Abstract
The taste of kimchi is greatly affected by the salt type used during fermentation. Here, we investigated the effects of salts with different mineral contents on the microbial community and metabolite profiles of fermented kimchi using multivariate statistical analysis. We evaluated different types of salt used to prepare kimchi, namely, solar salt aged for 1 year, solar salt aged for 3 years, dehydrated solar salt, and purified salt. The main microorganisms detected in kimchi were Weissella koreensis, Leuconostoc mesenteroides, and Latilactobacillus sakei. Leuconostoc and Weissella were mainly present in kimchi supplemented with solar salt. However, a high proportion of L. sakei was present in kimchi supplemented with purified salt and dehydrated salt. Additionally, using GC-MS-based metabolite analysis, we revealed that the content of free sugars, organic acids, and amino acids differed in kimchi fermented with different salt types. Therefore, we demonstrated that salt type had a pronounced effect on the resultant microbial community and the type and concentration of metabolites present in fermented kimchi.
Keywords: Kimchi, Solar salt, Metabolites, Microbial community, Lactic acid bacteria
Kimchi; Solar salt; Metabolites; Microbial community; Lactic acid bacteria.
1. Introduction
Kimchi is a traditional fermented Korean food. Kimchi fermentation is initiated by lactic acid bacteria (LAB), which affect the resultant flavor profile by producing organic acid, mannitol, CO2, and ethanol (Hong et al., 2013; Yun et al., 2014). The ingredients added during kimchi fermentation affect the growth of each LAB group, thereby altering the quality and flavor of the end product indirectly (Jung et al., 2012a, 2012b; Kim et al., 2004; Park et al., 2019). Therefore, the selection of ingredients is important to improve the sensory and nutritional qualities of kimchi. Other factors also driving the fermentation process are raw material, temperature, starter, and salt type (Kim et al., 2020; Lee et al., 2015; Song et al., 2021). In particular, the salt type greatly affects the kimchi fermentation process (Lee et al., 2018).
Salt is an important ingredient for improving the taste and shelf-life of fermented foods (Bautista-Gallego et al., 2013). Salt inhibits the growth of harmful microorganisms in kimchi via osmotic shock and regulates the selective growth of salt-resistant LAB. The optimal growth and acid production of LAB generally occur at a salt concentration of 2–3% or less (Hong et al., 2021; Kim et al., 2005). The salt types used in manufacturing kimchi are mainly purified salt and solar salt; however, other types of salts with various additions, such as roasted salt and bamboo salt, have been used (Chang et al., 2011; Kim and Kim, 2014).
In Korea, purified salt contains sodium chloride without any additives; it is cheap and safe to use, and it is most widely used in kimchi production (Lee et al., 2013). Solar salt is a crystallized substance obtained by the natural evaporation of seawater flowing into a salt field (Kim et al., 2005). In contrast with purified salt, it contains various minerals such as magnesium, calcium, sulfur, potassium, iron, and zinc (Gao et al., 2014). These minerals promote the growth of microorganisms during various fermentation processes such as those of soy sauce, kimchi, and fish sauce (Hahn, 2003; Kim et al., 2000). The type and concentration of salt used affect the taste and texture of kimchi (Chang et al., 2011; Lee et al., 2021) as well as its fermentation rate.
To further investigate the effect of salt concentration on fermented vegetables, we performed a non-targeted metabolite analysis using mass spectrometry along with multivariate statistical analysis (Lee et al., 2021; Seo et al., 2018; Yang et al., 2020). Although several studies have reported the effect of salt type on kimchi fermentation (Chang et al., 2011; Kim and Kim, 2014), few studies have reported the correlation between salt type and corresponding metabolites and microbial community (Kim et al., 2017). Also, previous studies have analyzed the effects of the effects of the addition of purified salt and solar salt to kimchi and the aging period of solar salt on kimchi. However, there were no studies on the effects of kimchi supplemented with dehydrated solar salt on metabolites or microbial community profiles compared to kimchi with purified salt and solar salt. Therefore, in this study, we investigated the effects of salts with different mineral contents on the microbial community and metabolite profiles of fermented kimchi using multivariate statistical analysis.
2. Materials and methods
2.1. Experimental materials
Kimchi cabbage and garlic were purchased from a western agricultural and fishery market in Gwangju (Republic of Korea). Red pepper powder (Geumchi, Gwangju, Korea) and purified salt (PS) were purchased from Hanju Salt (Ulsan, Korea). Solar salt aged for 1 year (SS1) and solar salt aged for 3 years (SS3) were purchased from Deojoeun Sogeum (Sinan, Korea), and dehydrated solar salt (DSS) was purchased from Solseomsikpum (Sinan, Korea). All the experimental analyses were performed using first-grade analytical reagents obtained from Daejung (Gyeonggi-do, Korea). The water and acetonitrile used for high-performance liquid chromatography (HPLC) were of chromatographic grade (Merck, Rahway, NJ, USA).
2.2. Kimchi preparation
To design four types of kimchi samples, brine was prepared with four salt types: SS1, SS3, DSS, and PS. Kimchi cabbage was soaked in brine at a salinity of 12% for 16 h. Subsequently, salted kimchi cabbage was washed with water three times, drained for 2 h, then cut into 3 × 3 cm pieces. Kimchi seasoning was prepared by mixing 16.7% garlic, 2.7% ginger, 23.3% red pepper powder, 20.0% salted shrimp, and 37.3% water. This seasoning was added to the salted cabbage at a ratio of 70:30 (cabbage:seasoning). The resultant test groups were labeled SS1K (kimchi with SS1), SS3K (kimchi with SS3), DSSK (kimchi with DSS), and PSK (kimchi with PS). Each kimchi sample (600 g) was separately packed in polyethylene film and sealed using a vacuum packaging machine (Airzero AZC-070; INTRISE, Ansan, Korea). The packed kimchi was stored at 6 °C for 6 weeks in a refrigerator, and its characteristics were analyzed at 1-week intervals.
2.3. Analysis of mineral contents
Homogenized kimchi samples (0.5 g) were decomposed in nitric acid (Dongwoo Fine-chem, Iksan, Korea) using a microwave system (MARS 5; CEM Corporation, Matthews, NC, USA) and then diluted to 50 mL (5 g of salt in 50 mL nitric acid). If dilution was required, the same nitric acid concentration as that of the sample was used. Samples were analyzed for mineral content using ICP-MS (Aurora M90; Bruker, Billerica, MA, USA) under the analysis conditions listed in Table 1.
Table 1.
Inductively coupled plasma mass spectrometry (ICP-MS) instrument operating parameters.
| Instrument Parameters | Settings | |
|---|---|---|
| Gas Flow Parameters (L/min) | Plasma Flow | 16.0 |
| Auxiliary Flow | 1.65 | |
| Sheath Gas | 0.22 | |
| Nebulizer Flow | 1.00 | |
| Torch Alignment (mm) | Sampling Depth | 6.5 |
| RF Setting | RF Power (kW) | 1.40 |
| Sample Introduction | Pump Rate (rpm) | 5 |
| Stabilization Delay (s) | 20 | |
| Quadrupole Scan | Scan Mode | Peak Hopping |
| Dwell Time (ms) | 10 | |
| Acquisition | Points/Peak | 1 |
| Scans/Replicated | 20 | |
| Replicates/Sample | 3 | |
| Ion Optics (volts) | First Extraction Lens | 0 |
| Second Extraction Lens | -40 | |
| Third Extraction Lens | -215 | |
| Corner Lens | -170 | |
| Mirror Lens Left | 40 | |
| Mirror Lens Right | 30 | |
| Mirror Lens Bottom | 30 | |
| Entrance Lens | 4 | |
| Fringe Bias | -2.7 | |
| Entrance Plate | -35 | |
| Pole Bias | -1.0 |
2.4. Analysis of pH, titratable acidity, and viable cell counts
Kimchi samples were juiced using a gauze after blending, and the pH value was measured using a pH meter (TitroLine 5000; SI Analytics GmbH, Mainz, Germany) at room temperature (24–26 °C). The total acidity was titrated with 0.1 N NaOH until a pH of 8.3 was reached. Titratable acidity was calculated as the percentage of lactic acid produced. The colony-forming units (CFUs) of total viable bacteria and LAB were counted using a 3M Petrifilm aerobic count plate and LAB count plate (3M, Saint Paul, MN, USA) according to the manufacturer’s instructions. Values were multiplied by the relevant dilution factors, and the results were expressed as log CFU/g.
2.5. Microbial community composition analysis
Total DNA was extracted from the samples using a PowerSoil DNA isolation kit (MO BIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. DNA concentration and purity were measured using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). In the amplicon PCR process, the V3–V4 region of the 16S rRNAgene was targeted, and MiSeq raw data were classified for each sample using the index sequence, as previous described (Lee et al., 2021). Polymerase chain reaction (PCR) was performed using the primers 16S V3 (5′-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG-3′) and 16S V4 (5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGA CTA CHV GGG TAT CTA ATC C-3′). The PCR protocol was as follows: initial denaturation at 95 °C for 2 min, followed by 30 cycles of denaturation at 95 °C for 20 s, annealing at 72 °C for 15 s, extension at 72 °C for 1 min, and a final extension for 5 min. Total DNA extracted from kimchi samples was subjected to PCR using the 16S V4 gene. Sequencing was conducted on the Mi-Seq platform (Illumina, San Diego, CA, USA) by Macrogen (Seoul, Korea). After eliminating sequencing errors as well as ambiguous and chimeric sequences, the CD-HIT-OTU (operational taxonomic unit) analysis program was used to calculate the species-level OTUs to cluster sequences with a similarity of 97% (Abarenkov et al., 2010). The OTU representative sequence was used to perform UCLUST (v1.2.22) in the reference database (SIVA DB) and to generate taxonomic assignments based on homology. Microbial communities were analyzed using the Ribosomal Database Project (RDP) classifiers in QIIME software (v.1.9.2) (Caporaso et al., 2010).
2.6. Analysis of free sugars
Ten grams of each homogenized kimchi sample was added to a 50 mL centrifuge tube and adjusted to a constant volume with distilled water. The sample solution (50 mL) was heated in a water bath at 85 °C for 25 min and then cooled to room temperature. After centrifugation at 3000 rpm for 10 min, 1 mL of the supernatant was filtered with a nylon membrane filter (0.45 μm, 25 mm, Whatman PTFE, Clifron, NG, USA), following which 6 μL of the supernatant was used for analysis. Free sugars were measured using a HPLC instrument (1260 Infinity; Agilent Technologies, Santa Clara, CA, USA) equipped with refractive index detectors. A carbohydrate column was used (Asahipak NH2P-50 4E; Shodex, Tokyo, Japan) at an oven temperature of 30 °C. The mobile phase consisted of 75% acetonitrile in water, dispensed at a flow rate of 1 mL/min. The free sugar content was estimated using standard curves of fructose, glucose, sucrose, maltose, and mannitol.
2.7. Gas chromatography coupled to mass spectrometry (GC-MS)-based metabolomic analysis
The GC-MS analysis and identification of metabolites from the samples were performed, as described previous study (Seo et al., 2020). GC-MS was used to identify the metabolites in kimchi samples. After freeze-drying, 100 μL of O-methoxyamine hydrochloride in pyridine solution (20 mg/mL) was added to each sample. All samples were vortexed for 30 s and then incubated at 30 °C for 90 min in the dark. A silylation process was performed by adding 50 μL of N-methyl-N-trimethylsilyl-trifluoroacetamide containing 1% trimethylchlorosilane. The samples were vortexed again for 30 s and then incubated at 37 °C for 30 min. Ten microliters of ribitol (0.5 mg/L) were added as an internal standard (IS). Samples were centrifuged at 13,000 rpm for 10 min, and the supernatants were subjected to GC-MS analysis. Quality control (QC) samples were prepared by pooling equal volumes (approximately 10 μL) of each sample before derivatization. To measure the performance and stability of the system, together with the reproducibility of the sample treatment procedure, QC samples were analyzed for every 10 test samples processed. The derivatized samples were analyzed using GC-MS (QP2020; Shimadzu, Kyoto, Japan). A fused silica Rtx-5MS capillary column (30 m × 0.25 mm ID; J&W Scientific, Folsom, CA, USA) was used for the separation of metabolites. The front inlet temperature was 230 °C. The column temperature was held at 80 °C for 2 min isothermally, then raised by 15 °C/min to 330 °C and held for 6 min isothermally. The transfer line and ion source temperatures were 250 °C and 200 °C, respectively. Ionization was achieved with a 70 eV electron beam. The flow rate of helium gas through the column was 1 mL/min. Twenty scans per second were recorded over the mass range of 85–500 m/z. Chromatograms and mass spectra were acquired using Shimadzu GC solution (Shimadzu, Kyoto, Japan).
2.8. Statistical analysis
The experimental results were expressed as means and standard deviations, derived from three repeated measurements from each kimchi samples. GC-MS data from the Shimadzu Postrun Analysis software were converted to a netCDF file and processed using MetAlign software for peak detection and alignment. The resulting data (CSV-format file) were imported into AIoutput software for peak identification and prediction. Partial least squares-discriminant analysis (PLS-DA) of the GC-MS data was performed to visualize the variance of metabolites using SIMCA-P v15.0 (Umetrics, Umea, Sweden). To compare the differences in metabolite production between the samples during the fermentation period, statistical analysis was performed using MetaboAnalyst v5.0, and the results were presented as a heatmap. An unmanaged pattern recognition method, principal component analysis (PCA), was used to identify outliers in the datasets. Correlation analysis between PCA and variables was performed using XLSTAT Premium v19.4 (Addinsoft, New York, NY, USA). This software was also used to perform one-way analysis of variance for determining statistical significance and to conduct Duncan’s multirange tests for detecting significant sample-to-sample differences (p < 0.05).
3. Results and discussion
3.1. Composition of minerals in various salts
The mineral content identified for each salt is presented in Table 2. The Na content was lower in the solar salts (SSs) than in the PS, with no significant difference between results for the two SSs aging periods. The levels of Mg and Mn, which are essential for LAB growth and metabolism (Kim et al., 2017; Raccach, 1998), were higher in SS and DDS than in the PS. The Ca content was high in SS, which is consistent with a previous study (Chang et al., 2011); furthermore, the Ca content of SS3 was higher than that of SS1. Mg and K, which produce a bitter taste (Ha and Park, 1998), decreased with the aging period of SS.
Table 2.
Mineral contents of the salts used in this study (Unit: mg/kg).
| Minerals | SS11 | SS3 | DSSK | PSK |
|---|---|---|---|---|
| Na | 334,887.15 ± 1206.47b,2,3 | 335,829.83 ± 325.49b | 323,810.65 ± 220.60c | 386,334.40 ± 1188.28a |
| Mg | 7069.25 ± 103.15b | 5150.13 ± 120.91c | 12,544.98 ± 77.71a | 261.94 ± 2.03d |
| K | 1906.54 ± 86.79c | 1339.76 ± 78.63d | 2141.19 ± 32.48b | 2778.57 ± 85.73a |
| Ca | 1129.36 ± 17.07b | 1893.14 ± 6.74a | 835.30 ± 12.90c | 465.21 ± 3.41d |
| Mn | 1.57 ± 0.13a | 1.26 ± 0.11b | 1.62 ± 0.15a | ND4 |
| Fe | 0.10 ± 0.03b | 0.21 ± 0.06a | 0.17 ± 0.05ab | ND |
| Zn | 0.02 ± 0.02ab | 0.02 ± 0.00ab | 0.05 ± 0.03a | ND |
| Sr | 21.90 ± 3.55a | 12.02 ± 2.00b | 6.58 ± 1.55c | 0.52 ± 0.11d |
SS1: solar salt aged 1 year; SS3: solar salt aged 3 years; DSS: dehydrated solar salt; PS: purified salt.
All values are the mean ± SD.
Mean sharing different letters in the same row (a–d) are significantly different (p < 0.05).
ND: not detected.
3.2. Changes in fermentation properties
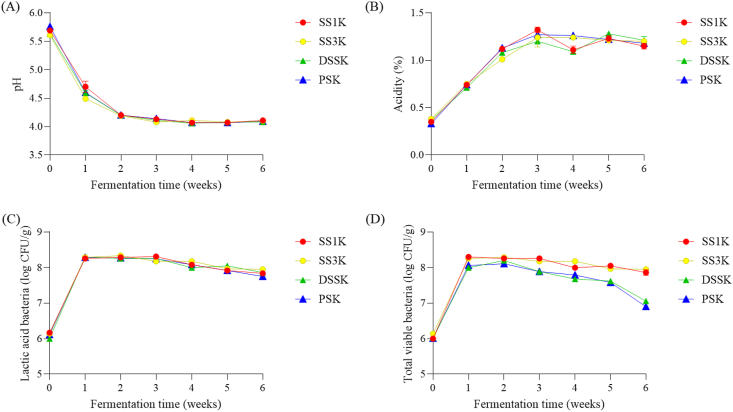
The pH, titratable acidity, number of total viable bacteria, and number of LAB of kimchi samples were monitored weekly for 6 weeks of fermentation (Figure 1). Changes in pH following supplementation with various salts are shown in Figure 1. The pH and titratable acidity are major quality indicators of fermentation that represent the ripening stage of kimchi. All samples displayed a decrease in pH as fermentation progressed. During initial fermentation, the pH of kimchi samples were as follows: SS1K 5.69 ± 0.02, SS3K 5.61 ± 0.03, DSSK 5.63 ± 0.02, and PSK 5.76 ± 0.02; at six weeks of fermentation, these pH values decreased to 4.11 ± 0.02, 4.10 ± 0.01, 4.08 ± 0.01, and 4.09 ± 0.01, respectively. There was no difference in pH changes among the salt types. In a study by Ku et al. (1988), the optimal flavor of kimchi was reached at a pH of 4.2–4.4. In this study, all types of kimchi reached a pH of 4.2–4.4 after 2 weeks of fermentation. The titratable acidity of kimchi samples obtained at the initial fermentation was 0.33–0.38% and increased to 1.15–1.21% by the end of fermentation. Again, there were no significant differences among treatment groups. These results contrast with those of Han et al. (2009), who reported that kimchi supplemented with SS fermented faster than kimchi supplemented with PS. However, the fermentation of kimchi is accelerated with sea salt because it contains a higher content of other inorganic ingredients, such as Ca and Mg, than PS, and the fermentation rate of kimchi appears to be affected by the inorganic ingredients contained in the salt. Similar results were reported by Kim et al. (2005).
Figure 1.
Changes in the fermentation properties of kimchi samples supplemented with different types of salt during 6 weeks of fermentation. (A) pH, (B) acidity (%), (C) lactic acid bacteria (log CFU/g), (D) total viable bacteria (log CFU/g).
Figure 1 shows the increase in the number of total viable bacteria and LAB during the fermentation of kimchi with different salt types. In general, microorganisms increase rapidly during initial fermentation but gradually decrease during late fermentation (Cho et al., 2006; Li et al., 2017). The number of total viable bacteria in our samples reached a maximum after 2 weeks of fermentation (range: 8.00–8.30 log CFU/g) and then decreased slightly during late fermentation. Moreover, the viability of LAB slightly decreased. After six weeks of fermentation, the number of viable cells for SS1K and SS3K was 7.86–7.95 log CFU/g, and 6.90–7.06 log CFU/g for PSK and DSSK, and the decrease in viable cells was large in PSK and DSSK. However, there was no difference between the various kimchi samples. These results are in agreement with a previous study (Lee et al., 2018) reporting that the number of viable bacteria was higher in kimchi supplemented with SS than in that supplemented with PS. One of the interesting observations from this previous study was that the proportion Leuconostoc species that are more sensitive to minerals such as Mg was significantly higher in the SS kimchi sample than in the PS kimchi sample. Therefore, the total number of bacteria was higher in kimchi containing SS with high Mg and Ca content than in kimchi containing PS.
3.3. Microbial community composition analysis
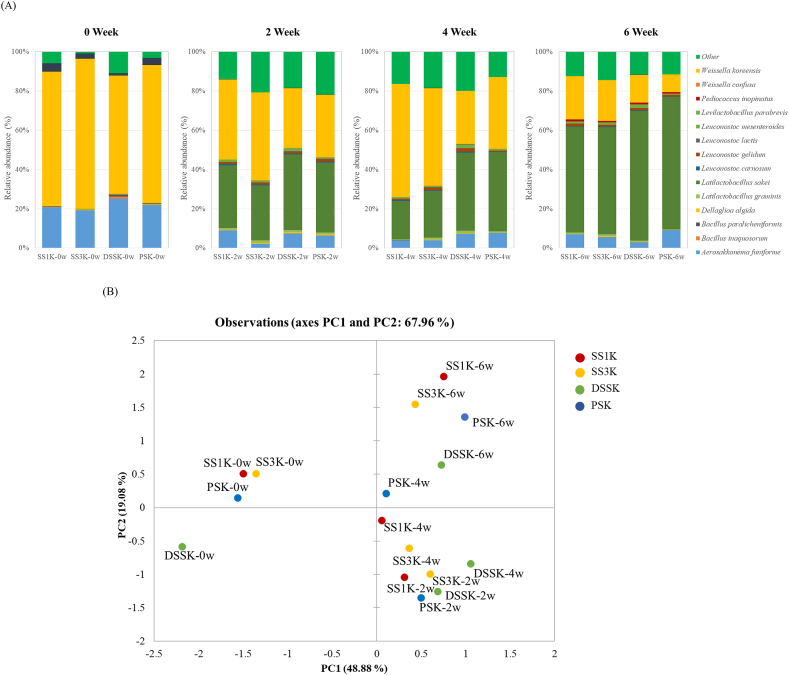
The microbial communities of kimchi supplemented with various salts (Figure 2A) showed a similar trend at the species level at the beginning of fermentation: Aerosakkonema funiforme was the most dominant species in all samples, followed by Weissella koreensis, and LAB were present in a small proportion. However, the levels of Weissella and Latilactobacillus increased rapidly in all samples after 1 week of fermentation, and Weissella continued to increase in SS1K and SS3K during the mid-fermentation period. By the end of fermentation, Latilactobacillus sakei was the predominant species, while Leuconostoc and Weissella decreased in all kimchi samples. Our results are similar to those of a previous study in which the ratio of Weissella began to decrease in the middle of the fermentation period because an increase in acidity inhibited the growth of this species (Fusco et al., 2015). Leuconostoc spp. are commonly used as kimchi starters because they improve the sensory characteristics and control the fermentation rate, whereas Latilactobacillus spp. cause over-ripening and quality deterioration (Jung et al., 2012a, 2012b; Lee et al., 2015, 2020b). In SS1K, the ratio of Weissella koreensis was twofold higher than that in DSSK at 4 weeks of fermentation, whereas the ratio of Latilactobacillus sakei was the highest in PSK. Therefore, these results demonstrated that SS significantly influenced the quality of kimchi compared to PS. Additionally, W. koreensis plays an important role in low-temperature kimchi fermentation (Mun and Chang, 2020) and is the dominant microorganism in the fermentation of kimchi supplemented with SS. A previous study has reported that kimchi supplemented with SS had a higher ratio of Leuconostoc spp. and Weissella spp. than that supplemented with PS (Kim et al., 2017). The microbial abundance and community composition in our kimchi samples differed according to the type of salt used. The two kimchi groups supplemented with SS (SS1K and SS3K) showed similar bacterial community profiles at the species level despite the different aging periods.
Figure 2.
Relative abundance of bacteria at the species level. (A) Principal component analysis (PCA) plot displaying the bacterial communities in each kimchi sample (B) as determined in the SILVA rRNA database. The kimchi samples were collected at 0, 2, 4, and 6 weeks. ‘Other’ indicates genera showing a percentage of reads <0.5% of the total reads in all the kimchi samples in species-level analyses.
PCA was performed to identify changes in the microbial community based on the salt type used during fermentation (Figure 2B). All samples displayed changes in the bacterial profiles until 6 weeks of fermentation. The salt type affected this bacterial composition as fermentation progressed in the PCA plot, with PC1 and PC2 accounting for 46.88% and 19.08% of the total variance, respectively. Since PC1 is the component that identifies changes during the fermentation period, it accounts for the largest variation in the original dataset (Seo et al., 2018; Song et al., 2021).
3.4. Changes in the free sugar content in kimchi supplemented with different salts
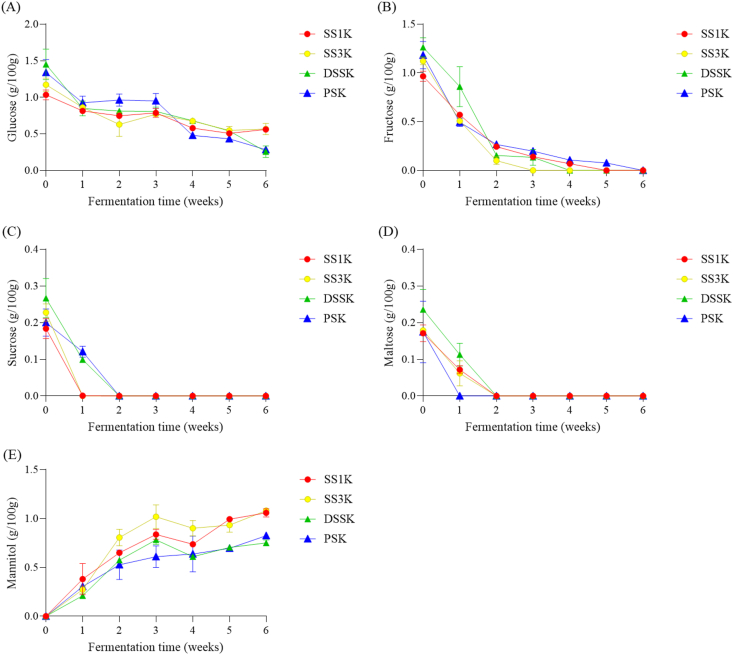
Free sugars play an important role as a carbon source for microorganisms during kimchi fermentation (Choi et al., 2019). Therefore, we investigated the effect of salt type on free sugars during kimchi fermentation (Figure 3). At the onset of fermentation, the main free sugars in each kimchi sample were glucose, sucrose, fructose, and maltose. As fermentation progressed, the concentrations of glucose, fructose, sucrose, and maltose decreased, while that of mannitol increased. The degradation of sucrose and fructose in SS1K and SS3K was faster than in DSSK and PSK. Mannitol, which was not detected at the beginning of fermentation, increased after 1 week of fermentation and significantly increased after 2 weeks, showing a significant difference between samples (p < 0.05). The concentration of mannitol was highest in kimchi supplemented with SS during the second week of fermentation. Mannitol content was particularly high in SS3K. This is likely due to the ratio of Weissella spp. being higher in SS1K and SS3K than in PSK. Mannitol is generally produced via the catalytic hydrogenation of fructose and sucrose (Wisselink et al., 2002). Hetero-fermentative LAB, such as Leuconostoc spp. and Weissella spp., convert fructose to mannitol, and Leuconostoc spp. have the highest mannitol production capacity among these LAB (Jung et al., 2012a, 2012b). Previous studies have reported that Weissella spp. and Lactobacillus spp. produce small amounts of mannitol (Carvalheiro et al., 2011; Lee et al., 2020a; Otgonbayar et al., 2011). Thus, mannitol content is high in kimchi with a high ratio of Weissella spp. Weissella koreensis, which can produce mannitol using a mixture of glucose and fructose.
Figure 3.
Changes in the free sugar contents of kimchi samples supplemented with different types of salt during 6 weeks of fermentation. (A) glucose, (B) fructose, (C) sucrose, (D) maltose, (E) mannitol.
3.5. Multivariate statistical analysis
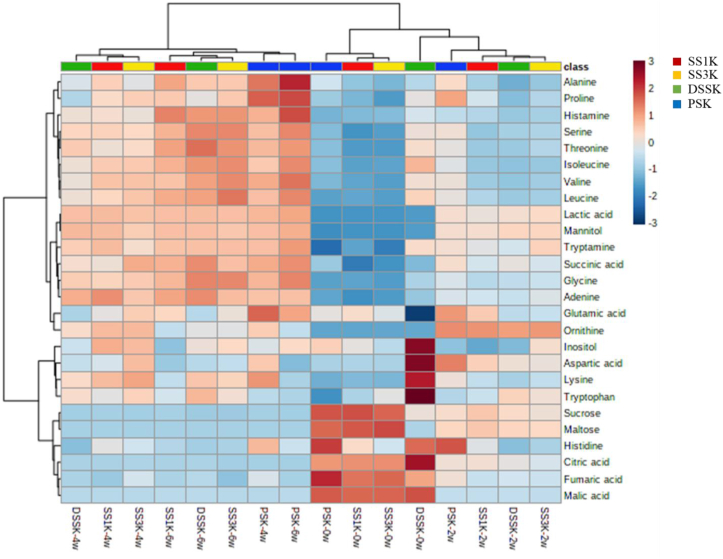
We constructed PCA plots and heatmaps showing the changes in the content of major metabolites according to the fermentation period were (Figures 4 and 5). The heatmap generated using the concentration of the 26 metabolite profiles revealed that the type of salt used during kimchi fermentation affects the concentration of metabolites (Figure 4). Most primary metabolite profiles showed similar trends in SS1K, SS3K, and PSK at the early stages of fermentation, whereas different trends were observed for DSSK. DSSK showed high concentration of glutamic acid, inositol, aspartic acid, lysine, tryptophan, and histidine. However, the changes in metabolite profiles varied among kimchi samples as fermentation progressed. By the end of fermentation, PSK produced a significantly higher concentration of amino acids than other samples did. A similar heatmap pattern was observed in samples supplemented with SSs, whereas they showed distinctly different patterns, especially in amino acid metabolites, compared to PSK.
Figure 4.
Heatmap showing the metabolite profiles in kimchi samples supplemented with different types of salt during 6 weeks of fermentation. The blue and red colors correspond to negative and positive correlations, respectively. The color intensity is proportional to the correlation coefficient.
Figure 5.
Principal component analysis (PCA) score plot derived from metabolite data of kimchi samples supplemented with different types of salt during 6 weeks of fermentation.
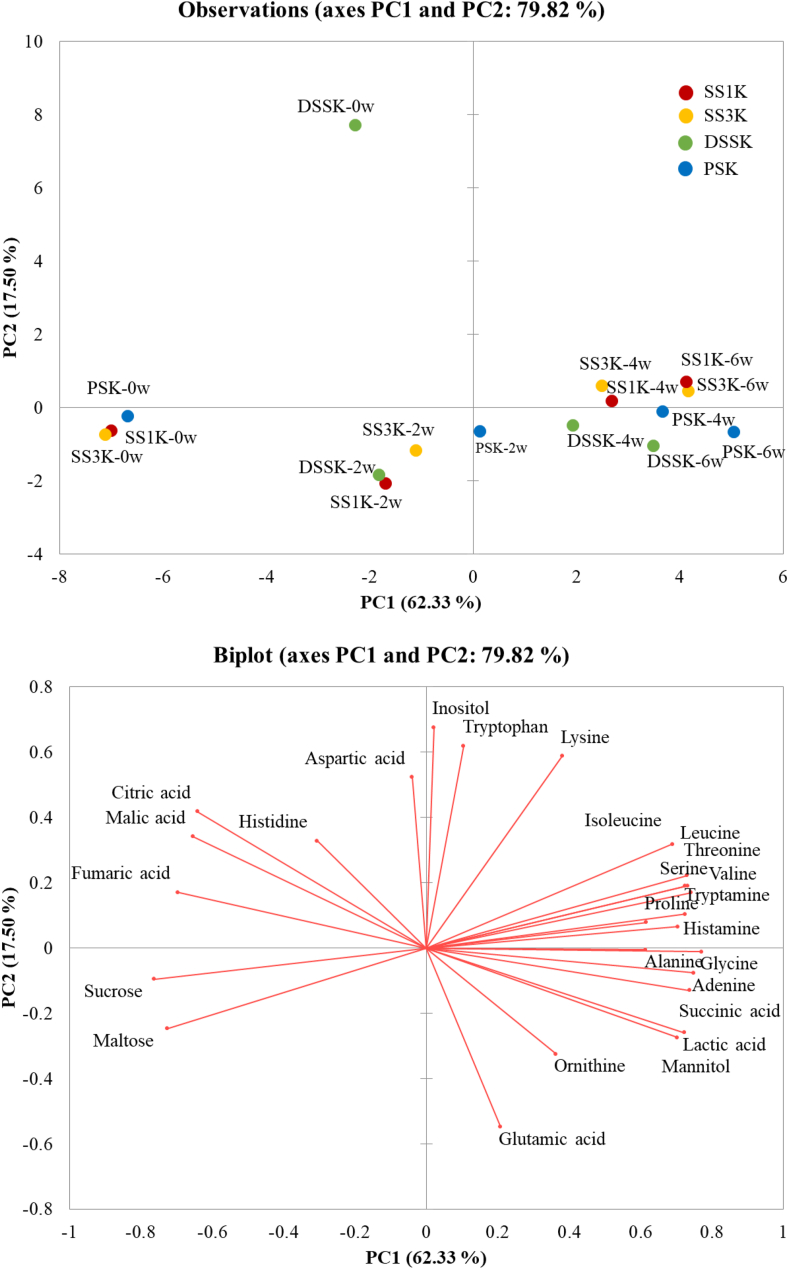
A PCA model was constructed using variables obtained from the metabolite data to identify the differences in metabolite profiles of the four kimchi samples for each fermentation step (Figure 5). PC1 and PC2 accounted for 62.33% and 17.50% of the total variance, respectively. Because PC1 accounts for changes in metabolite profiles, it accounts for the largest variability in the dataset (Wold et al., 1987). Therefore, quantitative information about the types of salts and metabolites was provided by these data. At the beginning of fermentation, SS1K, SS3K, and PSK were not discriminated in the PCA plot, and only DSSK was distinguished. This result showed a trend similar to that of the PCA plot of the microbial community. However, not all kimchi samples were discriminated in the PCA plot as fermentation progressed. Sucrose, maltose, fumaric acid, and citric acid contents which are related to the taste component showed a high correlation with the initial fermentation. Lactic acid, mannitol, glutamic acid, and other free amino acids showed highly positive correlations with late fermentation. The metabolite profile of kimchi changed according to the salt type and fermentation periods, which affected the PCA and heatmap analysis results. This is consistent with previous studies showing that the metabolite and sensory characteristics of kimchi differ with PS and SS supplementation (Chang et al., 2011; Lee et al., 2018).
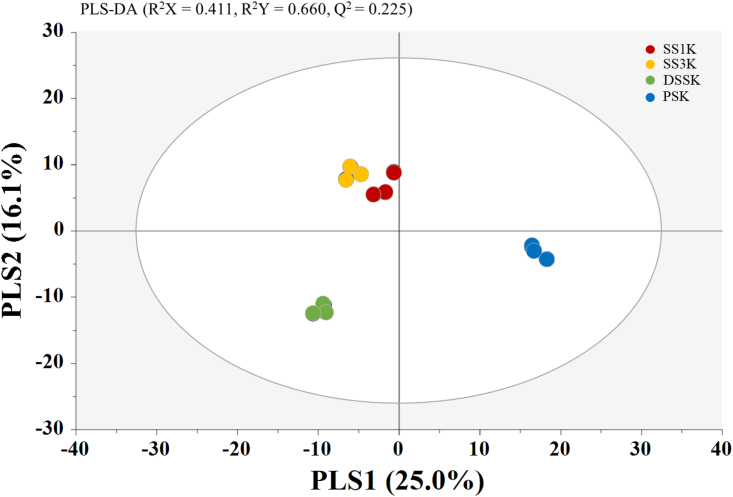
A supervised pattern recognition method, PLS-DA, was applied to the metabolite data analyzed by GC-MS at 6 weeks of fermentation to maximize the number of segregated kimchi samples (Figure 6). The PLS-DA score plot using data from 6 weeks of kimchi fermentation according to the type of salt clearly separated the R2X, R2Y, and Q2 values of PC1 and PC2 as 0.411, 0.660, and 0.225, respectively. SS1K and SS3K showed a similar trend in the PLS-DA plot at 6 weeks of fermentation, and DSSK and PSK were completely distinguished from kimchi supplemented with SSs (SS1K and SS3K). These results are consistent with those of a previous study reporting that a PLS-DA analysis showed that kimchi supplemented with PS and SS were significantly separated according to the salt type and fermentation time in the score plot with parameters (Kim et al., 2017).
Figure 6.
Partial least squares-discriminant analysis (PLS-DA) score plot obtained from gas chromatography coupled to mass spectrometry (GC-MS) data of kimchi samples supplemented with different types of salt at the 6 weeks of fermentation.
4. Conclusion
Several studies have reported the varying quality characteristics of kimchi according to the type of salt used. This study investigated the effects of four salt types on fermentation quality, composition of the microbial community, and metabolite profile during kimchi fermentation. We revealed that SS, PS and DSS supplementation produced were no differences in the fermentation properties of kimchi. SS promoted the dominance of Weissella bacteria during the initial fermentation stages. In the late fermentation period, the ratio of Latilactobacillus was high in kimchi supplemented with PS. Kimchi samples supplemented with SS showed different patterns with respect to amino acid metabolites compared to kimchi supplemented with PS. Our data therefore indicated that salt type changes the metabolite profile and microbial community of kimchi during fermentation. This study had some limitation. As a result of this study, metabolites results were derived by performing natural fermentation without using LAB starter. Because metabolites are affected according to the microbial community, it can be easy to understand the effect of various salts on the fermentation metabolites of kimchi when using a specific starter. Therefore, further studies are needed to comprehensively determine the effects of salt type on the mineral content, metabolites, and microbial community in kimchi.
Declarations
Author contribution statement
Mi-Ai Lee; Ye-Rang Yun: Analyzed and interpreted the data; Wrote the paper.
Yun-Jung Choi: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Ye-Sol Kim, Seo-Yeong Chon: Performed the experiments.
Young Bae Chung: Contributed reagents, materials, analysis tools or data.
Sung-Hee Park: Analyzed and interpreted the data.
Sung Gi Min: Conceived and designed the experiments.
Ho-Chul Yang: Conceived and designed the experiments; Performed the experiments.
Hye-Young Seo: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
Sung Gi Min and Hye-Young Seo were supported by Ministry of Science and ICT, Republic of Korea [KE2203-1 & KE2102-2-2].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abarenkov K., Tedersoo L., Nilsson R.H., Vellak K., Saar I., Veldre V., Parmasto E., Prous M., Aan A., Ots M. PlutoF-a web based workbench for ecological and taxonomic research, with an online implementation for fungal ITS sequences. Evol. Bioinf. Online. 2010;6:189–196. [Google Scholar]
- Bautista-Gallego J., Rantsiou K., Garrido-Fernández A., Cocolin L., Arroyo-López F.N. Salt reduction in vegetable fermentation: reality or desire. J. Food Sci. 2013;78:R1095–1100. doi: 10.1111/1750-3841.12170. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheiro F., Moniz P., Duarte L.C., Esteves M.P., Gírio F.M. Mannitol production by lactic acid bacteria grown in supplemented carob syrup. J. Ind. Microbiol. Biotechnol. 2011;38:221–227. doi: 10.1007/s10295-010-0823-5. [DOI] [PubMed] [Google Scholar]
- Chang J.Y., Kim I.C., Chang H.C. Effect of solar salt on the fermentation characteristics of kimchi. Korean J. Food Preserv. 2011;18:256–265. [Google Scholar]
- Cho J., Lee D., Yang C., Joen J., Kim J., Han H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol. Lett. 2006;257:262–267. doi: 10.1111/j.1574-6968.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Yong S., Lee M.J., Park S.J., Yun Y.R., Park S.H., Lee M.A. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT-Food Sci. Technol. 2019;105:118–126. [Google Scholar]
- Fusco V., Quero G.M., Cho G.S., Kabisch J., Meske D., Neve H., Bockelmann W., Franz C.M. The genus Weissella: taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015;6:155. doi: 10.3389/fmicb.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T.C., Cho J.Y., Feng L.Y., Saoraya C., Park S.Y., Auh C.K., Pai T.K., Ham K.S. Mineral-rich solar sea salt generates less oxidative stress in rats than mineral-deficient salt. Food Sci. Biotechnol. 2014;23:951–956. [Google Scholar]
- Ha J.O., Park K.Y. Comparison of mineral contents and external structure of various salts. J. Korean Soc. Food Sci. Nutr. 1998;27:413–418. [Google Scholar]
- Hahn Y.S. Effect of salt type and concentration on the growth of lactic acid bacteria isolated from kimchi. Korean J. Food Sci. Technol. 2003;35:743–747. [Google Scholar]
- Han G.J., Son A.R., Lee S.M., Jung J.K., Kim S.H., Park K.Y. Improved quality and increased in vitro anticancer effect of kimchi by using natural sea salt without bittern and baked (Guwun) salt. J. Korean Soc. Food Sci. Nutr. 2009;38:996–1002. [Google Scholar]
- Hong G.H., Lee S.Y., Park E.S., Park K.Y. Changes in microbial community by salt content in kimchi during fermentation. J. Korean Soc. Food Sci. Nutr. 2021;50:648–653. [Google Scholar]
- Hong Y., Yang H.S., Chang H.C., Kim H.Y. Comparison of bacterial community changes in fermenting kimchi at two different temperatures using a denaturing gradient gel electrophoresis analysis. J. Microbiol. Biotechnol. 2013;23:76–84. doi: 10.4014/jmb.1210.10002. [DOI] [PubMed] [Google Scholar]
- Jung H.J., Hong Y., Yang H.S., Chang H.C., Kim H.Y. Distribution of lactic acid bacteria in garlic (Allium sativum) and green onion (Allium fistulosum) using SDS-PAGE whole cell protein pattern comparison and 16S rRNA gene sequence analysis. Food Sci. Biotechnol. 2012;21:1457–1462. [Google Scholar]
- Jung J.Y., Lee S.H., Lee H.K., Seo H.Y., Park W.S., Jeon C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2012;153:378–387. doi: 10.1016/j.ijfoodmicro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kim D.M., Kim K.H. Growth of lactic acid bacteria and quality characteristics of Baechu kimchi prepared with various salts and concentration. J. Korean Soc. Food Cult. 2014;29:286–297. [Google Scholar]
- Kim D.W., Kim B.M., Lee H.J., Jang G.K., Song S.H., Lee J.I., Lee S.B., Shim J.M., Lee K.W., Kim J.H., Ham K.S., Chen F., Kim H.J. Effects of different salt treatments on the fermentation metabolites and bacterial profiles of kimchi. J. Food Sci. 2017;82:1124–1131. doi: 10.1111/1750-3841.13713. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Seo S.H., Park S.E., Lim Y.W., Roh E.W., Son H.S. Initial storage of kimchi at room temperature alters its microbial and metabolite profiles. LWT-Food Sci. Technol. 2020;134 [Google Scholar]
- Kim S.H., Kim S.J., Kim B.H., Kang S.G., Jung S.T. Fermentation of doenjang prepared with sea salts. Korean J. Food Sci. Technol. 2000;32:1365–1370. [Google Scholar]
- Kim S.J., Kim H.L., Ham K.S. Characterization of kimchi fermentation prepared with various salts. Korean J. Food Preserv. 2005;12:395–401. [Google Scholar]
- Kim T.W., Lee J.Y., Song H.S., Park J.H., Ji G.E., Kim H.Y. Isolation and identification of Weissella kimchi from green onion by cell protein pattern analysis. J. Microbiol. Biotechnol. 2004;14:105–109. [Google Scholar]
- Ku K.H., Kang K.O., Kim W.J. Some quality changes during fermentation of kimchi. Korean J. Food Sci. Technol. 1988;20:476–482. [Google Scholar]
- Lee H.M., Lee W.K., Jin J.H., Kim I.C. Physicochemical properties and microbial analysis of Korean solar salt and flower of salt. J. Korean Soc. Food Sci. Nutr. 2013;42:1115–1124. [Google Scholar]
- Lee J.J., Choi Y.J., Lee M.J., Park S.J., Oh S.J., Yun Y.R., Min S.G., Seo H.Y., Park S.H., Lee M.A. Effects of combining two lactic acid bacteria as a starter culture on model kimchi fermentation. Food Res. Int. 2020;136 doi: 10.1016/j.foodres.2020.109591. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Kim G.S., Baek A.H., Hwang H.S., Kwon D.Y., Kim S.G., Lee S.Y. Isolation and characterization of kimchi starters Leuconostoc mesenteroides PBio03 and Leuconostoc mesenteroides PBio104 for manufacture of commercial kimchi. J. Microbiol. Biotechnol. 2020;30:1060–1066. doi: 10.4014/jmb.2001.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Shim J.M., Kim D.W., Yao Z., Kim J.A., Kim H.J., Kim J.H. Effects of different types of salts on the growth of lactic acid bacteria and yeasts during kimchi fermentation. Food Sci. Biotechnol. 2018;27:489–498. doi: 10.1007/s10068-017-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.A., Choi Y.J., Lee H., Hwang S., Lee H.J., Park S.J., Chung Y.B., Yun Y.R., Park S.H., Min S., Kwon L.S., Seo H.Y. Influence of salinity on the microbial community composition and metabolite profile in kimchi. Fermentation. 2021;7:308. [Google Scholar]
- Lee M.E., Jang J.Y., Lee J.H., Park H.W., Choi H.J., Kim T.W. Starter cultures for kimchi fermentation. J. Microbiol. Biotechnol. 2015;25:559–568. doi: 10.4014/jmb.1501.01019. [DOI] [PubMed] [Google Scholar]
- Li Q., Kang J., Ma Z., Li X., Liu L., Hu X. Microbial succession and metabolite changes during traditional serofluid dish fermentation. LWT-Food Sci. Technol. 2017;84:771–779. [Google Scholar]
- Mun S.Y., Chang H.C. Characterization of Weissella koreensis SK isolated from kimchi fermented at low temperature (around 0 °C) based on complete genome sequence and corresponding phenotype. Microorganisms. 2020;8:1147. doi: 10.3390/microorganisms8081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otgonbayar G.E., Eom H.J., Kim B.S., Ko J.H., Han N.S. Mannitol production by Leuconostoc citreum KACC91348P isolated from kimchi. J. Microbiol. Biotechnol. 2011;21:968–971. doi: 10.4014/jmb.1105.05034. [DOI] [PubMed] [Google Scholar]
- Park S.E., Seo S.H., Kim E.J., Byun S., Na C.S., Son H.S. Changes of microbial community and metabolite in kimchi inoculated with different microbial community starters. Food Chem. 2019;274:558–565. doi: 10.1016/j.foodchem.2018.09.032. [DOI] [PubMed] [Google Scholar]
- Raccach M. Manganese and Lactic acid bacteria. J. Food Protect. 1998;48:895–898. doi: 10.4315/0362-028X-48.10.895. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Park S.E., Kim E.J., Cho K.M., Kwon S.J., Son H.S. Effect of fungi on metabolite changes in kimchi during fermentation. Molecules. 2020;25(21):5040. doi: 10.3390/molecules25215040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Park S.E., Kim E.J., Lee K.I., Na C.S., Son H.S. A GC-MS based metabolomics approach to determine the effect of salinity on kimchi. Food Res. Int. 2018;105:492–498. doi: 10.1016/j.foodres.2017.11.069. [DOI] [PubMed] [Google Scholar]
- Song H.S., Lee S.H., Ahn S.W., Kim J.Y., Rhee J.K., Roh S.W. Effects of the main ingredients of the fermented food, kimchi, on bacterial composition and metabolite profile. Food Res. Int. 2021;149 doi: 10.1016/j.foodres.2021.110668. [DOI] [PubMed] [Google Scholar]
- Wisselink H.W., Weusthuis R.A., Eggink G., Hugenholz J., Grobben G.J. Mannitol production by lactic acid bacteria: a review. Int. Dairy J. 2002;12:151–161. [Google Scholar]
- Wold S., Esbensen K., Geladi P. Principal component analysis. Chemometr. Intell. Lab. Syst. 1987;2:37–52. [Google Scholar]
- Yang X., Hu W., Xiu Z., Jiang A., Yang X., Saren G., Ji Y., Guan Y., Feng K. Effect of salt concentration on microbial communities, physicochemical properties and metabolite profile during spontaneous fermentation of Chinese northeast sauerkraut. J. Appl. Microbiol. 2020;129:1458–1471. doi: 10.1111/jam.14786. [DOI] [PubMed] [Google Scholar]
- Yun J.Y., Jeong J.K., Moon S.H., Park K.Y. Effects of brined baechu cabbage and seasoning on fermentation of kimchi. J. Korean Soc. Food Sci. Nutr. 2014;43:1081–1087. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.