Summary
Siglecs are a family of emerging glyco-immune checkpoints. Inhibiting them can enhance the functions of several types of immune cells, whereas engaging them can reduce hyper-inflammation and hyper-activation of immune functions. Siglec-sialoglycan interactions play an important role in modulating immunological functions during cancer, however, their roles in regulating immunological equilibrium during viral infections is less clear. In this review, we discuss the documented and potential roles of inhibitory Siglecs in balancing immune activation and tolerance during viral infections and consider how this balance could affect both the desired anti-viral immunological functions and the unwanted hyper- or chronic inflammation. Finally, we discuss the opportunities to target the Siglec immunological switches to reach an immunological balance during viral infections: inhibiting specific Siglec-sialoglycan interactions when maximum anti-viral immune responses are needed, or inducing other interactions when preventing excessive inflammation or reducing chronic immune activation are the goals.
Keywords: Viral infections, Siglec, Sialic acid, Immune activation, Immune tolerance, Immune surveillance, Glyco-immune checkpoints
An immunological equilibrium is needed during viral infections, and immune checkpoints could be a key to maintaining this equilibrium
The 2019 Coronavirus disease (COVID-19) pandemic made it clear that an immunological imbalance can be an independent and potent contributor to worse disease outcomes during acute viral infections.1 On the one hand, the lack of adequate immunological responses (such as interferon-mediated2) to acute viral infections can result in uncontrolled viral replication and worse outcomes.3 On the other hand, hyper-activation of immune responses can also lead to worse outcomes, as a side-effect of hyper-inflammation and its associated cytokine storm is tissue damage4 (Fig. 1). Well-coordinated innate and adaptive immune responses are also pivotal during chronic viral infections.5 During treated chronic viral infections, such as antiretroviral therapy (ART)-treated HIV infection, the state of chronic low-level immune activation/inflammation accompanied by immune exhaustion can lead to the development of inflammation-associated diseases6 and can contribute to viral persistence.7 Such immunological imbalances during acute and chronic viral infections likely involve multiple mechanisms, not all of which are well characterized. These mechanisms may include immunological senescence, pre-existing metabolic syndromes, co-infections, microbial dysbiosis/translocation, and imbalance of interactions at immune checkpoints. In this review, we focus on the imbalance in immune checkpoint interactions.
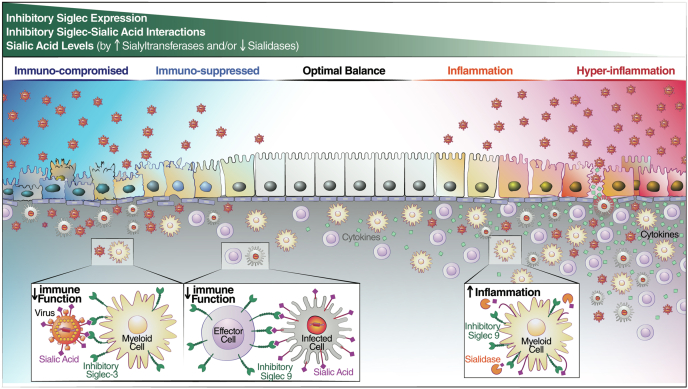
Fig. 1.
An immunological equilibrium is needed during viral infections and Siglecs could be a key to this equilibrium. The lack of adequate immune responses to viral infection can result in uncontrolled viral replication and worse disease outcome (left) whereas hyper-activation of immune response can also lead to worse outcome as a side-effect of hyperinflammation (right). Inhibitory Siglecs could regulate the crucial immune equilibrium by various mechanisms; 1) inhibitory Siglec expression, 2) maintaining the Siglec-Sialic acid interactions, 3) and/or by maintaining the balanced sialic acid levels on cell surface. The higher expression of inhibitory Siglecs on immune cells, higher expression of sialyltransferases in target cells, and/or lower activity of sialidases can lead to enhanced Siglec-sialic acid interactions and subsequently inhibit immune responses. On the other hand, the lower expression of inhibitory Siglecs in immune cells, lower expression of sialyltransferases in target cells, and/or higher sialidases activity can lower Siglec-sialic acid interactions and subsequently induce immune inflammation (top). Viruses can evade immune system by engaging the inhibitory Siglecs on immune cells either by the sialic acids present on its surface (such as the case of sialic acid on the surface of HBV that binds to Siglec-3 on myeloid cells) or by increasing the expression of sialic acid on infected cell (such as the case of α 2,3 sialic acid on the surface of HIV-infected cells that binds to Siglec-9 on NK cells). The sialic acids engage the inhibitory Siglecs and suppress the immune response against viral pathogen (left). On the other hand, viruses could induce hyper-inflammation by releasing Siglec dependent inhibition (such as the case of Siglec-9 ligands on neutrophils during COVID-19) (right).
Interactions between immune checkpoint molecules and their ligands are designed to regulate immune responses in order to limit collateral tissue damage.8 Unintended inhibition of these immune checkpoint interactions can lead to uncontrolled inflammation, which results in tissue damage.9 Inadvertent induction of immune checkpoint interactions can lead to immunological unresponsiveness, which helps virally-infected cells, or cancer cells, evade immune surveillance.10 Thus, striking the right balance between constructive inflammation and uncontrolled inflammation is essential to suppress the invader while protecting the host.
Siglecs are emerging glyco-immune checkpoints that modulate several immunological functions
Whereas most of the well-described immune checkpoints initiate their signal transduction via protein–protein interactions (such as PD1-PDL1 interactions), it recently became clear that there is another class of immune checkpoints that initiate signal transduction via protein-glycan (sugar) interactions. Immune cells express several types of sugar-binding proteins, called lectins, on their surface,11 and the binding between some of these lectins, i.e., those with inhibitory properties, and specific glycans (sugars) on the surface of cancer cells or virally-infected cells can induce potent inhibitory signal transduction cascades that reduce the ability of the immune cells to recognize and eliminate target cells.11 An example of lectins functioning as immune checkpoints is the Siglec family of cell surface receptors. Siglecs, sialic acid-binding immunoglobulin-like lectins are a family of immune regulatory lectins that recognize sialoglycans (sialic acid-containing glycomic structures) on target cells. Siglecs are expressed on multiple types of immune cells, including natural killer (NK) cells, T lymphocytes, neutrophils, macrophages, dendritic cells, and B cells.12 Most Siglecs inhibit immune functions through intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs).13
Siglecs can be divided based on their evolutionary conservation or their function. Evolutionarily, they divide into two groups: 1) an evolutionarily conserved group that contains Siglec-1 (CD169), Siglec-2 (CD22), Siglec-4 (Myelin-associated glycoprotein/MAG) and Siglec-15; and 2) an evolutionarily divergent group that contains the CD33-related Siglecs, Siglec-3 (CD33), Siglec-5, Siglec-6, Siglec-7, Siglec-8, Siglec-9, Siglec-10, Siglec-11, Siglec-12, Siglec-14 and Siglec-16. In mice, the CD33-related Siglecs are Siglec-3, Siglec-E, Siglec-F, Siglec-G and Siglec-H.14 Siglecs can also be divided based on their functions: inhibitory, activating, and non-signaling. Inhibitory Siglecs (Siglecs -2, 3, 5, 6, 7, 8, 9, 10, 11, and 12) contain an intracellular ITIM or an ITIM-like motif that is phosphorylated after receptor engagement and leads to the recruitment of either Src-homology 2 domain-containing phosphatase-1 (SHP-1) or SHP-2, which dephosphorylate downstream components of stimulatory pathways, thus inhibiting cell activation.15 Activating Siglecs (Siglec-14, -15, and -16) do not contain ITIM domains but have a positively charged residue in their transmembrane domain that enables them to complex with ITAM (immunoreceptor tyrosine-based activation motif) containing proteins, such as DAP10 or DAP12.13 This complex recruits protein kinases that can phosphorylate downstream targets, eventually triggering downstream activation pathways.13 Finally, non-signaling Siglecs (Siglecs-1 and -4) have a neutral transmembrane domain and lack fully signaling cytosolic motifs. Mostly, these Siglecs are involved in adhesion events.16 In this review article, we will focus on the inhibitory Siglecs as their functions are better characterized compared to those of activating Siglecs.
Siglecs exert their effects by interacting with sialic acid, either on a different cell (trans interaction) or the same cell (cis interaction). Each Siglec has a preference for the type of sialic acid it binds to, for example, Siglec-7 binds disialyl α2,8-sialogylcans and branched α2,6-sialoglycans, whereas Siglec-9 binds to α2,3-sialogylcans.17 The ability of Siglecs to alter the immune responses also depends on the levels of expression of sialic acid on the immune cell itself (cis) or the target cell (trans). These sialic acid levels depend on the balance between sialyltransferases and sialidases. Sialyltransferases are the enzymes that catalyze the transfer of sialic acid to galactose-containing substrates via various linkages; this transfer results in addition of sialic acid to the cell surface.18 In contrast, sialidases (neuraminidases) are enzymes that remove sialic acid from glycans.19 Sialidases can be endogenously expressed or expressed by pathogens and/or the microbiome. The likelihood of Siglec-sialic acid interactions therefore depends on the relative levels of expression of sialic acid, sialyltransferases, and sialidases. Thus, enhanced Siglec-sialic acid interactions, which subsequently inhibit immune responses, are due to high expression of inhibitory Siglecs on immune cells, high expression of sialyltransferases in target cells, and/or low activity of sialidases. On the other hand, poorer Siglec-sialic acid interactions, which subsequently induce inflammation, are due to low expression of inhibitory Siglecs on immune cells, low expression of sialyltransferases in target cells, and/or high sialidase activity (Fig. 1, top).
Despite the ubiquitous distribution of sialic acids on many cells, both cancer cells and viruses employ the dysregulation of sialic acid interactions to enhance disease progression. Cancer cells use hyper-sialylation (induction of cell-surface sialic acid) as an immune evasive strategy: enhancing inhibitory Siglec interactions ensures a permissive microenvironment that promotes tumor progression.20 Several viral infections (such as HSV-1, VZV, CMV, HTLV1, and HIV) alter the cell surface glycosylation of infected cells.15 These alterations (as in the case of HTLV and HIV) include the upregulation of sialic acid,16, 17, 18 similar to what occurs during oncogenesis. This suggests that cancer cells and virally-infected cells may use similar Siglec interactions to manipulate immune responses. In this review article, we will discuss the potential role of inhibitory Siglec interactions in regulating the balance between immune activation and tolerance during viral infections and how this balance could affect both the desired anti-viral immunological functions and the unwanted inflammation. We will start by discussing the role of elevated inhibitory Siglec interactions in inhibiting immunological functions (that are relevant to viral infections) during cancer; we will then transition to discussing the documented and potential role these Siglec interactions can play in inhibiting similar functions during viral infections. Next, we will review the documented and potential strategies for overcoming the immune inhibition and enhancing anti-viral immune functions. We will then discuss the potential role of diminished inhibitory Siglec interactions in inducing inflammation and tissue damage during viral infections and the possible strategies to overcome these effects. Finally, we will discuss the opportunities to target specific inhibitory Siglec interactions to reach an immunological balance during viral infections, such as by inhibiting specific interactions when maximum anti-viral immune responses are needed or inducing other interactions when preventing hyper-inflammation or reducing chronic immune activation are the goals.
The potential role of Siglec-sialic acid interactions in suppressing immunological functions during viral infections
Pathogens use Siglec-sialic acid interactions to facilitate infection. HIV and PRRSV contain sialic acids on their viral envelope glycoproteins. This sialic acid contributes to infectivity by binding to Siglec-1 on target cells and promoting trans infection.21, 22, 23 Influenza A virus also uses its lectin hemagglutinin to infect host cells by recognizing their α2,3 and α2,6 sialic acids. The Influenza A virus also carries a neuraminidase enzyme that cleaves off sialic acids from the target cell surface; this prevents the hemagglutinin from causing virion aggregation to the cell surface.24 However, whether Siglec-sialic acid interactions play any additional roles in virus–host interactions, such as modulating anti-viral cellular immunity is not clear. In this section, we will discuss the role of Siglec interactions in inhibiting immunological functions during cancer; we focus on those immunological functions that are relevant to viral infections. We will also discuss the documented and potential role of Siglec-mediated regulation of immunological functions during viral infections (Fig. 1, left). Finally, we will discuss documented and potential approaches to overcome the Siglec-mediated inhibition of these immunological functions relevant to viral infections.
Role of Siglec-sialic acid interactions in inhibiting the functions of immune cells
NK cells
NK cells play an important role in fighting viral infections,25 and their functions are significantly impacted by the Siglecs expressed on their cell surface, and on the binding of these Siglecs to sialic acids on target cells.26 In peripheral blood, almost all NK cells express Siglec-7,27 and a subset of the cytotoxic CD56dim NK cells also express Siglec-9.28 In addition, Siglec-3 can be detected on activated NK cells.29 Siglec expression is tissue-dependent, as NK cells from hepatocellular carcinoma (HCC) tissues express Siglec-10.30 In cancer pathology, an increase of sialoglycans on tumor cells inhibits the direct cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) by NK cells via engagement of Siglec-7.31 In addition, blocking Siglec-9 increases NK cell-mediated tumor cell killing.32,33
The role of Siglec interactions in inhibiting NK cytotoxicity against virally-infected cells is less clear. During some infectious states, the expression of specific Siglecs marks specific NK sub-populations. For example, during acute HIV infection, loss of Siglec-7 expression marks dysfunctional NK cells.34 During HIV and HBV infections, expression of Siglec-9 marks a highly cytotoxic and mature NK subpopulation. Beyond simply marking NK sub-populations, the expression of particular inhibitory Siglecs can alter the function of the NK cells. For example, the Siglec-9 molecule itself is inhibitory28,35; however, the Siglec-9+ subpopulation of NK cells (Siglec-9+ CD56dim cells) remains highly cytotoxic, despite expression of Siglec-9, likely because they express higher levels of several NK activating receptors and their lower levels of several other NK inhibitory receptors than do Siglec-9- NK cells.28,35 In effect, the high cytotoxicity of these Siglec-9+ CD56dim NK cells is being restrained by the inhibitory nature of the Siglec-9 molecule.28,35 Thus, inhibiting Siglec-9 on NK cells, or removing the sialic acid ligand on virally-infected cells, significantly enhances the susceptibility of infected cells to NK-mediated clearance.28,35 As mentioned above, several viruses alter the cell surface glycosylation of infected cells, and these alterations include the upregulation of α2-3 sialic acid. Thus upregulation enables infected cells to bind to Siglec-9 on NK cells and inhibit NK functions. Given the importance of NK cells in restricting viral infections, understanding how Siglec interactions inhibit their functions could lead to developing novel approaches to selectively enhance NK-mediated clearance of virally-infected cells.
T cells
Peripheral human T cells have low expression of Siglecs.36 However, as mentioned above, Siglec expression is tissue-specific and increases upon immune cell activation. For example, in tumor-infiltrating lymphocytes (TILs), Siglec-9, and also Siglecs -5, -7, and -10, are upregulated in individuals suffering from several cancers, including non-small cell lung cancer, epithelial ovarian cancer, colorectal cancer, and melanoma.37,38 In antigen-activated T cells, expression of Siglec-3 can be detected.29 The Siglec-9+ TILs are tumor-specific, and the inhibition of Siglec-9 signaling significantly increases T cell-mediated tumor cell killing.37,38
Although T cells activation can clearly upregulate Siglec expression in the context of cancer, it is unclear whether, during viral infections, tissue-infiltrating T cells also express Siglecs and whether these Siglec-expressing T cells contribute to the ability of virally infected cells to evade immune surveillance in tissues. It is also unclear whether non-classical T cells, such as γδ T cells and mucosal-associated invariant T (MAIT) cells, which can play an important role in restricting viral infections,39,40 express Siglecs (in blood or different tissues, at steady-state or during viral infections, with and without activation). Given the importance of tissue-specific immunity during viral infections (such as HIV and SARS-CoV-2 infections41,42), it will be important to understand the potential inhibitory impact of Siglec expression on tissue-infiltrating T cells during viral infections and how to overcome these potential inhibitory effects.
Myeloid cells
Monocytes, macrophages, and neutrophils express high levels of several signaling Siglecs, including Siglecs-3, -5, -7, -9, -10, -11,-14, -15, and -16.14 Siglec expression on these cells plays an important role in modulating their functions, including their anti-tumor activities. Often, engaging Siglec-sialic acid interactions impairs the immune cell function. For example, macrophages commonly express the inhibitory receptor Siglec-10; Siglec-10 binds to sialic acid on CD24 on cancer cells. This Siglec-10:CD24 interaction elicits inhibitory signaling in the macrophage, including the suppression of macrophage-mediated phagocytosis, which allows cancer cells to evade immune-mediated clearance mechanisms.43 Indeed, blocking CD24 (and thus the Siglec-sialic acid interaction), using a blocking antibody, reduced cancer growth and increased survival in an ovarian cancer mouse model.43 Consistently, the engagement between Siglec-9 on tumor-associated macrophages (TAMs) and sialic acid on MUC1 can polarize TAMs to a cancer-promoting, immunosuppressive M2 phenotype.44 Blocking Siglec-9/sialic acid interactions enhance myeloid cell function, similar to what occurs by blocking Siglec-9 on NK cells (see above). For example, blockade of Siglec-9 enhanced neutrophil activity against tumor cells in vitro, and mice deficient for Siglec-E, the murine homolog of human Siglecs-7 and -9, showed an enhanced immunosurveillance of tumors during the early stages of tumor establishment.33
Dendritic cells (DCs) express several signaling Siglecs, including Siglec-2, -3, -5, -6, -7, -9, -10, and -15.14 Siglecs interactions can modulate the functions of DCs. For example, engaging Siglec-E enables murine DCs to induce Treg cells, which then inhibit CD8+ effector T cells, reducing the anti-cancer capacities.45 Consistently, the binding between Siglec-9 on the surface of lipopolysaccharides (LPS)-stimulated human monocyte-derived DCs and α2,3 sialic acid has multiple, tumor-supportive effects: it alters these cells’ metabolism, reduces their expression of inflammatory cytokines, induces their expression of anti-inflammatory cytokines, and induces Treg differentiation at the expense of effector T cell differentiation.46
While little is known about the role of inhibitory Siglecs in modulating the anti-viral activities (such as phagocytosis) of myeloid cells, recent work suggests that Siglecs have an important role. For example, Siglec-3 on myeloid cells binds the α2,6-sialoglycans of hepatitis B virus (HBV) surface antigen (HBsAg), and this binding induces immunosuppression, which helps HBV to evade immunosurveillance.47 Several other viruses (such as HIV48) are heavily glycosylated by sialic acid and thus may be using this mechanism to evade immunosurveillance by myeloid cells; whether this becomes a common theme for additional viruses, or for virally-infected cells, remains to be determined.
B cells
B cells express Siglec-2,49 Siglec-6, and Siglec-10.50 There are several examples of the important roles that inhibitory Siglecs can play in regulating B cell activation and functions. First, the lack of Siglec-2 lowers the B cell receptor (BCR)-signaling threshold and leads to a pre-activated B cell phenotype.51 In mice, Siglec-G, the murine homolog of the human Siglec-10, inhibits BCR signaling.52 Finally, increased expression of Siglec-6 has been associated with the exhausted phenotype of tissue-like memory B-cells, and knock-down of Siglec-6 restores normal tissue-like memory B-cells function.53 It is possible that, during viral infections, such modulation of B cell activation and functions by Siglec interactions could impact their responses and ability to efficiently produce antibodies.
Approaches for blocking Siglec/sialoglycan interactions to enhance immune activation (Fig. 2A)
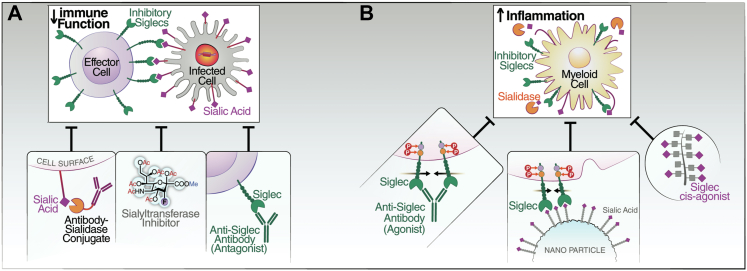
Fig. 2.
Strategies to manipulate Siglec-sialic-acid interactions. (A) Strategies which have been explored to enhance the inhibitory Siglec--mediated reduction of immunes response include 1) anti-Siglec antagonistic (blocking) antibody (tested against HIV- and HBV-infected cells), 2) sialyltransferase inhibitor to reduce the cell surface levels of sialic acids by inhibiting the enzyme involved in installing the sialic acid on cell surface, and 3) use of sialidase conjugated antibody recognizing the infected cell for targeted removal of sialic acids on infected cells (tested against HIV-infected cells). (B) Strategies have also been explored to suppress the excessive immune response which includes 1) engaging anti-Siglec antibodies (tested against SARS-CoV-2), 2) Sialic acid coated nanoparticles, and 3) cis-ligand agonist for Siglecs to keep Siglecs engaged (tested against SARS-CoV-2).
Blocking antibodies targeting Siglec receptors
Several Siglec blocking antibodies have been developed and tested in mice for their ability to prevent inhibitory Siglec-mediated inhibition of immune functions. However, the evaluation of the therapeutic utility of these antibodies has been hindered by the lack of direct human orthologues for many of the Siglecs expressed in and tested in mice. Nevertheless, several studies have tested human Siglec blocking antibodies in vitro and in vivo using humanized mouse models of various cancers. First, in vitro, a number of studies show that human blocking antibodies against Siglec-7 and Siglec-9 enhance anti-tumor immune activity.31,32,38,44 Second, in vivo, human blocking antibodies to Siglec-7 and Siglec-9 significantly reduced tumor burden in a humanized Siglec7+/9+/Siglec-Eknockout mouse model.54 Consistently, a Siglec-9 blocking antibody reduced tumor size in an ovarian cancer humanized mouse model.55 Antibodies against Siglec-2 (CD22) and Siglec-3 (CD33), both of which have mouse/human orthologs, have been used in humans studies but they were mainly used for delivering chemotherapeutics for lymphatic and myeloid hematological malignancies56,57 and not for their potential immuno-modulating, anti-cancer effects.
During viral infections, Siglec blocking antibodies are promising tools to enhance immune functions against virally-infected cells.28,47 Anti-Siglec-9 blocking antibodies enhanced NK cytotoxicity against HIV-infected targets28 and reversed the dysfunctionality of NK cells during HBV infection.35 A Siglec-3 blocking antibody inhibited the interactions between Siglec-3 on myeloid cells and sialic acid on HBV virions, and this blockage reversed HBV-induced immunosuppression.47
Importantly, Siglec-sialic acid interactions are important immune negative checkpoints against autoimmunity58,59; therefore, it is possible that blocking Siglecs can cause non-specific inflammation, as observed with the use of blocking antibodies against other immune negative checkpoints, such as PD1.60 There are notable differences between Siglecs and other immune negative checkpoints, such as PD1, such as the distribution of their expression (Siglecs are mainly expressed on myeloid cells whereas PD1 is expressed on T cells). Whether the distribution of checkpoint expression will influence the impact of checkpoint blockade on autoimmunity is not yet known. A recent clinical trial (NCT03822208) reported that blocking Siglec-3 was well-tolerated in humans. Such reports may indicate that blocking Siglecs could be safe; however, it will be important to carefully investigate the beneficial vs. detrimental effects of blocking Siglecs, especially those with no direct homologs in mice, such as Siglec-7 and Siglec-9. If toxicity is observed in vivo when blocking Siglecs using mono-specific antibodies, it is possible that bispecific or tri-specific antibodies that target the blocking antibody to specific immune cells and/or virally-infected cells will be needed to take advantage of the enhanced immune functions caused by Siglec blockade while avoiding potential non-specific inflammatory effects.
Sialyltransferase inhibitors
Sialyltransferases catalyze the transfer of sialic acid to other substrates, resulting in the addition of sialic acid to the cell surface. Several types of cancer cells and virally-infected cells exhibit hyper-sialylation on their surface, and removing this sialic acid can allow immune cells to recognize and respond against these cells.37 Accordingly, several strategies have been developed to inhibit the sialyltransferases. For example, sialyltransferase inhibitors enhance the susceptibility of myeloma cells to NK-mediated clearance in a Siglec-7-dependent manner.61 However, similar to the blocking antibodies, sialyltransferase inhibitors likely will need to target specific cancer or virally-infected cells to prevent off-target side effects.
Sialidase-antibody conjugates
For strategies that target Siglec interactions to have clinical utility, they likely need to be able to selectively block Siglec interactions on cancer cells or infected cells, but not on non-target cells. Towards developing such an approach, sialidase was conjugated to trastuzumab (Herceptin; an antibody against the HER2 receptor, overexpressed in HER2+ breast cancer cells). This trastuzumab-sialidase conjugate functioned as expected; it not only prevented Siglec/sialic acid-binding but also enhanced anti-tumor NK cell activity against HER2+ but not HER2– cells in vitro and in vivo.62,63 Consistently, sialidase conjugated to HIV-specific antibodies removed sialic acid specifically from the surface of HIV-infected cells, but not HIV-negative cells, and this removal significantly enhanced the susceptibility of HIV-infected cells to NK cell-mediated immune clearance in vitro.28 These promising early studies suggest that the conjugate approach has significant potential to improve the susceptibility of virally-infected cells to immune-mediated clearance. Further testing of this approach for feasibility and efficiency in in vivo systems is clearly warranted.
Siglec-sialic acid interactions in mediating hyper-inflammation
The potential role of Siglec-sialic acid interactions in inducing inflammation during viral infections
Siglecs can play an important role in regulating inflammatory responses by myeloid cells. In this section, we discuss the potential role of inhibitory Siglec interactions in modulating hyper-inflammation during viral infections (with a focus on cis interactions between Siglecs and sialic acid on myeloid cells) and how to overcome this effect (Fig. 1, right).
The binding of inhibitory Siglecs expressed on myeloid cells to sialic acid on the same cells (cis interaction) plays an important role in controlling the inflammatory properties or polarization of these cells upon stimulation. For example, upon TLR4 stimulation (by bacterial LPS), myeloid cells upregulate expression of endogenous sialidases.64 These sialidases remove sialic acids from the surface of the myeloid cells, thereby reducing the opportunities for inhibitory Siglec-sialic-acid interactions that could dampen myeloid cell function.64,65 While such mechanisms are likely important for maintaining a constructive immunological response to pathogens, they also may lead to a state of hyper or chronic inflammation, especially during viral infections associated with translocation of microbes from gut to blood, such as HIV and SARS-CoV-2.66,67 Indeed, it was recently shown that engaging the inhibitory effects of Siglec-9 interactions using cis agonist glycopolymers suppresses neutrophilic hyper-inflammation induced by SARS-CoV-2.68
In addition to the cis interactions, trans interactions between inhibitory Siglecs on myeloid cells and sialic acids on other cells or circulating proteins/lipids can lead to hyper-inflammation. Within the circulating glycome, sialic acid on circulating antibodies (immunoglobulin G; IgG) has been linked to strong anti-inflammatory responses.69 The exact mechanism of this action is not clear. One suggestion is that the binding of the sialic acid-containing glycans on the IgG to Siglecs on monocytes/macrophages initiates an inhibitory signal that leads to an anti-inflammatory response.70 IgG sialylation decreases during viral infections (such as chronic HIV infection, despite ART71); this reduction might reduce the opportunity for such anti-inflammatory sialic-acid/Siglec binding and thus promote inflammation. It is unclear whether sialidase expression by myeloid cells, sialic acid reduction on IgG, and/or Siglec interactions on myeloid cells during viral infections modulate hyper-inflammation during acute infections and/or chronic inflammation during chronic infections. However, strategies to enhance the cis - and/or trans-interactions of Siglecs on myeloid cells have the potential to reduce excessive inflammation during viral infections.
Beyond classic myeloid cells, microglial cells in the brain play an important role in regulating neuroinflammation and neurological functions. These microglial functions can be modified by Siglecs. For example, Siglec-2 reduces the inflammatory effects of microglial cells72 and modifies their phagocytosis capacity during aging.73 Siglec-11 on microglial cells reduces LPS-induced inflammation, phagocytosis, microglial cytotoxicity, and neurotoxicity.74,75 Finally, the expression of Siglec-E is neuroprotective.76 Several viral infections have been associated with neuroinflammation, including HIV and SARS-CoV-2, and understanding how inflammation in the CNS might be modified by Siglec interactions is an area in need of investigation. In HIV infection, even after long-term ART suppression,77 the neuro-inflammatory state likely causes cognitive dysfunction that impacts everyday functioning and increases morbidity and mortality among ART-suppressed HIV-infected individuals. SARS-CoV-2 infection can also induce a state of neuroinflammation and neurological symptoms78; however, unlike HIV infection, any long-term morbidity from the neurological effects of SARS-CoV-2 infection remains unknown. Examining how Siglecs, sialic acid, and neuraminidases in the central nervous system (CNS) may modulate virus-mediated neuro-inflammation could lead to novel approaches to reduce neuroinflammation during viral infections. However, modulating Siglec interactions in the CNS must be undertaken with great care, as microglial cells have multiple functions. For example, microglial-expressed Siglec-3 interacts with sialylated amyloid plaques, which reduces the ability of microglia to phagocytose the plaques, which may contribute to Alzheimer's disease progression.79, 80, 81
Approaches to engage Siglec/sialoglycan interactions to prevent hyper-inflammation (Fig. 2B)
Agonistic Siglec antibodies
Several antibodies engaging inhibitory Siglecs have been used to reduce inflammation. For example, an engaging Siglec-9 antibody suppresses LPS-induced inflammatory responses in human macrophages.82 Similarly, Siglec-7 engaged by an antibody lead to apoptosis of mast cells and reduced mast cell leukemia in mice.83 Finally, an engaging antibody to Siglec-F, the murine homolog of human Siglec-8, reduced inflammation in mice.84,85 Consistently, an agonistic Siglec-8 antibody inhibited IgE-independent mast cell activation and suppressed non-allergic airway inflammation,86 and reduced inflammation and COVID-19 severity in a mouse model.87 These antibodies, and other antibodies that activate inhibitory Siglec receptors, have the potential to be used to reduce the state of hyper-inflammation during acute viral infections and/or chronic inflammation during chronic inflammation.
Sialic acid mimetics, sialic acid-coated nanoparticles, and sialidase inhibitors
Other approaches to engage inhibitory Siglecs and reduce inflammation have been investigated, including lipid-conjugated glycopolypeptides that can be inserted into cell membranes and engage Siglec-9 receptors in cis conformation.88 This synthetic Siglec-9 agonist inhibited NETosis in neutrophils during COVID-19.68 Another approach in development is nanoparticles or liposomes coated with sialic acid. Such nanoparticles blocked LPS-mediated inflammation in a mouse model of sepsis.89 A third approach used sialidase inhibitors to prevent the removal of sialic acid by endogenous and/or exogenous sialidases. Indeed, sialidase inhibitors can protect mice from sepsis.90 Finally, conjugating Siglec ligands to an anti-receptor antibody can be used to suppress the excessive immune response in specific immune cells.91 Although only synthetic Siglec-9 agonists were tested in virus-related inflammatory settings, such approaches are promising tools for reducing inflammation during viral infections.
Conclusions and outstanding questions
Siglecs are immunological switches: when ‘off’, i.e., when their interactions are inhibited, they can enhance the functions of several types of immune cells that are central to anti-viral immunity; when ‘on’, i.e., when their interactions are engaged, they can reduce hyper-inflammation and hyper-activation of immune functions. Over the last few years, several tools and approaches have been developed to either block Siglec interactions or engage them. Some of these approaches have been used to enhance immune functions against viral infections (such as blocking antibodies and sialidase-antibody conjugates to enhance immune functions against HBV and HIV) or to prevent hyper-inflammation (such as synthetic Siglec-9 agonists to prevent hyper-inflammation during COVID-19). However, there is a need for extensive investigations to better understand not only the role of Siglec interactions in modulating immune functions during viral infections but also how to tailor Siglec-modulating approaches so that they enhance immune functions without inducing excessive inflammation, or they reduce excessive inflammation without inhibiting critical immune functions. These tailored approaches could include the development of blocking or engaging antibodies/molecules that are specific to immune cells or virally infected cells. Such tailored approaches might enable an optimal immunological equilibrium during viral infections by blocking specific Siglec interactions to enhance certain immune functions while simultaneously engaging other Siglec interactions to reduce excessive inflammation.
Search strategy and selection criteria
Data for this review were obtained from PubMed using the key words “Siglec”, “viral infection”, “Siglec engaging”, “Siglec blocking”, “Siglec in vivo”, “Siglec in cancer”. Articles published between 1980 and 2022 were included with particular emphasis on those published in the past five years.
Contributors
M.A.-M. and P.S. conceived and designed the review and wrote it. O.S.A. edited the review. All authors read and approved the final version of the manuscript.
Declaration of interests
All authors have nothing to disclose.
Acknowledgments
M.A.-M. is funded by the following grants: NIH (R01AI165079, R01NS117458, R01DK123733, and R01AG062383), Campbell Foundation award, Penn Center for AIDS Research (P30 AI 045008), and Bill & Melinda Gates Foundation. M.A.-M., O.S.A., and P.S. are members of the investigation team of the NIH-funded BEAT-HIV Martin Delaney Collaboratory to cure HIV-1 infection (UM1AI164570). The funders had no role in the review design or writing. We would also like to thank Rachel E. Locke, Ph.D., for providing comments.
References
- 1.Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadjadj J., Yatim N., Barnabei L., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandler N.G., Bosinger S.E., Estes J.D., et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511(7511):601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10(7):514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhen A., Rezek V., Youn C., et al. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J Clin Invest. 2017;127(1):260–268. doi: 10.1172/JCI89488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler N.G., Wand H., Roque A., et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katlama C., Deeks S.G., Autran B., et al. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013;381(9883):2109–2117. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel S.A., Minn A.J. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48(3):417–433. doi: 10.1016/j.immuni.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson D.B., Nebhan C.A., Moslehi J.J., Balko J.M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254–267. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RodrIguez E., Schetters S.T.T., van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018;18(3):204–211. doi: 10.1038/nri.2018.3. [DOI] [PubMed] [Google Scholar]
- 12.Bornhofft K.F., Goldammer T., Rebl A., Galuska S.P. Siglecs: a journey through the evolution of sialic acid-binding immunoglobulin-type lectins. Dev Comp Immunol. 2018;86:219–231. doi: 10.1016/j.dci.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Duan S., Paulson J.C. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365–395. doi: 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- 14.Varki A., Schnaar R.L., Crocker P.R. In: Essentials of glycobiology. Varki A., Cummings R.D., Esko J.D., et al., editors. Cold Spring Harbor (NY); 2015. I-type lectins; pp. 453–467. [Google Scholar]
- 15.Macauley M.S., Crocker P.R., Paulson J.C. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14(10):653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillai S., Netravali I.A., Cariappa A., Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Gil A., Schnaar R.L. Siglec ligands. Cells. 2021;10(5):1260. doi: 10.3390/cells10051260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kean E.L., Munster-Kuhnel A.K., Gerardy-Schahn R. CMP-sialic acid synthetase of the nucleus. Biochim Biophys Acta. 2004;1673(1–2):56–65. doi: 10.1016/j.bbagen.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Miyagi T., Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22(7):880–896. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues E., Macauley M.S. Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities. Cancers. 2018;10(6):207. doi: 10.3390/cancers10060207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delputte P.L., Nauwynck H.J. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J Virol. 2004;78(15):8094–8101. doi: 10.1128/JVI.78.15.8094-8101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izquierdo-Useros N., Lorizate M., Puertas M.C., et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10(12) doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderheijden N., Delputte P.L., Favoreel H.W., et al. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J Virol. 2003;77(15):8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd-Leotis L., Cummings R.D., Steinhauer D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int J Mol Sci. 2017;18(7):1541. doi: 10.3390/ijms18071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer Q., Ruckert T., Romagnani C. Natural killer cell specificity for viral infections. Nat Immunol. 2018;19(8):800–808. doi: 10.1038/s41590-018-0163-6. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y., Ma X., Su D., et al. The roles of Siglec7 and Siglec9 on natural killer cells in virus infection and tumour progression. J Immunol Res. 2020;2020 doi: 10.1155/2020/6243819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicoll G., Ni J., Liu D., et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem. 1999;274(48):34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 28.Adeniji O.S., Kuri-Cervantes L., Yu C., et al. Siglec-9 defines and restrains a natural killer subpopulation highly cytotoxic to HIV-infected cells. PLoS Pathog. 2021;17(11) doi: 10.1371/journal.ppat.1010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-Caselles T., Martinez-Esparza M., Perez-Oliva A.B., et al. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two isoforms of CD33 are generated by alternative splicing. J Leukoc Biol. 2006;79(1):46–58. doi: 10.1189/jlb.0205096. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P., Lu X., Tao K., et al. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. J Surg Res. 2015;194(1):107–113. doi: 10.1016/j.jss.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 31.Hudak J.E., Canham S.M., Bertozzi C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol. 2014;10(1):69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jandus C., Boligan K.F., Chijioke O., et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124(4):1810–1820. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laubli H., Pearce O.M., Schwarz F., et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci U S A. 2014;111(39):14211–14216. doi: 10.1073/pnas.1409580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunetta E., Fogli M., Varchetta S., et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114(18):3822–3830. doi: 10.1182/blood-2009-06-226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao D., Jiang X., Xu Y., et al. Decreased Siglec-9 expression on natural killer cell subset associated with persistent HBV replication. Front Immunol. 2018;9:1124. doi: 10.3389/fimmu.2018.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen D.H., Hurtado-Ziola N., Gagneux P., Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A. 2006;103(20):7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haas Q., Boligan K.F., Jandus C., et al. Siglec-9 regulates an effector memory CD8(+) T-cell subset that congregates in the melanoma tumor microenvironment. Cancer Immunol Res. 2019;7(5):707–718. doi: 10.1158/2326-6066.CIR-18-0505. [DOI] [PubMed] [Google Scholar]
- 38.Stanczak M.A., Siddiqui S.S., Trefny M.P., et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Invest. 2018;128(11):4912–4923. doi: 10.1172/JCI120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phetsouphanh C., Phalora P., Hackstein C.P., et al. Human MAIT cells respond to and suppress HIV-1. Elife. 2021;10 doi: 10.7554/eLife.50324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Massow G., Oh S., Lam A., Gustafsson K. Gamma delta T cells and their involvement in COVID-19 virus infections. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.741218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estes J.D., Kityo C., Ssali F., et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nature Med. 2017;23(11):1271–1276. doi: 10.1038/nm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabo P.A., Dogra P., Gray J.I., et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity. 2021;54(4):797–814.e6. doi: 10.1016/j.immuni.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barkal A.A., Brewer R.E., Markovic M., et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beatson R., Tajadura-Ortega V., Achkova D., et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. 2016;17(11):1273–1281. doi: 10.1038/ni.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perdicchio M., Ilarregui J.M., Verstege M.I., et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc Natl Acad Sci U S A. 2016;113(12):3329–3334. doi: 10.1073/pnas.1507706113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lübbers J., Eveline Li R.-J., Gorki F.S., et al. α2-3 Sialic acid binding and uptake by human monocyte-derived dendritic cells alters metabolism and cytokine release and initiates tolerizing T cell programming. Immunother Adv. 2021;1(1) doi: 10.1093/immadv/ltab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai T.Y., Huang M.T., Sung P.S., et al. SIGLEC-3 (CD33) serves as an immune checkpoint receptor for HBV infection. J Clin Invest. 2021;131(11) doi: 10.1172/JCI141965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panico M., Bouche L., Binet D., et al. Mapping the complete glycoproteome of virion-derived HIV-1 gp120 provides insights into broadly neutralizing antibody binding. Sci Rep. 2016;6 doi: 10.1038/srep32956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamenkovic I., Sgroi D., Aruffo A., Sy M.S., Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 50.Munday J., Kerr S., Ni J., et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355(Pt 2):489–497. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato S., Jansen P.J., Tedder T.F. CD19 and CD22 expression reciprocally regulates tyrosine phosphorylation of Vav protein during B lymphocyte signaling. Proc Natl Acad Sci U S A. 1997;94(24):13158–13162. doi: 10.1073/pnas.94.24.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann A., Kerr S., Jellusova J., et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8(7):695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 53.Kardava L., Moir S., Wang W., et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121(7):2614–2624. doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ibarlucea-Benitez I., Weitzenfeld P., Smith P., Ravetch J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2021;118(26) doi: 10.1073/pnas.2107424118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi H., Ho M., Adeniji O.S., et al. Development of Siglec-9 blocking antibody to enhance anti-tumor immunity. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.778989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hills R.K., Castaigne S., Appelbaum F.R., et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jabbour E., O'Brien S., Ravandi F., Kantarjian H. Monoclonal antibodies in acute lymphoblastic leukemia. Blood. 2015;125(26):4010–4016. doi: 10.1182/blood-2014-08-596403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz F., Pearce O.M., Wang X., et al. Siglec receptors impact mammalian lifespan by modulating oxidative stress. Elife. 2015;4 doi: 10.7554/eLife.06184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varki A., Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su C., Wang H., Liu Y., et al. Adverse effects of anti-PD-1/PD-L1 therapy in non-small cell lung cancer. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.554313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daly J., Sarkar S., Natoni A., et al. Targeting hypersialylation in multiple myeloma represents a novel approach to enhance NK cell-mediated tumor responses. Blood Adv. 2022;6(11):3352–3366. doi: 10.1182/bloodadvances.2021006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao H., Woods E.C., Vukojicic P., Bertozzi C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A. 2016;113(37):10304–10309. doi: 10.1073/pnas.1608069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray M.A., Stanczak M.A., Mantuano N.R., et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat Chem Biol. 2020;16(12):1376–1384. doi: 10.1038/s41589-020-0622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G.Y., Brown N.K., Wu W., et al. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. Elife. 2014;3 doi: 10.7554/eLife.04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varchetta S., Brunetta E., Roberto A., et al. Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0045821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamers M.M., Beumer J., van der Vaart J., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner J.R. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 68.Delaveris C.S., Wilk A.J., Riley N.M., et al. Synthetic Siglec-9 agonists inhibit neutrophil activation associated with COVID-19. ACS Cent Sci. 2021;7(4):650–657. doi: 10.1021/acscentsci.0c01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cobb B.A. The history of IgG glycosylation and where we are now. Glycobiology. 2020;30(4):202–213. doi: 10.1093/glycob/cwz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murugesan G., Weigle B., Crocker P.R. Siglec and anti-Siglec therapies. Curr Opin Chem Biol. 2021;62:34–42. doi: 10.1016/j.cbpa.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Vadrevu S.K., Trbojevic-Akmacic I., Kossenkov A.V., et al. Frontline science: plasma and immunoglobulin G galactosylation associate with HIV persistence during antiretroviral therapy. J Leukoc Biol. 2018;104(3):461–471. doi: 10.1002/JLB.3HI1217-500R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mott R.T., Ait-Ghezala G., Town T., et al. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46(4):369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- 73.Pluvinage J.V., Haney M.S., Smith B.A.H., et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature. 2019;568(7751):187–192. doi: 10.1038/s41586-019-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Neumann H. Alleviation of neurotoxicity by microglial human Siglec-11. J Neurosci. 2010;30(9):3482–3488. doi: 10.1523/JNEUROSCI.3940-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linnartz B., Wang Y., Neumann H. Microglial immunoreceptor tyrosine-based activation and inhibition motif signaling in neuroinflammation. Int J Alzheimer's Dis. 2010;2010 doi: 10.4061/2010/587463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Claude J., Linnartz-Gerlach B., Kudin A.P., Kunz W.S., Neumann H. Microglial CD33-related Siglec-E inhibits neurotoxicity by preventing the phagocytosis-associated oxidative burst. J Neurosci. 2013;33(46):18270–18276. doi: 10.1523/JNEUROSCI.2211-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heaton R.K., Franklin D.R., Ellis R.J., et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rehmani R., Segan S., Maddika S.R., Lei Y.W., Broka A. Spectrum of neurologic & neuroimaging manifestation in COVID-19. Brain Behav Immun Health. 2021;13 doi: 10.1016/j.bbih.2021.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Estus S., Shaw B.C., Devanney N., Katsumata Y., Press E.E., Fardo D.W. Evaluation of CD33 as a genetic risk factor for Alzheimer's disease. Acta Neuropathol. 2019;138(2):187–199. doi: 10.1007/s00401-019-02000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Griciuc A., Serrano-Pozo A., Parrado A.R., et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78(4):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malik M., Simpson J.F., Parikh I., et al. CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing. J Neurosci. 2013;33(33):13320–13325. doi: 10.1523/JNEUROSCI.1224-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu S., Zhu X., You N., et al. The fab fragment of a human anti-Siglec-9 monoclonal antibody suppresses LPS-induced inflammatory responses in human macrophages. Front Immunol. 2016;7:649. doi: 10.3389/fimmu.2016.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Landolina N., Zaffran I., Smiljkovic D., et al. Activation of Siglec-7 results in inhibition of in vitro and in vivo growth of human mast cell leukemia cells. Pharm Res. 2020;158 doi: 10.1016/j.phrs.2020.104682. [DOI] [PubMed] [Google Scholar]
- 84.Song D.J., Cho J.Y., Lee S.Y., et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183(8):5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zimmermann N., McBride M.L., Yamada Y., et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63(9):1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schanin J., Gebremeskel S., Korver W., et al. A monoclonal antibody to Siglec-8 suppresses non-allergic airway inflammation and inhibits IgE-independent mast cell activation. Mucosal Immunol. 2021;14(2):366–376. doi: 10.1038/s41385-020-00336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gebremeskel S., Schanin J., Coyle K.M., et al. Mast cell and eosinophil activation are associated with COVID-19 and TLR-mediated viral inflammation: implications for an anti-Siglec-8 antibody. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.650331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delaveris C.S., Chiu S.H., Riley N.M., Bertozzi C.R. Modulation of immune cell reactivity with cis-binding Siglec agonists. Proc Natl Acad Sci U S A. 2021;118(3) doi: 10.1073/pnas.2012408118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spence S., Greene M.K., Fay F., et al. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med. 2015;7(303):303ra140. doi: 10.1126/scitranslmed.aab3459. [DOI] [PubMed] [Google Scholar]
- 90.Chen G.Y., Chen X., King S., et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. 2011;29(5):428–435. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Islam M., Arlian B.M., Pfrengle F., Duan S., Smith S.A., Paulson J.C. Suppressing immune responses using Siglec ligand-decorated anti-receptor antibodies. J Am Chem Soc. 2022;144(21):9302–9311. doi: 10.1021/jacs.2c00922. [DOI] [PMC free article] [PubMed] [Google Scholar]