Abstract
Susceptibility of mice to Leishmania major is associated with an insufficient NK cell-mediated innate immune response. We analyzed the expression of NK cell-activating chemokines in vivo during the first days of infection in resistant and susceptible mice. The mRNA expression of gamma interferon-inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), and lymphotactin was upregulated 1 day after infection in the draining lymph nodes of resistant C57BL/6 mice but not in those of susceptible BALB/c mice. In vivo local treatment of BALB/c mice with recombinant IP-10 shortly after infection resulted in an enhanced NK cell activity in the draining lymph node. The data suggest that although the recruitment of NK cells is normal in susceptible mice, the lack of NK cell-activating chemokines is a factor resulting in a suboptimal NK cell-mediated defense.
Cutaneous infection of mice with Leishmania major is a well-established experimental model of chronic disease caused by an intracellular parasite (for review, see reference 25). In this infection model, most strains of mice, including C57BL/6, develop a Th1-dominated immune response which is associated with healing (2, 7). Conversely, some strains like BALB/c succumb to the infection. In these susceptible animals, the immune response is dominated by Th2 cells. Cumulative evidence suggests that the basis for the respective Th-cell adaptation is laid very early, i.e., during the first 24 to 48 h in the draining lymph node (LN) (5, 11, 13, 27, 30). We found that, in susceptible mice, parasites disseminate very rapidly to visceral organs, while containment of parasites in the draining LN is characteristic of resistant mouse strains (11). This early parasite containment was found to depend on natural killer (NK) cells. Accordingly, measures which activate NK cells, such as in vivo treatment with interleukin 12 (IL-12), poly(I-C), or alpha or beta interferon (IFN-α/β), were all found to induce parasite containment in susceptible mice (5, 11). These findings, together with those of other studies (27), present strong evidence for the instrumental role of LN NK cells in the development of protective immunity against the parasite.
Peripheral LN cells provide the environment for the generation of a specific immune response after antigen exposure in the periphery (20). The attraction of leukocytes into LN is essential for the host response to infection. The process of leukocyte recruitment is controlled by chemokines, which are chemotactic cytokines belonging to a superfamily of polypeptide mediators (16, 34). Recent evidence suggests that the pleiotropic and redundant effects of chemokines can be grouped according to their biological effects. Thus monocyte chemoattractant protein 1 (MCP-1), MCP-2, MCP-3, RANTES, macrophage inflammatory protien 1α (MIP-1α), and MIP-1β all have been reported to be chemotactic and activating for NK cells (1, 15, 17, 33). Gamma interferon-inducible protein 10 (IP-10) and lymphotactin (Ltn) act on both T cells and activated NK cells (18). Although most of the data presented above are derived from experiments with human chemokines, the data so far available concerning murine chemokines showed that the activating effect of chemokines on NK cells and the production of chemokines by NK cells are similar in the murine system (8). In the present study, we asked whether differences in the production of chemokines by LN can be correlated with the resistant or susceptible phenotype. In previous studies, L. major was shown to induce the expression of MCP-1 both in vitro (24) and in vivo (19). The early production of chemokines in LNs draining a site of infection has not been investigated so far. Similarly, there are no published data available concerning the in vivo production and possible in vivo action of NK cell-activating chemokines.
Specific-pathogen-free 8- to 12-week-old female BALB/c and C57BL/6 mice (Charles River Breeding Laboratories, Sulzfeld, Germany) were infected subcutaneously in both hind footpads with 2 × 106 stationary-phase L. major promastigotes (MHOM/IL/81/FEBNI) as described elsewhere (12, 32). Popliteal LNs (pLNs) were removed aseptically 1, 2, or 3 days after infection and from noninfected animals as a control. Single-cell suspensions of pLNs were washed with phosphate-buffered saline (PBS), and total RNA was extracted by using the standard guanidinium thiocyanate-phenol-chloroform extraction method as described previously (4). The expression of chemokine mRNA was determined and quantified by the RiboQuant RNase protection assay system (Pharmingen, San Diego, Calif.). The use of the 32P-labeled anti-sense mCK-5 Multi-Probe template set of this assay system allows comparative analysis of mRNA expression of a whole set of chemokine species. A Phosphor-Imager (BAS 2000; Fuji Photo Film Co., Tokyo, Japan) with TINA 2.0 software was used to measure and analyze the expression intensity of the chemokine mRNA species. Comparison of the expression of a given chemokine mRNA species with the expression of the housekeeping genes coding for L32 or glyceraldehyde-3-phosphate dehydrogenase allows the quantitative analysis of mRNA expression. In our experiments, the expression intensity for a given chemokine mRNA species was calculated as a percentage of L32 gene expression. As a sham infection, we injected PBS and latex particles into the footpads of mice. These treatments did not induce significant chemokine mRNA expression in the pLN (data not shown).
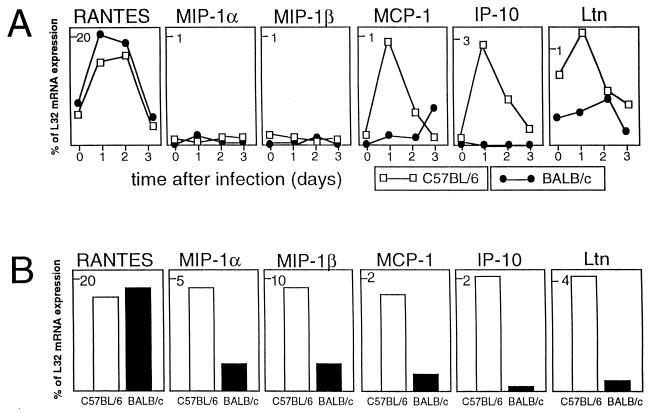
L. major infection induces the expression of RANTES on a high level in the pLNs of both resistant and susceptible mice.
The gene expression of RANTES, a chemokine with chemotactic activity on NK cells and Th1 cells, was upregulated rapidly after L. major infection (Fig. 1A). The high expression of RANTES was restricted to the first 2 days of infection. This expression pattern was very similar in both mouse strains.
FIG. 1.
(A) Chemokine mRNA expression by pLN cells in the early phase of footpad infection with L. major. The level of expression for chemokine mRNA species is given as a percentage of expression of the L32 housekeeping gene. (B) Chemokine mRNA expression of pLN cells after restimulation with L. major antigen in vitro. pLNs were removed on day 1 after infection, and the cells were incubated for 18 h in vitro in the presence of L. major lysate. The data shown are from one representative experiment of three performed.
In addition to the expression of chemokine genes by freshly isolated pLN cells, we also investigated the potential of in vivo-primed pLN cells to express chemokine genes upon restimulation with L. major in vitro. Single-cell suspensions from pLNs taken on day 1 after infection were plated at a concentration of 106/ml in tissue culture plates (Greiner, Nürtingen, Germany) in a volume of 10 ml of RPMI 1640 medium supplemented with 2 mM l-glutamine, 10 mM HEPES buffer, 100 μg of penicillin per ml, 160 μg of gentamicin per ml, 13 mM NaHCO3, 50 μM 2-mercaptoethanol and 10% fetal calf serum (Sigma Chemical Co., Deisenhofen, Germany). Total RNA was extracted after incubation of the cells at 37°C in a 5% CO2 humidified atmosphere for 18 h with L. major lysate (5 × 105 promastigotes/ml) as described previously (10). This treatment did not result in increased RANTES mRNA expression (Fig. 1B); the level of expression remained on the same high level during the in vitro culture.
MIP-1α and MIP-1β are expressed by pLN cells only after in vitro restimulation.
There was no upregulation of the expression of MIP-1α and MIP-1β in freshly isolated pLN cells during the first 3 days after L. major infection (Fig. 1A). However, cells isolated from pLN 1 day after infection expressed the mRNA for these chemokines after in vitro restimulation with L. major (Fig. 1B). The level of the in vitro gene expression was markedly different between the two mouse strains; pLN cells from resistant C57BL/6 mice expressed both chemokines on a significantly higher level than cells from susceptible BALB/c animals (Fig. 1B). A possible explanation for the high in vitro expression versus the lack of expression in vivo is the difference in antigen load. Only a few parasites can be found in the draining LN 1 day after infection, while the antigen doses used in vitro are high. The in vitro restimulation data indicate the potential of pLN cells from C57BL/6 mice to produce MIP-1α and MIP-1β upon exposure to L. major, and it is noteworthy that the expression of both chemokines correlates with the resistant phenotype.
Resistant mice express MCP-1, IP-10, and Ltn in pLNs early after infection with L. major.
The expression of the MCP-1, IP-10, and Ltn genes was rapidly upregulated after L. major infection in the pLNs of resistant C57BL/6 mice (Fig. 1A). This increased gene expression was transient and was restricted to the very early phase of infection. The expression of these genes returned to normal after 2 to 3 days of infection. There was no significant upregulation of MCP-1, IP-10, and Ltn expression in the pLNs of susceptible BALB/c mice (Fig. 1A). In vitro restimulation resulted in a significantly higher expression of Ltn mRNA in cultures of pLN cells from C57BL/6 mice, but not in those from BALB/c mice (Fig. 1B). Again, the high expression of this chemokine clearly correlates with the resistance phenotype.
Similarly to RANTES, the expression of mRNA for MCP-1 and IP-10 was not increased after in vitro restimulation of pLN cells with L. major antigen (Fig. 1B). These findings suggest that the in vivo exposure of the pLN cells to Leishmania is sufficient to induce the expression of these chemokine genes.
L. major infection induces significantly higher NK cell cytotoxic activity in pLNs of resistant mice compared to susceptible animals.
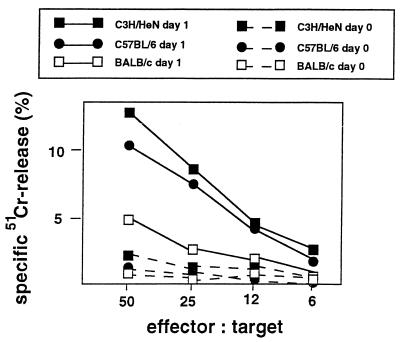
Given the known NK cell-activating potential of chemokines such as MCP-1, MIP-1α, MIP-1β, IP-10, and Ltn (18, 33), it could be expected that pLN cells from resistant C57BL/6 mice early after L. major infection have a higher NK cell activity than cells from susceptible BALB/c mice. We analyzed the NK cell cytotoxic activity in a standard 4-h 51Cr-release cytotoxicity assay by using YAC-1 target cells as described earlier (10). Briefly, 104 51Cr-labeled target cells were added to the LN cells in a total of 200 μl in U-bottom microtiter plates at effector/target ratios of 50:1, 25:1, 12:1, and 6:1. The plates were incubated for 4 h at 37°C in an atmosphere of air containing 5% CO2. pLN cells of noninfected C57BL/6 and BALB/c mice displayed no significant NK cell cytotoxic activity (Fig. 2). L. major infection resulted in the rapid induction of NK cell cytotoxic activity in pLN cells of both mouse strains (Fig. 2). However, pLN cells from resistant C57BL/6 mice indeed had a significantly higher NK cell activity than pLN cells from susceptible BALB/c mice (Fig. 2). Infection with L. major induced similarly high NK cell activity as well in C3H/HeN mice, another resistant mouse strain tested (Fig. 2).
FIG. 2.
NK cell cytotoxic activity of pLN cells from C57BL/6, C3H/HeN, and BALB/c mice on day 1 after footpad infection with L. major. NK cytotoxic activity against YAC-1 target cells was measured in a 4-h 51Cr-release assay. The data shown are from one representative experiment of three performed.
We then asked whether the higher NK cell activity in C57BL/6 mice reflects a higher number of NK cells recruited to the pLN in this mouse strain. MCP-1, RANTES, MIP-1α, and MIP-1β all have been reported to exert chemotactic activity on NK cells (1, 15, 17, 33). We analyzed the recruitment of NK cells into the draining pLN by flow cytometry on a FACS-Calibur with CellQuest software (Becton Dickinson & Co., Mountain View, Calif.). NK cells were analyzed by using a fluorescein isothiocyanate-labeled monoclonal antibody to the pan-NK cell marker DX5 (Pharmingen). This antigen is expressed on the surface of NK cells from both C57BL/6 and BALB/c mice (21). As expected, pLNs in noninfected C57BL/6 and BALB/c mice were found to contain few DX5+ cells: the ratio of DX5+ NK cells was 1 to 2% in both mouse strains. The proportion of DX5+ cells increased to 3 to 4% in both mouse strains on day 1 after L. major infection (not shown). These data indicate that the levels of L. major-induced recruitment of NK cells in the pLN are similar in both mouse strains. Therefore, the low NK cell activity of pLN in L. major-infected BALB/c mice cannot be simply explained by the low numbers of NK cells. It is rather the level of activation of NK cells which is possibly different in resistant versus susceptible mice.
RANTES mRNA is expressed 1 day after infection in both BALB/c and C57BL/6 mice on a relatively high level. RANTES has been reported to exert chemotactic and a limited stimulatory activity on NK cells (33). In this respect, the expression of IP-10 and Ltn in resistant but not in susceptible mice is of special interest, since these chemokines have been reported to have a stimulating effect only on preactivated NK cells (18). Therefore, RANTES may lead to recruitment and to initial low-level activation of NK cells in both mouse strains. Subsequent expression of IP-10 and Ltn can then lead to further activation of these cells in resistant C57BL/6 mice, but not in susceptible BALB/c mice.
In vivo treatment of infected BALB/c mice with rIP-10 enhances NK cell activity in draining LN.
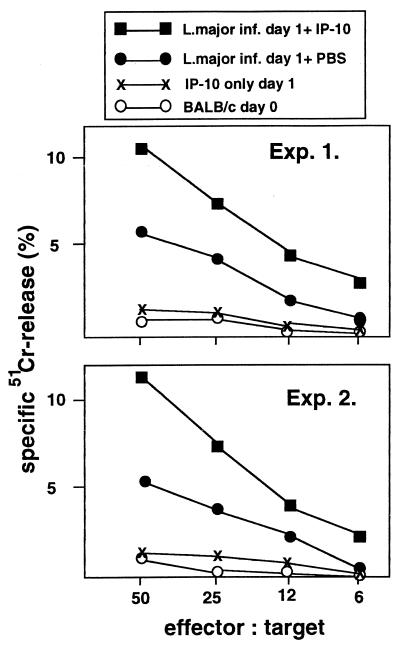
If a low infection-induced production of chemokines lies behind the low NK cell activation of pLN cells in BALB/c mice, treatment of mice with an NK cell-activating chemokine should enhance the NK cell activity in these animals. Therefore, we tried to rescue the NK cell activity of pLN cells from infected BALB/c mice by local in vivo treatment with IP-10. BALB/c mice were injected in the infected footpad with 0.5 μg of murine recombinant IP-10 (rIP-10) (R&D Systems, Wiesbaden, Germany) in 20 μl of PBS 4 h after challenge with L. major. Control mice received 20 μl of PBS in the infected footpad. The pLN was removed 24 h after infection, and the cells were assayed for their cytotoxic activity against YAC-1 target cells. This in vivo treatment of the infected footpad with IP-10 indeed resulted in an enhanced NK cell cytotoxic activity (Fig. 3), comparable to that of resistant mice. These data clearly demonstrate the in vivo NK cell-activating potential of IP-10 in L. major-infected mice. The data also support our view that the low expression of NK cell-activating chemokines, such as IP-10, is involved in the suboptimal early activation of NK cells in the draining LNs of BALB/c mice infected with L. major. However, IP-10 alone, i.e., without L. major infection, did not result in increased NK cell activity in the pLN (Fig. 3). Therefore, the in vivo effect of IP-10 is dependent on other infection-induced factors. RANTES, a chemokine with NK cell-activating potential (33), could be one of those factors, since RANTES mRNA expression was highly upregulated after L. major infection (Fig. 1). This is in agreement with the finding that only activated NK cells respond to IP-10 (18). The data suggest that the lack of IP-10 expression is at least one of the factors to result in a suboptimal NK cell activation in susceptible BALB/c mice despite an initial low-level activation of NK cells early after infection. In vitro treatment of pLN cells from infected BALB/c mice with rIP-10, however, did not lead to enhanced NK cell activity (not shown).
FIG. 3.
Effect of in vivo treatment with rIP-10 on NK cell cytotoxic activity. BALB/c mice were injected with 2 × 106 L. major promastigotes in the left hind footpad. Infected mice were injected in the left hind footpad with 0.5 μg of murine rIP-10 4 h after challenge with L. major. Control mice received an injection of PBS in the infected footpad. Uninfected BALB/c mice were given an injection of 0.5 μg of murine rIP-10 in the left hind footpad. pLN cells were removed 24 h after infection and assayed for their NK cell cytotoxic activity against YAC-1 target cells.
Recently, the increase in the number of IFN-γ-producing cells in the liver of Leishmania donovani-infected mice after blockade of CTLA-4 was reported to correlate with the enhanced expression of IP-10 (22). In the livers of L. donovani-infected mice, NK cells have been suggested to play a prominent role in IFN-γ production and host defense (6), and NK cells have been shown to utilize CD28/B-7 mediated pathways during their activation (9). These data are consistent with our finding that IP-10 upregulates the NK cell function in mice infected with Leishmania. Previous studies have suggested that the suboptimal production of or response to immunoregulators such as IL-12, IFN-α/β, and type 2 nitric oxide synthase may cause the dysfunction of the early defense machinery operative on day 1 after infection with L. major (5, 28). Our data suggest that the lack of early production of chemokines also contributes to the insufficient activation of NK cells in susceptible animals.
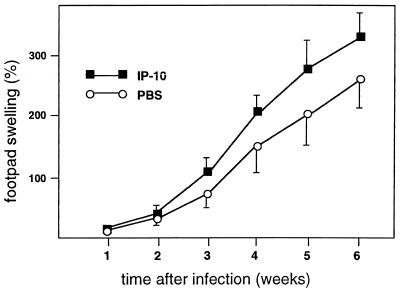
The synthesis of RANTES, MIP-1α, and MIP-1β was reported to be associated with a Th1 type of immune response (29). These chemokines have also been found to be chemotactic for Th1 cells (31). Moreover, CXCR3, the receptor for IP-10, was reported to be expressed not only by NK cells, but also by activated T cells (3, 14, 23, 26). Therefore, in addition to the activation of NK cells, the early production of RANTES, MIP-1α, MIP-1β, and, particularly, IP-10 may also contribute to the resistance to L. major by affecting the circulating population of activated T cells. Therefore, we tested the effect of IP-10 treatment on the course of L. major infection in BALB/c mice. A single injection of 0.5 μg into the footpad 4 h after infection, a treatment regimen which resulted in enhanced NK cell activity, did not protect the mice from the disease. The IP-10 treatment actually led to a slight exacerbation of lesion development (Fig. 4). This finding does not support the role of IP-10 in the protective immunity. However, one should be cautious with the interpretation of these findings in the absence of data concerning the dose dependency, time kinetics, and pleiotropic action of IP-10 in vivo. For example, the expression of CXCR3 was demonstrated not only on Th1 cells but also on Th2 cells (26). Moreover, although a lower concentration of IP-10 is required to attract Th1 cells, Th2 cells can also respond to IP-10 if this chemokine is applied in a higher concentration (26). The dose of 0.5 μg used in our experiments may be high enough to attract Th2 cells. Application of neutralizing anti-IP-10 antibodies in resistant C57BL/6 mice could possibly clarify the role of IP-10 in the resistance to L. major.
FIG. 4.
Effect of IP-10 on lesion development in mice infected with L. major. BALB/c mice were injected with 2 × 106 L. major promastigotes in the left hind footpad. The mice were injected in the infected footpad with 0.5 μg of murine rIP-10 4 h after challenge with L. major. Control mice received an injection of PBS in the infected footpad. Footpad swelling was measured with a metric caliper. The data shown are from one representative experiment of two performed.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 263 and SFB 367).
We thank H. Fickenscher for help with the data analysis, Nicole Jahnke and Milena Lipkowski for the fluorescence-activated cell sorter analysis, and Helmut Laufs for critical reading of the manuscript.
REFERENCES
- 1.Bianchi G, Sozzani S, Zlotnik A, Mantovani A, Allavena P. Migratory response of human natural killer cells to lymphotactin. Eur J Immunol. 1996;26:816–824. doi: 10.1002/eji.1830261260. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C, Röllinghoff M. The immune response to Leishmania: mechanisms of parasite control and evasion. Int J Parasitol. 1998;28:121–134. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 3.Bonecchi R, Bianchi G, Bordignon P P, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray P A, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Röllinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 6.Engwerda C R, Murphy M L, Cotterell S E, Smelt S C, Kaye P M. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol. 1998;28:669–680. doi: 10.1002/(SICI)1521-4141(199802)28:02<669::AID-IMMU669>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Etges R, Müller I. Progressive disease or protective immunity to Leishmania major infection: the result of a network of stimulatory and inhibitory interactions. J Mol Med. 1998;76:372–390. doi: 10.1007/s001090050230. [DOI] [PubMed] [Google Scholar]
- 8.Hedick J A, Saylor V, Figueora D, Mizoue L, Xu Y, Menon S, Abrams J, Handels T, Zlotnik A. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J Immunol. 1997;158:1533–1540. [PubMed] [Google Scholar]
- 9.Hunter C A, Ellis-Neyer L, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 10.Laskay T, Röllinghoff M, Solbach W. Natural killer cells participate in the early defense against Leishmania major infection in mice. Eur J Immunol. 1993;23:2237–2241. doi: 10.1002/eji.1830230928. [DOI] [PubMed] [Google Scholar]
- 11.Laskay T, Diefenbach A, Röllinghoff M, Solbach W. Early parasite containment is decisive for resistance to Leishmania major infection. Eur J Immunol. 1995;25:2220–2227. doi: 10.1002/eji.1830250816. [DOI] [PubMed] [Google Scholar]
- 12.Laskay T, Wittmann I, Diefenbach A, Röllinghoff M, Solbach W. Control of Leishmania major infection in BALB/c mice by inhibition of early lymphocyte entry into peripheral lymph nodes. J Immunol. 1997;158:1246–1253. [PubMed] [Google Scholar]
- 13.Leiby D A, Schreiber R D, Nacy C A. IFN-γ produced in vivo during the first two days is critical for resolution of murine Leishmania major infections. Microb Pathog. 1993;14:495–500. doi: 10.1006/mpat.1993.1049. [DOI] [PubMed] [Google Scholar]
- 14.Loetscher M, Gerber B, Loetscher P, Jones S A, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156:322–327. [PubMed] [Google Scholar]
- 16.Luster A D. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 17.Maghazachi A A, Al-Aoukaty A, Schall T J. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. J Immunol. 1994;153:4969–4977. [PubMed] [Google Scholar]
- 18.Maghazachi A A, Skalhegg B S, Rolstad B, Al-Aoukaty A. Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J. 1997;11:765–774. doi: 10.1096/fasebj.11.10.9271361. [DOI] [PubMed] [Google Scholar]
- 19.Moll H. The role of chemokines and accessory cells in the immunoregulation of cutaneous leishmaniasis. Behring Inst Mitt. 1997;99:73–78. [PubMed] [Google Scholar]
- 20.Mondino A, Khoruts A, Jenkins M K. The anatomy of T-cell activation and tolerance. Proc Natl Acad Sci USA. 1996;93:2245–2252. doi: 10.1073/pnas.93.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore T A, von Frieeden-Jeffry U, Murray R, Zlotnik A. Inhibition of γδ T cell development and early thymocyte maturation in IL-7 −/− mice. J Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 22.Murphy M L, Cotterell S E, Gorak P M, Engwerda C R, Kaye P M. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen Leishmania donovani. J Immunol. 1998;161:4153–4160. [PubMed] [Google Scholar]
- 23.Qin S, Rottman J B, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch A E, Moser B, Mackay C R. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Investig. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racoosin E L, Beverley S M. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp Parasitol. 1997;85:283–295. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- 25.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharton T M, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharton-Kersten T M, Sher A. Role of natural killer cells in innate resistance to protozoan infections. Curr Opin Immunol. 1997;9:44–51. doi: 10.1016/s0952-7915(97)80157-4. [DOI] [PubMed] [Google Scholar]
- 29.Schrum S, Probst P, Fleischer B, Zipfel P F. Synthesis of the CC-chemokines MIP-1α, MIP-1β, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–3604. [PubMed] [Google Scholar]
- 30.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 31.Siveke J T, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 32.Solbach W, Forberg K, Kammerer E, Bogdan C, Röllinghoff M. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J Immunol. 1986;137:702–707. [PubMed] [Google Scholar]
- 33.Taub D D, Sayers T J, Carter C R D, Ortaldo J R. α and β chemokines induce NK cell migration and enhance NK-mediated lysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 34.Vaddi K, Keller M, Newton R C. The chemokine facts book. San Diego, Calif: Academic Press, Harcourt Brace & Company, Publishers; 1997. [Google Scholar]