Abstract
The sperm whale lives in most deep ice-free waters of the globe. It was targeted during two periods of whaling peaking in the 1840’s and 1960’s. Using a habitat suitability model, we extrapolated estimates of abundance from visual and acoustic surveys to give a global estimate of 736,053 sperm whales (CV = 0.218) in 1993. Estimates of trends in the post-whaling era suggest that: whaling, by affecting the sex ratio and/or the social cohesion of females, reduced recovery rates well after whaling ceased; preferentially-targeted adult males show the best evidence of recovery, presumably due to recruitment from breeding populations; several decades post-whaling, sperm whale populations not facing much human impact are recovering slowly, but populations may be declining in areas with substantial anthropogenic footprint. A theta-logistic population model enhanced to simulate spatial structure and the non-removal impacts of whaling indicated a pre-whaling population of 1,949,698 (CV = 0.178) in 1710 being reduced by whaling, and then then recovering a little to about 844,761 (CV = 0.209) in 2022. There is much uncertainty about these numbers and trends. A larger population estimate than produced by a similar analysis in 2002 is principally due to a better assessment of ascertainment bias.
Subject terms: Ecology, Zoology, Ocean sciences
Introduction
The sperm whale Physeter macrocephalus, is the largest of the toothed whales. It is a deep diver, and uses most of the ice-free waters of the globe greater than 1000 m deep1. Sperm whales are thought to be important elements in marine ecosystems because of their numbers, size, wide-distribution, and ability to recycle nutrients from the depth to the photic zone2,3. The hunting of sperm whales was economically significant and global in scale, and used two principal methodologies4,5. Killing from open boats began in 1712, peaked in about 1840, and continued into the 1920’s. ‘Modern’ whalers, with larger engine-powered whaling vessels, harpoon guns and other technological aids, first targeted sperm whales in the early twentieth century, although the hunt became much more intense between 1950–19706. Following the International Whaling Commission’s moratorium on commercial whaling in 1986 there has been very little whaling for sperm whales (an average of 12 whales per year7.
In 2002, one of us produced a global estimate of sperm whale population size in year 2000 by extrapolating estimates from visual surveys of about 24% of sperm whale habitat8. This estimate (360,000 whales; CV = 0.36) was then used together with catch data to construct a historical trajectory of sperm whale populations. This population estimate and trajectory have been widely cited, despite the paper stating: “the global estimates may be more imprecise than is suggested by the calculated CV” and the “CV itself is somewhat disappointingly large”8.
Here, 20 years later, we update and improve the global population estimate and trajectory, as well as reviewing results on trends in population size since the end of whaling. Improvements include: the inclusion of a number of more recent surveys, some of new areas, and usually using more developed field and analytical methodology9; better consideration of the correction for availability bias, the probability that a whale on the survey line is counted, g(0)10; the inclusion of acoustic surveys11,12; the addition of habitat suitability modelling to better extrapolate from surveyed areas to a global estimate13,14; a more considered estimate of the potential maximal rate of increase15; new information on historical catch data6,16,17; incorporation of spatial structure into the population model18 as well as the demographic consequences of social disruption caused by whaling19.
Additionally, we examine trends in sperm whale since the end of substantial whaling in about 1983 (when catches fell below 500 per year6). Sperm whale abundance would be expected to have increased with the end of significant removals from a depleted population due to density-dependent effects, but some analyses have found no indication of this, perhaps due to the lingering non-removal effects of whaling on sperm whale demography, and/or the effects of more recent threats such as ocean noise, ship strikes and entrapment in fishing gear19–24. We review such studies to suggest global post-whaling trends.
The primary goals of this paper are to estimate: the current (2022) size of the global sperm whale population, the pre-whaling (1711) population, the three-generation decline (IUCN criterion for listing25), post-whaling trends in population size, and to plot an estimate of the population’s historical trajectory.
Results
Surveys
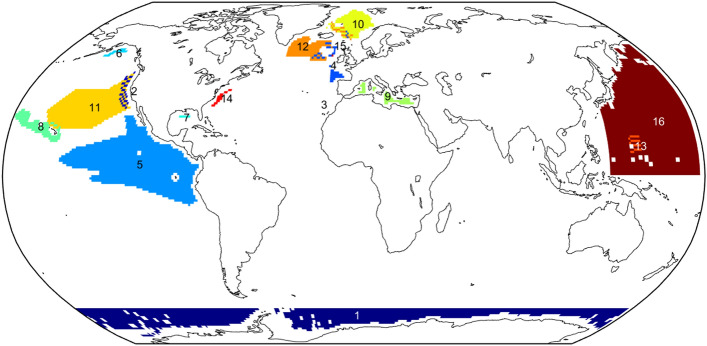
Surveys which estimate sperm whale population size are listed in Table 1 and mapped in Fig. 1. They cover a total of 73,681,588 km2, 24.0% of the global sperm whale habitat (306,594,122 km2), with 0.8% of the global habitat included in two different surveys (see Fig. 1).
Table 1.
Population estimates for sperm whales used to construct global population estimate.
| Region | Years | Type | g(0) | Area 1000 km2 | Population | CV | Whales/1000 km2 | Source | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Antarctic | 1985–1991 | Ship visual | 0.60 | 15,234 | 17,500 | 0.33 | 1.15 | 26 |
| 2 | California current | 2014 | Ship visual | Varied | 859 | 2281 | 0.57 | 2.66 | 20 |
| 3 | Canary islands | 2009–2010 | Ship acoustic | 0.92 | 33 | 224 | 0.32 | 6.88 | 27 |
| 4 | Eastern North Atlantic | 2005–2007 | Ship visual | 0.60 | 1086 | 5445 | 0.37 | 5.02 | 28 |
| 5 | Eastern tropical Pacific | 1986–1990 | Ship visual | 0.60 | 18,166 | 37,777 | 0.37 | 2.08 | 29 |
| 6 | Gulf of Alaska | 2015 | Ship visual | 0.60 | 266 | 575 | 0.52 | 2.16 | 30 |
| 7 | Gulf of Mexico | 2017–2018 | Ship visual | 0.60 | 209 | 1967 | 0.36 | 9.41 | 31 |
| 8 | Hawaiian islands | 2017 | Ship visual | 0.62 | 2126 | 5095 | 0.56 | 2.40 | 32 |
| 9 | Mediterranean sea | 2003–2013 | Ship acoustic | 0.92 | 1093 | 1842 | 0.23 | 1.69 | 12 |
| 10 | Northeast Atlantic | 2002–2007 | Ship visual | 0.60 | 1494 | 13,557 | 0.34 | 9.07 | 33 |
| 11 | Northeast Pacific | 1997 | Ship acoustic | 1.00 | 7670 | 32,068 | 0.36 | 4.18 | 34 |
| 12 | Northern Atlantic | 2007 | Ship visual | 0.57 | 2111 | 12,268 | 0.33 | 5.81 | 35 |
| 13 | Northern Mariana islands | 2007 | Ship visual | 0.60 | 563 | 1175 | 0.67 | 2.09 | 36 |
| 14 | Northwest Atlantic | 2016 | Ship and aerial, visual | 0.61 | 412 | 4349 | 0.28 | 10.55 | 37,38 |
| 15 | Offshore Ireland | 2015–2016 | Ship acoustic | 1.00 | 45 | 380 | 0.04 | 8.43 | 11 |
| 16 | Western North Pacific | 1982–1996 | Ship visual | 0.60 | 24,752 | 43,027 | 0.31 | 1.74 | 39 |
The correction for availability bias (i.e. whales being under water), g(0), is as given in the paper or, if not given, the assumed generic value (italics). The survey areas exclude waters less than 1000 m deep.
Figure 1.
Sperm whale surveys used to produce global population estimates. Numbers by each colour reference surveys listed in Table 1. Where surveys overlap the colours are pixelated. Unsurveyed waters, waters less than 1000 m deep and land are uncoloured. Map created using MATLAB2021b (mathworks.com).
As sperm whales are more easily heard than seen, acoustic surveys generally needed very little correction for availability bias. In contrast, all visual surveys were corrected using g(0) either by the researchers themselves, or, for the 8 surveys where this was not done, using a generic default value of g(0) = 0.60.
Habitat suitability
The relationships between 14 predictor variables and sperm whale density across the different surveys are shown in Supplementary Fig. S1, together with linear and quadratic best fit regressions. No variable is an extremely good predictor of sperm whale density, but several (such as depth, salinity, phosphates and nitrates) seem to show a relationship.
Stepwise regression of sperm whale density from each survey on the predictor variables (weighting by survey area) retained three variables, without including any quadratic terms. The retained predictor variables were salinity, average nitrogen and depth (expressed as a negative number relative to the surface):
Thus, the prediction is that sperm whales are more abundant when waters are lower in salinity and nitrates, and shallower (but more than 1000 m deep). This relationship has an adjusted R2 = 0.561, indicating some predictive ability. A similar analysis weighting all surveys equally rather than by area included most predictor variables and was consequently overfitted.
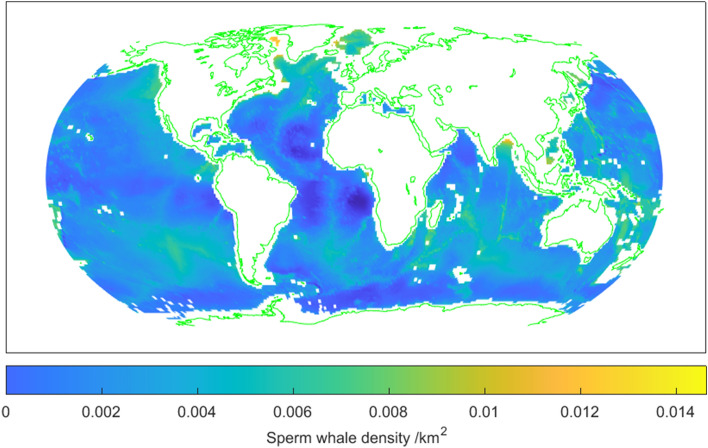
The predicted global distribution of sperm whales using the weighted stepwise regression is shown in Fig. 2. The plot seems mostly reasonable (for instance it is generally similar to the pattern in Fig. 8.2.38 of40 which was produced using very general and largely qualitative classes of habitat preference). However, there are some small-scale anomalies, such as some high predicted densities in the high Arctic, and negative densities off west Africa.
Figure 2.
Predicted density of sperm whales from weighted stepwise regression of survey densities on oceanographic variables. Waters less than 1000 m deep and land are uncoloured. Map created using MATLAB2021b (mathworks.com).
Densities and populations
Estimated sperm whale densities in the survey areas varied from 1.15 whales/1000 km2 in the Antarctic to 10.55 whales/1000 km2 off the US continental shelf in the northwest Atlantic (Table 1). The mean density over all survey areas was 2.31 whales/1000 km2 (CV = 0.137).
Extrapolating these densities to the global sperm whale habitat by area gives an estimated 706,932 whales at a density of 2.31 whales/1000 km2 (CV for both density and population = 0.218) in 1993. The year 1993 is the mean year of the surveys in Table 1, weighting each survey by the area of its study area. Extrapolating using the oceanographic predictor variables gives 736,053 whales globally at a density of 2.40 whales/1000 km2 (CV = 0.218). Estimates for the North Atlantic, North Pacific and Southern Oceans are presented in Table 2.
Table 2.
Population and density estimates for sperm whales in different ocean areas in 1993, extrapolating from survey data using weighted stepwise regression of survey densities on oceanographic variables.
| Ocean | Area 1000 km2 | Population | CV | Density/1000 km2 |
|---|---|---|---|---|
| Global | 306,594 | 736,053 | 0.22 | 2.40 |
| North Atlantic | 37,229 | 92,085 | 0.38 | 2.47 |
| North Pacific | 72,160 | 177,555 | 0.20 | 2.46 |
| Southern Hemisphere | 187,984 | 443,311 | 0.23 | 2.36 |
The equator is a latitudinal boundary of the ocean areas.
Post-whaling trends in population size
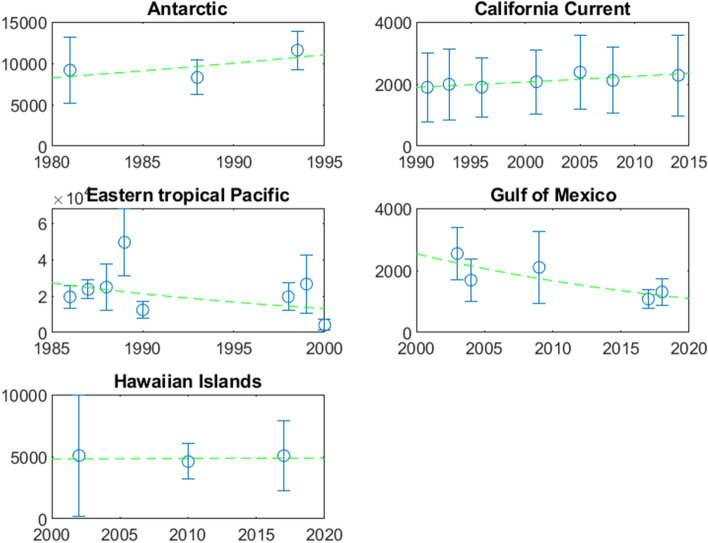
The estimated population trends across five sets of visual surveys are listed in Table 3, and illustrated in Fig. 3. Rates of increase vary from − 0.049/yr in the eastern tropical Pacific to + 0.019/yr in the Antarctic. We had originally planned a meta-analysis of these results using mixed or Bayesian hierarchical models, but the data sets tell of several scenarios. Thus, they are not replicate studies of the same phenomenon, and not suitable for such analyses. Here is a synopsis of the scenarios:
Table 3.
Studies used in post-whaling trend analysis.
| Region | Years | Data | Method (years of data) | Area 1000 km2 | Trend/yr | SE | Source |
|---|---|---|---|---|---|---|---|
| Antarctic | 1978–1998 | Ship visual | Abundance estimates (3) | 15,234 | 0.019 | 0.022 | 26 |
| California current | 1991–2014 | Ship visual | Abundance estimates (7) | 859 | 0.009 | 0.003* | 20 |
| Eastern tropical Pacific | 1986–2000 | Ship visual | Abundance estimates (8) | 18,166 | − 0.049 | 0.043 | 41 |
| Gulf of Mexico | 2003–2018 | Ship visual | Abundance estimates (5) | 209 | − 0.042 | 0.015 | 31 |
| Hawaiian islands | 2002–2017 | Ship visual | Abundance estimates (3) | 2126 | 0.001 | 0.009 | 32 |
| Galápagos | 1985–1995 | Ship sightings | Sighting rates (6) | ~ 65 | − 0.090 | 0.086* | 19 |
| Galápagos | 1985–1995 | Photo-identifications | Mark-recapture (9) | ~ 65 | − 0.200 | 0.064† | 19 |
| Guadeloupe | 2001–2013 | Photo-identifications | Mark-recapture (13) | ~ 1.4 | − 0.062 | 0.030† | 42 |
| Dominica-Guadeloupe | 2005–2015 | Photo-identifications | Count of individuals (10) | ~ 4.6 | − 0.195 | 0.059† | 43 |
*Calculated using weighted regression from presented data.
†Calculated from presented confidence interval.
Figure 3.
Trends in the estimated populations of sperm whales in five study areas where repeated surveys covered at least 10 years and there were at least 3 such surveys. Error bars indicate standard errors, and trend lines are shown (see Table 3 for further details).
The eastern tropical Pacific analysis indicates a population decline of about 4%/yr over a large area in the years following the end of whaling (1981 in Peru44), although there is uncertainty. The mark-recapture and sighting estimates from the Galápagos Islands, in the heart of the eastern Tropical Pacific, also show declines during that period, although some of the changes were likely caused by redistribution of the whales19. However, these declines, together with low calving rates and few large males off the Galápagos in the years following the end of whaling19, provide evidence for the demographic effects of social disruption caused by whaling. They also indicate the scale—around 5% per year—and duration—perhaps 15 years—of such effects, which are incorporated in the parameterizing of the population model (Table 4).
Table 4.
Parameters of population model.
| Parameter | Explanation | Best value | Range for sensitivity analysis | Assumed distribution | Source/Comments |
|---|---|---|---|---|---|
| n(1993) | Population in year 1993 | 736,053 | 422,995–1,049,111 | Normal (CV = 0.218) | Extrapolation from surveys |
| r | Maximum potential rate of increase | 0.0096/yr | 0.0046–0.0146 | Uniform [0.0046–0.0146] | 15 |
| θ | Shape of density-dependent function | 2.39 | 1.0–18.17 | Taken from {1.00, 1.55, 2.39, 4.55, 18.17} | 45Adding intermediate (on a log scale) values of 1.55 and 4.55 |
| f(1712–1899) | Correction for earlier catch | 1.1 | 1.05–1.15 | Uniform [1.05–1.15] | 17 |
| f(1900–1986) | Correction for later catch | 1.05 | 1.01–1.20 | Uniform [1.01–1.20] | Based on6 |
| K | Number of subpopulations | 8 | 1–15 | Taken from 1:15 | K = 10 and α = 0.7 gave an expansion of whaling efforts to new populations in the early 1800’s in line with the historical record |
| α | Proportion of original size when exploitation moves to next population | 0.7 | 0.5–0.8 | Uniform [0.5–0.8] | |
| q | Reduction in rate of increase due to social disruption of whaling | 0.05/yr | 0.0–0.1 | Normal (SE = 0.02) | Based upon decrease in Eastern Tropical Pacific post whaling |
| T | Number of years over which social disruption occurs | 15 yr | 2–25 | Taken from {2, 4, 8, 16, 32} | Based upon decrease in Eastern Tropical Pacific post whaling. Effects documented in elephants46 |
In contrast, the time series for the California Current and Hawaiian Islands indicate a slow increase in more recent years. These estimated rates of increase are within 1SD of the estimated maximum potential rate of increase for a sperm whale population of about 1% per year15. These study areas are more removed from intense whaling than the whales in the eastern tropical Pacific, both by later starts of the survey time series (1991 and 2002), and by an earlier end to intense whaling in the central and eastern North Pacific (early 1970’s). Thus, the indication is that about two decades after the end of whaling the demographic consequences of social disruption have largely dissipated.
The clearest sign of a post-whaling increase is from the Antarctic, where there are only mature males as females and immature males are restricted to latitudes less than about 50o1. This increase would be expected as mature males were the most heavily affected by modern whaling, and recruitment into their depleted polar habitat from less affected low-latitude breeding populations should lead to a rebound following the end of whaling19. Unfortunately, we do not have good trend data for any other largely-male habitat.
In the smaller study areas, the Gulf of Mexico and eastern Caribbean, ship surveys and mark-recapture studies indicate declines in abundance since 2000 (Table 3). This suggests that in nearshore areas with substantial anthropogenic impact, sperm whale populations are, in current times, in some jeopardy.
However, as a general caution, we stress that the estimated trends indicated in Table 3 are mostly either very small or very uncertain, or both.
Historical trajectory
The historical trajectory of the global sperm whale population using the 1993 estimate extrapolated from surveys, the annual reported catches, and the best estimates of population parameters is shown in Fig. 4, along with twenty of the trajectories with randomly-chosen parameter values. (Eight of the 1000 runs with randomly-chosen parameter values gave invalid 1711 population estimates.) Declines during the two major phases of sperm whaling, peaking in the 1840’s and 1960’s, are clear, and a recovery beginning in the 1990’s is indicated. The variety of population trends in the 21st Century in the random-parameter trajectories shows our uncertainty about this recovery. Depending on the length and nature of the effects of social disruption caused by whaling, the current trend can be positive—if social cohesion has recovered or was not greatly affected by whaling—or only just beginning to level out—if the social disruption of whaling had a major and long-lasting effect on sperm whale demography.
Figure 4.
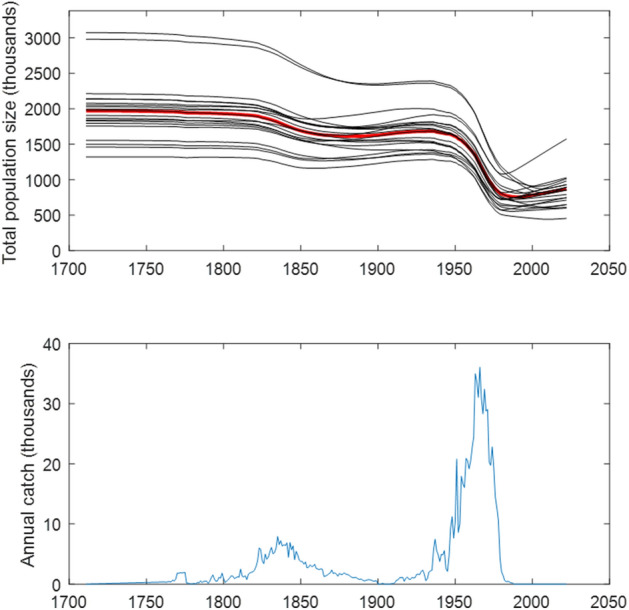
Upper: Estimated trajectories of global sperm whale population using best estimates for model parameters in red, and 20 trajectories using randomly chosen but reasonable parameter estimates in black. Lower: Reported annual global sperm whale catch.
Using the best parameter estimates, with the random parameter runs providing confidence intervals, the population in 1711 is estimated as 1,949,698 (SE = 335,054; CV = 0.178; 95% CI 1,296,811–2,575,057; see Supplementary Fig. S2). In 2022, the estimated population is 844,761 (SE = 171,206; CV = 0.209; 95% CI 481,901–1,153,459). The three-generation decline (1940–2022) is estimated as 0.4819 (SE = 0.1035; CV = 0.215; 95% CI 0.2631–0.6696; see Supplementary Fig. S2). The estimated overall decline from 1711–2022 is 0.5667 (SE = 0.1172; CV = 0.212; 95% CI 0.2843–0.7555).
The sensitivity analysis (Supplementary Fig. S3) indicates that these estimates and the trajectory are most affected by the estimate of 1993 population size, n(1993), as by well as the significance and duration of social disruption caused by whaling (q and T), and least by the catch corrections (f).
Discussion
We estimate a global population of 736,053 sperm whales at a density of 2.40 whales/km2 (CV = 0.218) in 1993. The CV, assessing confidence in this estimate, includes uncertainty due to sampling issues in each population survey, uncertainty about the availability bias, g(0), and extrapolation from sampled areas to the global habitat for sperm whales. The elements of the CV estimate are themselves uncertain, but not obviously biased.
However, there are some potential sources of error and bias not included in the population and CV estimates. Perhaps the most serious is that the survey areas are largely in the northern hemisphere (with the principal exception of the Antarctic, unique sperm whale habitat). In the extrapolation process we assume that variation in sperm whale density among study areas in the northern hemisphere is representative of differences between the hemispheres in sperm whale habitat suitability, given the oceanographic variables that we included. As different ocean basins have fundamental contrasts in their oceanography, this may be an optimistic assumption. But, for now, it is untested.
Two other issues are probably less serious. First, we excluded waters less than 1000 m deep, even though sperm whales, especially male sperm whales, are known sometimes to use such waters47. However, densities are typically lower than in deeper waters37, and the global area of such habitat (roughly 400–1000 m deep) is low compared with deeper waters. Secondly, the different surveys are treated as independent samples to obtain the habitat suitability prediction function used to extrapolate globally, but there is some overlap. However, only 3.6% of the grid points used were included in two surveys (none in three or more), so, given the overall uncertainty in the global population estimate, the effect of double counting is likely insignificant.
Compared with the earlier population estimate for year 20008, our estimate for 1993 is 96% higher (706,932 vs. 361,400). A number of the surveys used to estimate populations, especially the larger and more heavily-weighted ones, are in both studies. The great difference is that the default availability bias for visual ship surveys was assumed to be g(0) = 0.87 in8, while several more recent estimates consistently suggest g(0) = 0.60. If applied to Whitehead’s8 global estimate, g(0) = 0.60 would have given an estimated population of 524,030, 1.3 SDs lower and so not significantly different (at P < 0.05) from our current 1993 estimate. There are other potential causes of the higher estimate in this paper. Even though the overall survey coverage is not changed from the surveys used by8 (24% of global sperm whale habitat), the more recent estimates indicate higher densities, perhaps because of better survey techniques. Additionally, the extrapolation to global habitat suitability using environmental variables suggests that the surveyed areas were generally slightly less suitable habitat than the oceans as a whole.
There are several ways to improve the estimate of recent sperm whale abundance. Improving the survey coverage, especially in the southern hemisphere, is most obvious. While visual surveys have been the primary source of abundance estimates (Table 1), they are logistically and financially demanding, especially in waters far from land. Acoustic surveys are becoming more common, and procedures for analysis have been developed in recent years so that they seem to produce more precise estimates than visual surveys with comparable effort34. Methods for surveying acoustically from autonomous vessels and drifting buoys are under development48,49 and these should considerably reduce costs and the feasibility of large-scale acoustic surveys in a wide range of habitats and conditions. Satellite imagery is another potential solution to the problem of surveying far offshore50. However, unless there is a full global survey, there needs to be extrapolation to unsurveyed areas. Here we used habitat suitability mapping to make this extrapolation, but it was carried out in a rather crude way, considering population survey areas (of very varied sizes) as independent units. There has been more sophisticated fine-scale habitat mapping of sperm habitat in some, relatively small and usually near-shore, ocean areas13,14,51, but the results are not consistent. Larger-scale habitat studies, including offshore waters in different oceans, and including environmental variables which might be more relevant for sperm whales (e.g. measures of the abundance of cephalopods, the primary sperm whale prey), could make for a more satisfactory extrapolation to unsurveyed areas.
Our review of studies of trends in sperm whale populations over the roughly 40 years since the end of whaling did not identify a single clear trend. Instead, several processes seemed to be affecting the population dynamics of the sperm whales now largely free from the whaling of the preceding 270 years: that whaling causes social disruption and other lingering effects which can reverse the expected population increase following exploitation; that such declines may be of the order of − 5%/yr and last for a decade or two; that, once these effects have run their course, sperm whale populations largely free from other adverse impacts may increase at about the maximum theoretical rate of increase, + 1%/yr15; that populations of adult male sperm whales, which were particularly targeted by modern whalers, have, since the end of whaling, been increasing more quickly than the general population; and that in areas of substantial anthropogenic impact sperm whale populations may be declining.
The support for these processes is not very strong. They are mostly based on one or two estimates of trend, most of which are not statistically significantly different from zero at P < 0.05. With their very low reproductive rates, in the absence of substantial immigration positive trends in sperm whale populations are constrained to be very low, with the exception of mature male populations recovering from near eradication through recruitment. There are indications of such increases for males in the Antarctic and California Current20,26, but no sign of recovery in the population of large male sperm whales off southwestern Australia between surveys in 1968–1978 and 200921. Declines can be larger in magnitude than increases, and the largely independent estimates of decline in the eastern Caribbean are concerning42,43, as is that in the Gulf of Mexico31. The nearly isolated population of sperm whales in the Mediterranean Sea is also believed to be at risk52, although no good trend data are currently available.
We are now nearly 40 years since the end of whaling and the opportunity for learning more about how sperm whale populations responded to whaling’s immediate aftermath has largely gone. Moving forward, we can obtain a clearer view of current trends using the methods currently available—ship surveys, mark recapture analyses, acoustic recordings—preferably with greater intensity, wider geographic and temporal scope and standardized methodology, as well as new methods, such as acoustic surveys from drifters and autonomous vehicles as well as satellite images.
Our population model gives estimates of the current, 2022, population (844,761), the initial, 1711, population (1,949,698), the overall, 1711–2022, decline (57%), and the 3-generation, 1940–2022, decline (48%). All these estimates have a similar proportional error with a CV ~ 0.2. This level of error is estimated from reasonable (to us) estimates of uncertainty in the input parameters.
However, not included is uncertainty about the form of model. While the theta-logistic is often used as a default population model53, there are alternatives. However, as variation in the parameters r and θ include most realistic forms of density dependence, and sperm whales do not seem to have reached the very low abundance levels where inverse density dependence (the Allee effect) can come into play and complicate the recruitment-abundance relationship, the theta-logistic seems a reasonable model, and its innate inaccuracies are likely dwarfed by those in the input parameters. However, variation in carrying capacity (n(1711) in our formulation) is a potentially important issue54. There seems to have been considerable variation in sperm whale population size over evolutionary time55, which might indicate that there is also variation in global carrying capacity over the ecological time scales considered here.
We made two modifications to the theta-logistic to mimic seemingly important elements of sperm whale population structure: spatial structure and the demographic consequences of the social disruption caused by whaling. There is very strong evidence for spatial structure18 and good evidence for social disruption having population-level effects. However, the realism of our modelling of these effects is less clear. Spatially, it would be better to use the actual geographical structures of sperm whales and whaling (e.g. ocean basins), for which we would need a breakdown of catches by area for each year, which is not currently available for the entire whaling time series. Sperm whale populations seem more structured by culture than geography and ideally should be managed at the level of the cultural clan56. However, we have no way to break down catches by clan, so this seems impossible. A linear decline in rate of increase with proportional catch over the previous T years may be a good way to model the effects of social disruption, but we don’t know this. However, we suspect, but do not know, that the simplicities of our model in these respects are less important than uncertainty in the model parameters which we do include.
Based upon the sensitivity analysis (Supplementary Fig. S3) we surmise that uncertainty about the historical trajectory of the global sperm whale population is: most strongly caused by uncertainty in the estimates of the 1993 population (n(1993)) and the effects of social disruption (q); somewhat due to uncertainty in the maximum rate of increase (r), the shape of the density dependent function (θ) and the duration of the effects of social disruption (T). Uncertainty in the catch corrections and parameters determining the spatio-temporal progression of whaling (f, K, α) seem to have lesser importance. This suggests that in order to improve the precision of the historical trajectory, effort on reducing uncertainty about post-whaling population size and the significance of social disruption will have greatest payoffs.
Conclusion
There are about 850,000 sperm whales now, down from about two million before the start of whaling in 1712, so a decline of approximately 57% in 310 years caused by substantial open-boat whaling which peaked in the 1840’s and massive modern whaling primarily between 1950 and 1975. Time series data collected since whaling ended in the 1980’s suggest several factors. Intense whaling seems to have caused social disruption and related effects, reducing recovery rates for a decade or two afterwards. However, populations of adult males which were particularly targeted in the most recent phases of whaling show better evidence of recovery, presumably due to recruitment from less exploited breeding populations. With whaling 30–40 years in the past, some sperm whale populations in relatively undisturbed areas show modest signs of recovery, but others, living with more intense anthropogenic pressures, appear to be declining. There is considerable uncertainty about these conclusions. We need better data on the size of sperm whale populations today, as well as their trends.
Methods
Selection of surveys and extraction of data
We selected published surveys that produced estimates of sperm whale population size or density (see Supplementary Information for methodology; surveys listed in Table 1). We extracted: the type of survey (ship, aerial; acoustic, visual), the years of data collection; the coordinates of the boundary of the study area; the estimates of g(0) and CV (g(0)) used to correct for availability bias, if given; and an estimate of sperm whale population or density in study area with CV. From these we calculated for each survey the survey area with waters greater than 1000 m deep (typical shallow depth limit of sperm whales3). When no value of g(0) was used (8 ship visual surveys) we corrected the population/density estimate using an assumed generic value of g(0) and recalculated the CV to include uncertainty in g(0) (as in Eq. 1 of8). Three ship visual surveys did calculate a single g(0) estimate: 0.62 (CV 0.35)32; 0.57 (CV 0.28)35; 0.61 (CV 0.25)37. These are consistent and suggest a generic g(0) = 0.60 (CV 0.29), also agreeing with g(0) = 0.60 estimated from pooled surveys in the California Current10.
Global habitat of sperm whales
To extrapolate sperm whale densities from surveyed study areas to the sperm whales’ global habitat, we created a one-degree latitude by one-degree longitude grid. We removed the following grid points as not being prime sperm whale habitat1,3,40: points on land or with central depths less than 1000 m; largely ice-covered points in the Beaufort Sea, and the waters north of Svalbard and Russia; the Black Sea and Red Sea both of which have shallow entrances that appear not to be traversable by sperm whales.
Generally, food abundance is a good predictor of species distribution. However, this is not possible for sperm whales as we have no good measures of the abundance or distribution of most of their prey, deep-water squid57. Instead, oceanographic measures have been used to describe sperm whale distributions over various spatial scales with a moderate level of success13,14. We follow this approach. Measures that might predict sperm whale density were collected for each grid point, some at just the surface, others at the surface, 500 m depth, 1000 m depth or an average of the measures at the different depths (Supplementary Table S2). Water depth was the strongest predictor in Mediterranean encounters, when compared to slope and distance to shore13. Temperature and salinity have been used as predictors for the distribution of fish and larger marine animals, which could translate into prey availability and thus density for sperm whales58,59. Primary productivity and dissolved oxygen generally dictate the biomass of wildlife in an area, while nitrate and phosphate levels limit the amount of primary productivity in an area60. Eddy kinetic energy is a measure of the dynamism of physical oceanography which is becoming a commonly used predictor of cetacean habitat61. We did not use: latitude and longitude as these primarily describe the general geographic distribution of the study areas, and geographic aggregates of sperm whale catches62 as these proved to have no predictive power. The mean values of the 14 predictor measures were calculated over calendar months for each grid point, and then over the grid points in each study area.
To obtain predictors of the sperm whale density at each grid point, we then made quadratic regressions of the density of sperm whales in each study area (i), d(i), on the mean values of the predictor measures, weighting each study area by its surface area. Because the surveys were conducted over different time periods, the densities were corrected based on the estimated trajectory of global sperm whale populations by multiplying d(i) by the ratio of the global population in 1993 over that in the mid-year of the survey (as in Fig. 4). Predictor variables were selected using forward stepwise selection based upon reduction in AIC.
Sperm whale population size
The population of sperm whales globally, N, was then calculated as follows:
| 1 |
where a{k} are the parameters of the regression; the summation is over k, the grid points; d(k) is the estimated sperm whale density at grid point k from the habitat suitability model; and a(k) is the area of the 1° cell centred on grid point k. Population estimates for other ocean areas (North Atlantic, North Pacific, Southern Hemisphere) were calculated similarly.
The CVs of these population estimates were calculated following the methodology in8, (although there is an error in Eq. (3) of8 such that the squareroot symbol covers both the numerator and denominator rather than just the numerator). The error due to uncertain density estimates for the different surveys is:
| 2 |
This is combined with the uncertainty in the extrapolation process (output from the linear models), CV(extrap.), to give an overall CV for the population estimate:
| 3 |
Post-whaling trend in population size
We compiled a database of series of surveys producing population estimates of the same study area during the period 1978 (by which time most commercial sperm whaling had ceased) and 2022. Each series had to span at least 10 years, and all of the surveys in the series had to be comparable in terms of area covered throughout the time span. There also had to have been at least 3 surveys for a data set to be included.
The data consisted of the survey area, A, the estimated population in area A in year y (for multi-year surveys, y would be the midpoint of the data collection years), nE(A,y), and the provided CV of that estimate, CV(nE(A,y)). The data series used for these analyses are summarized in Table 3.
For each survey area, A, we calculated the trend in logarithmic population size, r(A), over time using weighted linear regression:
| 4 |
Table 3 also includes other published estimates of sperm whale population trends, from sighting rates or mark-recapture analyses of photoidentification data, with these estimates also having to span at least 10 years of data collection, and include data collected in three or more different years.
Population trajectory
To examine possible trajectories of the global sperm whale population following the start of commercial whaling in 1712, we used a variant of the theta-logistic, a population model that has been employed in other recent analyses of the population trajectories of large cetaceans45,63. The theta-logistic model is:
| 5 |
Here, n(y) is the population of sperm whales in year y, r is the maximum potential rate of increase of a sperm whale population, and θ describes how the rate of increase varies with population size relative to its basal level before whaling in 1711, n(1711). The recorded catch in year y is c(y) and f(y) is a correction for bias in recorded catches.
Whaling reduced the proportion of large breeding males64, likely disrupted the social cohesion of the females3, and may have had other lingering effects which reduced pregnancy or survival, and thus the rate of increase. Poaching has been found to reduce the reproductive output of African elephants, Loxodonta Africana, which have a similar social system to the sperm whales3, and this effect lingers well beyond the effective cessation of poaching46. There is some evidence for these effects of what we call “social disruption” on sperm whale population dynamics20,46,65. We added a term to the theta-logistic to account for such effects:
| 6 |
Here, is the proportion of the population killed over the last T years, and q is the reduction in the rate of increase when almost all the whales have been killed. This reduction is modelled to fall linearly as the proportion killed declines to zero.
The global sperm whale population has some geographic structure18. Females appear to rarely move between ocean basins, and males seem to largely stay within one basin. Furthermore, sperm whaling was progressive, moving from ocean area to ocean area as numbers were depleted4. We model this by assuming K largely separate sperm whale subpopulations of equal size. Exploitation in 1712 starts in subpopulation 1 and moves to subpopulations 1 and 2 when the population 1 falls to α% of its initial value, and so on for the other ocean areas. The catch in each year in each area being exploited is pro-rated by the sizes of the different subpopulations being exploited. The population model for subpopulation k, which is one of the KE subpopulations being exploited in year y, is:
| 7 |
where the estimated catch in year y in subpopulation k is given by:
Global catch data for sperm whales have been compiled by year for the periods 1762–179916, 1800–189917 and 1900–19996 (Fig. 4). This catch record is not complete. There are no compiled data on sperm whaling between its inception in 17124 and 1761. We filled this in by extrapolating a linear trend between the catch in 1711 (0 whales) and that in 1762 (351 whales). Catch records for the 18th and 19th Century are based on extrapolations from oil production records, and are not corrected for whales struck and lost that died, which will produce a downward bias in estimates of catches of the order of a few percent16,17. The 20th Century catch records are also likely downward biased by an unknown, but likely quite small, amount, primarily due to falsification of records6.
For any choice of population and exploitation parameters (n(1711), r, θ, {f}, K, α, q, T) a population trajectory can be calculated, giving a population size in 1993, n(1993). We have an estimate of the population in 1993 from the first part of this paper, but not in 1711. Thus, given values of the other parameters, the catch history {c}, and a value of n(1993), we found the value of n(1711) (using the “fzero” function of MATLAB2021b) so that the trajectory reached n(1993) in year 1993.
The best estimates, assumed distributions, and range of acceptable values for each of the parameters of the population model are given and justified in Table 4.
Using these parameter estimates, we ran the model in three ways: with the best estimate of each parameter; in a sensitivity analysis, using a range of values for each parameter in turn (the range given in Table 4) with the best estimates of the other parameters; and using random but reasonable values for all parameters (see Table 4 for assumed distribution of each) in each of 1,000 runs. Runs producing invalid (infinite or unreal) population estimates in 1711 were discarded.
The primary outputs of the population model were the estimated trajectory from 1711 to 2022, the current population (n(2022)), the estimated population in 1711 before the commencement of commercial whaling, and n(2022)/n(1940). Assuming three generations equals about 82 years66, n(2022)/n(1940) gives the proportional change in population size over 3 generations (the IUCN Red List measure for population trends). The proportional decline from pre-whaling numbers (i.e. 1711) to 2022 was also calculated.
Supplementary Information
Acknowledgements
Thanks to Jay Barlow and Ana Eguiguren for providing data, and suggestions for analysis.
Author contributions
H.W. conceived the study. H.W. and M.S. collated the data, designed the analysis, and carried it out. H.W. and M.S. wrote and reviewed the manuscript.
Data availability
All data analysed during this study are included or referenced in this published article and its Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24107-7.
References
- 1.Rice DW. Sperm whale Physeter macrocephalus Linnaeus, 1758. In: Ridgway SH, Harrison R, editors. Handbook of Marine Mammals. Academic Press; 1989. pp. 177–233. [Google Scholar]
- 2.Lavery TJ, et al. Iron defecation by sperm whales stimulates carbon export in the Southern Ocean. Proc. R. Soc. B. 2010;277:3527–3531. doi: 10.1098/rspb.2010.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead H. Sperm Whales: Social Evolution in the Ocean. Chicago University Press; 2003. [Google Scholar]
- 4.Starbuck A. History of the American Whale Fishery from its Earliest Inception to the Year 1876. United States Commission on Fish and Fisheries, Report of the Commissioner for 1875-1876. United States Government Printing Office; 1878. [Google Scholar]
- 5.Tønnessen JN, Johnsen AO. The History of Modern Whaling. University of California Press; 1982. [Google Scholar]
- 6.Rocha RC, Clapham PJ, Ivashchenko YV. Emptying the oceans: A summary of industrial whaling catches in the 20th century. Mar. Fish. Rev. 2014;76:37–48. [Google Scholar]
- 7.International Whaling Commission. Total Catches Since the Moratorium came into place in 1985. https://iwc.int/total-catches Accessed 4 Nov 2022 (2022).
- 8.Whitehead H. Estimates of the current global population size and historical trajectory for sperm whales. Mar. Ecol. Prog. Ser. 2002;242:295–304. [Google Scholar]
- 9.Kaschner K, Quick NJ, Jewell R, Williams R, Harris CM. Global coverage of cetacean line-transect surveys: Status quo, data gaps and future challenges. PLoS ONE. 2012;7:e44075. doi: 10.1371/journal.pone.0044075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow, J. Cetacean abundance in the California Current estimated from ship-based line-transect surveys in 1991–2014. Report LJ-16–01 (2016).
- 11.Gordon J, et al. A first acoustic density estimate for sperm whales in Irish offshore waters. J. Cetacean Res. Manag. 2020;21:123–133. [Google Scholar]
- 12.Lewis T, et al. Abundance estimates for sperm whales in the Mediterranean Sea from acoustic line-transect surveys. J. Cetacean Res. Manag. 2018;18:103–117. [Google Scholar]
- 13.Pace DS, et al. Habitat suitability modeling in different sperm whale social groups. J. Wildlife Manag. 2018;82:1062–1073. [Google Scholar]
- 14.Eguiguren A, et al. Historical and contemporary habitat use of sperm whales around the Galapagos Archipelago: Implications for conservation. Aquat. Conserv. 2021;31:1466–1481. [Google Scholar]
- 15.Chiquet RA, Ma B, Ackleh AS, Pal N, Sidorovskaia N. Demographic analysis of sperm whales using matrix population models. Ecol. Model. 2013;248:71–79. [Google Scholar]
- 16.Best, P. B. Estimating the landed catch of sperm whales in the eighteenth century. Meeting Document SC/56/IA5- suppl, Scientific Committee, International Whaling Commission (2005).
- 17.Best, P. B. Estimating the landed catch of sperm whales in the nineteenth century. Meeting Document SC/56/IA5, Scientific Committee, International Whaling Commission (2005).
- 18.Alexander A, et al. What influences the worldwide genetic structure of sperm whales (Physeter macrocephalus)? Mol. Ecol. 2016;25:2754–2772. doi: 10.1111/mec.13638. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead H, Christal J, Dufault S. Past and distant whaling and the rapid decline of sperm whales off the Galápagos Islands. Conserv. Biol. 1997;11:1387–1396. [Google Scholar]
- 20.Moore, J. E. & Barlow, J. Population abundance and trend estimates for beaked whales and sperm whales in the California Current from ship-based visual line-transect survey data, 1991–2014. NOAA-TM-NMFS-SWFSC-585 (2017).
- 21.Carroll G, Hedley S, Bannister J, Ensor P, Harcourt R. No evidence for recovery in the population of sperm whale bulls off Western Australia, 30 years post-whaling. Endanger. Species Res. 2014;24:33–43. [Google Scholar]
- 22.Weilgart LS. The impacts of anthropogenic noise on cetaceans and implications for management. Can. J. Zool. 2007;85:1091–1116. [Google Scholar]
- 23.Haase B, Félix F. A note on the incidental mortality of sperm whales (Physeter macrocephalus) in Ecuador. Rep. Int. Whal. Comm. 1994;15:481–483. [Google Scholar]
- 24.Laist DW, Knowlton AR, Mead JG, Collet AS, Podesta M. Collisions between ships and whales. Mar. Mamm. Sci. 2001;17:35–75. [Google Scholar]
- 25.Taylor, B. L., Chivers, S. J., Larese, J. & Perrin, W. F. Generation length and percent mature estimates for IUCN assessments of cetaceans. NOAA, NMFS, Southwest Fisheries Science Center Administrative Report LJ-07--01 Vol. 21 (2007).
- 26.Branch TA, Butterworth DS. Estimates of abundance south of 60oS for cetacean species sighted frequently on the 1978/79 to 1997/98 IWC/IDCR SOWER sighting surveys. J. Cetacean Res. Manag. 2001;3:251–270. [Google Scholar]
- 27.Fais A, et al. Abundance and distribution of sperm whales in the Canary Islands: Can sperm whales in the archipelago sustain the current level of ship-strike mortalities? PLoS ONE. 2016;11:e0150660. doi: 10.1371/journal.pone.0150660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogan E, et al. Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Res. 2017;II(141):8–19. [Google Scholar]
- 29.Wade PR, Gerrodette T. Estimates of cetacean abundance and distribution in the eastern tropical Pacific. Rep. Int. Whal. Comm. 1993;43:477–493. [Google Scholar]
- 30.Rone BK, Zerbini AN, Douglas AB, Weller DW, Clapham PJ. Abundance and distribution of cetaceans in the Gulf of Alaska. Mar. Biol. 2017;164:1–23. [Google Scholar]
- 31.Garrison, L., Ortega-Ortiz, J. & Rappucci, G. Abundance of marine mammals in waters of the U.S. Gulf of Mexico during the summers of 2017 and 2018. PRD Contribution: #PRD-2020–07 (2020).
- 32.Bradford, A. L., Oleson, E. M., Forney, K. A., Moore, J. E. & Barlow, J. Line-transect abundance estimates of cetaceans in US Waters around the Hawaiian Islands in 2002, 2010 and 2017. NOAA Technical Memorandum NMFS-PIFSC-115 (2021).
- 33.Leonard, D. M. & Øien, N. I. Estimated abundances of cetacean species in the Northeast Atlantic from two multiyear surveys conducted by Norwegian vessels between 2002–2013. NAMMCO Scientific Publications11 (2020).
- 34.Barlow J, Taylor BL. Estimates of sperm whale abundance in the northeastern temperate Pacific from a combined acoustic and visual survey. Mar. Mamm. Sci. 2005;21:429–445. [Google Scholar]
- 35.Pike, D. G. et al. Estimates of the abundance of cetaceans in the Central North Atlantic from the T-NASS Icelandic and Faroese ship surveys conducted in 2007. NAMMCO Scientific Publications11 (2019).
- 36.Fulling GL, Thorson PH, Rivers J. Distribution and abundance estimates for cetaceans in the waters off Guam and the Commonwealth of the Northern Mariana Islands. Pac. Sci. 2011;65:321–343. [Google Scholar]
- 37.Palka, D. Cetacean abundance in the US Northwestern Atlantic Ocean: summer 2016. Northeast Fisheries Science Center Reference Document 20–05 (2020).
- 38.Hayes, H. S. A., Elizabeth, J., Katherine, M.-F. & E, R. P. US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments—2019. NOAA Technical Memorandum NMFS-NE-264 (2020).
- 39.Kato, H. & Miyashita, T. Current status of the North Pacific sperm whales and its preliminary abundance estimates. Paper presented to Scientific Committee of International Whaling Commission SC/50/CAWS2 (2000).
- 40.Kaschner, K. Modelling and mapping resource overlap between marine mammals and fisheries on a global scale. PhD thesis, University of British Columbia (2004).
- 41.Gerrodette, T. & Forcada, J. Estimates of abundance of western/southern spotted, whitebelly spinner, striped and common dolphins and pilot, sperm and Bryde’s whales in the eastern tropical Pacific Ocean. SWFSC Administrative Report LJ-02–20 (2002).
- 42.Rinaldi C, Rinaldi R, Laine J, Barbraud C. Population dynamics of sperm whales (Physeter macrocephalus) in Guadeloupe, French Caribbean: A mark-recapture study from 2001 to 2013. Mar. Mamm. Sci. 2021;37:1391–1405. [Google Scholar]
- 43.Gero S, Whitehead H. Critical decline of the eastern Caribbean sperm whale population. PLoS One. 2016;11(11):e0162019. doi: 10.1371/journal.pone.0162019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramirez P. Captura de cachalote en Paita: 1976–1981. Boletin de Lima. 1989;63:81–88. [Google Scholar]
- 45.Monnahan CC, Branch TA, Punt AE. Do ship strikes threaten the recovery of endangered eastern North Pacific blue whales? Mar. Mamm. Sci. 2015;31:279–297. [Google Scholar]
- 46.Gobush KS, Mutayoba BM, Wasser SK. Long-term impacts of poaching on relatedness, stress physiology and reproductive output of adult female African elephants. Conserv. Biol. 2008;22:1590–1599. doi: 10.1111/j.1523-1739.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- 47.Scott TM, Sadove SS. Sperm whale, Physeter macrocephalus, sightings in the shallow shelf waters off Long Island New York. Mar. Mamm. Sci. 1997;13:317–321. [Google Scholar]
- 48.Klinck H, et al. Near-real-time acoustic monitoring of beaked whales and other cetaceans using a Seaglider™. PLoS ONE. 2012;7:e36128. doi: 10.1371/journal.pone.0036128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barlow J, Moore JE, McCullough JLK, Griffiths ET. Acoustic-based estimates of Cuvier’s beaked whale (Ziphius cavirostris) density and abundance along the US West Coast from drifting hydrophone recorders. Mar. Mamm. Sci. 2022;38:517–538. [Google Scholar]
- 50.Borowicz A, et al. Aerial-trained deep learning networks for surveying cetaceans from satellite imagery. PLoS ONE. 2019;14:e0212532. doi: 10.1371/journal.pone.0212532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirotta E, Matthiopoulos J, MacKenzie M, Scott-Hayward L, Rendell L. Modelling sperm whale habitat preference: A novel approach combining transect and follow data. Mar. Ecol. Prog. Ser. 2011;436:257–272. [Google Scholar]
- 52.Notarbartolo-Di-Sciara G. Sperm whales, Physeter macrocephalus, in the Mediterranean Sea: A summary of status, threats and conservation recommendations. Aquat. Conserv. 2014;24:4–10. [Google Scholar]
- 53.Sibly RM, Barker D, Denham MC, Hone J, Pagel M. On the regulation of populations of mammals, birds, fish and insects. Science. 2005;309:607–610. doi: 10.1126/science.1110760. [DOI] [PubMed] [Google Scholar]
- 54.Clark F, Brook BW, Delean S, Reşit Akçakaya H, Bradshaw CJA. The theta-logistic is unreliable for modelling most census data. Methods. Ecol. Evol. 2010;1:253–262. [Google Scholar]
- 55.Warren WC, et al. The novel evolution of the sperm whale genome. Genome Biol. Evol. 2017;9:3260–3264. doi: 10.1093/gbe/evx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brakes P, et al. Animal cultures matter for conservation. Science. 2019;363:1032–1034. doi: 10.1126/science.aaw3557. [DOI] [PubMed] [Google Scholar]
- 57.Jaquet N. How spatial and temporal scales influence understanding of sperm whale distribution: A review. Mamm. Rev. 1996;26:51–65. [Google Scholar]
- 58.Castillo J, Barbieri MA, Gonzalez A. Relationships between sea surface temperature, salinity and pelagic fish distribution off northern Chile. ICES J. Mar. Sci. 1996;53:139–146. [Google Scholar]
- 59.Thorson JT, Ianelli JN, Kotwicki S. The relative influence of temperature and size-structure on fish distribution shifts: A case-study on walleye pollock in the Bering Sea. Fish Fish. 2017;18:1073–1084. [Google Scholar]
- 60.Saito MA, et al. Multiple nutrient stresses at intersecting Pacific Ocean biomes detected by protein biomarkers. Science. 2014;345:1173–1177. doi: 10.1126/science.1256450. [DOI] [PubMed] [Google Scholar]
- 61.Mannocci L, et al. Assessing cetacean surveys throughout the Mediterranean Sea: A gap analysis in environmental space. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-19842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith TD, Reeves RR, Josephson EA, Lund JN. Spatial and seasonal distribution of American whaling and whales in the age of sail. PLoS ONE. 2012;7:e34905. doi: 10.1371/journal.pone.0034905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker CS, Clapham PJ. Modelling the past and future of whales and whaling. Trends Ecol. Evol. 2004;19:365–371. doi: 10.1016/j.tree.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Botkin DB, Schimel DS, Wu LS, Little WS. Some comments on density dependent factors in sperm whale populations. Rep. Int. Whal. Comm. 1980;2:83–88. [Google Scholar]
- 65.Clarke R, Aguayo A, Paliza O. Pregnancy rates of sperm whales in the southeast Pacific between 1959 and 1962 and a comparison with those from Paita, Peru between 1975 and 1977. Rep. Int. Whal. Comm. 1980;2:151–158. [Google Scholar]
- 66.Taylor, B. L. et al.Physeter macrocephalus (amended version of 2008 assessment). The IUCN Red List of Threatened Species 2019: e. T41755A160983555 (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are included or referenced in this published article and its Supplementary Information.