Abstract
The split left coronary artery (LCA) is an anomaly of coronary arteries connection related to the aorta, presenting more often in patients who underwent invasive coronary angiography compared to coronary computed tomography angiography. Although this anomaly causes no hemodynamic impairment, failure to recognize may lead to incorrect diagnosis and prolonged procedures during acute myocardial infarction resulting in serious complications. We report 2 cases of split left coronary artery presenting with acute myocardial infarction who underwent primary percutaneous coronary interventions (pPCI) with excellent outcomes. In the both cases, electrocardiogram demonstrated ST-segment elevation and cardiac biomarkers were increased. Also, before coronary angiography in both patients echocardiographic examination was performed revealing hypokinesis who corresponded with the territory of occluded coronary arteries. During invasive further coronary examinations split left artery was found, besides the culprit lesion in the left anterior descending artery (LAD). Successful percutaneous stenting was performed on LAD achieving TIMI flow grade 3 in both cases. Prompt recognition of split LCA in the setting of acute myocardial infarction during pPCI, it is essential to achieve appropriate treatment and avoid potential clinical consequences.
Keywords: Split coronary artery, Artery anomalies
Introduction
Congenital coronary artery anomalies (CAAs) have a prevalence of 0.21%-5.79% [1]. Based on embryological-anatomical classification, one of anomalies of coronary arteries is split coronary left artery, which is found in 0.41% of patients who undergo coronary angiography [[1], [2], [3]]. This anomaly in contrast to other CAAs is found more often by invasive coronary angiography (ICA) compared to coronary computed tomography angiography (CCTA) [4]. The anomaly, denoting separate left-sided origins of both main arteries may be challenging during invasive treatment of acute myocardial infarction [5].
We present 2 cases with split left coronary artery and acute myocardial infarction who underwent primary percutaneous coronary interventions.
Case presentation no. 1
A 50-year-old, male, presented with typical chest pain in last 2 hours prior to admission. Regarding risk factors for coronary artery disease, patient referred that was an active smoker.
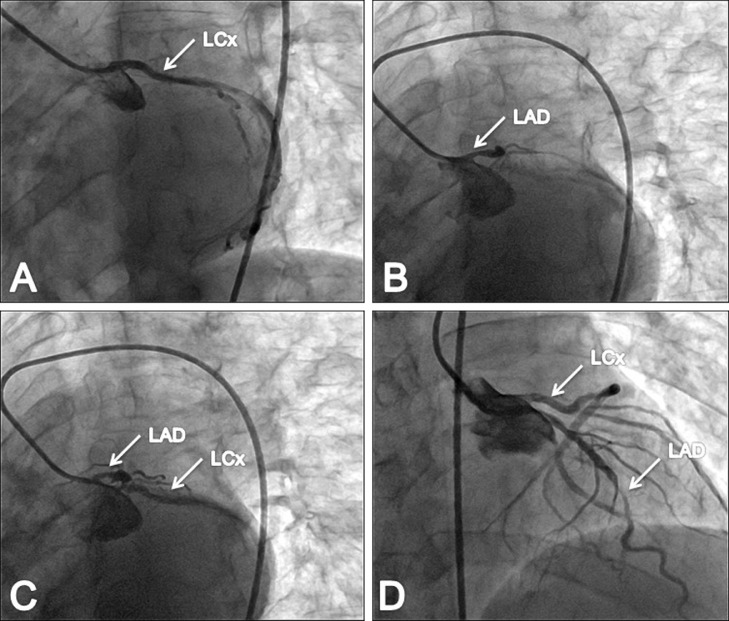
Electrocardiogram on presentation demonstrated ST-segment elevation in leads V1-V4. Cardiac biomarkers were as follows: initial cardiac troponin (cTn) level of 0.14 ng/mL (0-0.02 ng/mL), creatine kinase (CK) level of 2080 U/L (38-171 U/L), and creatine kinase-MB (CK-MB) level of 210ng/mL (5-25 ng/mL). Echocardiography examination revealed hypokinesis of mid anteroseptal segment, apical anterior segment, apical septal segment, and apex with low left ventricular systolic function. Urgent coronary angiography was performed. The right coronary system was engaged first and it showed no significant lesions. During the effort to engage the left coronary system in the left anterior oblique caudal (LAO-caudal) projection it was possible to visualize only the left circumflex artery (LCx) but not the left anterior descending artery (LAD) (Fig. 1A). In the same projection, by gently pulling the catheter back and testing injections of contrast, we were able to identify separate origin of LAD (Figs. 1B and C). In the right anterior oblique cranial (RAO-cranial) projection a separate origin of LAD and LCx was demonstrated, and culprit lesion in mid-LAD (subocclusion) was identified (Fig. 1D). Subsequently, LAD was engaged and percutaneous intervention was performed with a drug-eluting stent achieving TIMI flow grade 3 (Figs. 2A and B).
Fig. 1.
Coronary angiography showing split left coronary arteries (case no.1). (A) Separately engaged LCx (arrow). (B) Separately engaged LAD (arrow). (C) Separate ostia for LAD and LCx (arrows). (D) Culprit lesion in mid-LAD (arrows).
Fig. 2.
Primary percutaneous coronary intervention in LAD (case no.1). (A) Stent deployment. (B) The final angiographic result.
Case presentation no. 2
A 63-year-old male presented to emergency department complaining of chest pain, an hour and half before admission to hospital. He had history of hypertension and smoking. Electrocardiogram showed infero-lateral acute changes. Initial cardiac troponin (cTn), creatine kinase (CK) and creatine kinase-MB (CK-MB) levels were 0.10 ng/mL (0-0.02 ng/mL), 2510 U/L (38-171 U/L) and 253ng/mL (5-25 ng/mL), respectively. Transthoracic echocardiography revealed hypokinesis of apical anterior segment, apical septal segment, apical inferior segment, and the apex with left ventricular ejection fraction of 50%. Coronary angiography showed no significant stenosis in the right coronary artery. The left main coronary artery was split into the left anterior descending artery (LAD) and the left circumflex artery (LCx) (Figs. 3A and B). Since there was no common left main coronary artery, LAD was separately engaged in posterior anterior cranial (PA-cranial) projection and LCx in left anterior oblique cranial (LAO-cranial) projection, confirming the culprit lesion (occlusion) in distal LAD (Figs. 3C and D). Finally, it performed PCI in the LAD with a drug-eluting stent which resulted in TIMI flow grade 3 (Figs. 4A and B).
Fig. 3.
Coronary angiography showing absence of left main coronary artery (case no. 2). (A and B) LAD and LCx arising from separate ostia in the left coronary sinus (arrows). (C) Separately engaged LAD detecting culprit lesion (arrow). (D) Separately engaged LCx (arrow).
Fig. 4.
Primary percutaneous coronary intervention in LAD (case no 2). (A) The stent balloon is inflated, deploying the stent. (B) The final angiographic result.
Discussion
Based on invasive further examinations, computed tomography (CT) and data form autopsies coronary arteries congenital anomalies are presented with a prevalence of 0.21%-5.79% [1,2]. Absent left main coronary trunk (split left coronary artery) is classified among anomalies of coronary arteries (CAs) connection to the aorta/systemic circulation. Split origin of LAD and LCx is found in 0.41% of patients who underwent coronary angiography [[1], [2], [3]]. CAs in humans, are meant that originate during angiogenesis from the endothelium [6]. Members of the vascular endothelial growth factor (VEGF) family are regulators of endothelial cells (ECs) penetration to the aorta [7]. The VEGF-C mediates the establishment of the peritruncal vessels that surround and are anastomosed with aorta, morphological steps that are required for CA stem patterning [8]. Thereby, abnormal CA stem patterning results from failure to complete either morphological steps.
Most of these anomalies are asymptomatic, may reveal with serious clinical outcomes as coronary artery disease, heart failure, heart rhythm disturbances or sudden cardiac death [[2], [3]]. Clinical manifestations depend on long courses of coronary arteries, angulation, intramural courses, vasospasm, endothelial injury, and the compression of the aberrant artery [[9], [10]]. Usually not existing left main coronary artery (LMCA) causes no hemodynamic harm, is considered benign, failure being aware may lead to prolonged procedures, in particular during acute coronary syndromes or inaccurate diagnosis with a possibility of resulting in serious complications [[11], [12]]. When the LAD and LCx arise from separate ostia in the left coronary cusp, separately engagement either of the LCx or the LAD with only one branch visualized may be confused with an occluded companion branch.
Coronary anomalies can be detected with both imaging modalities, ICA and CCTA. The split LCA was the most frequently observed anomaly, occurring in 36% of the patients who underwent ICA, while occurred in only 11.6% of patients who underwent CCTA [4].
Therefore, the clinical challenge of coronary anomalies presented with acute myocardial infarction is understanding of their anatomical variability towards a correct diagnosis and early treatment [[13], [14]].
Conclusions
During acute coronary syndromes, in order of achieving optimal treatment, on time detection of split LCA is essential, which will help in avoiding potential consequences too. Therefore, coronary anomalies should be kept in mind, as overlooking them may lead to coronary artery disease misdiagnosis.
Declarations
This manuscript or essential parts of it have not been previously published or are under consideration by another journal, in English or another language.
Availability of data and materials
All data from this study are included.
Authors’ contribution
XK was the first author. XK, AB, HÇ and DK prepared the final manuscript. All authors contributed to data collection and read and approved the final manuscript.
Ethics approval and consent to participate
The Approval of our local ethics committee for publication was obtained.
Patient consent
Written informed consent was obtained from patients for the publication of these 2 case reports and any accompanying images. A copy of the written consents is available for review by the Editor-in-Chief of this journal.
Footnotes
Competing Interests: The authors have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Pérez-Pomares JM, de la Pompa JL, Franco D, Henderson D, Ho SY, Houyel L, et al. Congenital coronary artery anomalies: a bridge from embryology to anatomy and pathophysiology—a position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc Res. 2016;109(2):204–216. doi: 10.1093/cvr/cvv251. [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21(1):28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 3.Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation Diagn. 2007;115(10):1296–1305. doi: 10.1161/CIRCULATIONAHA.106.618082. [DOI] [PubMed] [Google Scholar]
- 4.Ghadri JR, Kazakauskaite E, Braunschweig S, Burger IA, Frank M, Fiechter M, et al. Congenital coronary anomalies detected by computed tomography compared to invasive coronary angiography. BMC Cardiovasc Disord. 2014;14(1):1–10. doi: 10.1186/1471-2261-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong MY, Shin DH, Kwon JH, Chang WS, Choi KU, Song YA, et al. Anomalous separate origin of left anterior descending coronary artery: presented as acute anterior myocardial infarction. Korean Circ J. 2013;43(6):408–410. doi: 10.4070/kcj.2013.43.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramaj D, Lai J, Monzidelis C, Reddy S. Coronary artery development: origin, malformations, and translational vascular reparative therapy. J Cardiovasc Pharmacol Ther. 2018;23(4):292–300. doi: 10.1177/1074248418769633. [DOI] [PubMed] [Google Scholar]
- 7.Silva-Junior GO, Miranda WS, Mandarim-de-Lacerda CA. Origin and development of coronary arteries. Int J Morphol. 2009;27(3):891–898. [Google Scholar]
- 8.Chen HI, Poduri A, Numi H, Kivela R, Saharinen P, McKay AS, et al. VEGF-C and aortic cardiomyocytes guide coronary artery stem development. J Clin Invest. 2014;24(11):4899–4914. doi: 10.1172/JCI77483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opolski MP, Pregowski J, Kruk M, Witkowski A, Kwiecinska S, Lubjenska E, et al. Prevalence and characteristics of coronary anomalies originating from the opposite sinus of Valsalva in 8,522 patients referred for coronary computed tomography angiography. Am J Cardiol. 2013;111(9):1361–1367. doi: 10.1016/j.amjcard.2013.01.280. [DOI] [PubMed] [Google Scholar]
- 10.Yurtdaş M, Gülen O. Anomalous origin of the right coronary artery from the left anterior descending artery: review of the literature. Cardiol J. 2012;19(2):122–129. [PubMed] [Google Scholar]
- 11.Angelini P. Coronary artery anomalies—current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. 2002;29(4):271–278. [PMC free article] [PubMed] [Google Scholar]
- 12.Serota H, Barth CW, 3rd, Seuc CA, Vandormael M, Aguirre F, Kern MJ, et al. Rapid identification of the course of anomalous coronary arteries in adults: the ”dot and eye” method. Am J Cardiol. 1990;65(13):891–898. doi: 10.1016/0002-9149(90)91432-6. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Zhu C, Guo Y, Zhang M, Li J. A rare case of complex anomalous origin of coronary arteries with severe atherosclerosis. Chin Med J (Engl) 2014;127(24):4297–4298. [PubMed] [Google Scholar]
- 14.Marchesini J, Campo G, Righi R, Benea G, Ferrari R. Coronary artery anomalies presenting with ST-segment elevation myocardial infraction. Clin Pract. 2011;1(4):e107. doi: 10.4081/cp.2011.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data from this study are included.