Abstract
Background
Immunotherapy has been proved to have a large effect on extensive‐stage small cell lung cancer, but the role of immunotherapy in limited‐stage small‐cell lung cancer (LS‐SCLC) is still unknown.
Methods
A retrospective study of six patients with LS‐SCLC who were treated with neoadjuvant chemoimmunotherapy (durvalumab plus etoposide combined with cisplatin) was performed. Patients were evaluated by the safety, feasibility and pathologic responses of neoadjuvant chemoimmunotherapy.
Results
Neoadjuvant durvalumab combined chemotherapy was associated with few immediate adverse events and did not delay planned surgery. All patients achieved partial pathologic response (pPR) instead of major pathologic response, or pathologic complete response. No association was observed between programmed death‐ligand 1 expression in tumor specimens and the pathologic response. However, tumors with high expression of immune cells such as CD4+ T cells, CD8+ T cells and FoxP3+ Tregs tended to have better pathologic responses than tumors with low expression of immune cells.
Conclusions
Neoadjuvant durvalumab combined chemotherapy could induce pPR with few side effects in resectable LS‐SCLC. The immune cells in the tumor microenvironment might play an important role in neoadjuvant chemoimmunotherapy in resectable LS‐SCLC.
Keywords: limited‐stage small‐cell lung cancer (LS‐SCLC), neoadjuvant chemoimmunotherapy, partial pathologic response, PD‐L1, surgery
Neoadjuvant durvalumab plus chemotherapy could induce partial pathologic response with few side effects in resectable limited‐stage small‐cell lung cancer (LS‐SCLC). The immune cells such as CD4+ T cells, CD8+ T cells and FoxP3+ Tregs in tumor microenvironment might play an important role in neoadjuvant chemoimmunotherapy in resectable LS‐SCLC.

BACKGROUND
Small‐cell lung cancer (SCLC) is a highly malignant tumor, accounts for 10–15% of all lung cancer pathologic types, and is divided into limited‐stage small‐cell lung cancer (LS‐SCLC) and extensive‐stage small‐cell lung cancer (ES‐SCLC). 1 , 2 Chemoradiotherapy was considered to be the main treatment for SCLC for a long time, but the risk of recurrence and metastasis remained high. However, recent retrospective studies have shown that the survival of early‐stage SCLC with systemic chemotherapy after surgery is comparable to that of early‐stage non‐small‐cell lung cancer (NSCLC). 3 Some data even show that the efficacy of surgery plus chemotherapy for stages II and IIIA SCLC is comparable to that of surgery for NSCLC of the corresponding stages. For the efficacy of surgery is far better than that of nonsurgical treatment, 4 the role of surgical treatment of LS‐SCLC is underestimated in clinical practice. In addition to regular radiochemotherapy, immunotherapies that block the immune inhibition of programmed death 1 (PD‐1) protein or programmed death‐ligand 1 (PD‐L1) have a huge effect in ES‐SCLC, 5 , 6 , 7 and neoadjuvant chemoimmunotherapy induced a major pathologic response (mPR) or even a pathologic complete response (PCR) in local advanced NSCLC in various clinical studies. 8 , 9 , 10 Therefore, based on the significant effect in ES‐SCLC and local advanced NSCLC, neoadjuvant chemoimmunotherapy and radical surgery for LS‐SCLC might have the advantage of improving prognosis. 5 , 11
Durvalumab is a recombinant humanized anti‐PD‐L1 monoclonal antibody that blocks interactions between PD‐1 and its ligands, and previous clinical trials have shown that durvalumab achieved a good effect in ES‐SCLC with few side effects. 11 , 12 Durvalumab was therefore approved in China for ES‐SCLC by the Chinese Center for Drug Evaluation in 2018, but its role in LS‐SCLC is still unknown. 7 , 13 The safety and feasibility of neoadjuvant chemoimmunotherapy in local advanced NSCLC patients have also been proved in several studies, 9 , 14 but there have been no studies reported on neoadjuvant chemoimmunotherapy in LS‐SCLC. Herein, we characterized the pathologic features of neoadjuvant chemoimmunotherapy in patients with LS‐SCLC, report the clinical factors that might influence the pathologic response, and aim to provide the basis for improved treatment of LS‐SCLC.
METHODS
Patient selection and data collection
We performed a retrospective study of six patients with LS‐SCLC. All of these patients were in good physical condition and were willing to have surgery at the initial diagnosis. In view of previous studies that suggested the value of surgery in LS‐SCLC and the fact that immunotherapy has demonstrated great pathological benefits in locally advanced NSCLC and ES‐SCLC, all of these patients were strongly in favor of neoadjuvant chemoimmunotherapy and surgical treatment after sufficient preoperative communication. All procedures performed in this study were in accordance with the Declaration of Helsinki and approved by the Tianjin Medical University Cancer Hospital Institutional Review Board.
All patients received two cycles of neoadjuvant chemoimmunotherapy (i.e. intravenous durvalumab plus chemotherapy [etoposide combined with cisplatin, EP] every 3 weeks) followed by R0 resections (4–6 weeks after the last dose of chemoimmunotherapy), then received two cycles of adjuvant chemoimmunotherapy after surgery. After four cycles of chemoimmunotherapy, all the patients received maintenance treatment of durvalumab alone for 1 year and had regular reviews including chest and abdominal CT, tumor markers, and brain MRI every 2–4 months (Figure 1). Safety was evaluated by the severity of adverse effects and feasibility was evaluated by the time of pre‐operative preparation and post‐operative recovery. The treatment effects of tumors were divided into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD) based on the Response Evaluation Criteria in Solid Tumors, version 1.1. 15 , 16
FIGURE 1.
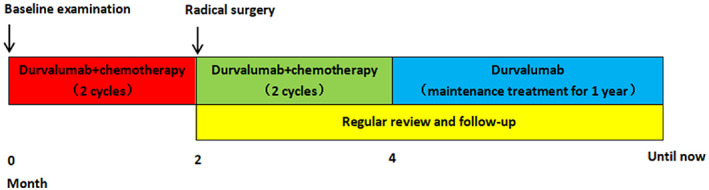
Timelines of patients
Gross pathologic examination and histologic assessment
All tumor tissues were sectioned and each tumor slide was assessed. Two pathologists evaluated the average percentage of residual viable tumor cells (RVT), which was determined by the ratio of tumor area to tumor bed area in all slides. Hematoxylin and eosin (HE) stained slides from tumors were assessed histologically based on the immune‐related pathologic response criteria (irPRC), 17 which defined the tumor regression bed as a major feature of pathologic response, specifically accompanied by fibrosis with neovascularization and immune cell proliferation. In this system, the tumor bed is defined as the regression bed, the RVT, and the necrosis. Tumors were grouped as having a PCR (absence of any viable invasive tumor cells), mPR (%RVT ≤ 10%), partial pathologic response (pPR, 10% < %RVT < 90%) and no pathologic response (nPR, %RVT ≥ 90%) according to the %RVT.
Immunohistochemistry
The primary tumors were made into consecutive slides of 5 μm thickness, and all tissue sections were deparaffinized, rehydrated, and pretreated for antigen retrieval. PD‐L1 was analyzed by immunohistochemistry using the Monoclonal Rabbit Anti‐Human PD‐L1 clone SP263 (Ventana, Roche). Furthermore, fluorescence staining was conducted on immune cells with primary antibodies (CD4 [Clone EPR6588, ab133616; Abcam], CD8 [Clone EPR22483‐288, ab245118; Abcam], and FoxP3[Clone 236A/E7, ab20034; Abcam]). The cell densities of these immune cells in resected tumors were calculated.
Statistical analysis
All data were analyzed using SPSS 23.0 (IBM Corporation). The correlation between clinicopathological factors and pathologic response was conducted by the Pearson's correlation coefficient test, all p values were based on a two‐sided hypothesis, and p < 0.05 was considered statistically significant.
RESULTS
Safety and feasibility
Six patients who were diagnosed with resectable LS‐SCLC received neoadjuvant chemoimmunotherapy and R0 resections in our department from July 2020 to July 2021. All patients underwent baseline tumor staging and were clinically staging IIIA–IIIB (resectable IIIB, T3 or T4) preoperatively. The clinicopathological characteristics of all patients are listed in Table 1. The median age of the patients was 52.17 ± 7.91 (40–60) years, and 66.7% (4/6) were male and long‐term smokers. Neoadjuvant durvalumab combined EP did not induce any severe toxic effects in patients, and all patients were discharged from hospital within 1 week after surgery without severe surgical complications. The median time between the last administration of chemoimmunotherapy and radical resection was 35.2 (range 30–44) days, and no surgical delays occurred. Until September 2022, after a median of 18 (range 7–23) months of postoperative follow‐up, 83.3% (5/6) of patients were alive. One patient died 7 months after surgery because of severe pneumonia induced by bacterial infection (patient 1). No patients were diagnosed with any tumor relapses during the follow‐up time.
TABLE 1.
Clinicopathological characteristics of all patients
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age/sex | 58/M | 45/F | 60/M | 56/M | 40/F | 54/M |
| Smoking index | 300 | – | 400 | 600 | – | 800 |
| Pre‐neoadjuvant radiographic size (cm) | 2.4 | 5.6 | 1.5 | 2.6 | 5.4 | 3.1 |
| TNM classification | T2aN2M0 | T3N2M0 | T1bN2M0 | T1cN2M0 | T3N2M0 | T2aN2M0 |
| Clinical stage | IIIA | IIIB | IIIA | IIIA | IIIB | IIIA |
| Effect of neoadjuvant therapy | PR | PR | SD | PR | PR | SD |
| Gross pathologic size (cm) | 1.5 | 1.0 | 2.0 | 1.0 | 1.8 | 3.0 |
| Pathological stage | IIB | IIIA | IA2 | IIIA | IIIA | IIIA |
| %RVT in primary tumor | 25% | 58% | 72% | 54% | 30% | 80% |
| Pathologic response | pPR | pPR | pPR | pPR | pPR | pPR |
| Cell density of CD4+ T cells (/mm2) | 1103 | 38 | 333 | 31 | 2148 | 129 |
| Cell density of CD8+ T cells (/mm2) | 906 | 169 | 204 | 8 | 402 | 30 |
| Cell density of FoxP3+ Tregs (/mm2) | 170 | 22 | 56 | 6 | 115 | 11 |
| %PD‐L1 in tumor cells | 5 | 6 | 0 | 3 | 0 | 0 |
| %PD‐L1 in immune cells | 42 | 40 | 25 | 20 | 20 | 3 |
| Type of resection | Single lobectomy | Complex lobectomy | Single lobectomy | Single lobectomy | Complex lobectomy | Bilobectomy |
| Surgical approach | OPEN | VATS | OPEN | VATS | OPEN | OPEN |
| Time between neoadjuvant therapy and surgery (days) | 35 | 33 | 44 | 31 | 30 | 38 |
| Postoperative hospital stay (days) | 4 | 4 | 5 | 3 | 6 | 5 |
| Follow‐up time (months) | 7 | 23 | 22 | 19 | 17 | 17 |
| Survival status | No | Yes | Yes | Yes | Yes | Yes |
| Relapse status | No | No | No | No | No | No |
Abbreviations: OPEN, open thoracotomy; VATS, video‐assisted thoracic surgery.
Features of pathologic response in primary tumors
The RVT differed in various cases. The %RVT incresed from 25% (patient 1) to 80% (patient 6), with a median percentage of 53 ± 22%. Although there was no PCR or mPR in primary tumors, all achieved pPR in postoperative tumor specimens with no nPR, and infiltrating lymphocytes were widely distributed in tumor microenvironments (Figure 2). As Figure 3 shows, the radiographic tumor sizes before neoadjuvant chemoimmunotherapy had no relationship with %RVT in resected tumors, and no associations between PD‐L1 expression and %RVT were found, while both PD‐L1‐positive and PD‐L1‐negative tumors achieved pPR. However, tumors with radiographic PR had a better pathologic response (%RVT 42 ± 17%) than tumors with SD (%RVT 76 ± 6%) (p = 0.006) (Figure 4).
FIGURE 2.

Representative pathologic responses to neoadjuvant durvalumab plus chemotherapy in primary tumor specimens of LS‐SCLC (patient 5). (a) The characteristics of pathologic response at ×20 magnifications. (b) The characteristics of pathologic response at ×100 magnifications. The white dotted line separates the tumor cells from the degenerated tissues. The left side indicates the tumor cells, the right side indicates the degenerated tissues such as lymphocytes
FIGURE 3.

Correlation of pathologic response with pre‐neoadjuvant radiographic tumor size (A) and the PD‐L1 expressions of the primary tumor (B, C). Each dot indicates one patient
FIGURE 4.

Patterns of radiologic and pathologic response to neoadjuvant chemoimmunotherapy. Left column: patient 5 (PR), 30% of RVT in the resected specimen; right column, patient 6 (SD), 80% of RVT in the resected specimen. (A, C) Chest CT imaging of patient 5 before and after the administration of neoadjuvant chemoimmunotherapy. (E) Representative sections of tumor specimens after HE staining in patient 5. (B, D) Chest CT imaging of patient 6 before and after the administration of neoadjuvant chemoimmunotherapy. (F) Representative sections of tumor specimens after HE staining in patient 6. The black star indicates the RVT and the black arrow indicates the lymphocytes. Magnifications×20
Fluorescence staining of immune cells
Fluorescence staining was performed to explore the variations of immune cells (CD4+ T cells, CD8+ T cells, and FoxP3+ Tregs) after neoadjuvant chemoimmunotherapy, and the cell densities of these immune cells in the tumor microenvironments were analyzed. The scatter diagram in Figure 5 indicates that the inflamed tumor microenvironment after neoadjuvant chemoimmunotherapy showed pathological benefit, and the immunofluorescence staining showed that tumors with abundant CD4+, CD8+, and FoxP3+ Tregs tended to have a lower %RVT after neoadjuvant chemoimmunotherapy (Figures 6, 7, 8).
FIGURE 5.
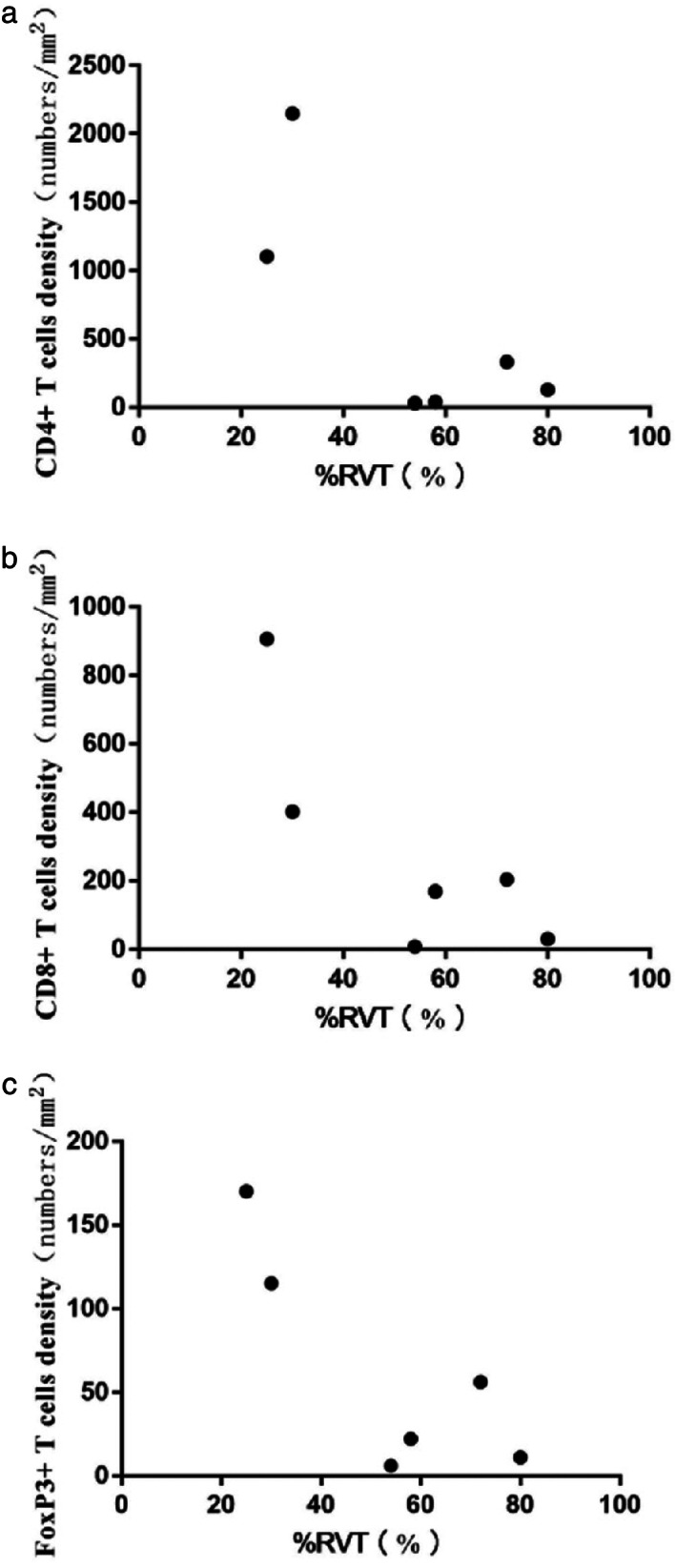
Scatter diagram of the correlation of immune cells with %RVT. (a) Correlation of CD4+ T cells with %RVT. (b) Correlation of CD8+ T cells with %RVT. (c) Correlation of FoxP3+ Tregs with %RVT
FIGURE 6.

Immunofluorescent staining of immune cells to neoadjuvant chemoimmunotherapy in resected primary tumors. The orange fluorescence indicates the CD4+ T cells
FIGURE 7.

Immunofluorescent staining of immune cells to neoadjuvant chemoimmunotherapy in resected primary tumors. The green fluorescence indicates the CD8+ T cells
FIGURE 8.

Immunofluorescent staining of immune cells to neoadjuvant chemoimmunotherapy in resected primary tumors. The pink fluorescence indicates the FoxP3+ Tregs
DISCUSSION
SCLC is an extremely malignant type of lung cancer and is not sensitive to conventional treatment such as chemoradiotherapy. 18 , 19 Chemotherapy alone had low effectiveness in ES‐SCLC, whereas the combination with immunotherapy significantly improved the survival rate in ES‐SCLC. 18 , 20 , 21 , 22 Radical surgery plus adjuvant chemotherapy is the routine treatment for limited T1‐2N0 LS‐SCLC, but the long‐term prognosis is still unsatisfactory. 20 , 23 , 24 Nowadays immunotherapy combined with chemotherapy provides the synergistic effect in local‐advanced NSCLC, 8 and thus provides a basis for the application of immunotherapy in LS‐SCLC.
As reported before, neoadjuvant chemotherapy promoted earlier elimination of micrometastatic diseases, reduced the surgery risks, and improved tolerability to treatment in patients with NSCLC. 10 , 25 In this study, the neoadjuvant durvalumab plus chemotherapy (EP) in patients with staging IIIA–IIIB LS‐SCLC resulted in few adverse events and did not delay the anticipated surgery. Only one patient encountered severe pneumonia induced by the bacteria infection 7 months after surgery, which had no direct relationship with the chemoimmunotherapy. The evaluation of the pathologic response ratio after neoadjuvant therapy allowed the early estimation of curative efficacy, and potentially predicts disease‐free (DFS) and overall survival (OS). 26 Clinical trials reported that neoadjuvant chemoimmunotherapy achieved a mPR in 46–83% and a PCR in 38–56% of patients with NSCLC, 8 , 14 but chemoimmunotherapy hardly achieved mPR in LS‐SCLC, as shown in our study. However, Li et al. 27 reported PCR after receiving neoadjuvant durvalumab combined chemotherapy in one LS‐SCLC patient, and Yan et al. 28 reported that neoadjuvant atezolizumab combined with chemotherapy significantly improved PCR in LS‐SCLC without unknown adverse events and no surgical delays. These two studies also provide hope for neoadjuvant chemoimmunotherapy in LS‐SCLC. All patients in this study had less than 90% RVT in tumor beds and achieved pPR, consistent with the phenomena of immunologic activation and tumor necrosis. No tumor relapses occurred during the follow‐up period. The prognosis statistics and the efficacy of neoadjuvant chemoimmunotherapy for LS‐SCLC needs to be further assessed in the future.
We also studied the dynamic changes in response to neoadjuvant chemoimmunotherapy, such as the changes in tumor size. Of these six patients, four achieved radiographic PR and two achieved radiographic SD. The tumors with PR had a better pathologic response than tumors with SD (p = 0.006), which indicates that the pathologic regression after chemoimmunotherapy was consistent with the radiographic changes. The PD‐L1 expression levels in tumor cells were extremely low in all patients, and the PD‐L1 expression had no significant relationship with the pathologic response (%RVT), indicating that PD‐L1 expression might not be a good predictor for pathologic response in LS‐SCLC.
As reported in previous research, the therapeutic effect of immunotherapy was closely related to the tumor's immune microenvironment. If the immune cells in tumor's microenvironment were in the state of extreme deficiency, the immune checkpoint inhibitors could hardly come into play. 29 , 30 Our study revealed that the immune cells, including CD4+ T cells, CD8+ T cells, and FoxP3+ Tregs, could influence the pathologic response. Tumors with higher expression of immune cells presented a lower %RVT, indicating that the inflamed tumor environment increased the pathologic response and played a key role in immunotherapy in LS‐SCLC.
The study had some drawbacks. First, the sample size was small, which might influence the statistical data. Second, only a short postoperative follow‐up period was included due to time limitations, thus the prognosis of all patients needs to be evaluated in the future. However, the study preliminarily confirmed the safety and feasibility of radical surgery after neoadjuvant durvalumab plus chemotherapy (EP) in IIIA–IIIB LS‐SCLC for the first time, and also confirmed that tumors with radiographic PR presented a better pathologic response to neoadjuvant chemoimmunotherapy, which will be of great value in screening out patients who are not suitable for surgery in the future. It is necessary to continue long‐term studies to evaluate whether or not the pPR could translate into prolonged DFS or OS, and the relationship between %RVT and prognosis.
CONCLUSION
In summary, this study found that neoadjuvant durvalumab plus chemotherapy achieved pPR with few side effects in resectable LS‐SCLC. More significantly, we confirmed that the inflamed tumor microenvironment was associated with a lower %RVT in primary tumors, indicating that the immune cells might play an important role in chemoimmunotherapy in LS‐SCLC. These findings will help surgeons to recognize patients who are sensitive to neoadjuvant chemoimmunotherapy and therefore develop a personalized treatment plan for resectable LS‐SCLC.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: Meng Lu and Jian You. Methodology: Li‐sha Qi and Ya‐lei Wang. Investigation: Meng Lu and Li‐sha Qi. Formal analysis: Ran Zhang and Xiao‐xuan Sun. Resources: Ran Zhang and Jian You. Writing – original draft: Meng Lu and Ran Zhang. Writing – reviewing and editing: Meng Lu, Xiao‐xuan Sun, and Jian You. Visualization: Li‐sha Qi. Supervision: Xiao‐xuan Sun. Funding acquisition: Jian You.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.
Lu M, Zhang R, Qi L, Wang Y, Sun X, You J. Pathologic responses to neoadjuvant chemoimmunotherapy in primary limited‐stage small‐cell lung cancer. Thorac Cancer. 2022;13(22):3208–3216. 10.1111/1759-7714.14679
Meng Lu and Ran Zhang contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. [DOI] [PubMed] [Google Scholar]
- 2. Abdelhamid K, Kakourou A, Degrauwe N, Nikolopoulou A, Bouchaab H, Peters S, et al. Small‐cell lung cancer: management and novelties. Rev Med Suisse. 2020;16(695):1079–85. [PubMed] [Google Scholar]
- 3. Luchtenborg M, Riaz SP, Lim E, et al. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998‐2009. Thorax. 2014;69(3):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the case for lobectomy in stages I, II, and IIIA small‐cell lung cancer using the National Cancer Data Base. J Thorac Oncol. 2015;10(2):316–23. [DOI] [PubMed] [Google Scholar]
- 5. Calles A, Aguado G, Sandoval C, Álvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol. 2019;21(8):961–76. [DOI] [PubMed] [Google Scholar]
- 6. Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small‐cell lung cancer. Nat Rev Clin Oncol. 2020;17(5):300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paz‐Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum‐etoposide versus platinum‐etoposide in first‐line treatment of extensive‐stage small‐cell lung cancer (CASPIAN): a randomised, controlled, open‐label, phase 3 trial. Lancet. 2019;394(10212):1929–39. [DOI] [PubMed] [Google Scholar]
- 8. Gentzler RD, Riley DO, Martin LW. Striving toward improved outcomes for surgically resectable non‐small cell lung cancer: the promise and challenges of neoadjuvant immunotherapy. Curr Oncol Rep. 2020;22(11):109. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Yan B, Xu F, Hui Z, Zhao G, Liu J, et al. Neoadjuvant chemoimmunotherapy in resectable stage IIIA/IIIB non‐small cell lung cancer. Transl Lung Cancer Res. 2021;10(5):2193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahern E, Solomon BJ, Hui R, et al. Neoadjuvant immunotherapy for non‐small cell lung cancer: right drugs, right patient, right time? J Immunother Cancer. 2021;9(6):e2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esposito G, Palumbo G, Carillio G, Manzo A, Montanino A, Sforza V, et al. Immunotherapy in small cell lung cancer. Cancer. 2020;12(9):2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death −1 (PD‐1) and ligand (PD‐L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84–106. [DOI] [PubMed] [Google Scholar]
- 14. Shukla N, Hanna N. Neoadjuvant and adjuvant immunotherapy in early‐stage non‐small cell lung cancer. Lung Cancer (Auckland). 2021;12:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz LH, Litiere S, de Vries E, et al. RECIST 1.1‐update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti‐PD‐1 in resected non‐small‐cell lung carcinoma: a proposal for quantitative immune‐related pathologic response criteria (irPRC). Ann Oncol. 2018;29(8):1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Namikawa S, Den T, Kimura M, Kusagawa M. The role of surgical resection and the effects of neo‐adjuvant therapy in the management of small cell lung cancer. 1994;24(4):342–6. [DOI] [PubMed] [Google Scholar]
- 19. Wada H, Yokomise H, Tanaka F, Hirata T, Fukuse T, Bando T, et al. Surgical treatment of small cell carcinoma of the lung: advantage of preoperative chemotherapy. Lung Cancer. 1995;13(1):45–56. [DOI] [PubMed] [Google Scholar]
- 20. Li S, Jin K, Pan Y, Wu C, Ren S, Jiang G, et al. Role of surgery in a case‐control study of patients with clinical stage IIIA small cell lung cancer. J Thorac Dis. 2021;13(5):2738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsiouprou I, Zaharias A, Spyratos D. The role of immunotherapy in extensive stage small‐cell lung cancer: a review of the literature. Can Respir J. 2019;2019:6860432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konala VM, Madhira BR, Ashraf S, Graziano S. Use of immunotherapy in extensive‐stage small cell lung cancer. Oncology. 2020;98(11):749–54. [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Zheng H, Gao W, Jiang GN, Xie HK, Chen C, et al. Is neoadjuvant chemotherapy mandatory for limited‐disease small‐cell lung cancer? Interact Cardiovasc Thorac Surg. 2014;19(6):887–93. [DOI] [PubMed] [Google Scholar]
- 24. Stish BJ, Hallemeier CL, Olivier KR, Harmsen WS, Allen MS, Garces YI. Long‐term outcomes and patterns of failure following surgical resection of small. Clin Lung Cancer. 2015;6(5):e67–73. [DOI] [PubMed] [Google Scholar]
- 25. Ling Y, Li N, Li L, Guo C, Wei J, Yuan P, et al. Different pathologic responses to neoadjuvant anti‐PD‐1 in primary squamous lung cancer and regional lymph nodes. NPJ Precision Oncol. 2020;4(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weissferdt A, Pataer A, Vaporciyan AA, Correa AM, Sepesi B, Moran CA, et al. Agreement on major pathological response in NSCLC patients receiving neoadjuvant chemotherapy. Clin Lung Cancer. 2020;21(4):341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z, Zhang B, Yang F, Yang L. Pathological complete response to radical surgery after receiving durvalumab plus neoadjuvant chemotherapy for 1 limited‐stage small cell lung cancer patient: a case report. Transl Cancer Res. 2022;11(4):973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan X, Duan H, Shi L, Li H, Tong L, Liu Z. Neoadjuvant attelizumab combined chemotherapy in limited‐stage and resectable small cell lung cancer: a multicentric, single‐arm and open study. J Clin Oncol. 2022;40(16_suppl):e20615. [Google Scholar]
- 29. Rowshanravan B, Halliday N, Sansom DM. CTLA‐4: a moving target in immunotherapy. Blood. 2018;131(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koh J, Hur JY, Lee KY, Kim MS, Heo JY, Ku BM, et al. Regulatory (FoxP3[+]) T cells and TGF‐beta predict the response to anti‐PD‐1 immunotherapy in patients with non‐small cell lung cancer. Sci Rep. 2020;10(1):18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


