rates during the pandemic in Brazil. 46 This analysis may support an action by Brazilian authorities to consider special strategies to better protect populations more vulnerable to incident dementia.
There are some limitations in this study. Due to the high frequency of missing data of some variables we used to define SCD in this sample, many individuals were excluded from the analysis. However, the sociodemographic and clinical characteristics of the excluded individuals were similar to those of the analyzed subsample, which suggests that these findings may have national representativeness, and a sensitivity analysis confirmed that missing data were not substantial (Table S6). In addition, data collected must be carefully interpreted. As information of each participant was self‐reported, the possibility of a memory bias cannot be excluded and may have impacted the results. Because data were self‐reported, individuals may over‐ or underestimate their cognitive complaint according to their mood level at the time of data collection. Another limitation is related to SCD definition. The exclusion of depression to fulfill SCD criteria is a matter of debate, since depression can cause cognitive complaints and also may indicate an earlier manifestation of Alzheimer's pathology. 47 , 48 Nevertheless, the association of depressive symptoms and SCD could help improve the understanding of the causes of cognitive complaints in Brazil.
In conclusion, SCD is frequent in Brazil, varying according to sex, age, and country region. Individuals with SCD presented a higher frequency of low education, hearing loss, physical inactivity, and psychological distress when compared with cognitively unimpaired individuals. Hearing loss complaint was the most robust factor associated with SCD. Findings presented here can provide valuable insights for public health strategies across the country that may mitigate the negative effects of preventable risk factors for dementia in this population.
CONFLICT OF INTEREST
None.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENT
This work was supported by an Alzheimer's Association Grant (AACSFD‐22‐928689). RMC is funded by the Alzheimer's Association Grant (AARGD‐21‐846545).
Borelli WV, Zimmer ER, Bieger A, et al. Subjective cognitive decline in Brazil: Prevalence and association with dementia modifiable risk factors in a population‐based study. Alzheimer's Dement. 2022;14:e12368. 10.1002/dad2.12368
REFERENCES
- 1. GBD 2016 Dementia Collaborators . Global, regional, and national burden of Alzheimer's disease and other dementias, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Risk reduction of cognitive decline and dementia: WHO Guidelines. 2019. [PubMed]
- 3. Borelli WV, Leotti VB, Strelow MZ, Chaves MLF, Castilhos RM. Preventable risk factors of dementia: population attributable fractions in a Brazilian population‐based study. The Lancet Regional Health. 2022;11:100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jessen F, Kleineidam L, Wolfsgruber S, et al. Prediction of dementia of Alzheimer type by different types of subjective cognitive decline. Alzheimers Dement. 2020;16(12):1745‐1749. [DOI] [PubMed] [Google Scholar]
- 5. Jack CR, Jr , Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand. 2014;130(6):439‐451. [DOI] [PubMed] [Google Scholar]
- 8. Roheger M, Hennersdorf XS, Riemann S, Flöel A, Meinzer M. A systematic review and network meta‐analysis of interventions for subjective cognitive decline. 2021:e12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Röhr S, Pabst A, Riedel‐Heller SG, et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res Ther. 2020;12(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alzheimer's disease international . The state of the art of dementia research: new frontiers. World Alzheimer Report 2018. 2018. https://www.alzint.org/u/WorldAlzheimerReport2018.pdf [Google Scholar]
- 11. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wen C, Hu H, Ou YN, et al. Risk factors for subjective cognitive decline: the CABLE study. Transl Psychiatry. 2021;11(1):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IBGE. BRASIL. Mudança Demográfica No Brasil No Início Do Século XXI – Subsídios Para as Projeções Da População ; 2015.
- 14. Macinko J, Vaz de Melo Mambrini J, Bof de Andrade F, Drumond Andrade FC, Lazalde GE, Lima‐Costa MF. Life‐course risk factors are associated with activity of daily living disability in older adults. Eur J Public Health. 2021;31(3):520‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lima‐Costa MF, de Andrade FB. The Brazilian Longitudinal Study of Aging (ELSI‐Brazil): objectives and Design. Am J Epidemiol. 2018;187(7):1345‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lima‐Costa MF, de Melo Mambrini JV, Bof de Andrade F, et al. Cohort profile: the Brazilian Longitudinal Study of Ageing (ELSI‐Brazil). Int J Epidemiol. 2022:dyac132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13(3):296‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caramelli P, Carthery‐Goulart MT, Porto CS, Charchat‐Fichman H, Nitrini R. Category fluency as a screening test for Alzheimer disease in illiterate and literate patients. Alzheimer Dis Assoc Disord. 2007;21(1):65‐67. [DOI] [PubMed] [Google Scholar]
- 20. Alegret M, Peretó M, Pérez A, et al. The role of verb fluency in the detection of early cognitive impairment in Alzheimer's disease. J Alzheimers Dis. 2018;62(2):611‐619. [DOI] [PubMed] [Google Scholar]
- 21. McDonnell M, Dill L, Panos S, et al. Verbal fluency as a screening tool for mild cognitive impairment. Int Psychogeriatr. 2020;32(9):1055‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Website. https://books.google.com/books/about/Caracterist%C3%ADcas_%C3%A9tnico_raciais_da_popu.html?hl=&id=STlPTcefEt8C
- 23. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention . Subjective Cognitive Decline—A Public Health Issue. Alzheimer's Disease and Healthy Aging. https://www.cdc.gov/aging/agingdata/docs/subjective‐cognitive‐decline‐508.pdf. 2019.
- 25. Hao L, Wang X, Zhang L, et al. Prevalence, risk factors, and complaints screening tool exploration of subjective cognitive decline in a large cohort of the Chinese population. J Alzheimers Dis. 2017;60(2):371‐388. [DOI] [PubMed] [Google Scholar]
- 26. Slot RER, Sikkes SAM, Berkhof J, et al. Subjective cognitive decline and rates of incident Alzheimer's disease and non‐Alzheimer's disease dementia. Alzheimers Dement. 2019;15(3):465‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414‐422. [DOI] [PubMed] [Google Scholar]
- 28. van Harten AC, Mielke MM, Swenson‐Dravis DM, et al. Subjective cognitive decline and risk of MCI: the mayo clinic study of aging. Neurology. 2018;91(4):e300‐e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences between women and men in incidence rates of dementia and Alzheimer's disease. J Alzheimers Dis. 2018;64(4):1077‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niu H, Álvarez‐Álvarez I, Guillén‐Grima F, Aguinaga‐Ontoso I. Prevalence and incidence of Alzheimer's disease in Europe: a meta‐analysis. Neurologia. 2017;32(8):523‐532. [DOI] [PubMed] [Google Scholar]
- 31. Seedat S, Scott KM, Angermeyer MC, et al. Cross‐national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66(7):785‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blay SL, Fillenbaum GG, Mello MF, et al. 12‐month prevalence and concomitants of DSM‐IV depression and anxiety disorders in two violence‐prone cities in Brazil. J Affect Disord. 2018;232:204‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early‐onset hypertension, and risk of dementia. Neurology. 2017;89(18):1886‐1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azad NA, Al Bugami M, Loy‐English I. Gender differences in dementia risk factors. Gend Med. 2007;4(2):120‐129. [DOI] [PubMed] [Google Scholar]
- 35. Anstey KJ, Peters R, Mortby ME, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population‐based cohort spanning 20‐76 years. Sci Rep. 2021;11(1):7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Curhan SG, Willett WC, Grodstein F, Curhan GC. Longitudinal study of hearing loss and subjective cognitive function decline in men. Alzheimers Dement. 2019;15(4):525‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Curhan SG, Willett WC, Grodstein F, Curhan GC. Longitudinal study of self‐reported hearing loss and subjective cognitive function decline in women. Alzheimers Dement. 2020;16(4):610‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization . World Report on Hearing. World Health Organization; 2021. https://www.who.int/publications/i/item/world‐report‐on‐hearing 2021. [Google Scholar]
- 39. Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age‐related hearing loss to late‐life depression and cognitive decline. Am J Psychiatry. 2018;175(3):215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. Am J Public Health. 2016;106(10):1820‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salinas J, Beiser AS, Samra JK, et al. Association of loneliness with 10‐year dementia risk and early markers of vulnerability for neurocognitive decline. Neurology. 2022;98(13):e1337‐e1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim JH, Ahn JH, Min CY, Yoo DM, Choi HG. Association between sleep quality and subjective cognitive decline: evidence from a community health survey. Sleep Med. 2021;83:123‐131. [DOI] [PubMed] [Google Scholar]
- 43. Sabia S, Fayosse A, Dumurgier J, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement. 2018;4:510‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hone T, Stokes J, Trajman A, et al. Racial and socioeconomic disparities in multimorbidity and associated healthcare utilisation and outcomes in Brazil: a cross‐sectional analysis of three million individuals. BMC Public Health. 2021;21(1):1287. 10.1186/s12889-021-11328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID‐19 in Brazil: a cross‐sectional observational study. Lancet Glob Health. 2020;8(8):e1018‐e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gonzales MM, Samra J, O'Donnell A, et al. Association of midlife depressive symptoms with regional amyloid‐β and Tau in the Framingham heart study. J Alzheimers Dis. 2021;82(1):249‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halahakoon DC, Lewis G, Roiser JP. Cognitive impairment and depression‐cause, consequence, or coincidence. JAMA Psychiatry. 2019;76(3):239‐240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


