Abstract
For most, if not all, organisms, iron (Fe) is an essential element. In response to the nutritional requirement for Fe, bacteria evolved complex systems to acquire the element from the environment. The genes encoding these systems are often coordinately regulated in response to the Fe concentration. Recent investigations revealed that Bordetella avium, a respiratory pathogen of birds, expressed a number of Fe-regulated genes (T. D. Connell, A. Dickenson, A. J. Martone, K. T. Militello, M. J. Filiatraut, M. L. Hayman, and J. Pitula, Infect. Immun. 66:3597–3605, 1998). By using manganese selection on an engineered strain of B. avium that carried an Fe-regulated alkaline phosphatase reporter gene, a mutant was obtained that was affected in expression of Fe-regulated genes. To determine if Fe-dependent regulation in B. avium was mediated by a fur-like gene, a fragment of the B. avium chromosome, corresponding to the fur locus of B. pertussis, was cloned by PCR. Sequencing revealed that the fragment from B. avium encoded a polypeptide with 92% identity to the Fur protein of B. pertussis. In vivo experiments showed that the cloned gene complemented H1780, a fur mutant of Escherichia coli. Southern hybridizations and PCRs demonstrated that the manganese mutant had a deletion of 2 to 3 kbp of nucleotide sequence in the region located immediately 5′ of the fur open reading frame. A spontaneous PCR-derived mutant of the B. avium fur gene was isolated that encoded a Fur protein in which a histidine was substituted for an arginine at amino acid position 18 (R18H). Genetic analysis showed that the R18H mutant gene when cloned into a low-copy-number vector did not complement the fur mutation in H1780. However, the R18H mutant gene was able to complement the fur mutation when cloned into a high-copy-number vector. The cloned wild-type fur gene will be useful as a genetic tool to identify Fur-regulated genes in the B. avium chromosome.
Most, if not all, living organisms require iron (Fe) for growth. Fe, an element which is very abundant in the environment, is usually quite accessible to free-living bacteria. The situation is very different when bacterial pathogens are considered. Successful establishment of infection by bacterial pathogens requires that the organisms acquire iron directly from the cells, tissues, and fluids of the infected host (39). To inhibit bacterial colonization, the host utilizes a variety of mechanisms to deny the pathogen easy access to the element (39). In response to these selective pressures, bacterial pathogens evolved very efficient molecular mechanisms to assess the availability of Fe within the microenvironments of the host and to coordinately regulate the expression of those genes which are required for expression of Fe uptake systems (22).
The coordinate regulation of Fe acquisition systems has been well characterized in Escherichia coli (6, 15, 16, 33). In that bacterium, Fe-regulated gene expression is mediated by Fur, a DNA-binding protein that represses Fur-dependent promoters. In general, the Fur regulator is responsible for coordinated regulation of Fe-regulated proteins in an inverse relationship to the local concentration of Fe. The Fur protein of E. coli has been well characterized (8, 15, 29, 34, 38). It has been established that E. coli Fur binds Fe. When complexed with the element, FurFe has binding affinity for specific nucleotide sequences known as Fur boxes that are located proximal to Fur-regulated promoters. Binding of FurFe to the Fur box prevents transcription, most likely by interfering with the ability of RNA polymerase to bind to the promoter. Alternatively, binding of Fur may physically block the processivity of RNA polymerase (22). In the absence of Fe, there occurs in Fur an allosteric change that reduces its binding affinity for Fur boxes (34). In this case, the promoter is derepressed and the mRNA of the Fur-dependent gene is synthesized. In the presence of adequate amounts of Fe, Fur-regulated genes are repressed; under Fe-limiting conditions, Fur-regulated genes are expressed.
In addition to E. coli, fur genes have been identified in Salmonella sp. (10), Neisseria sp. (5, 18, 35), Pseudomonas sp. (25, 37), Campylobacter jejuni (36), Yersinia pestis (32), and Bordetella pertussis (4, 7). A common theme with these and other bacteria in which the fur genes have been mutated is that many, but not all, of the Fe-regulated genes do not respond to the local concentration of Fe (4, 7, 14, 24). Since regulation is decoupled from the Fe concentration, expression of fur-dependent genes in fur mutants is constitutive.
Bordetella avium is a respiratory pathogen of birds that has a predilection for ciliated epithelial cells of the trachea. Infection with B. avium produces anorexia, exudative conjunctivitis, sneezing, and a serous discharge from the nares (28). The symptoms elicited by infection of birds by B. avium are similar to those produced by infection of humans with B. pertussis. Although the expression of several outer membrane proteins and extracellular molecules is known to be regulated in B. pertussis (3, 13, 17, 26) and Bordetella bronchiseptica (1, 2, 11, 12) in response to Fe, few studies have been done to identify genes that encode Fe-regulated proteins of B. avium. Recently, a mutant of B. avium 4169 was isolated that contained a transposon (TnphoA) insertion into an Fe-regulated gene (9). Quantitative analysis of the mutant, which was designated Tnpho6, demonstrated that the PhoA fusion protein encoded by the inserted gene was regulated in a coordinate manner with the local concentration of Fe: i.e., the mutant expressed high levels of alkaline phosphatase activity when grown under Fe-limiting conditions, but much less alkaline phosphatase activity when cultured under Fe-replete conditions. We hypothesized that the expression of the Fe-regulated gene in strain Tnpho6 was likely under the control of a Fur-like regulator.
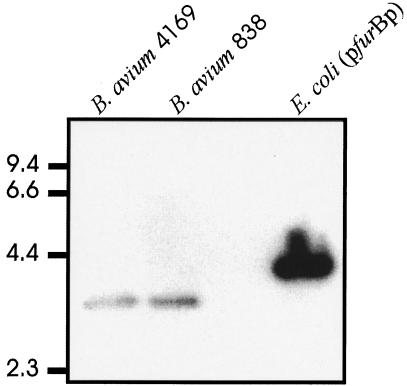
Both genotypic and phenotypic lines of evidence have suggested the presence of an active fur gene in B. avium. Initial experiments with Southern hybridizations of chromosomal DNA demonstrated that B. avium 4169 and 838 had homology to the fur gene of B. pertussis (4, 7) (Fig. 1). Further evidence for a B. avium fur gene was suggested by isolation of a Fur-like mutant of B. avium. Silver et al. (31) and Hantke (16) demonstrated that E. coli fur mutants can be obtained by selection for spontaneous resistance to Mn2+. Although the molecular mechanism is not known, Hantke (16) suggests that Mn2+ stimulates the cell to accumulate higher than tolerable concentrations of Fe. Cells acquiring mutations in fur potentially lose their ability to tightly regulate the fur-dependent Fe uptake systems. As a result, fur mutants, unlike cells with the wild-type allele, do not accumulate toxic levels of Fe. This technique has been used to map the Fur protein of Vibrio cholerae for amino acids that are necessary for regulatory activity (20). To determine if manganese selection would be useful for isolating deregulated mutants of B. avium, strain Tnpho6 was plated on Luria-Bertani agar containing 50 μM Fe chelator Desferol (CIBA-GEIGY, Basel, Switzerland) and 30 mM MnCl2. After overnight incubation at 37°C, 61 robustly growing colonies were replica plated onto brain-heart infusion (BHI) agar (Difco, Detroit, Mich.) containing 36 μM FeSO4 and screened for expression of the alkaline phosphatase reporter gene by using the chromogenic indicator bromo-chloro-indolylphosphate (BCIP) (Sigma Chemical Co., St. Louis, Mo.) (9). Under these growth conditions, the reporter gene in a fur-proficient strain would be expected to be repressed, while a strain harboring a mutant fur gene would express significant levels of alkaline phosphatase activity. Tnpho6Mn, one of seven mutants that showed the appropriate phenotype, was chosen for further study. When Tnpho6Mn was measured for alkaline phosphatase activity, the mutant was found to express over 18-fold greater enzymatic activity than the parental strain Tnpho6 when both strains were cultured in Fe-replete medium (Table 1). These results were consistent with a mutation in a fur-like regulatory gene.
FIG. 1.
Hybridization of B. avium 4169 and 838 chromosomal DNA with a cloned copy of B. pertussis fur (4). pfurBp contains a PCR-derived insert containing the entire ORF of the fur gene of B. pertussis. Chromosomal DNAs of 4169 and 838 were digested with RsaI; pfurBp was linearized with KpnI. Moderate-stringency conditions (0.5× SSC [1× SSC is 0.15 M NaCl plus 0.15 M sodium citrate] at 65°C) were used for the Southern hybridization. Molecular sizes are in kilobase pairs.
TABLE 1.
Derepression of the Fe-regulated alkaline phosphatase reporter gene in the B. avium Mn-resistant mutant Tnpho6Mn
| Straina | Alkaline phosphatase activityb
|
|
|---|---|---|
| +Fe | −Fe | |
| 4169 | 0.006 (0.0004) | 0 (0) |
| Tnpho6 | 0.021 (0.001) | 0.092 (0.018) |
| Tnpho6Mn | 0.391 (0.008) | 0.095 (0.006) |
| Tnpho6(fur) | 0.178 (0.016) | 0.092 (0.001) |
| Tnpho6Mn(pRK415) | 0.452 (0.012) | 0.146 (0.021) |
| Tnpho6Mn(pRKBav) | 0.051 (0.003) | 0.132 (0.018) |
Tnpho6, a mutant strain derived from 4169, has a TnphoA insertion into an Fe-regulated gene (9); Tnpho6Mn is a manganese-resistant mutant of Tnpho6; the fur gene in Tnpho6(fur) was inactivated by integration of ptr5-1 at the fur locus by homologous recombination. pRKBav encodes the wild-type fur gene of 4169; pRK415 (19) is a low-copy-number vector used for construction of pRKBav.
Cells were cultured in BHI broth containing either 36 μM FeSO4 to produce an Fe-replete medium (+Fe) or in BHI broth containing 100 μM EDDHA to produce an Fe-limiting medium (−Fe) (9). Alkaline phosphatase activities were calculated according to the rate of hydrolysis of p-nitrophenyl phosphate (27). Specific activities are reported as micromoles of p-nitrophenyl phosphate hydrolyzed per minute per OD600 unit. The results are the average from three independent cultures; the standard deviations are in parentheses.
To clone the gene from B. avium that had fur-like properties, we took advantage of the published sequence of the fur gene of B. pertussis (4, 7). Two synthetic oligonucleotides with homology to the 5′ end (fur-6, 5′-GGGGTACCATGAGCGACCAAAGCGAA-3′ [KpnI site underlined]) and the 3′ end (fur-7, 5′-GAAGATCTTCAGCGGCCCTTCTGACA-3′ [BglII site underlined]) of the open reading frame (ORF) of the B. pertussis fur gene were used as heterologous primers in a PCR (reaction conditions: 45 s at 92°C, 45 s at 45°C, and 60 s at 72°C for 30 cycles; Perkin-Elmer DNA thermal cycler 480) to amplify the corresponding locus of the B. avium chromosome. With these two primers, a 500-bp fragment was amplified from B. avium 4169 (9). By using the terminal KpnI and BglII restriction sites that were incorporated into the DNA during amplification, the fragment was directionally ligated into KpnI and BamHI sites of the expression vector pBluescriptSKII+ (Stratagene, La Jolla, Calif.). A clone confirmed by restriction mapping to contain an DNA fragment of the appropriate size was designated p67-1. Nucleotide sequencing of the insert of p67-1 revealed that the fragment had significant homology to the B. pertussis fur gene (data not shown). To clone the wild-type copy of the PCR-amplified sequence, a cosmid library of B. avium 4169 chromosomal DNA was screened by colony blot hybridization with the 32P-labeled 500-bp KpnI-BamHI insert of p67-1 as a hybridization probe. Moderate-stringency conditions (0.5× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA; pH 7.7], 0.1% sodium dodecyl sulfate, 5 mM sodium pyrophosphate at 65°C) were used to avoid hybridization of the fur-like sequences of the p67-1 insert to the chromosomal copy of the fur gene in the E. coli host cells. pf2-1, a cosmid clone with homology to the probe, was isolated from the library. A synthetic oligonucleotide (fur-9, 5′-TATCGAAAAGCGTCAGC-3′) homologous to internal sequences of the fur-like gene in p67-1 was used to sequence outward toward the 5′ end of the ORF of pf2-1, while a second synthetic oligonucleotide (fur-10, 5′-TCAGCGATCAGGGCGCGA-3′) homologous to the opposite strand was used to sequence toward the 3′ end of the ORF in the plasmid. Sequencing revealed a 417-bp ORF that encoded a predicted polypeptide of 139 amino acids (Fig. 2). Although the nucleotide sequences of the fur-like gene of pf2-1 had only 84% identity to B. pertussis fur, the amino acid sequences of the predicted polypeptides were over 92% identical (Fig. 3). Comparisons of the amino acid sequence of the predicted polypeptide to the Fur proteins of E. coli and V. cholerae suggests that the B. avium protein is a member of the Fur family (Fig. 3).
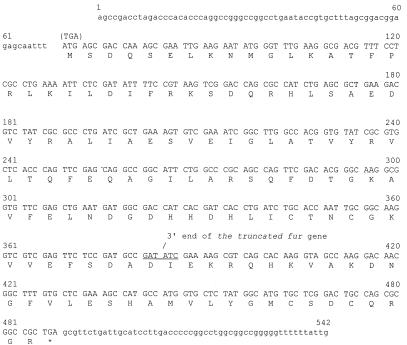
FIG. 2.
Nucleotide sequence of the fur gene of B. avium 4169. The ORF of the fur gene is in uppercase, while the noncoding flanking sequences are in lowercase. The amino acid sequence of the predicted Fur protein is shown below the nucleotide sequence in the single-letter amino acid code. A truncated fur gene used to engineer the fur mutation in Tnpho6(fur) was comprised of nucleotides 70 to 384, in which the initiation codon at nucleotide 70 was replaced with a nonsense codon (TGA). The internal EcoRV site used to produce the 3′ truncation of the mutant fur is underlined. The location of the 3′ end of the truncated fur gene is denoted by a slash.
FIG. 3.
Comparison of amino acid sequences of the Fur proteins produced by E. coli (15), V. cholerae (21), B. pertussis (4), and B. avium 4169. The single-letter amino acid code is used. Amino acids which are conserved in the Fur proteins of the four species are denoted by asterisks. The arginine at amino acid position 18 that was substituted for with a histidine in the PCR-derived B. avium fur mutant R18H is underlined.
Genetic complementation was used to demonstrate that the gene from pf2-1 encoded a functional Fur protein. A fragment of pf2-1 from 67 bp upstream of the ATG initiation codon, which included a putative ribosomal binding site to 20 bp downstream of the TGA stop codon, was amplified by PCR with the synthetic oligonucleotide primers fur-13 (5′-GGAATTCCCGACCTAGACCCACACC-3′ [EcoRI site is underlined]) and fur-14 (5′-CGGGATCCCAAGGATGCAATCAGAACGC-3′ [BamHI site is underlined]). The insert was directionally ligated into pBluescriptKS− (Stratagene) at the EcoRI and BamHI sites which placed the ORF of the fur-like gene under control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible lac promoter. Restriction mapping was used to confirm that the resulting plasmid, pBav, contained the expected insert. Initial genetic complementation studies were simplified by use of E. coli H1780 (16), a strain in which the promoter of the Fur-regulated fiu gene was fused to a promoterless lacZ gene (16). Since the fur gene of H1780 has been inactivated, the fiu-lacZ reporter gene is unregulated and constitutive. Introduction of a cloned E. coli fur gene into H1780 reestablishes Fe-dependent regulation of the fiu-lacZ reporter gene. When pBav was introduced into H1780 and the cells were grown in BHI broth containing 36 μM FeSO4 and 1 mM IPTG, the expression of the fiu-lacZ reporter gene was highly repressed (Table 2). These data demonstrated that the fur-like gene from B. avium was capable of regulating the Fur-dependent fiu promoter in H1780. pBluescriptKS− is a very-high-copy-number vector. Since gene dosage may have affected the outcome of the complementation, an identical fragment containing the fur-like gene of B. avium was ligated into the low-copy-number vector pRK415 (19) to produce pRKBav. As was observed for pBav, the gene cloned into pRKBav complemented the fur mutation in H1780 (Table 2). Similar experiments were done to determine if the cloned gene in pRKBav would complement the regulatory defect in Tnpho6Mn. Introduction of the plasmid into Tnpho6Mn restored the ability of the mutant to repress the Fe-dependent reporter gene when the cells were cultured under Fe-replete conditions (Table 1). Based upon these results, the fur-like gene isolated from 4169 was determined to be the functional fur gene of B. avium.
TABLE 2.
Genetic complementation of E. coli H1780 (fur mutant) with wild-type and mutant (R18H) fur genes of B. avium
| Plasmida | fur phenotype | Plasmid copy number | β-Galactosidase activityb |
|---|---|---|---|
| pRK415 | Low | 1,481 (273) | |
| pBluescriptKS− | High | 1,600 (185) | |
| pRKBav | Wild type | Low | 121 (2) |
| pBav | Wild type | High | 45 (18) |
| pfurR18H | Mutant | High | 15 (9) |
| pRKfurR18H | Mutant | Low | 1,514 (330) |
pfurR18H encodes the PCR-derived mutant fur gene encoding a predicted Fur polypeptide with an R18H substitution; pBluescriptKS− was used as the vector for cloning pfurR18H. pRKfurR18H encodes the same mutant Fur, but the mutant gene is cloned into pRK415.
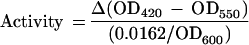 |
To confirm that the deregulated phenotype of Tnpho6Mn resulted from a mutation in fur, an isogenic mutation was engineered in the parental strain Tnpho6. PCR was used to produce a mutant fur gene for this purpose. Primers were synthesized that amplified fur sequences in which the ATG initiation codon was replaced with a TGA nonsense codon (Bavd-1, 5′-GGGAATTCTGAAGCGACCAAAGCGAATTG-3′ [EcoRI site underlined]; Bavd-2, 5′-CGGGATCCGCGCACGCTTTTCGATA-3′; amplification conditions: 30 s at 92°C, 45 s at 45°C, and 60 s at 72°C for 30 cycles) (Fig. 2). Digestion of the amplified fragment with EcoRI produced an EcoRI-cohesive end at the 5′ terminus. Subsequent digestion with EcoRV which hydrolyzes the DNA at a site within the ORF was used to remove the DNA encoding the last 34 codons of fur from the fragment. The digested fragment was ligated into the mobilizable vector p1910 (unpublished data; a gift of Scott Stibitz). The plasmid, denoted ptr5-1, was conjugated into B. avium Tnpho6. Since p1910 does not replicate in B. avium, plating the transconjugants on BHI agar containing 200 μg of ampicillin per ml selected for clones in which ptr5-1 had integrated into the fur locus by homologous recombination. The single site recombination resulted in a gene duplication in which a fur gene containing the TGA mutation at the original initiation codon was separated by plasmid sequences from a second copy of the fur gene having the 3′ truncation (data not shown). With this arrangement of sequences, neither copy of fur in Tnpho6(fur) should express a wild-type Fur protein. Growth experiments with Tnpho6(fur) confirmed that fur was required to regulate the Fe-dependent alkaline phosphatase reporter gene (Table 1). When cultured in Fe-replete medium, the alkaline phosphatase reporter gene in Tnpho6 was strongly repressed. In contrast, Tnpho6(fur) exhibited high levels of alkaline phosphatase activity when cultured under identical conditions of Fe availability.
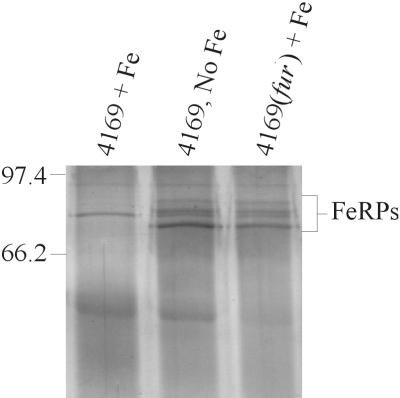
In other bacterial species, a number of genes are regulated by fur in response to the local concentration of Fe. To determine whether fur regulated the expression of B. avium genes other than the transposon-inserted Fe-regulated gene in Tnpho6, a mutation in fur identical to the mutation in Tnpho6(fur) was engineered in the wild-type strain, 4169. Previous studies have shown that culture of 4169 in Fe-limited medium stimulated expression of at least four Fe-regulated outer membrane proteins (FeRPs) with molecular masses of 84, 90, 91.5, and 95 kDa (9). Supplementation of the Fe-limited medium with 36 μM FeSO4 resulted in a coordinate loss of expression of the four FeRPs by 4169. To determine whether the expression of these four proteins was regulated by fur, 4169(fur) was cultured in Fe-limited and Fe-replete media. Analysis of the outer membrane protein profiles of the cells demonstrated that the FeRPs were expressed by 4169(fur) irrespective of the concentration of Fe in the medium (Fig. 4). This result is consistent with a model in which the FeRPs are regulated by fur.
FIG. 4.
Expression of the Fe-regulated outer membrane proteins by 4169 and 4169(fur). To produce Fe-replete conditions, BHI was supplemented with 36 μM FeSO4; Fe-limiting conditions were produced by supplementation of BHI with 100 μM EDDHA. The positions of the FeRPs are designated. Molecular masses are in kilodaltons.
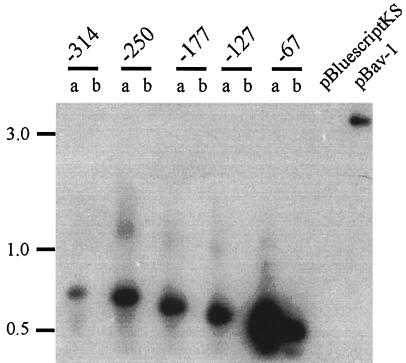
Because it was deemed likely that the mutation in Tnpho6Mn was located within the fur gene, the mutant fur was cloned from the strain by PCR. The reaction conditions were identical to those used to amplify the wild-type fur gene from pf2-1. The amplified fragment was ligated into pBluescriptKS− to produce the recombinant plasmid pBavMn. Contrary to expectations, nucleotide sequencing of the fur gene in pBavMn revealed that the gene was identical in nucleotide sequence to the wild-type fur gene in pBav. These results suggested that the mutation that affected fur regulation in Tnpho6Mn was located outside of the ORF of the gene. Preliminary results from PCR analysis of Tnpho6Mn were consistent with a model that the mutation was most likely a deletion of upstream sequences. To confirm this hypothesis, synthetic oligonucleotides corresponding to sequences located 314, 250, 177, 127, and 67 bp upstream of the ATG initiation codon of fur were synthesized and used in combination with oligonucleotide fur-14 in PCRs to amplify fragments from 4169 and Tnpho6Mn that contained the fur ORF with various amounts of upstream sequence. Results from Southern hybridizations of the amplified DNAs demonstrated that none of the combinations of oligonucleotides, with the exception of the oligonucleotide which was homologous to sequences located 67 bp upstream of the fur ORF, amplified a fur-containing fragment from Tnpho6Mn (Fig. 5). The failure to amplify fragments from Tnpho6Mn was attributed to a loss of sequences to which the oligonucleotides would have annealed. All combinations of oligonucleotides amplified fragments of expected size from 4169, each of which hybridized to fur sequences. To estimate the extent of the upstream deletion in Tnpho6Mn, an additional Southern hybridization was performed with chromosomal DNA of 4169 and Tnpho6Mn by using the fur ORF as a hybridization probe. The pattern of hybridizations indicated a loss of 2 to 3 kbp of DNA in the Tnpho6Mn chromosome (Fig. 6). We interpret these data as highly suggestive that the deletion removed upstream sequences which are required for full expression of the fur gene in Tnpho6Mn. To our knowledge, this is the first report demonstrating that fur mutants harboring significant deletions of nucleotide sequence can be derived by Mn selection.
FIG. 5.
Southern hybridization of PCR amplifications of B. avium 4169 and Tnpho6Mn. DNA fragments containing the fur ORF and various lengths of sequences located 5′ to the gene (314, 250, 177, 127, or 67 nucleotides upstream of the ATG initiation codon of fur) were PCR amplified from chromosomal DNA of 4169 and Tnpho6Mn by using the appropriate synthetic oligonucleotides as 5′ primers. A common oligonucleotide primer (fur-14) corresponding to the 3′ end of the ORF of fur was used in all reactions. Amplified DNA was hybridized to a DNA fragment obtained from pBav-1 which contained the ORF of fur. The pattern of hybridization observed for the amplified DNA indicated that a region of DNA located 5′ to fur in 4169 was absent in Tnpho6Mn. The pattern of amplified DNA fragments derived from Tnpho6 was identical to the pattern derived from 4169 (data not shown). a, 4169; b, Tnpho6Mn. Molecular sizes are in kilobase pairs.
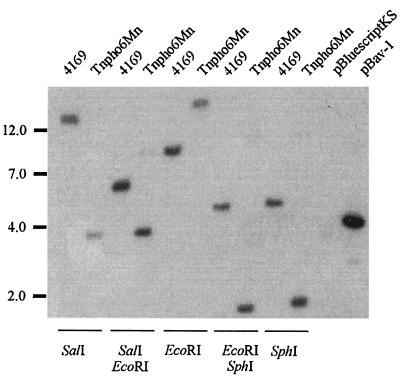
FIG. 6.
Southern hybridization of B. avium 4169 and Tnpho6Mn. Chromosomal DNA was singly and doubly digested with the restriction endonucleases SalI, EcoRI, and SphI, as indicated. A DNA fragment corresponding to the ORF of fur obtained from pBav was utilized as the hybridization probe. The pattern of hybridization suggested a loss of 2 to 3 kbp of nucleotide sequence in the chromosome of Tnpho6Mn proximal to the region of fur. Molecular sizes are in kilobase pairs.
While cloning the wild-type regulatory gene, a mutant fur gene was obtained as a result of a spontaneous PCR-derived nucleotide misincorporation. Sequencing revealed that the mutant fur gene encoded an arginine-for-histidine substitution at amino acid position 18 in the predicted Fur polypeptide (i.e., R18H) (Fig. 3). When the predicted amino acid sequences of Fur polypeptides from several species were compared, it was found that an arginine at amino acid position 18 was highly conserved. To determine if the arginine-to-histidine substitution in the mutant Fur affected regulatory activity of the encoded protein, the gene was cloned into pBluescriptKS− and introduced into H1780. Introduction of the R18H-encoding plasmid into H1780 complemented the fur mutation (see pfurR18H in Table 2). However, when the mutant R18H gene was cloned into pRK415, a low-copy-number vector, no complementation was detected (see pRKfurR18H in Table 2). These results were consistent with a model in which the mutant fur gene expressed a Fur protein with residual regulatory activity. The higher level of expression of the mutant Fur from pfurR18H compensated for its lower activity. Whether the amino acid substitution affects the ability of the protein to bind Fe or the ability of the regulatory protein to bind to specific nucleotide sequences located proximal to Fur-regulated genes (i.e., Fur boxes) has yet to be determined.
This study confirmed that a fur gene is involved in regulation of at least five Fe-regulated genes in B. avium (the four outer membrane proteins and the protein encoded by the TnphoA-inserted gene in Tnpho6). Current research in our laboratory is focused on identifying all Fur-regulated genes in the bacterium. While some of these Fur-dependent genes will undoubtedly be involved in routine Fe metabolism, it is likely that the fur regulator may have additional roles in B. avium, including controlling expression of genes involved in virulence. An analogous situation is found in Corynebacterium diphtheriae, which coordinately regulates expression of diphtheria toxin through the activity of dtxR, a gene encoding a regulatory protein with properties similar to Fur (30).
Acknowledgments
This work was supported by funds made available to T.D.C. from the School of Medicine and Biomedical Sciences, State University of New York at Buffalo.
REFERENCES
- 1.Armstrong S K, Clements M O. Isolation and characterization of Bordetella bronchiseptica mutants deficient in siderophore activity. J Bacteriol. 1993;175:1144–1152. doi: 10.1128/jb.175.4.1144-1152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beall B, Hoenes T. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology. 1997;143:135–145. doi: 10.1099/00221287-143-1-135. [DOI] [PubMed] [Google Scholar]
- 3.Beall B, Sanden G N. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology. 1995;141:3193–3205. doi: 10.1099/13500872-141-12-3193. [DOI] [PubMed] [Google Scholar]
- 4.Beall B W, Sanden G N. Cloning and initial characterization of the Bordetella pertussis fur gene. Curr Microbiol. 1995;30:223–226. doi: 10.1007/BF00293637. [DOI] [PubMed] [Google Scholar]
- 5.Berish S A, Subbarao S, Chen C-Y, Trees D L, Morse S A. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect Immun. 1993;61:4599–4606. doi: 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun V, Hantke K, Koster W. Bacterial iron transport: mechanisms, genetics, and regulation. Met Ions Biol Syst. 1998;35:67–145. [PubMed] [Google Scholar]
- 7.Brickman T J, Armstrong S K. Bordetella pertussis fur gene restores iron repressibility of siderophore and protein expression to deregulated Bordetella bronchiseptica mutants. J Bacteriol. 1995;177:268–270. doi: 10.1128/jb.177.1.268-270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderwood S B, Mekalanos J J. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operon fusion plasmid. J Bacteriol. 1988;170:1015–1017. doi: 10.1128/jb.170.2.1015-1017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell T D, Dickenson A, Martone A J, Militello K T, Filiatraut M J, Hayman M L, Pitula J. Iron starvation of Bordetella avium stimulates expression of five outer membrane proteins and regulates a gene involved in acquiring iron from serum. Infect Immun. 1998;66:3597–3605. doi: 10.1128/iai.66.8.3597-3605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst J F, Bennett R L, Rothfield L I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135:928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giardina P C, Foster L A, Toth S I, Roe B A, Dyer D W. Identification of alcA, a Bordetella bronchiseptica gene necessary for alcaligin production. Gene. 1995;167:133–136. doi: 10.1016/0378-1119(95)00659-1. [DOI] [PubMed] [Google Scholar]
- 12.Giardina P C, Foster L A, Toth S I, Roe B A, Dyer D W. Analysis of the alcABC operon encoding alcaligin biosynthesis enzymes in Bordetella bronchiseptica. Gene. 1997;194:19–24. doi: 10.1016/s0378-1119(97)00094-2. [DOI] [PubMed] [Google Scholar]
- 13.Graeff-Wohlleben H, Killat S, Banemann A, Guiso N, Gross R. Cloning and characterization of an Mn-containing superoxide dismutase (SodA) of Bordetella pertussis. J Bacteriol. 1997;179:2194–2201. doi: 10.1128/jb.179.7.2194-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 15.Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli. Mol Gen Genet. 1984;197:288–294. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- 16.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 17.Kang H Y, Brickman T J, Beaumont F C, Armstrong S K. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J Bacteriol. 1996;178:4877–4884. doi: 10.1128/jb.178.16.4877-4884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karkhoff-Schweizer R R, Schryvers A B, Schweizer H P. Cloning and sequence analysis of the fur gene encoding an iron-regulatory protein of Neisseria meningitidis. Gene. 1994;141:139–140. doi: 10.1016/0378-1119(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 19.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 20.Lam M S, Litwin C M, Carroll P A, Calderwood S B. Vibrio cholerae fur mutations associated with loss of repressor activity: implications for the structural-functional relationships of fur. J Bacteriol. 1994;176:5108–5115. doi: 10.1128/jb.176.16.5108-5115.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litwin C M, Boyko S A, Calderwood S B. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992;174:1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller V L, Mekalanos J J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci USA. 1984;81:3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prince R W, Cox C D, Vasil M L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993;175:2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince R W, Storey D G, Vasil A I, Vasil M L. Regulation of toxA and regA by the Escherichia coli fur gene and identification of a Fur homologue in Pseudomonas aeruginosa PA103 and PA01. Mol Microbiol. 1991;5:2823–2831. doi: 10.1111/j.1365-2958.1991.tb01991.x. [DOI] [PubMed] [Google Scholar]
- 26.Redhead K, Hill T. Acquisition of iron from transferrin by Bordetella pertussis. FEMS Microbiol Lett. 1991;61:303–307. doi: 10.1016/0378-1097(91)90570-z. [DOI] [PubMed] [Google Scholar]
- 27.Russo T A, Singh G. An extraintestinal, pathogenic isolate of Escherichia coli (O4/K54/H5) can produce a group 1 capsule which is divergently regulated from its constitutively produced group 2, K54 capsular polysaccharide. J Bacteriol. 1993;175:7617–7623. doi: 10.1128/jb.175.23.7617-7623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saif Y M, Moorhead P D, Dearth R N, Jackwood D J. Observations of Alcaligenes faecalis infection in turkeys. Avian Dis. 1980;24:665–684. [PubMed] [Google Scholar]
- 29.Schaffer S, Hantke K, Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200:110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt M P, Holmes R K. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect Immun. 1991;59:1899–1904. doi: 10.1128/iai.59.6.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silver S, Johnseine P, Whitney E, Clark D. Manganese-resistant mutants of Escherichia coli: physiological and genetic studies. J Bacteriol. 1972;110:186–195. doi: 10.1128/jb.110.1.186-195.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staggs T M, Perry R D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991;173:417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stojiljkovic I, Baumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 34.Stojiljkovic I, Hantke K. Functional domains of the Escherichia coli ferric uptake regulator protein (Fur) Mol Gen Genet. 1995;247:199–205. doi: 10.1007/BF00705650. [DOI] [PubMed] [Google Scholar]
- 35.Thomas C E, Sparling P F. Identification and cloning of a fur homologue from Neisseria meningitidis. Mol Microbiol. 1994;11:725–737. doi: 10.1111/j.1365-2958.1994.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 36.van Vliet A H M, Wooldridge K G, Ketley J M. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J Bacteriol. 1998;180:5291–5298. doi: 10.1128/jb.180.20.5291-5298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venturi V, Ottevanger C, Bracke M, Weisbeek P. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol Microbiol. 1995;15:1081–1093. doi: 10.1111/j.1365-2958.1995.tb02283.x. [DOI] [PubMed] [Google Scholar]
- 38.Wee S, Neilands J B, Bittner M L, Hemming B C, Haymore B L, Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Metals. 1988;1:62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]