Graphical abstract
Keywords: Nucleoside modifications, Methylations, Acetylation, P. capsici, ROS, P. nigrum
Abstract
The efforts to signify the relevance of tRNA modifications were always within the limits of prokaryotes, humans, and some fewer model plant systems. The story of tRNA modifications in higher plants is still overlooked, especially in non-model spice crops. Stress causes alterations in tRNA modifications to facilitate the downstream functions of tRNAs. The present study was done to identify and better understand the fate of tRNA nucleoside modifications during biotic stress response in a widely used spice crop called Black pepper. We have uncovered the various tRNA nucleoside modifications present in black pepper. Methylations were the predominant nucleoside modifications in black pepper tRNAs.
Furthermore, the different methyltransferase gene candidates implicated in catalyzing tRNA nucleoside methylations in black pepper were also identified. The LC-MS profile showed that certain tRNA nucleoside modifications showed varied abundance upon P. capsici infection. The N4-acetylcytidine (ac4C) nucleoside modification has shown a constant hike at 24 and 48 hpi. At the same time, some nucleoside modifications have exhibited a time-dependent abundance. Altogether our study suggests that tRNA modifications and the expression of associated enzymes are altered during biotic stress regulation.
1. Introduction
The transfer RNAs (tRNAs) are known as adaptor molecules that are implicated in the process of protein translation. A mature tRNA requires extensive post-transcriptional modifications to execute its normal functions in a cell. The modifications vary from simple addition or substitution of functional groups to complex biosynthetic reactions [34], [6], [19]. However, research on tRNA modifications began decades ago when Holley et al. [25] noticed the presence of unusual nucleosides like inosinic acid, l-methylinosinic acid, and 5,6-dihydro uridylic acid; the investigations on its significance are still growing.
The tRNA modifications confer structural stability and integrity to tRNAs and facilitate the efficient translation of proteins [15]. The tRNA modifications are prevalent at the wobble position of tRNAs to enhance the codon-anticodon pairing [29], [51]. Moreover, the tRNA modifications in the anticodon loop regulate the translational rate of specific genes during adversities [12], [18]. Whereas certain modifications in the variable loop, the D arm, and the T arm confer structural stability to tRNAs [15]. Interestingly, the abundance of these tRNA modifications is altered in respone to various environmental stimuli [38], [15]. For example, in humans, the modification levels are dynamically altered during diverse physiological conditions like cancer [14], [18], neurological disorders [3], [5] etc. Whereas in plants, the 2′-O-methyladenosine (Am) nucleoside was abundantly found during salt stress. In Rice, the overexpression of OsTRM13- methyltransferase implicated in the synthesis of Am showed higher resistance to salt stress [59,60]. The tRNA modifications: Am, Cm, m1A and m7G and their cognate methyltransferases (MTases), were upregulated during abiotic stresses in Rice and Arabidopsis [59,60]. Another study in Rice demonstrated that a tRNA His guanylyltransferase- AET1 aid the plant to withstand high temperatures [10]. But, most of the studies done in plants to understand the implication of tRNA modification in stress response were confined to abiotic stress. The investigations on the fate of tRNA modification profiles reprogrammed during a biotic attack are scanty. A study has demonstrated that a nonfunctional SUPPRESSOR OF CSB3 9 (SCS9) protein which codes a MTase, has enhanced disease susceptibility in plants, showing the functional importance of tRNA modifications in biotic stress regulation [45].
In plants, the pathogen attack builds up reactive oxygen species (ROS) in the cells, which ultimately causes oxidative stress or damage to the cells [53]. According to previous reports in model organisms like yeast, oxidative stress alters the tRNA modification profile [8]. Although tRNA modifications and how they respond to stress are well probed in organisms like bacteria, yeast and humans, the chore of tRNA modifications during biotic stress in higher plants is least explored. The lack of such studies is mainly due to the complexity of defining tRNA modifications using conventional genetic and biochemical methods in higher systems. In such circumstances, the availability of omics data can be exploited to study more about tRNA modifications and associated enzymes [55].
In the current study, we investigated how Phytophthora capsici infection affected the tRNA modification profile of black pepper. The study identified the different tRNA nucleoside modifications in black pepper and the enzyme gene candidates involved in the modification pathways. Additionally, the expression of genes associated with tRNA modification in P. capsici-infected and uninfected black pepper was studied. The study explained the impact of stress on tRNA modifications and their cognate enzymes implicated in stress response in black pepper. An attempt to investigate the various tRNA modifications and associated genes during pathogen attack in a non-model spice crop like black pepper can also aid in annotating its obscured functions in higher plants.
2. Results
2.1. Detection of ROS generation and cell death during P. capsici infection using DAB, NBT and trypan blue staining
During pathogen infection, the plants undergo a series of chemical reactions, and the release of ROS is one among them. Generally, Hydrogen peroxide (H2O2) and Super oxide (O−) accumulation are considered markers of ROS burst. In black pepper during P. capsici attack, the outburst of H2O2, O− and related cell death was detected using 3,3′-diaminobenzidine (DAB), Nitro tetrazolium blue (NBT) and Trypan blue staining, respectively (Fig. 1). The trypan blue stained cell death occurred in the infected leaves. DAB staining has shown the accumulation of H2O2 in pathogen-infected leaves. Similarly, the release of superoxide radicals was also seen more in pathogen-challenged leaves when reacted with NBT.
Fig. 1.

Histochemical staining for the detection of ROS. Trypan blue staining for indicating cell death during pathogen, 3,3′-diaminobenzidine, staining for Hydrogen peroxide detection, Nitro tetrazolium blue staining for superoxide radical detection. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. tRNA isolation
The tRNAs extracted from the total RNA samples were run on the 7.5 % urea gel, as shown in Fig. 2. The red rectangle box indicates the 60–90 nt tRNA band. These tRNAs were excised and used for further analysis.
Fig. 2.

Urea PAGE analysis of tRNA isolated from black pepper.
2.3. Identification of modified tRNA nucleosides of black pepper
The LC-MS analysis of tRNAs was done at Arraystar, USA, to identify the various tRNA modifications present in black pepper. Interestingly, from the fifty-two standard modifications used for the analysis, a maximum of fifty different tRNA nucleoside modifications were detected. These fifty modifications include (Supplementary Table 1) twenty ‘U’ derivatives, eight ‘A’ derivatives, ten ‘C’ derivatives and five ‘G’ derivatives.
2.4. Identification of methylation modifications enzymes from black pepper
Our analysis revealed that the nucleoside methylations catalyzed by MTases were predominantly found in black pepper. So, further study was done to extract the MTase candidate genes from black pepper. Fifty-nine MTase candidates were identified from black pepper based on protein sequence homology with thirteen MTase sequences from yeast, twenty-two from Arabidopsis and twenty-two from Rice, and also from the NR classification of black pepper transcriptome data using local tblastn in BioEdit (Table 1).
Table 1.
Table showing the identified fifty-nine MTase candidates from black pepper.
| Black Pepper MTase Protein | Yeast Candidate Gene | E-value | Arabidopsis Candidate Gene | E-value | Rice Candidate Gene | E-value |
|---|---|---|---|---|---|---|
| Unigene10018_All | Trm1p | 1.00E−56 | At3g02320 | 2.00E−163 | LOC_Os03g57280 | 7.00E−158 |
| At5g15810 | 2.00E−170 | LOC_Os10g21360 | 6.00E−149 | |||
| Unigene10022_All | Trm1p | 8.00E−26 | At3g02320 | 1.00E−118 | LOC_Os03g57280 | 2.00E−118 |
| At5g15810 | 2.00E−120 | LOC_Os10g21360 | 1.00E−83 | |||
| Unigene10021_All | Trm1p | 3.00E−33 | At3g02320 | 4.00E−107 | LOC_Os03g57280 | 5.00E−105 |
| At5g15810 | 8.00E−113 | LOC_Os10g21360 | 1.00E−100 | |||
| CL1469.Contig5_All | Trm1p | 3.00E−33 | At3g02320 | 7.00E−107 | LOC_Os03g57280 | 7.00E−105 |
| At5g15810 | 4.00E−111 | LOC_Os10g21360 | 1.00E−100 | |||
| CL1469.Contig4_All | Trm1p | 1.00E−29 | At5g15810 | 7.00E−105 | LOC_Os03g57280 | 2.00E−98 |
| At3g02320 | 1.00E−100 | LOC_Os10g21360 | 4.00E−95 | |||
| Unigene6901_All | Trm2 | 8.00E−27 | At3g21300 | 0 | LOC_Os01g09750 | 0 |
| At2g28450 | 2.00E−17 | LOC_Os04g01480 | 1.00E−18 | |||
| LOC_Os01g29409 | 1.00E−10 | |||||
| LOC_Os02g39370 | 1.00E−06 | |||||
| Unigene285_All | Trm2 | 9.00E−13 | At2g28450 | 0 | LOC_Os04g01480 | 0 |
| At3g21300 | 7.00E−21 | LOC_Os01g09750 | 4.00E−09 | |||
| Unigene3795_All | Trm5p | 2.00E−51 | At3g56120 | 9.00E−116 | LOC_Os01g29409 | 2.00E−56 |
| At3g21300 | 3.00E−09 | LOC_Os02g39370 | 7.00E−98 | |||
| At4g04670 | 4.00E−08 | |||||
| At4g27340 | 2.00E−52 | |||||
| CL8815.Contig2_All | Trm5p | 2.00E−53 | At4g27340 | 7.00E−134 | LOC_Os01g29409 | 1.00E−135 |
| At3g21300 | 9.00E−06 | LOC_Os01g09750 | 2.00E−06 | |||
| At3g56120 | 7.00E−60 | LOC_Os02g39370 | 4.00E−43 | |||
| At4g04670 | 2.00E−10 | |||||
| CL839.Contig3_All | Trm5p | 1.00E−07 | At4g04670 | 9.00E−134 | LOC_Os01g29409 | 7.00E−09 |
| At3g56120 | 5.00E−09 | LOC_Os02g39370 | 6.00E−09 | |||
| At4g27340 | 1.00E−09 | |||||
| CL1131.Contig3_All | Trm7p | 3.00E−36 | At4g25730 | 3.00E−175 | LOC_Os05g49230 | 0 |
| At5g01230 | 2.00E−34 | LOC_Os06g49140 | 4.00E−35 | |||
| At5g13830 | 2.00E−17 | LOC_Os09g27270 | 2.00E−52 | |||
| LOC_Os03g60750 | 4.00E−17 | |||||
| Unigene10222_All | Trm8p | 5.00E−69 | At5g24840 | 2.00E−106 | LOC_Os06g12990 | 1.00E−109 |
| At5g17660 | 3.00E−11 | LOC_Os01g35170 | 1.00E−14 | |||
| Unigene10020_All | Trm1p | 3.00E−33 | At3g02320 | 7.00E−94 | LOC_Os03g57280 | 2.00E−90 |
| At5g15810 | 1.00E−97 | LOC_Os10g21360 | 1.00E−77 | |||
| Unigene16681_All | Trm1p | 1.00E−20 | At3g02320 | 2.00E−72 | LOC_Os03g57280 | 1.00E−69 |
| At5g15810 | 8.00E−76 | LOC_Os10g21360 | 2.00E−58 | |||
| Unigene7198_All | Trm1p | 9.00E−09 | At3g02320 | 6.00E−11 | LOC_Os03g57280 | 3.00E−10 |
| At5g15810 | 7.00E−11 | LOC_Os10g21360 | 1.00E−12 | |||
| CL10116.Contig2_All | Trm1p | 2.00E−08 | At3g02320 | 2.00E−06 | LOC_Os03g57280 | 8.00E−06 |
| At5g15810 | 7.00E−08 | LOC_Os10g21360 | 2.00E−09 | |||
| CL10116.Contig1_All | Trm1p | 2.00E−08 | At3g02320 | 2.00E−06 | LOC_Os03g57280 | 8.00E−06 |
| At5g15810 | 7.00E−08 | LOC_Os10g21360 | 2.00E−09 | |||
| Unigene16680_All | Trm1p | 9.00E−06 | At3g02320 | 4.00E−27 | LOC_Os03g57280 | 5.00E−26 |
| At5g15810 | 9.00E−29 | LOC_Os10g21360 | 3.00E−19 | |||
| Unigene10017_All | – | – | At3g02320 | 3.00E−42 | LOC_Os03g57280 | 7.00E−36 |
| At5g15810 | 2.00E−38 | LOC_Os10g21360 | 3.00E−32 | |||
| Unigene10019_All | – | – | At3g02320 | 1.00E−35 | LOC_Os03g57280 | 2.00E−14 |
| At5g15810 | 6.00E−34 | LOC_Os10g21360 | 5.00E−31 | |||
| CL1469.Contig3_All | – | – | At3g02320 | 1.00E−35 | LOC_Os03g57280 | 2.00E−14 |
| At5g15810 | 6.00E−34 | LOC_Os10g21360 | 5.00E−31 | |||
| Unigene22464_All | At3g02320 | 4.00E−15 | LOC_Os03g57280 | 7.00E−17 | ||
| At5g15810 | 5.00E−16 | |||||
| CL1469.Contig6_All | At3g02320 | 7.00E−15 | LOC_Os03g57280 | 9E−17 | ||
| At5g15810 | 1.00E−14 | |||||
| Unigene22463_All | At3g02320 | 1.00E−06 | LOC_Os03g57280 | 3.00E−08 | ||
| At5g15810 | 5.00E−08 | |||||
| CL1469.Contig1_All | At5g15810 | 8.00E−07 | LOC_Os03g57280 | 2.00E−08 | ||
| CL2435.Contig5_All | At3g21300 | 1.00E−08 | LOC_Os01g09750 | 5.00E−10 | ||
| CL8815.Contig1_All | Trm5p | 2.00E−45 | At3g21300 | 9.00E−06 | LOC_Os01g29409 | 2.00E−16 |
| At3g56120 | 9.00E−57 | LOC_Os02g39370 | 3.00E−40 | |||
| At4g27340 | 3.00E−90 | |||||
| At4g04670 | 2.00E−09 | |||||
| CL3255.Contig2_All | Trm4p | 3.00E−21 | At4g40000 | 4.00E−09 | LOC_Os09g29630 | 1.00E−15 |
| At2g22400 | 7.00E−39 | LOC_Os08g37780 | 5.00E−39 | |||
| Unigene10101_All | Trm4p | 7.00E−11 | At4g40000 | 5.00E−07 | LOC_Os09g29630 | 2.00E−11 |
| At2g22400 | 9.00E−10 | LOC_Os08g37780 | 8.00E−11 | |||
| Unigene3766_All | Trm5p | 2.00E−10 | At3g56120 | 3.00E−41 | LOC_Os01g29409 | 4.00E−09 |
| At4g27340 | 2.00E−07 | LOC_Os02g39370 | 2.00E−42 | |||
| CL9819.Contig1_All | At4g27340 | 3.00E−46 | LOC_Os01g29409 | 1.00E−27 | ||
| Unigene22693_All | Trm7p | 4.00E−37 | At5g01230 | 5.00E−67 | LOC_Os06g49140 | 5.00E−65 |
| At4g25730 | 3.00E−15 | LOC_Os05g49230 | 8.00E−17 | |||
| At5g13830 | 4.00E−07 | LOC_Os09g27270 | 6.00E−14 | |||
| LOC_Os03g60750 | 4.00E−06 | |||||
| CL166.Contig5_All | Trm7p | 6.00E−12 | At5g01230 | 8.00E−11 | LOC_Os06g49140 | 1.00E−11 |
| At4g25730 | 5.00E−15 | LOC_Os05g49230 | 1.00E−14 | |||
| At5g13830 | 3.00E−66 | LOC_Os09g27270 | 3.00E−14 | |||
| LOC_Os03g60750 | 5.00E−76 | |||||
| CL8431.Contig2_All | LOC_Os05g49230 | 8.00E−08 | ||||
| Unigene15091_All | Trm140p | 9.00E−07 | LOC_Os05g49230 | 8.00E−06 | ||
| CL7021.Contig3_All | Trm8p | 4.00E−10 | At5g24840 | 1.00E−12 | LOC_Os06g12990 | 1.00E−12 |
| At5g17660 | 1.00E−86 | LOC_Os01g35170 | 5.00E−90 | |||
| CL6988.Contig2_All | LOC_Os02g51490 | 1.00E−06 |
2.5. Phylogenetic and conserved motif analysis of MTase in Black pepper
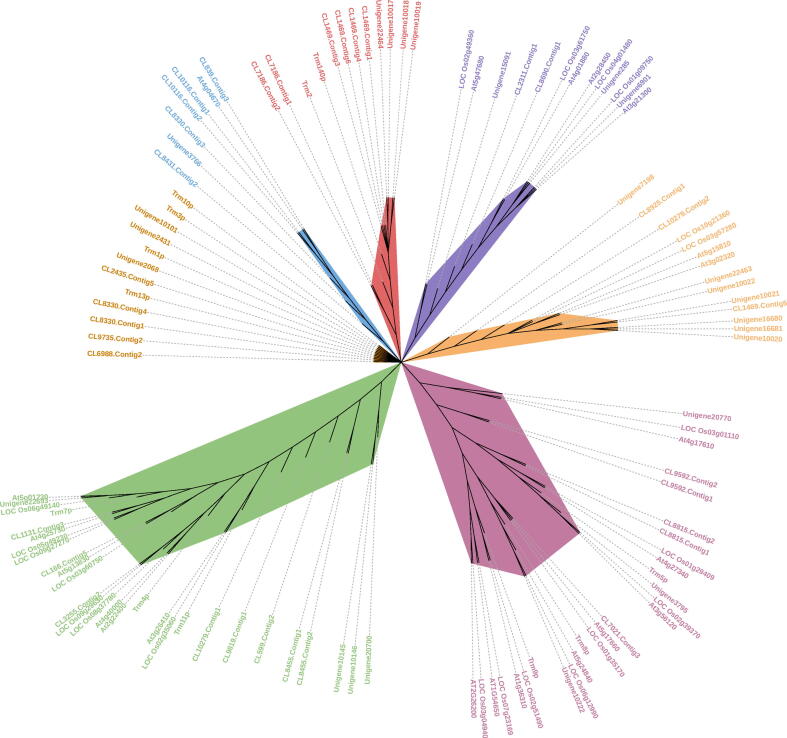
A phylogenetic tree was constructed to study the evolutionary significance of the fifty-nine MTase candidates. The tree has shown that the candidates have clustered into closely related groups. The phylogenetic tree has been clustered into six groups (Fig. 3). Group I include the candidate genes of black pepper needed for m7G and mcm5U methylation in Arabidopsis, Yeast and Rice, the group II consist of m3C and m5U candidates. The group III consists of the MTases of m5C, m2G and Cm. Group IV and V include Am, m1G, m5U and m1G, m22G, respectively. Group VI possess the candidates for Am and Gm modifications.
Fig. 3.
The phylogenetic tree of MTase candidates from Black pepper. Unrooted phylogenetic tree of MTase candidates from Black pepper, Oryza sativa (Rice), Arabidopsis thaliana and Saccharomyces cerevisiae (Yeast) [Phylogenetic relationship among various MTases candidates from Black pepper, O. sativa, A. thaliana and S. cerevisiae] by employing the maximum-likelihood method based on Jones-Taylor-Thornton (JTT) Protein substitution model. The six groups of Black pepper genes clustered together were annotated with green lines for group I, Opera Mauve (light pinkish purple) lines for group II, light orange lines for group III, medium purple lines for group IV, red lines for group V and steel blue lines for group VI respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Further, the obtained sequences were aligned in MEME for visualizing the conserved domain in the MTase candidates in black pepper. Eleven candidates from black pepper shared conserved domains with the MTases from Arabidopsis, Rice and Yeast. Based on the conserved domain analysis of black pepper, Yeast, Arabidopsis and Rice datasets, the MEME analysis yielded three groups (Fig. 4).
Fig. 4.
The Conserved motif analysis of group I, group II and group III MTase candidate genes from black pepper. The X-axis indicates the position of each residue within the motifs identified from black pepper; Y-axis indicates bit score values. The degree of conservation within the group of proteins studied was denoted by the size of the residue letter. The table below each illustration is the translated protein sequence from black pepper, protein sequence from Yeast, Arabidopsis and Rice for each member, with the name of the protein, starting position, the p-value of the conserved motif and the whole motif sequence.
The first group has shown aspartic acid (D) conservation in the 9th position. Moreover, a motif of APG from the 13th position was also seen in group I. The contigs in group I were candidates of tRNA (cytidine32/guanosine34-2′-O)-methyltransferase and Ado Met-dependent rRNA MTase SPB1. In group II, amino acid residues were conserved throughout all the sequences. This group's contigs and unigenes of black pepper represented tRNA (guanine26-N2/guanine27-N2)-dimethyltransferase. Conserved motifs like DFY at the 14th to 16th position and FV at the 20th position were present in group II. The other amino acid residues which have shown conservation in all enzyme candidates were K, P, S, K, S, K, Y, C, C, S and H. Moreover, the most significant number of conserved amino acid residues were distributed between the Lysine (K) at the 2nd position and 31st position.
Furthermore, in group III, the longest motif PDPHFK, among all three groups, was found along with a GG- double glycine motif. Additionally, this group found the longest motif between two Leucine residues. Moreover, a prominence of D, P and G were found in the three groups. Thus, the conserved motif analysis by MEME has confirmed the presence of the conserved catalytic domain of MTases.
2.6. Comparison of MTase gene expression during P. capsici infection in black pepper
The gene expression of MTase candidate genes during P. capsici challenge was done to understand the impact of biotic stress on tRNA nucleoside methylations. The FPKM of fifty-nine MTases were compared with the control to understand the fate of these genes during the pathogen challenge (Fig. 5). The study revealed the MTase gene expression during P. capsici infection and uninfected black pepper samples showed differential expression, among which eleven genes were significantly expressed (p ≤ 0.05) (Fig. 6).
Fig. 5.
The Bubble plot showing the differential expression of MTase candidates in Black pepper during P. capsici infection (Pn_IL) in comparison with control (Pn_CL). FDR (False Discovery Rate) control is the statistical method used here for the normalization of FPKM values.
Fig. 6.
The eleven MTase candidates in Black pepper show significant differential expression (p ≤ 0.05) during P. capsici infection. The blue colour represents low-expressed MTase candidate genes, and the red colour represents highly expressed MTase candidate genes. Pn_CL control; Pn_IL P. capsici infected samples. The FPKM values were used to plot the heatmap. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The significantly regulated contigs include the one which represents MTases like tRNA (guanine-N(7)-)-methyltransferase subunit WDR4-like protein, which is needed for the formation of N7-methylguanine at position 46 (m7G46) in tRNA [39], tRNA (cytidine(34)-2′-O)-methyltransferase (TrmL) which is involved in the methylation reaction of 34th position of C to Cm, Cm to Cmnm5U and also involved in the methylation of Cmnm5U to Cmnm5Um [4], the tRNA (guanine(37)-N1)-methyltransferase (TRM5)1-like protein which methylates the N1 position of guanosine-37 near to the anticodon loop. Moreover, it catalyzes the initial step in the biosynthesis of wybutosine (yW), a modified base that is essential for the proper decoding of tRNA anticodons [7] and tRNA (guanine(26)-N(2))-dimethyltransferase execute two successive methylations of guanosine (G) to N2-methylguanosine (m2G) and N2-methylguanosine (m2G) to N2, N2-dimethylguanosine (m22G). These gene candidates were significantly upregulated in infected samples. Altogether the enzymes involved in the formation of Guanosine and Cytosine derivatives like m7G, m1G, m2G, m22G, Cm, Cmnm5U, and Cmnm5Um are significantly upregulated in the infected leaf sample. Moreover, phosphoethanolamine N-methyltransferase, which is a SAM-dependent methyltransferase and some candidates of tRNA (adenine(58)-N(1))-methyltransferase catalytic subunit TRMT61A- tRNA, which catalyzes the formation of N1-methyladenine at position 58 (m1A58) in the initiator methionyl-tRNA were down-regulated in infected leaf. The expression of enzyme gene candidates, which showed significant differential expression in infected samples, was validated by real-time qRT PCR analysis with mock-treated and 24 hpi plants. The qRT PCR analysis was consistent with the FPKM values (Fig. 7).
Fig. 7.
The qRT-PCR analysis of gene candidates shown significant differential expression during 24 hpi. The x-axis is the hours post infection, MSL is the mock leaf, and 24 hpi is 24 h post-infection with P. capsici. While performing the unpaired test, p values less than 0.05 are summarised with one asterisk 0.001 are summarized with three asterisks, and p values less than 0.0001 are summarized with four asterisks, ns means non-significant p-value summarised greater than 0.05.
2.7. Comparison of tRNA modification profile of P. capsici infected vs uninfected black pepper
The present study investigated how the composition and abundance of tRNA-modified nucleosides change in response to P. capsici infection in black pepper. LC-MS analysis can detect and quantify tRNA nucleoside modification and was used to identify and quantify the tRNA modification during stress response (Fig. 8).
Fig. 8.
The heatmap shows the tRNA modification profile comparison in uninfected, 24 hpi and 48 hpi black pepper leaf samples. MRM (Multi reaction monitoring) peaks of modified nucleoside were extracted and normalized to the quantity of the tRNA purified. The 24SL and 48SL are the 24, and 48 hpi P. capsici infected samples, and groups 24MSL and 48MSL are the 24 h and 48 h mock-treated samples.
The LC-MS analysis has shown that the modifications, namely, N4-acetylcytidine (ac4C), 5-formyl-2′-O-methylcytidine (f5Cm), 5-methyluridine (m5U), 5-methoxyuridine (mo5U) and 5-methoxycarbonylmethyl-2′-o-methyluridine (mcm5Um) have significantly increased in infected samples. This differential abundance indicates the impact of biotic stress on certain tRNA modifications. Moreover, the increase in fold change of these modifications was ≥ 1.5. The N4-acetylcytidine (ac4C) was the most abundantly found modification in 24 hpi samples followed by 5-formyl-2′-O-methylcytidine (f5Cm) compared to the uninfected samples.
Surprisingly, besides the abundance of ac4C, a similar trend was not observed at 48 hpi. Some tRNA modifications like 5-taurinomethyluridine(tm5U), pseudouridine (Ψ), 2′-O-methyluridine (Um), 3′-O-methyluridine (3′-OMeU), 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A), 5-carboxymethyluridine (cm5U), 3-methylcytidine (m3C), 3′-O-methylcytidine (3′-OmeC), 2′-O-methylcytidine (Cm), 3-methyluridine (m3U), Queuosine (Q), 5-formyl-2′-O-methylcytidine (f5Cm) were significantly increased at 48 hpi. The increase of tm5U modification was more than sevenfold during 48 hpi and least found during 24 hpi, indicating a time-dependent reprogramming of tRNA modifications in black pepper during stress regulation. Moreover, the modifications like N4-acetylcytidine (ac4C), and the f5Cm modification, have shown consistent abundance in the 24 hpi and 48 hpi samples.
Further, LC-MS was used to confirm the abundance of methylations whose potential MTase genes were upregulated during P. capsici infection. The results showed that the intermediates and final products of the reactions catalyzed by upregulated MTases were increased during P. capsici infection. The nucleoside profile of Cm, m2G, m2,2G whose corresponding MTases were upregulated during P. capsici infection has shown an increase at 48 hpi. Though the nucleoside profile of m7G was not consistent with the expression of its cognate MTase, N2, N2,7-trimethylguanosine (m2,2,7G), which are the by-products of the m7G reactions were increased upon pathogen infection.
Whereas other four modifications, 5-methyluridine (m5U), 5-formyl-2′-O-methylcytidine (f5Cm), 5-methoxyuridine (mo5U) and 5-methoxycarbonylmethyl-2′-o-methyluridine (mcm5Um) have shown a significant difference. But the commonly seen Gm modification in plants like Arabidopsis and Rice [59,60] were not or feebly detected in black pepper.
2.8. Real-time analysis of enzymes involved in ac4C modification
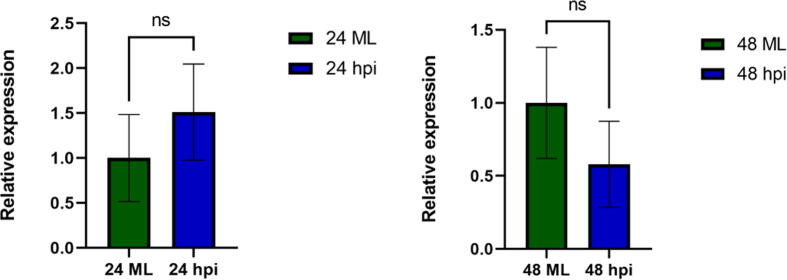
The abundance of ac4C in the LC-MS analysis during P. capsici infection has led us to investigate the crucial enzyme, N-acetyltransferase 10 (NAT10). NAT10 is involved in the acylation of tRNA nucleoside cytidine (C) to N4-acetylcytidine (ac4C) [16]. The qRT-PCR analysis has shown that the NAT10 expression was increased at 24 hpi but later decreased at 48 hpi (Fig. 9).
Fig. 9.
The qRT-PCR analysis of NAT10 expression during 24 hpi and 48 hpi.
The qRT-PCR analysis of gene candidates showed significant differential expression during 24 hpi. The x-axis is the hours post infection where 24 ML is the mock leaf, and 24 hpi is 24 h post-infection with P. capsici and 48ML is the mock leaf, and 48 hpi is 48 h post-infection with P. capsici. While performing the unpaired test, the p-value ns means non-significant p-value summarised greater than 0.05.
3. Discussion
Black pepper is a well-known spice crop, fostered with various health benefits and has been used for ages. The globally used spice crop is exposed to various biotic and abiotic threats. Among them, the infection caused by P. capsici is the most devastating threat to black pepper cultivation. Plants respond to infections by reprogramming their molecular and cellular mechanisms. The outburst of ROS is one of the primary levels of defence opted by the plants during pathogen invasion [21]. The accumulation of ROS and associated cell death in black pepper during P. capsici challenge were detected using histochemical methods like Trypan blue, DAB and NBT, as these are the standards to detect ROS accumulation. Even in other plants like strawberries, pumpkins, etc., the same procedure was used to visualize ROS outbursts after pathogen challenges [46]. Interestingly, studies in yeast demonstrated that under oxidative stress, the tRNA modifications are dynamically altered [11], [42]. The present study is the first attempt to unveil the impact of stress in tRNA modifications in a non-model spice crop like black pepper. Despite several studies that have revealed the molecular level changes during pathogen attack in black pepper, we attempted to uncover the status of tRNA modifications during P.capsici infection. The study has explored the various tRNA nucleoside modifications, corresponding enzymes in black pepper and their response to pathogen attacks.
The technical intricacies in tRNA isolation and purification hinder thorough research on tRNA modification in non-model plants. The size-specific separation of tRNAs using Urea polyacrylamide gel electrophoresis is a facile and widely accepted method for tRNA studies in higher organisms [35]. Further, an LC-MS approach was used to scrutiny the alterations in the tRNA modification profile of black pepper during P. capsici infection. Though LC-MS cannot predict the exact positions of modifications, it is mainly used for the qualitative and quantitative analysis of nucleoside modifications under different cellular conditions [56]. For example, the LC-MS accurately quantified the abundance of different tRNA nucleoside modifications like Cm, m5C and m22G modifications in S. cerevisiae during oxidative stress induced by H2O2 [8], [49]. Our study identified fifty different modifications in black pepper, whereas similar studies in Arabidopsis and Rice have predicted twenty-one and twenty-two different nucleosides, respectively [59,60].
Furthermore, our analysis showed that most of the modifications in black pepper tRNA were methylations catalyzed by enzymes called MTases. Methylations are the products of complex pathways requiring various enzymes and protein complexes. For instance, the mcm52U modification reaction in yeast needs nearly 25 gene products [11], [57]. So later, we investigated the various tRNA MTase gene candidates in black pepper. But the biochemical and molecular extraction of the entire set of MTases in a non-model spice crop like black pepper using conventional methods brings many challenges. So, we relied on the omics datasets of black pepper to predict the MTases gene candidates in black pepper [41], [31], [32]. Previous studies in yeast, Arabidopsis, Rice etc., have used the protein sequence homology-based extraction of tRNA modification gene candidates from the omics datasets [11]. Similarly, we extracted the MTase gene candidates from the publicly available black pepper transcriptome based on sequence homology with yeast, Rice and Arabidopsis. Further, the bioinformatic characterization of the black pepper MTases shown the presence of multiple conserved motifs compared to yeast, Arabidopsis and Rice, which may be crucial in rendering catalytic activity to the enzymes [22].
Similar to modifications, corresponding enzymes that catalyze these reactions also have a crucial role in stress response [43], [44]. In line with this during P. capsici infection, the MTase candidate genes involved in the methylation of Guanine, Cytosine and their derivatives were upregulated in black pepper. Predominantly, the candidate genes associated with the methylation of m7G, Cm, Cmnm5U, Cmnm5Um, m1G, Wybutosine, m1G, m2G and m22G were significantly upregulated, which suggests the importance of tRNA methylations during the stress response. Intriguingly, we noted the increase of corresponding nucleoside modifications and some of their intermediates in the LC-MS profile of pathogen-challenged black pepper. Our results were in partial agreement with previous reports in Rice and Arabidopsis where the tRNA modifications and their related MTases gene expression of tRNA nucleosides namely Cm (2′-O-methylcytidine), m1 A (1- methyladenosine), and m7 G (7-methylguanosine) were implicated in plant stress regulation [59,60]. Surprisingly in black pepper, the differential analysis of MTases during P. capsici infection didn't signify the enzymes responsible for Am modification which was increased in Arabidopsis during biotic stress.
Furthermore, the LC-MS profile demonstrated that N4-acetylcytidine (ac4C) was the most abundant modification present in black pepper during P. capsici infection at 24 hpi. The ac4C modification is known to present at the wobble position of tRNA Met in E.coli [33], tRNAGln, tRNAGlu, tRNALys, tRNAPro, and tRNASer from halobacteria species [37]. So, the absence of ac4C disturbs the correct codon-anticodon pairing. Additionally, in humans, the significance of ac4C during stress response was noted during several diseases [27]. However, in plants, the ac4C modification was previously known for enhancing translation efficiency [2]; for the first time, the current study reports the abundance of ac4C modification in P. capsici infection hints its implication in stress regulation. The synthesis of ac4C modification is catalyzed by the enzyme- N-acetyltransferase 10 (NAT10) or its homologous enzyme [28]. The qRT analysis showed an increase in gene expression during 24hpi. This observation support the increase of ac4C abundance at 24 hpi. As mentioned earlier, the expression of enzymes associated with modification is very critical in the determine the presence of corresponding modification in the cell. For example, the down regulation of TRMT61B gene which catalyses m1A58 in three mt-tRNAs Leu, Ser, and Lys has resulted in the decrease of corresponding modifications in Alzheimer′s disease [13].
The f5Cm modification that is present at the 34th position of tRNA Leu [26] was the second most abundant modification at 24 hpi. Further, during 48hr post infection, the tm5U modification was significantly elevated. The presence of tm(5)U modification was reported at the 34th wobble position of tRNA Leu, tRNA Trp and tRNA Lys, tRNA Gln, tRNA Glu [50]. In humans, the lack of tm5U modification in 34th wobble position leads to MELAS (Mitochondrial Encephalomyopathy, Lactic Acidosis and Stroke-like episodes) disease [29], [30]. However, the enzymes involved in the formation of tm5U modification are not reported yet [20], [47]. Nevertheless, there exists a temporal alteration in the modification profile, the most abundant modifications ac4C, tand m5U, f5Cm were found in the wobble position of multiple tRNAs. The modifications present at the wobble position of the anticodon loop are crucial in the codon-anticodon pairing, thereby facilitating the translation. The significant increase of such modifications during pathogen infection can be an adaptive response chosen by the plant to enhance the translation during stress. Similarly, in yeast the modifications of tRNALysUUU, tRNAGlnUUG and tRNAGluUUC at the wobble positions were increased during high temperatures and restored at normal conditions [1].
Nevertheless, we have identified and quantified an extensive set of tRNA nucleotides, and we never intend to claim that the tRNA modifications, which were not detected in the study, are not present in black pepper; also the possibility of missing their detection in the LC-MS approach cannot be ignored. In an LC-MS study in yeast could not detect 2′-O-ribosyladenosine phosphate (Ar(p)) moreover, the 5-Carbamoylmethyl-2′-O-methyluridine (ncm5Um) was uncertainly detected by collision-induced dissociation (CID) due to weak signal strength [48]. Furthermore, the LC-MS data cannot map the exact position of modification in tRNAs. But mapping these abundant modifications in black pepper can highly assist in confirming the functional relevance of these modification in stress response. Here we assume that the modifications which have a regulatory role in defence regulation may lie in the anticodon region to enhance the translation during the stress response. Studies have shown that the modifications in the anticodon regions are crucial during the translation of stress-responsive proteins [9], [27].
In conclusion, we identified a wide range of tRNA modifications in black pepper, a non-model spice crop which is extensively used for its taste and other medicinal benifits. Among the various predicted tRNA modifications, methylations were predominant in black pepper. Since MTases catalyze the methylations, we further annotated the tRNA MTase gene candidates from the publicly available black pepper transcriptome. Further, comparison of MTase candidate gene expression during P. capsici- black pepper interaction showed their significant upregulation. Our study revealed the differential expression of tRNA modifications and cognate enzymes during P. capsici infection in black pepper. Moreover, the LC/MS profile of black pepper tRNA modification during P. capsici infection demonstrated a time-dependent variation in the profile. Since, to date, the pathways of tRNA nucleoside modification are not fully depicted even in model organisms, our study is the first attempt to outline the story of tRNA modifications and their role in stress response in a non-model spice crop like black pepper. So, our future efforts will be to overcome the present limitations of the study by isolating the individual tRNAs and mapping the modifications associated with them in black pepper. Additionally, to validate the direct role of tRNA modification in stress response. Similar studies can assist researchers in predicting the hidden functions of tRNA modifications in other higher plants.
4. Materials and methods
4.1. Plant inoculation by the pathogen
Black pepper young, healthy plantlets were inoculated with a pure culture of P. capsici collected from the Department of Plant Pathology, College of Agriculture, Vellayani. Kerala, India. The P. capsici culture was maintained in Potato Dextrose Agar at 28 °C. The 48 hr old P. capsici culture plug was used to inoculate the abaxial side of black pepper leaves. The inoculation procedure was done according to Kattupalli et al. [31], [32]. The uninfected plants were mock-inoculated with PDA plugs with sterile water. Both the infected and the uninfected plants were maintained under the same environmental conditions. The leaf above the infected leaf (systemic leaves) from both uninfected and infected plants in triplicates were harvested at different time intervals like 24 hpi and 48 hpi for further studies.
4.2. DAB, NBT and trypan blue staining
The pathogen infection in plants leads to the release of several reactive oxygen species. DAB staining [17], [52], NBT Staining [36], [23] were done for detecting the release of H2O2 and O− respectively whereas Trypan blue staining was carried out to evaluate cell death as previously described with slight modifications [24].
Briefly, the P. capsici inoculated leaf samples were immersed in DAB solution and kept for shaking for about 4–5 h. Later the sample was treated with a bleaching solution (ethanol: acetic acid: glycerol = 3:1:1) in a water bath at 95 °C for 15 min. The step was repeated by immersing the leaf samples in fresh bleaching solution until the chlorophyll was fully bleached out and then visualized under a light microscope. The detection of O−, which is generated by the activity of NADH dehydrogenase was done by NBT staining. NBT was dissolved in Sodium Phosphate Buffer (pH 7.5), then the leaves were immersed in the solution and incubated in a shaker for 3–4 h at 27 °C. Later, NBT was removed by adding 100 % ethanol and kept in the water bath for 10–15 min at 90 °C and then visualized under the light microscope. Trypan blue staining solution was prepared as previously mentioned by Heese and team (6 vol of ethanol, 1 vol of water, 1 vol of lactic acid, 1 vol of glycerol, 1 vol of phenol and 0.067 % wt/vol trypan blue) [24]. The stain is then added to the leaf sample and heated in a boiling water bath for 10 min or till the leaves were completely devoid of chlorophyll. Later, the samples were washed with 1X Phosphate-buffered saline (PBS). A set of three young plants were used as biological replicates, and leaves were sampled from each plant for all microscopic analysis.
4.3. Total RNA isolation
The leaf samples were collected from the uninfected (mock-infected at 24 h and 48 h) and infected samples at 24 and 48 hpi. Total RNA was isolated from all the samples using the miRVana Total RNA isolation kit (Thermo Fisher) according to the manufacturer’s protocol. Briefly, 1 gm of freeze-dried leaf samples were homogenized with lysis buffer and RNA isolation aid. The mixture was centrifuged at 12,000 rpm for 5 min, 4 °C. The supernatant was collected then 1/10th volume of miRNA homogenate additive and an equal amount of acid phenol: chloroform (Thermo Fisher) were added. The upper aqueous phase was retrieved, and 100 % ethanol was added and allowed to pass through a filter cartridge provided by the manufacturer (Thermo Fisher). The flow-through was discarded, and the filter cartridges were washed with miRNA wash solution I and then with miRNA wash solution II. The filter cartridge was transferred to a fresh collection tube, and RNA was recovered with 100 µL of preheated (95 °C) elution buffer or nuclease-free water. The quality and quantity of thus obtained total RNA samples were assessed by absorbance measurements at 230, 260, and 280 nm in a NanoDrop™1000 spectrophotometer (Colibri, Germany). The integrity of total RNA was checked by 1.2 % (w/v) Agarose Gel Electrophoresis. The isolated total RNA with quality and integrity was submitted to Arraystar, USA for LC-MS analysis.
4.4. tRNA isolation
tRNA was isolated from total RNA samples by Urea-PAGE electrophoresis. The total RNA for each sample was separated by 7.5 % PAGE (29:1 acrylamide: bisacrylamide) containing 7 M urea. The 60–90 nt tRNA band was excised from the gel and then extracted 0.3 M NH4Ac and precipitated with glycogen and ethanol. Purified tRNA was quantified using Qubit RNA HS Assay kit (ThermoFisher, Q32855) and proceeded to further downstream analysis.
4.5. tRNA digestion and LC-MS analysis
Purified tRNA was hydrolyzed to single nucleosides and dephosphorylated by a 50 μL enzyme mix (10 U Benzonase (Sigma), 0.1 U Phosphodiesterase I (US Biological), 1U Alkaline Phosphatase (NEB)]. Pre-treated nucleosides solution was deproteinized using Satorius 10,000-Da MWCO spin filter. Then the reaction was incubated at 37 °C for 3 h. Parallelly, a 10,000-Da MWCO spin filter (Satorius) was rinsed by adding 300 µL of deionized water and centrifuging for 5 min at 16,000 g at 4 °C. The hydrolyzed RNA sample was transferred to the rinsed spin filter and centrifuged for 10 min at 16,000 g at 4 °C. The filtrate was collected for downstream LC-MS analysis. The single nucleosides mixtures from black pepper tRNA were injected into the LC-MS system and set up the HPLC condition according to solvent gradient (Solution A, HPLC-grade water with the relevant amount of formic acid to obtain a final formic acid concentration of 0.1 % (vol/vol); Solution B, 100 % acetonitrile with the appropriate amount of formic acid to achieve a final formic acid concentration of 0.1 % (vol/vol).
4.6. Transcriptome-wide extraction of tRNA methyltransferase candidates from black pepper
The protein sequences of known tRNA nucleoside MTases from S. cerevisiae, Arabidopsis (https://www.arabidopsis.org/) and Rice (https://rice.plantbiology.msu.edu/index.shtml) were considered as query sequences. These query sequences were used to retrieve black pepper MTase gene homologs with tblastn search using bioedit software (https://bioedit.software.informer.com/). Along with these, using NR and Swissport annotations, we have curated all the MTase genes from the black pepper transcriptome. All obtained sequences were translated into all six frames using Geneious software (https://www.geneious.com/) for further analysis. A cut-off e-value was set as 1.0E−6 for the initial identification of candidate genes. A phylogenetic tree of MTases was constructed with the Maximum likelihood method in MEGA X software, bootstrap analysis was performed with 1000 iterations. Protein sequences were manually verified by protein domain analysis on NCBI-CDD (Conserved Domain Database) website (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The logos and conserved motifs were identified by MEME online searching engine (https://meme-suite.org/tools/meme) with default parameters setup.
4.7. Gene expression analysis of MTase candidate genes
The spatial expression pattern of MTases were analyzed by the comparative analysis between control and P. capsici challenged transcriptomes of black pepper plants (SRX853366- PnIL; SRA050094- PnCL). The expression levels of each MTase candidate unigenes were taken by their corresponding FPKM (fragments per kilobase per million mapped reads). The raw reads from the mRNA transcriptomes were submitted in the NCBI Sequence Read Archive [NCBI: SRX853366 (PNIL) and SRA050094 (PNCL)] treated and control transcriptome which was previously done by our lab were used for the analysis. The calculation of Unigene expression uses FPKM (RPKM) method (Fragments Per kb per Million reads), the formula is shown below.
| FPKM = (10^6 C)/(NL/10^3) |
Set FPKM(A) to be the expression of Unigene A, and C to be number of fragments that uniquely aligned to Unigene A, N to be total number of fragments that uniquely aligned to all Unigenes, and L to be the base number in the CDS of Unigene A. The FPKM method is able to eliminate the influence of different gene length and sequencing level on the calculation of gene expression. Therefore, the calculated gene expression can be directly used for comparing the difference of gene expression between samples. A heatmap representing all the MTases genes in uninfected and infected samples was generated using the edge R package. 1 µg of total RNA was used for cDNA synthesis using from high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time quantitative reverse-transcription (qRT-PCR) was performed using ABI Quantstudio using POWER SYBR Green qPCR Master Mix (Applied Biosystems) according to the manufacturer's instructions. Each qRT-PCR reaction was conducted in 10 µL volumes containing 1 µL of cDNA (5 ng), 5 µL of SYBR green and 5 ρmol of forward and reverse primers with the following conditions: 40 cycles at 95 °C for 15 s and 65 °C for 15 s. Negative PCR controls (Non template control- NTC) were prepared to detect possible contamination. The primers used were shown in Supplementary Table 2. The expression of these genes was compared with that of endogenous control gene 5.8 s rRNA. Relative mRNA ratios were calculated by 2−ΔΔCT [40]. The experiments were done in triplicates.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
A.U acknowledges the Department of Science and Technology (DST) – JRF & SRF. S.B acknowledges CSIR, P.V, K.D and E.V.S greatly acknowledge the Department of Biotechnology (DBT), Government of India.
Funding
This work was supported by the Rajiv Gandhi Center for Biotechnology and the Department of Science and Technology, Govt. of India under the DST/INSPIRE Fellowship (DST/INSPIRE FELLOWSHIP 2015/IF150352).
List of author contributions
A.U and E.V.S conceived the research plans and designed the experiments. A.U, K.D performed the wet-lab experiments, and S.B assisted the same. A.U, K.D and P.V did in silico experiments and did data analysis. A.U wrote the article with the contributions of all the authors; S.B, P.V and K.D made critical revisions. E.V.S supervised and complemented the writing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2022.11.002.
Contributor Information
Aswathi Usha, Email: aswathiu@rgcb.res.in.
Divya Kattupalli, Email: divya.kattupalli9@gmail.com.
Pooja Viswam, Email: poojaviswam@rgcb.res.in.
Sruthi Bharathan, Email: sruthibharathan@rgcb.res.in.
Eppurath Vasudevan Soniya, Email: evsoniya@rgcb.res.in.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Alings F., Sarin L.P., Fufezan C., Drexler H.C., Leidel S.A. An evolutionary approach uncovers a diverse response of tRNA 2-thiolation to elevated temperatures in yeast. RNA. 2015;21(2):202–212. doi: 10.1261/rna.048199.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atanasova L. Modified nucleosides in plant transfer RNA. Comptes Rendus de l Academie Bulgare des Sciences. 2011;64:67. [Google Scholar]
- 3.Bednářová A., Hanna M., Durham I., VanCleave T., England A., Chaudhuri A., et al. Lost in translation: defects in transfer RNA modifications and neurological disorders. Front Mol Neurosci. 2017;10:135. doi: 10.3389/fnmol.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benítez-Páez A., Villarroya M., Douthwaite S., Gabaldón T., Armengod M.E. YibK is the 2’-O-methyltransferase TrmL that modifies the wobble nucleotide in Escherichia coli tRNA(Leu) isoacceptors. RNA (New York, N.Y.) 2010;16:2131–2143. doi: 10.1261/RNA.2245910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco S., Dietmann S., Flores J.V., Hussain S., Kutter C., Humphreys P., et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2020;33(18):2020–2039. doi: 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boccaletto P., MacHnicka M.A., Purta E., Pitkowski P., Baginski B., Wirecki T.K., et al. MODOMICS: A database of RNA modification pathways. 2017 update. Nucl Acids Res. 2018;46(D1):D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brulé H., Elliott M., Redlak M., Zehner Z.E., Holmes W.M. Isolation and characterization of the human tRNA-(N1G37) methyltransferase (TRM5) and comparison to the Escherichia coli TrmD protein. Biochemistry. 2004;43(28):9243–9255. doi: 10.1021/BI049671Q. [DOI] [PubMed] [Google Scholar]
- 8.Chan CT, Pang YLJ, Deng W, Babu IR, Dyavaiah M, Begley TJ, Dedon PC. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun 2012;3(1):1-9. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed]
- 9.Chan C., Pham P., Dedon P.C., Begley T.J. Lifestyle modifications: coordinating the tRNA epitranscriptome with codon bias to adapt translation during stress responses. Genome Biol. 2018;19(1):1–11. doi: 10.1186/s13059-018-1611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K., Guo T., Li X.M., Zhang Y.M., Yang Y.B., Ye W.W., et al. Translational Regulation of Plant Response to High Temperature by a Dual-Function tRNAHis Guanylyltransferase in Rice. Mol Plant. 2019;12(8):1123–1142. doi: 10.1016/J.MOLP.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen P., Jäger G., Zheng B. Transfer RNA modifications and genes for modifying enzymes in Arabidopsis thaliana. BMC Plant Biol. 2010;10(1):201. doi: 10.1186/1471-2229-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou H.J., Donnard E., Gustafsson H.T., Garber M., Rando O.J. Transcriptome-wide Analysis of Roles for tRNA Modifications in Translational Regulation. Mol Cell. 2017;68(5):978–992.e4. doi: 10.1016/J.MOLCEL.2017.11.002/ATTACHMENT/033AAC85-987F-49D4-8128-B3740560756D/MMC10.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chujo T., Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18(12):2269–2276. doi: 10.1261/rna.035600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Close P., Bose D., Chariot A., Leidel S.A. Cancer and noncoding RNAs. Academic Press; 2018. Dynamic regulation of tRNA modifications in cancer; pp. 163–186. [DOI] [Google Scholar]
- 15.Motorin Y., Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010;49(24):4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 16.Dalhat M.H., Altayb H.N., Khan M.I., Choudhry H. Structural insights of human N-acetyltransferase 10 and identification of its potential novel inhibitors. Scientific Rep. 2021;11(1):1–10. doi: 10.1038/s41598-021-84908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daudi A, O'brien JA, Author BP. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012;2(18). doi:10.21769/bioprotoc.263. [PMC free article] [PubMed]
- 18.Delaunay S., Frye M. RNA modifications regulating cell fate in cancer. Nat Cell Biol. 2019;21(5):552–559. doi: 10.1038/s41556-019-0319-0. [DOI] [PubMed] [Google Scholar]
- 19.Engelke D.R., Hopper A.K. Modified View of tRNA: Stability amid Sequence Diversity. Mol Cell. 2006;21(2):144–145. doi: 10.1016/j.molcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Fakruddin M, Wei F-Y, Scorrano L, Suzuki T, Tomizawa K. Defective Mitochondrial tRNA Taurine Modification Activates Global Proteostress and Leads to Mitochondrial Disease Data and Software Availability GSE98332; 2018. doi:10.1016/j.celrep.2017.12.051. [DOI] [PubMed]
- 21.Fones H., Preston G.M. Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS Microbiol Lett. 2012;327(1):1–8. doi: 10.1111/J.1574-6968.2011.02449.X. [DOI] [PubMed] [Google Scholar]
- 22.Gregorio J., Hernández-Bernal A.F., Cordoba E., León P. Characterization of evolutionarily conserved motifs involved in activity and regulation of the ABA-INSENSITIVE (ABI) 4 transcription factor. Mol Plant. 2014;7(2):422–436. doi: 10.1093/mp/sst132. [DOI] [PubMed] [Google Scholar]
- 23.Grellet Bournonville C.F., Díaz-Ricci J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem Anal. 2011;22(3):268–271. doi: 10.1002/PCA.1275. [DOI] [PubMed] [Google Scholar]
- 24.Heese Heese A., Hann D.R., Gimenez-Ibanez S., Jones A.M., He K., Li J., et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci. 2007;104(29):12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holley R.W., Everett G.A., Madison J.T., Zamir A. Nucleotide sequences in the yeast alanine transfer ribonucleic acid. J Biol Chem. 1965;240(5):2122. [PubMed] [Google Scholar]
- 26.de Barros Jean-Paul Païs, Keith Gérard, El Adlouni Chakib, Glasser Anne-Lise, Mack Gérard, Dirheimer Guy, Desgrès Jean. 2′- 0 -Methyl-5-Formylcytidine (F 5 Cm), a New Modified Nucleotide at the ‘wobble’ Position of Two Cytoplasmic tRNAs Leu (NAA) from Bovine Liver. Nucl Acids Res. 1996;24(8):1489–1496. doi: 10.1093/nar/24.8.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin G, Xu M, Zou M, Duan S. The Processing, Gene Regulation, Biological Functions, and Clinical Relevance of N4-Acetylcytidine on RNA: A Systematic Review. In Molecular Therapy – Nucleic Acids (vol. 20, pp. 13–24). Cell Press; 2020. doi:10.1016/j.omtn.2020.01.037. [DOI] [PMC free article] [PubMed]
- 28.Johansson M.J.O., Byström A.S. The Saccharomyces cerevisiae TAN1 gene is required for N 4-acetylcytidine formation in tRNA. RNA. 2004;10(4):712–719. doi: 10.1261/rna.5198204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamble A.S., Kumbhar B.V., Sambhare S.B., Bavi R.S., Sonawane K.D. Conformational preferences of modified nucleoside 5-taurinomethyluridine, τm5U occur at ‘wobble’34th position in the anticodon loop of tRNA. Cell Biochem Biophys. 2015;71(3):1589–1603. doi: 10.1007/s12013-014-0382. [DOI] [PubMed] [Google Scholar]
- 30.Kamble A.S., Fandilolu P.M., Sambhare S.B., Sonawane K.D. Idiosyncratic recognition of UUG/UUA codons by modified nucleoside 5-taurinomethyluridine, τm5U present at ‘wobble’position in anticodon loop of tRNALeu: A molecular modeling approach. PLoS ONE. 2017;12(4):e0176756. doi: 10.1371/journal.pone.0176756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kattupalli D., Srinivasan A., Soniya E.V. A Genome-Wide Analysis of Pathogenesis-Related Protein-1 (PR-1) Genes from Piper nigrum Reveals Its Critical Role during Phytophthora capsici Infection. Genes. 2021;12(7):1007. doi: 10.3390/genes12071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kattupalli D, Pinski A, Sreekumar S, Usha A, Girija A, Beckmann M, Mur LAJ, Eppurathu Vasudevan S. Non-Targeted Metabolite Profiling Reveals Host Metabolomic Reprogramming during the Interaction of Black Pepper with Phytophthora capsici. Int J Mol Sci 2021;22:11433. doi:10.3390/IJMS222111433. [DOI] [PMC free article] [PubMed]
- 33.Murao K, Yahagi T, Von Minden DL, McCloskey JA, Nishimura S. Characterization of C + located in the first position of the anticodon of Escherichia coli tRNA Met as N 4 –acetylcytidine. Biochim Biophys Acta 1972;262:209–213. doi:10.1016/0005-2787(72)90234-1. [PubMed]
- 34.Koh CS, Sarin LP. Transfer RNA modification and infection – Implications for pathogenicity and host responses. Biochimica et Biophysica Acta - Gene Regulatory Mechanisms (Vol. 1861, Issue 4, pp. 419–432). Elsevier B.V. doi:10.1016/j.bbagrm.2018.01.015. [DOI] [PubMed]
- 35.Köhrer C., RajBhandary U.L. The Many applications of acid urea polyacrylamide gel electrophoresis to studies of tRNAs and aminoacyl-tRNA synthetases. Methods. 2008;44(2):129–138. doi: 10.1016/j.ymeth.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar D, Yusuf M, Singh P, Sardar M, Sarin N. Histochemical Detection of Superoxide and H2O2 Accumulation in Brassica juncea Seedlings. Bio-protocol 2014;4(8). 10.21769/BIOPROTOC.1108.
- 37.Kumbhar B.V., Kamble A.D., Sonawane K.D. Conformational preferences of modified nucleoside N (4)-acetylcytidine, ac4C occur at the “wobble” 34th position in the anticodon loop of tRNA. Cell Biochem Biophys. 2013;66(3):797–816. doi: 10.1007/s12013-013-9525-8. [DOI] [PubMed] [Google Scholar]
- 38.Laxman S., Sutter B.M., Wu X., Kumar S., Guo X., Trudgian D.C., et al. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell. 2013;154(2):416–429. doi: 10.1016/j.cell.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin S., Liu Q., Lelyveld V.S., Choe J., Szostak J.W., Gregory R.I. Mettl1/Wdr4-Mediated m 7 G tRNA Methylome Is Required for Normal mRNA Translation and Embryonic Stem Cell Self-Renewal and Differentiation. Mol Cell. 2018;71(2):244–255.e5. doi: 10.1016/J.MOLCEL.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method Methods (San Diego, Calif) 2001;25(4):402–408. doi: 10.1006/METH.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Mahadevan C., Krishnan A., Saraswathy G.G., Surendran A., Jaleel A., Sakuntala M. Transcriptome-assisted label-free quantitative proteomics analysis reveals novel insights into Piper nigrum—Phytophthora capsici Phytopathosystem. Front Plant Sci. 2016;7:785. doi: 10.3389/fpls.2016.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nawrot B., Sochacka E., Düchler M. tRNA structural and functional changes induced by oxidative stress. Cell Mol Life Sci. 2011;68(24):4023–4032. doi: 10.1007/s00018-011-0773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira M., Francisco S., Varanda A.S., Santos M., Santos M.A.S., Soares A.R. Impact of tRNA Modifications and tRNA-Modifying Enzymes on Proteostasis and Human Disease. Int J Mol Sci. 2018;19(12) doi: 10.3390/IJMS19123738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramírez V., González B., López A., Castelló M.J., Gil M.J., Zheng B., et al. A 2’-O-Methyltransferase Responsible for Transfer RNA Anticodon Modification Is Pivotal for Resistance to Pseudomonas syringae DC3000 in Arabidopsis. Molecular Plant-Microbe Interactions: MPMI. 2018;31(12):1323–1336. doi: 10.1094/mpmi-06-18-0148-r. [DOI] [PubMed] [Google Scholar]
- 45.Salazar S.M., Castagnaro A.P., Arias M.E., Chalfoun N., Tonello U., Díaz Ricci J.C. Induction of a defense response in strawberry mediated by an avirulent strain of Colletotrichum. Eur J Plant Pathol. 2007;117(2):109–122. doi: 10.1007/s10658-006-9075-7. [DOI] [Google Scholar]
- 46.Steiner R.E., Ibba M. Bridging the Gap between tRNA Modifications and the Respiratory Chain. Biochemistry. 2018;57(18):2565–2566. doi: 10.1021/ACS.BIOCHEM.8B00377. [DOI] [PubMed] [Google Scholar]
- 47.Su D., Chan C.T.Y., Gu C., Lim K.S., Chionh Y.H., McBee M.E., et al. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat Protoc. 2014;9(4):828–841. doi: 10.1038/NPROT.2014.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su D, Chan CTY, Gu C, Lim KS, Chionh YH, McBee ME, Russell BS, Babu IR, Begley TJ, Dedon PC. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat Protocols 2014;9(4):828–841. doi:10.1038/nprot.2014.047. [DOI] [PMC free article] [PubMed]
- 49.Suzuki T, Suzuki T, Wada T, Saigo K, Watanabe K. Novel taurine-containing uridine derivatives and mitochondrial human diseases. Nucleic acids symposium series (Vol. 1, No. 1, pp. 257-258). Oxford University Press; 2001. doi:10.1093/nass/1.1.257. [DOI] [PubMed]
- 50.Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat Rev Mol Cell Biol 2021;22(6):375–392. doi:10.1038/s41580-021-00342-0. [DOI] [PubMed]
- 51.Thordal-Christensen H., Zhang Z., Wei Y., Collinge D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997;11(6):1187–1194. doi: 10.1046/J.1365-313X.1997.11061187.X. [DOI] [Google Scholar]
- 52.Torres MA, Jones JD, Dangl JL. Reactive oxygen species signalling in response to pathogens. Plant Physiol 2006;141(2):373-378. doi:10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed]
- 53.Wang Y., Pang C., Li X., Hu Z., Lv Z., Zheng B., et al. Identification of tRNA nucleoside modification genes critical for stress response and development in Rice and Arabidopsis. BMC Plant Biol. 2017 doi: 10.1186/s12870-017-1206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y., Li D., Gao J., Li X., Zhang R., Jin X., et al. The 2′-O-methyladenosine nucleoside modification gene OsTRM13 positively regulates salt stress tolerance in rice. J Exp Bot. 2017;68(7):1479–1491. doi: 10.1093/jxb/erx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Crécy‐Lagard, Valérie Identification of genes encoding tRNA modification enzymes by comparative genomics. Methods in enzymology. 2007:153–183. doi: 10.1016/S0076-6879(07)25007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su Dan, Clement TY Chan, Chen Gu, Kok Seong Lim, Yok Hian Chionh, Megan E McBee, et al. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nature Protocols. 2014;9:828–841. doi: 10.1038/nprot.2014.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bo Huang, Jian Lu, Anders S. Byström. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14(10):2183–2194. doi: 10.1261/rna.1184108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.